Improvement in Corrosion Resistance and Interfacial Contact Resistance Properties of 316L Stainless Steel by Coating with Cr, Ti Co-Doped Amorphous Carbon Films in the Environment of the PEMFCs
Abstract
1. Introduction
2. Results and Discussion
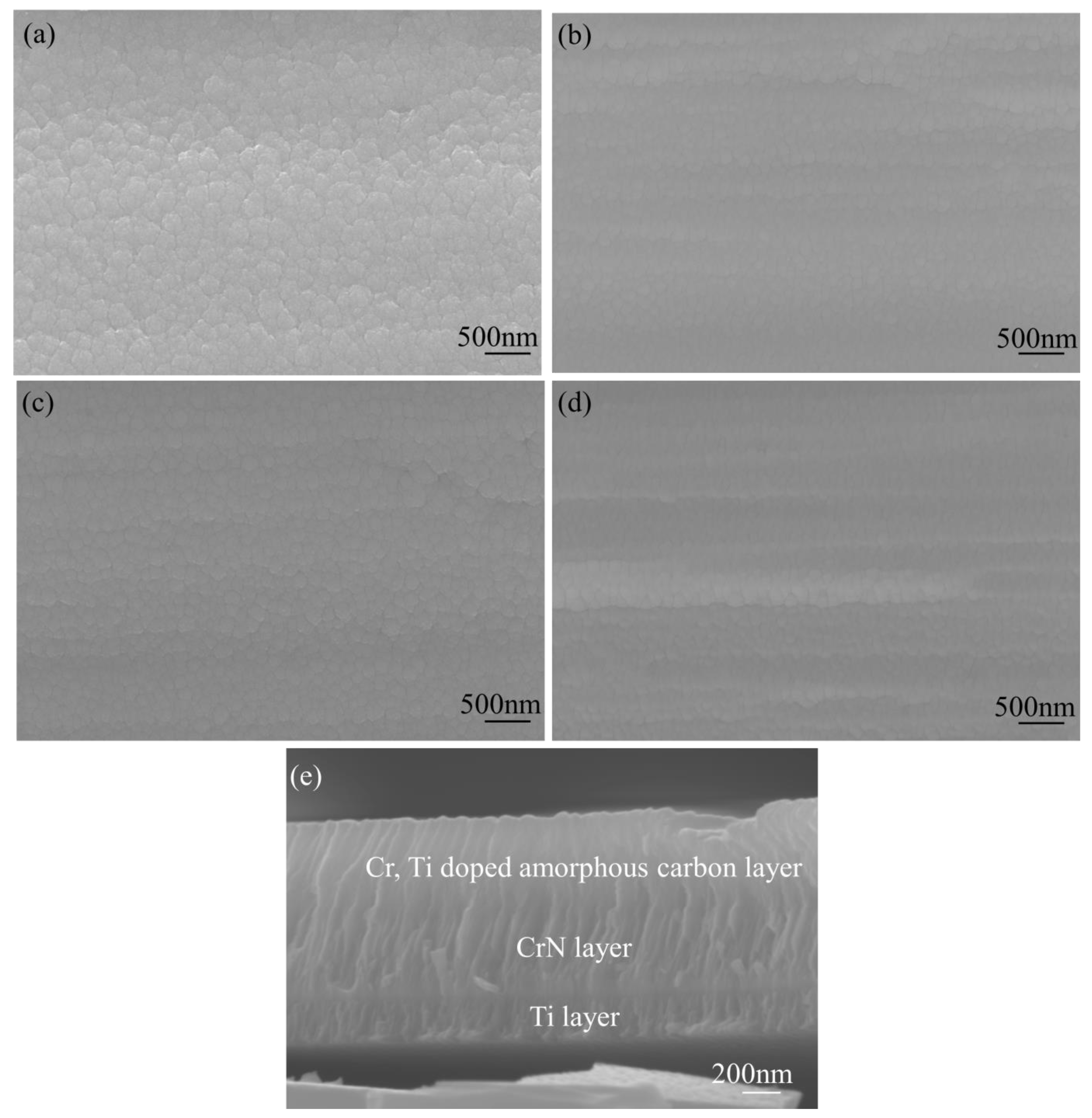
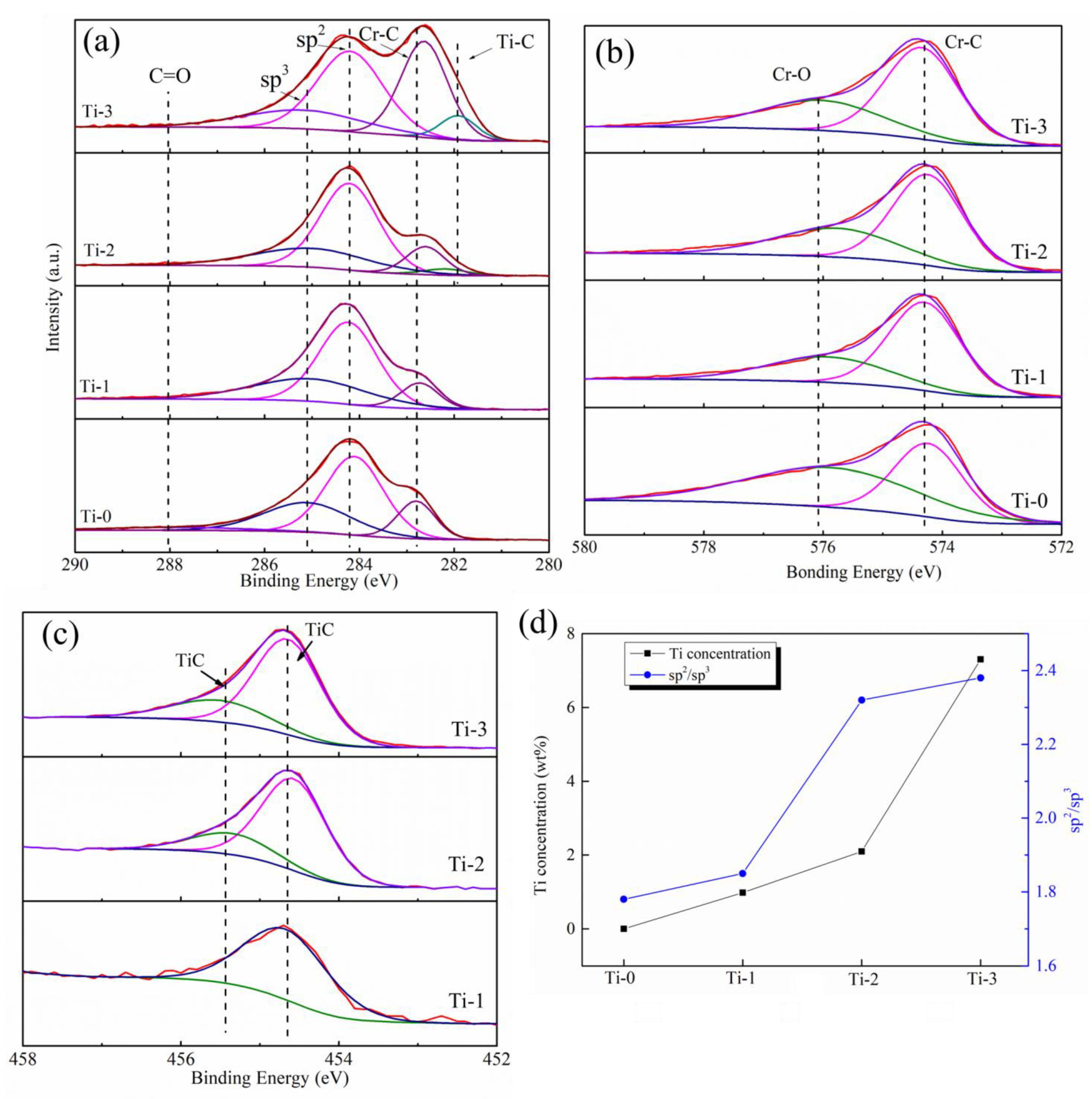
2.1. Morphology
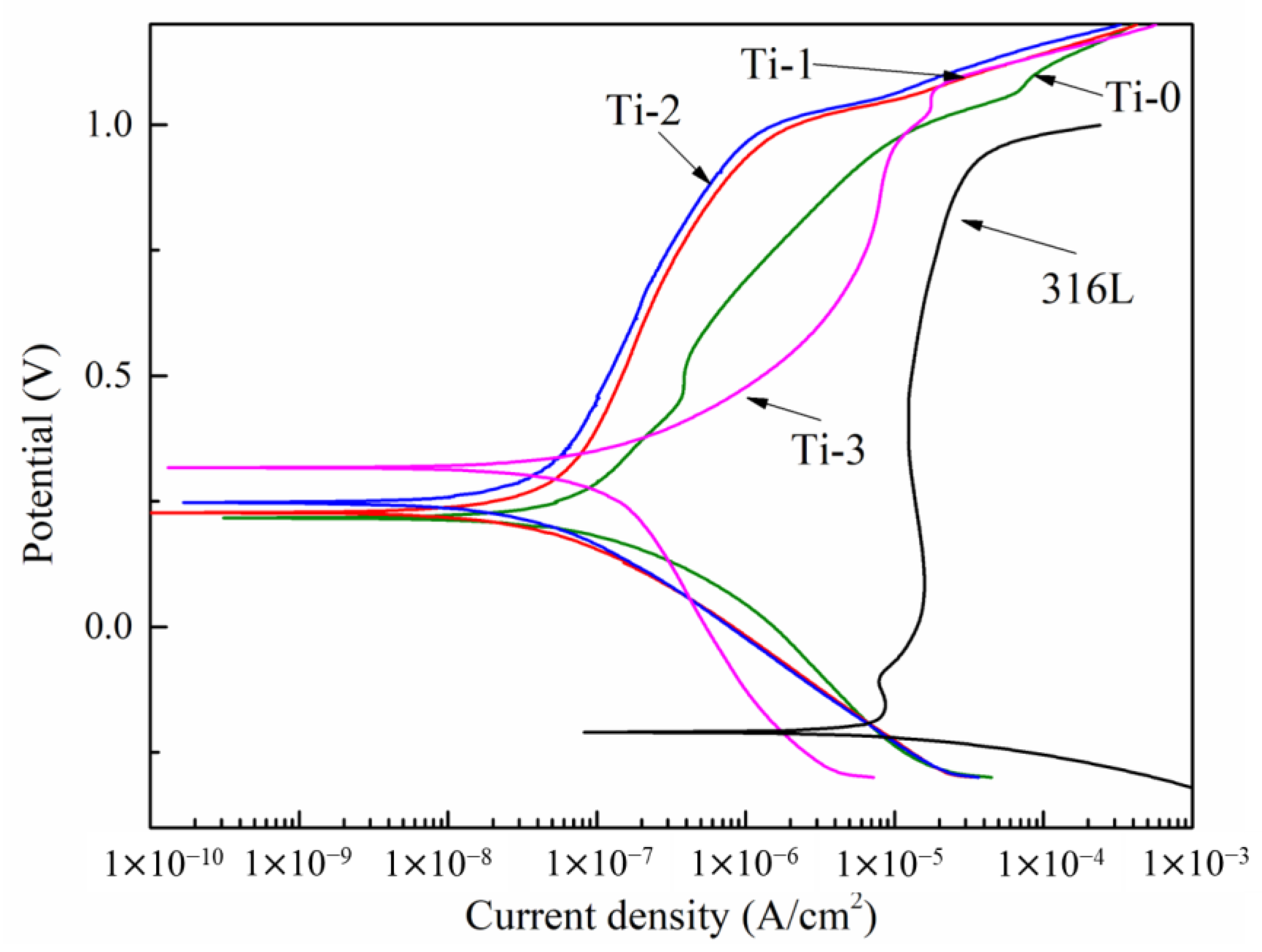
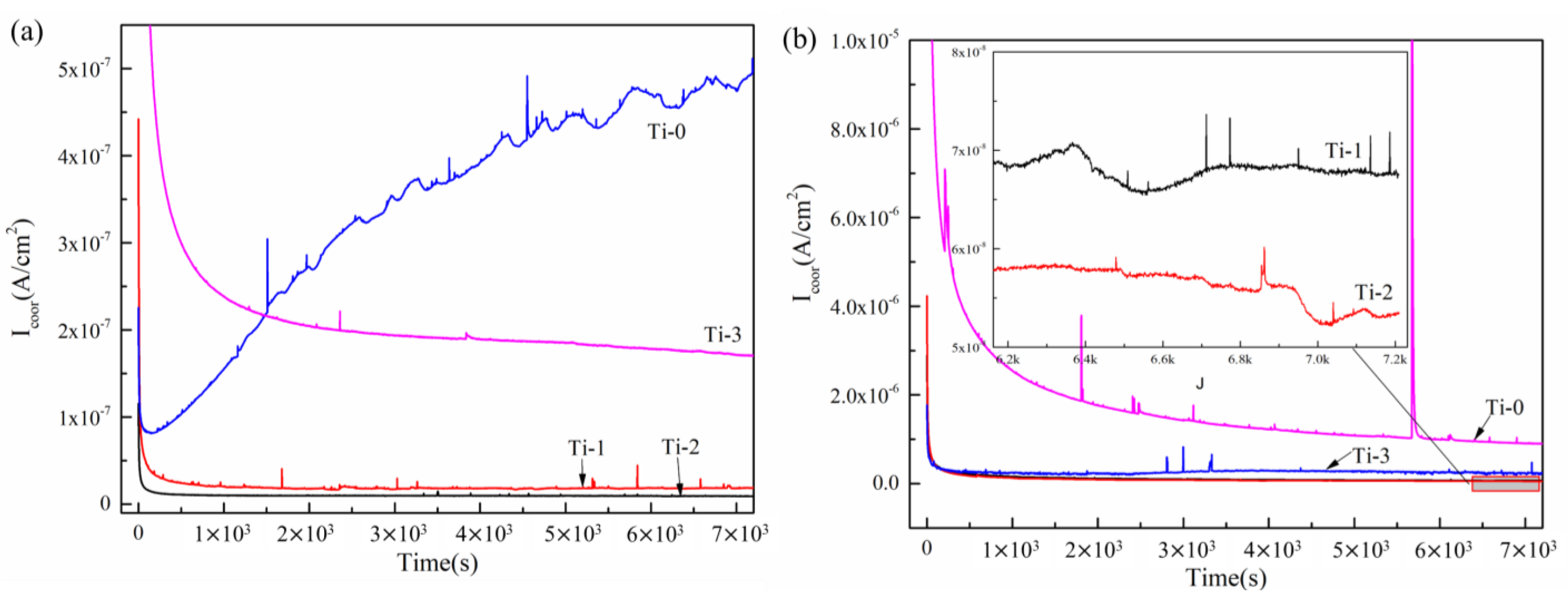
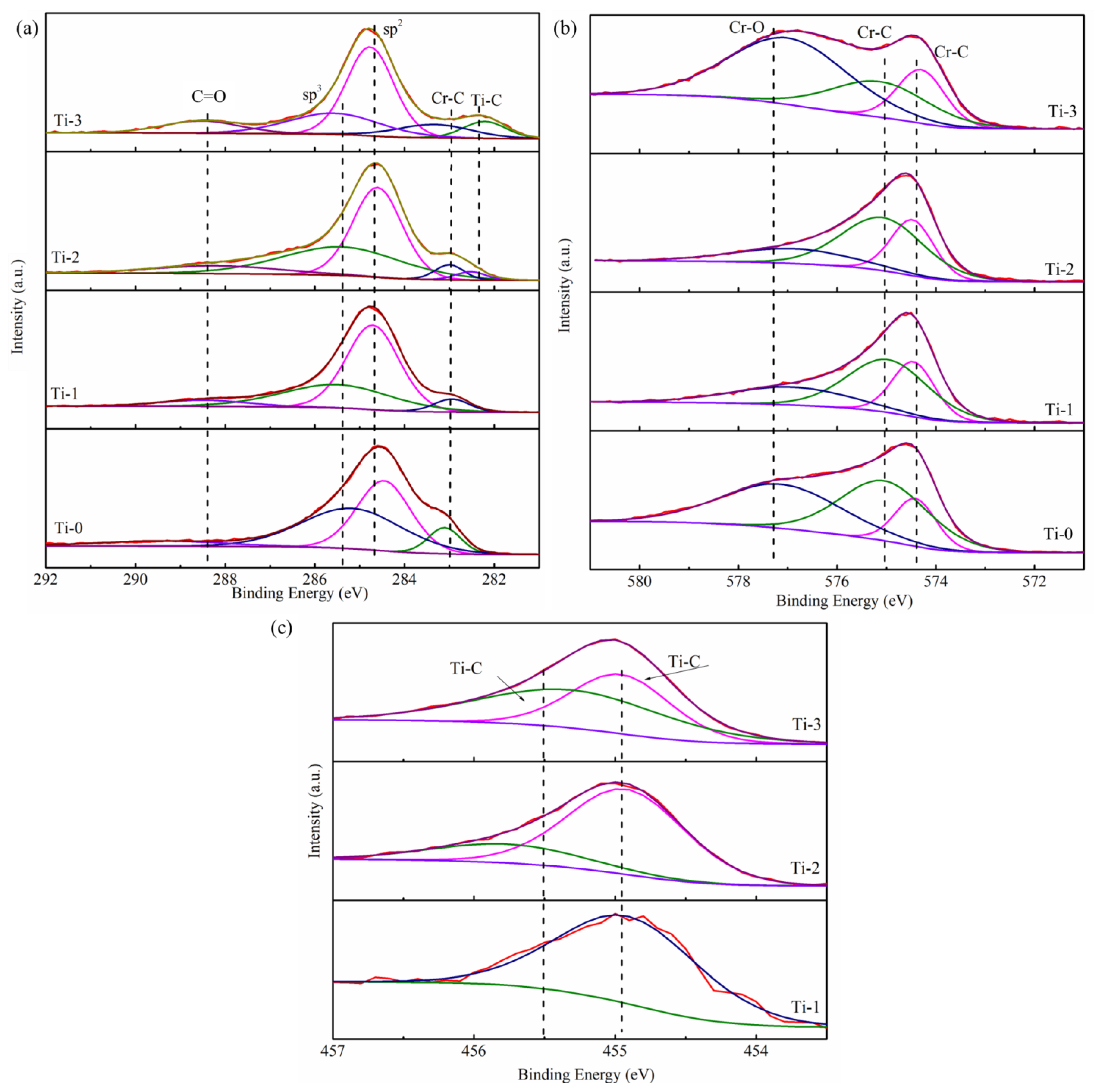
2.2. The Electrochemical Corrosion Behavior
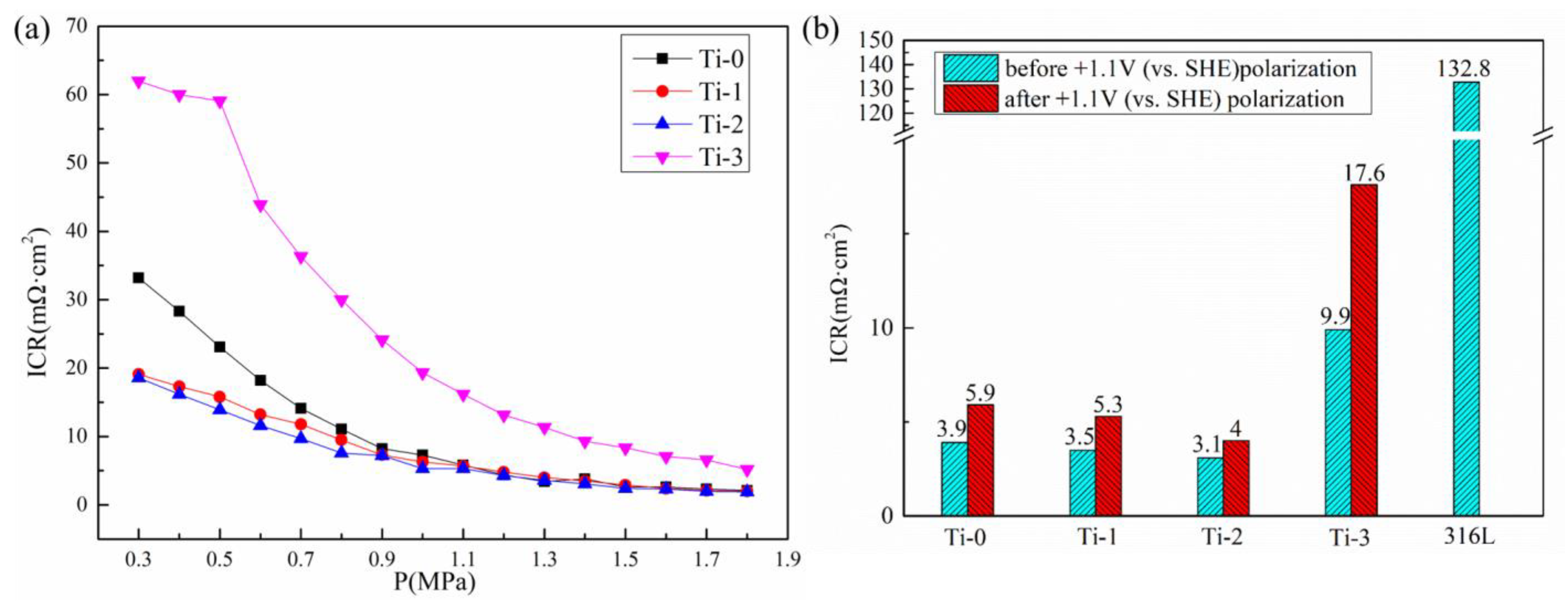
2.3. Interfacial Contact Resistance (ICR)
3. Experimental Section
3.1. Sample Preparation
3.2. Samples Characterization
3.3. Electrochemical Corrosion
3.4. Interfacial Contact Resistance (ICR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jin, J.; Zhang, J.; Hu, M.; Li, X. Investigation of high potential corrosion protection with titanium carbonitride coating on 316L stainless steel bipolar plates. Corros. Sci. 2021, 191, 109757. [Google Scholar] [CrossRef]
- Yang, L.X.; Liu, R.J.; Wang, Y.; Liu, H.J.; Zeng, C.L.; Fu, C. Growth of nanocrystalline β-Nb2N coating on 430 ferritic stainless steel bipolar plates of PEMFCs by disproportionation reaction of Nb(IV) ions in molten salt. Corros. Sci. 2020, 174, 108862. [Google Scholar] [CrossRef]
- Tang, A.; Crisci, L.; Bonville, L.; Jankovic, J. An overview of bipolar plates in proton exchange membrane fuel cells. J. Renew. Sustain. Energy 2021, 13, 022701. [Google Scholar] [CrossRef]
