Ethanol Extract of Rosa laevigata Michx. Fruit Inhibits Inflammatory Responses through NF-κB/MAPK Signaling Pathways via AMPK Activation in RAW 264.7 Macrophages
Abstract
1. Introduction
2. Results
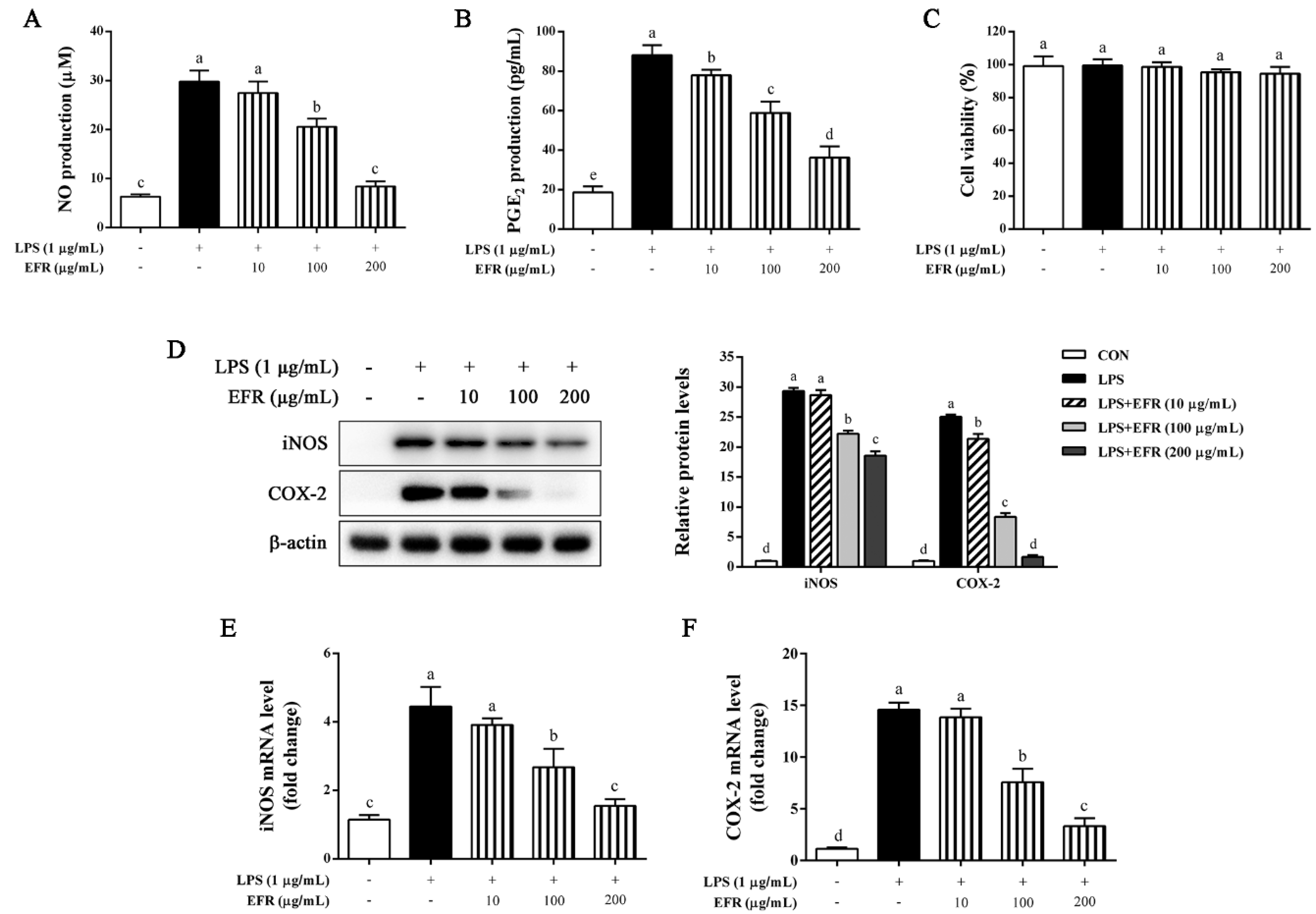
2.1. EFR Inhibits LPS-Induced Production of NO and PGE2 via the Suppression of iNOS and COX-2 Expression