- Forouzanmehr, M.; Kashyzadeh, K.R.; Borjali, A.; Ivanov, A.; Jafarnode, M.; Gan, T.H.; Wang, B.; Chizari, M. Detection and Analysis of Corrosion and Contact Resistance Faults of TiN and CrN Coatings on 410 Stainless Steel as Bipolar Plates in PEM Fuel Cells. Sensors 2022, 22, 750. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, X.-W.; Liu, S.; Wang, X.-Z.; Fu, X.-Z.; Luo, J.-L. High corrosion resistance of reduced graphene oxide coated 316L stainless steel bipolar plate for proton exchange membrane fuel cell prepared by a facile method. Mater. Chem. Phys. 2022, 290, 126663. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L. Characterization and properties of NbN–Nb bilayer formed on titanium for bipolar plates. Mater. Chem. Phys. 2022, 290, 126628. [Google Scholar] [CrossRef]
- Wu, S.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Yang, W.; Zhan, J. Low-cost graphite coated copper as bipolar plates of proton exchange membrane fuel cells for corrosion protection. Fuel Cells 2021, 21, 502–511. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Tao, Y.; Cao, R.; Kou, X.; Tian, X. Investigation of corrosion properties with Ni–P/TiNO coating on aluminum alloy bipolar plates in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2022, 47, 22165–22179. [Google Scholar] [CrossRef]
- Yan, W.; Zhao, Y.; Zhang, Y.; Li, Q.; Chen, S.; Yuan, H.; Zhang, Z.; Chen, L.; Luo, J.; Pang, P.; et al. Corrosion-resistant and interfacial conductive AlTiVCrMo high-entropy alloy and (AlTiVCrMo)Nx high-entropy ceramics coatings for surface modification of bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2022, 527, 231217. [Google Scholar] [CrossRef]
- Chanda, U.K.; Behera, A.; Roy, S.; Pati, S. Evaluation of Ni-Cr-P coatings electrodeposited on low carbon steel bipolar plates for polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2018, 43, 23430–23440. [Google Scholar] [CrossRef]
- Chen, M.; Ding, J.C.; Kwon, S.-H.; Wang, Q.; Zhang, S. Corrosion resistance and conductivity of NbN-coated 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2022, 196, 110042. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Zhang, B.; Lv, J.; Zhang, J. Nano-Cr2N dominated films with high conductivity and strong corrosion resistance for Ti bipolar plates. Mater. Des. 2022, 224, 111305. [Google Scholar] [CrossRef]
- Yu, F.; Wang, K.; Cui, L.; Wang, S.; Hou, M.; Xiong, F.; Zou, R.; Gao, P.; Peng, H.; Liu, Z. Vertical-Graphene-Reinforced Titanium Alloy Bipolar Plates in Fuel Cells. Adv Mater. 2022, 34, e2110565. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Li, S.; Wen, Z.; Ji, S. Surface diffusion modification AISI 304SS stainless steel as bipolar plate material for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 1140–1144. [Google Scholar] [CrossRef]
- Xu, M.; Kang, S.; Lu, J.; Yan, X.; Chen, T.; Wang, Z. Properties of a Plasma-Nitrided Coating and a CrNx Coating on the Stainless Steel Bipolar Plate of PEMFC. Coatings 2020, 10, 183. [Google Scholar] [CrossRef]