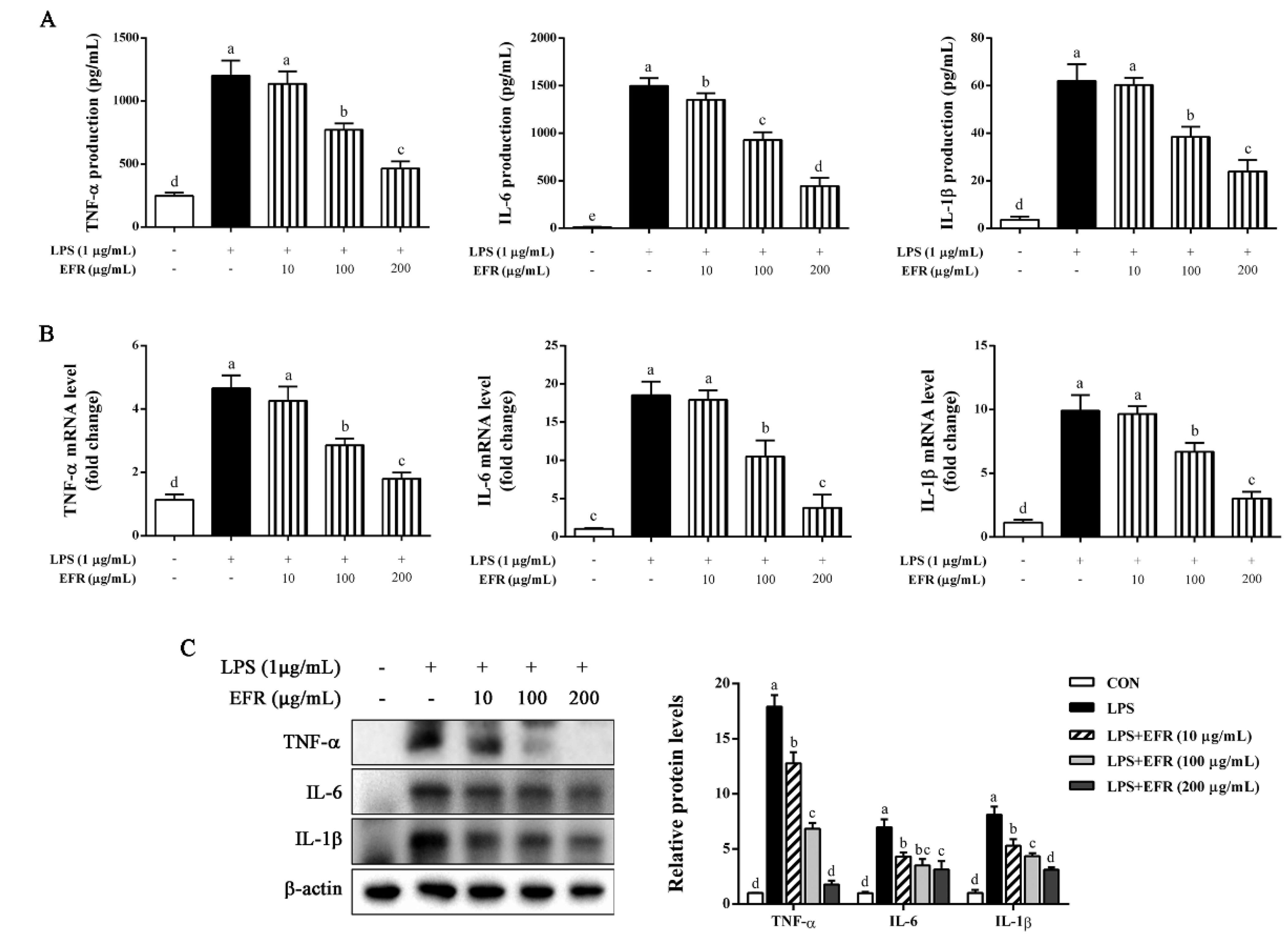
2.2. EFR Suppresses LPS-Induced Production of Inflammatory Cytokines
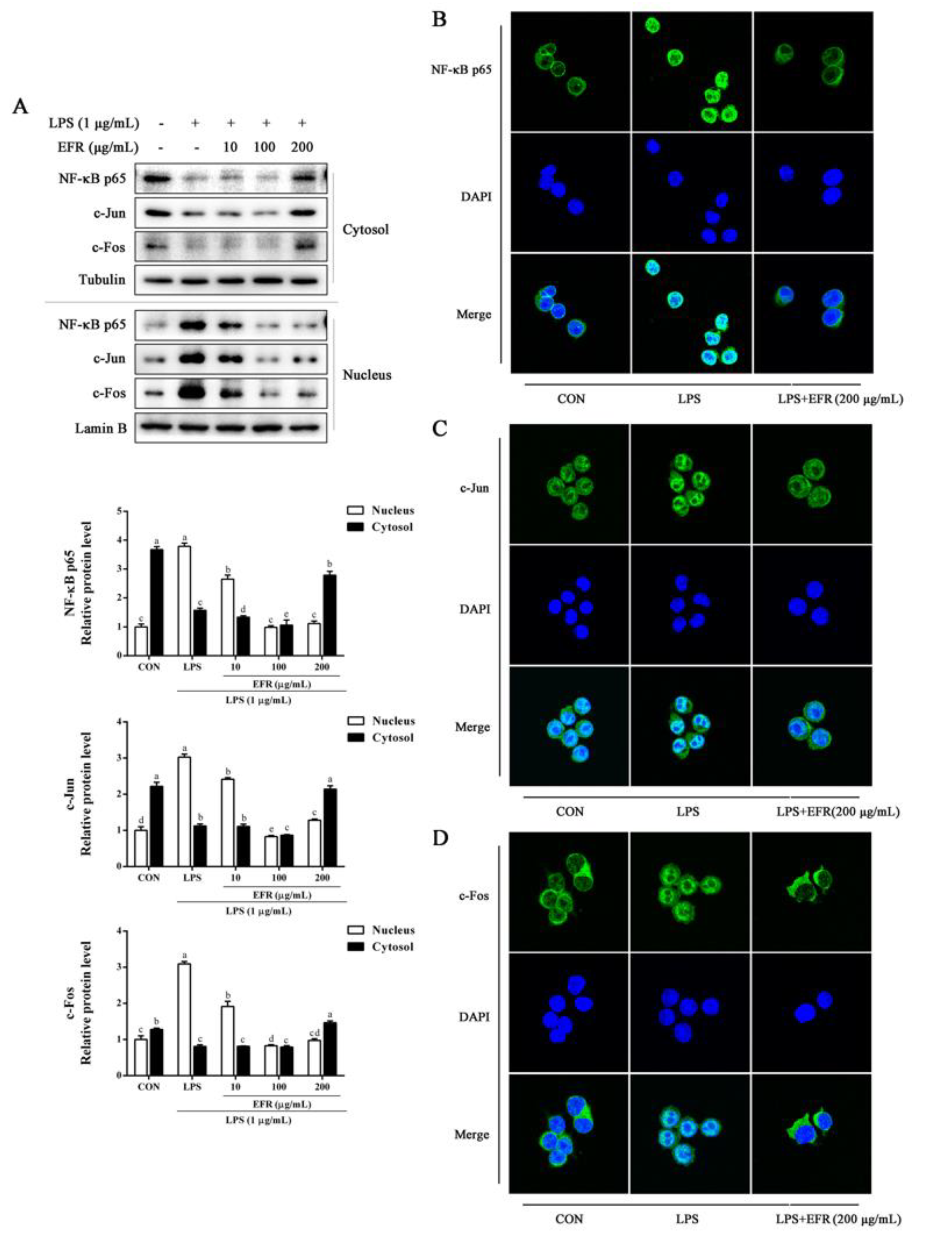
2.3. EFR Inhibits LPS-Induced