- Shu, R.; Paschalidou, E.-M.; Rao, S.G.; Lu, J.; Greczynski, G.; Lewin, E.; Nyholm, L.; le Febvrier, A.; Eklund, P. Microstructure and mechanical, electrical, and electrochemical properties of sputter-deposited multicomponent (TiNbZrTa)Nx coatings. Surf. Coat. Technol. 2020, 389, 125651. [Google Scholar] [CrossRef]
- Peng, S.; Xu, J.; Li, Z.; Jiang, S.; Munroe, P.; Xie, Z.-H.; Lu, H. A reactive-sputter-deposited TiSiN nanocomposite coating for the protection of metallic bipolar plates in proton exchange membrane fuel cells. Ceram. Int. 2020, 46, 2743–2757. [Google Scholar] [CrossRef]
- Taherian, R.; Mohammadi, M.; Samiei, Z. Investigation of graphite conductive adhesive coated on SS316L used for bipolar plates of proton-exchange membrane fuel cell. J. Adhes. Sci. Technol. 2021, 36, 1094–1111. [Google Scholar] [CrossRef]
- Alaefour, I.; Shahgaldi, S.; Zhao, J.; Li, X. Synthesis and Ex-Situ characterizations of diamond-like carbon coatings for metallic bipolar plates in PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 11059–11070. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Yu, S.; Liu, Y.; Shi, B.; Hu, E.; Hei, H. Investigation of hydrophobic and anti-corrosive behavior of Cr-DLC film on stainless steel bipolar plate. Diam. Relat. Mater. 2022, 129, 109352. [Google Scholar] [CrossRef]
- Wongpanya, P.; Wongpinij, T.; Photongkam, P.; Siritapetawee, J. Improvement in corrosion resistance of 316L stainless steel in simulated body fluid mixed with antiplatelet drugs by coating with Ti-doped DLC films for application in biomaterials. Corros. Sci. 2022, 208, 110611. [Google Scholar] [CrossRef]