Nuclear Translocation of NF-κB p65 and AP-1
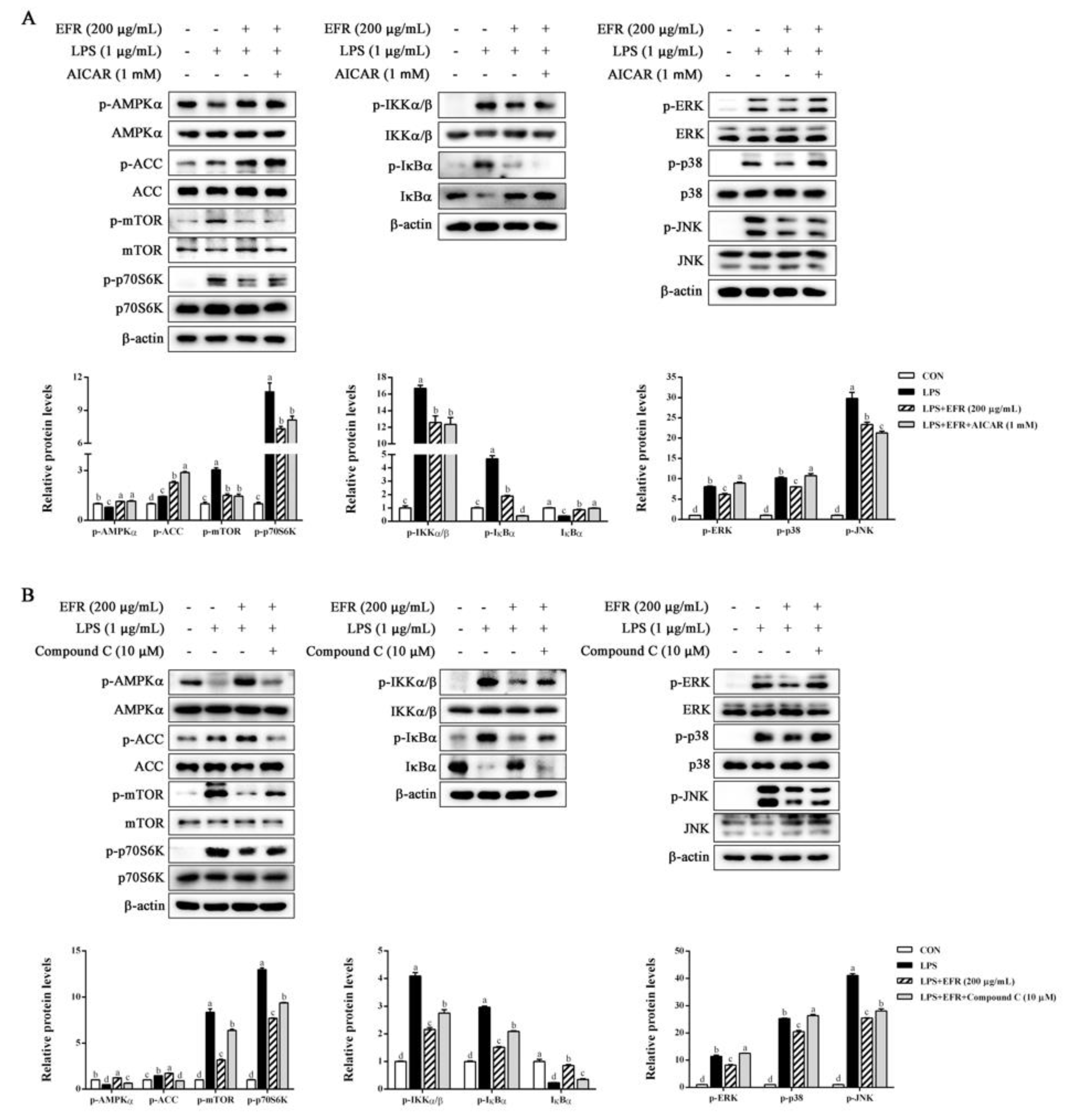
2.4. EFR Inhibits LPS-Induced Activation of NF-κB/MAPK Signaling Pathways