- Wongpanya, P.; Pintitraratibodee, N.; Thumanu, K.; Euaruksakul, C. Improvement of corrosion resistance and biocompatibility of 316L stainless steel for joint replacement application by Ti-doped and Ti-interlayered DLC films. Surf. Coat. Technol. 2021, 425, 127734. [Google Scholar] [CrossRef]
- Meng, W.; Zhu, H.; Wang, X.; Li, G.; Fan, Y.; Sun, D.; Kong, F. Electrochemical Behavior and Surface Conductivity of C/TiC Nanocomposite Coating on Titanium for PEMFC Bipolar Plate. Metals 2022, 12, 771. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, B.; Shao, W.; Shi, J. Majorization of GLC properties by the introduction of silver nanowires as conductive framework for metal bipolar plates. Appl. Surf. Sci. 2020, 533, 147493. [Google Scholar] [CrossRef]
- Jia, Q.; Mu, Z.; Zhang, X.; Zhang, B.; Liu, R.; Gao, K.; Yu, Y.; Lai, Z.; Zhang, J. Electronic conductive and corrosion mechanisms of dual nanostructure CuCr-doped hydrogenated carbon films for SS316L bipolar plates. Mater. Today Chem. 2021, 21, 100521. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.-T.; Li, Z.-X.; Wang, Y.-F.; Li, H.-Z.; Lei, J.-J. Corrosion and conductivity behavior of titanium-doped amorphous carbon film coated SS316L in the environment of PEMFCs. Mater. Chem. Phys. 2022, 276, 125234. [Google Scholar] [CrossRef]
- Hou, K.; Yi, P.; Li, X.; Peng, L.; Lai, X. The effect of Cr doped in amorphous carbon films on electrical conductivity: Characterization and mechanism. Int. J. Hydrogen Energy 2021, 46, 30841–30852. [Google Scholar] [CrossRef]
- Zhang, D.; Yi, P.; Peng, L.; Lai, X.; Pu, J. Amorphous carbon films doped with silver and chromium to achieve ultra-low interfacial electrical resistance and long-term durability in the application of proton exchange membrane fuel cells. Carbon 2019, 145, 333–344. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R. Deposition and characterization of Ti- and W-containing diamond-like carbon films by plasma source ion implantation. Surf. Coat. Technol. 2003, 169–170, 287–290. [Google Scholar] [CrossRef]
- Schroeder, G.F.A.; Bruinink, A.; Hauert, R.; Mayer, J.; Wintermantel, E. Titanium containing amorphous hydrogenated carbon films (a-C: H/Ti): Surface analysis and evaluation of cellular reactions using bone marrow cell cultures in vitro. Biomaterials 2001, 21, 449–456. [Google Scholar] [CrossRef]
- Lewin, E.; Wilhelmsson, O.; Jansson, U. Nanocomposite nc-TiC/a-C thin films for electrical contact applications. J. Appl. Phys. 2006, 100, 054303. [Google Scholar] [CrossRef]
- Wang, C.B.; Fu, Z.Q.; Yue, W.; Peng, Z.J.; Yu, X.; Lin, S.S.; Dai, M.J. Influence of Target Current on the Structure of Ti-Doped DLC Films. Adv. Mater. Res. 2010, 105–106, 451–454. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, P.; Sun, L.; Liu, L.; Xu, X.; Li, W.; Li, X.; Lee, K.-R.; Wang, A. Microstructure and property evolution of diamond-like carbon films co-doped by Al and Ti with different ratios. Surf. Coat. Technol. 2019, 361, 83–90. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A. Deposition and properties of Al-containing diamond-like carbon films by a hybrid ion beam sources. J. Alloy. Compd. 2011, 509, 4626–4631. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Wang, Y.; Li, H.; Li, Z. Evaluation of vacuum heat-treated α-C films for surface protection of metal bipolar plates used in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 22983–22997. [Google Scholar] [CrossRef]
- Ferrari, A.C. Determination of bonding in diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2002, 11, 1053–1061. [Google Scholar] [CrossRef]
- Lee, W.-J.; Yun, E.-Y.; Lee, H.-B.-R.; Hong, S.W.; Kwon, S.-H. Ultrathin effective TiN protective films prepared by plasma-enhanced atomic layer deposition for high performance metallic bipolar plates of polymer electrolyte membrane fuel cells. Appl. Surf. Sci. 2020, 519, 146215. [Google Scholar] [CrossRef]
- Mi, B.; Wang, H.; Wang, Q.; Cai, J.; Qin, Z.; Chen, Z. Corrosion resistance and contact resistance properties of Cr-doped amorphous carbon films deposited under different carbon target current on the 316L stainless steel bipolar plate for PEMFC. Vacuum 2022, 203, 111263. [Google Scholar] [CrossRef]
- Wang, H.; Turner, J.A. Ferritic stainless steels as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2004, 128, 193–200. [Google Scholar] [CrossRef]



| Samples | Steel | Ti-0 | Ti-1 | Ti-2 | Ti-3 |
|---|---|---|---|---|---|
| Icorr (μA/cm2) | 69.8 | 0.076 | 0.066 | 0.057 | 0.152 |
| Ecorr (mV vs. SCE) | −216 | 203 | 227 | 247 | 317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, B.; Wang, Q.; Xu, Y.; Qin, Z.; Chen, Z.; Wang, H. Improvement in Corrosion Resistance and Interfacial Contact Resistance Properties of 316L Stainless Steel by Coating with Cr, Ti Co-Doped Amorphous Carbon Films in the Environment of the PEMFCs. Molecules 2023, 28, 2821. https://doi.org/10.3390/molecules28062821
Mi B, Wang Q, Xu Y, Qin Z, Chen Z, Wang H. Improvement in Corrosion Resistance and Interfacial Contact Resistance Properties of 316L Stainless Steel by Coating with Cr, Ti Co-Doped Amorphous Carbon Films in the Environment of the PEMFCs. Molecules. 2023; 28(6):2821. https://doi.org/10.3390/molecules28062821
Chicago/Turabian StyleMi, Baosen, Quan Wang, Yuhao Xu, Ziwei Qin, Zhuo Chen, and Hongbin Wang. 2023. "Improvement in Corrosion Resistance and Interfacial Contact Resistance Properties of 316L Stainless Steel by Coating with Cr, Ti Co-Doped Amorphous Carbon Films in the Environment of the PEMFCs" Molecules 28, no. 6: 2821. https://doi.org/10.3390/molecules28062821
APA StyleMi, B., Wang, Q., Xu, Y., Qin, Z., Chen, Z., & Wang, H. (2023). Improvement in Corrosion Resistance and Interfacial Contact Resistance Properties of 316L Stainless Steel by Coating with Cr, Ti Co-Doped Amorphous Carbon Films in the Environment of the PEMFCs. Molecules, 28(6), 2821. https://doi.org/10.3390/molecules28062821