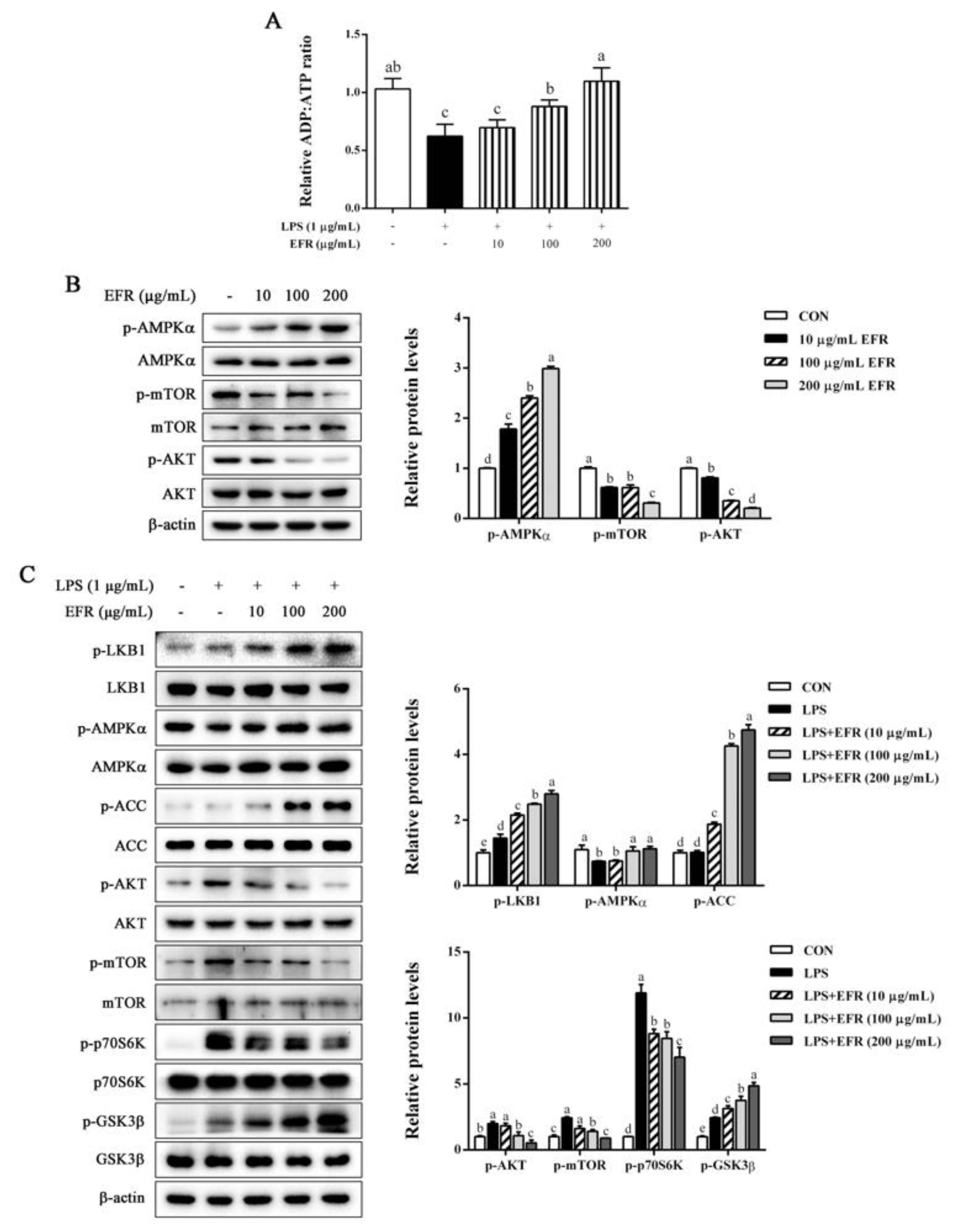
2.5. EFR Activates AMPK by up-Regulating the ADP:ATP Ratio
2.6. AMPK Activation Is Necessary for EFR-Mediated Suppression of NF-κB/MAPK Signaling Pathways
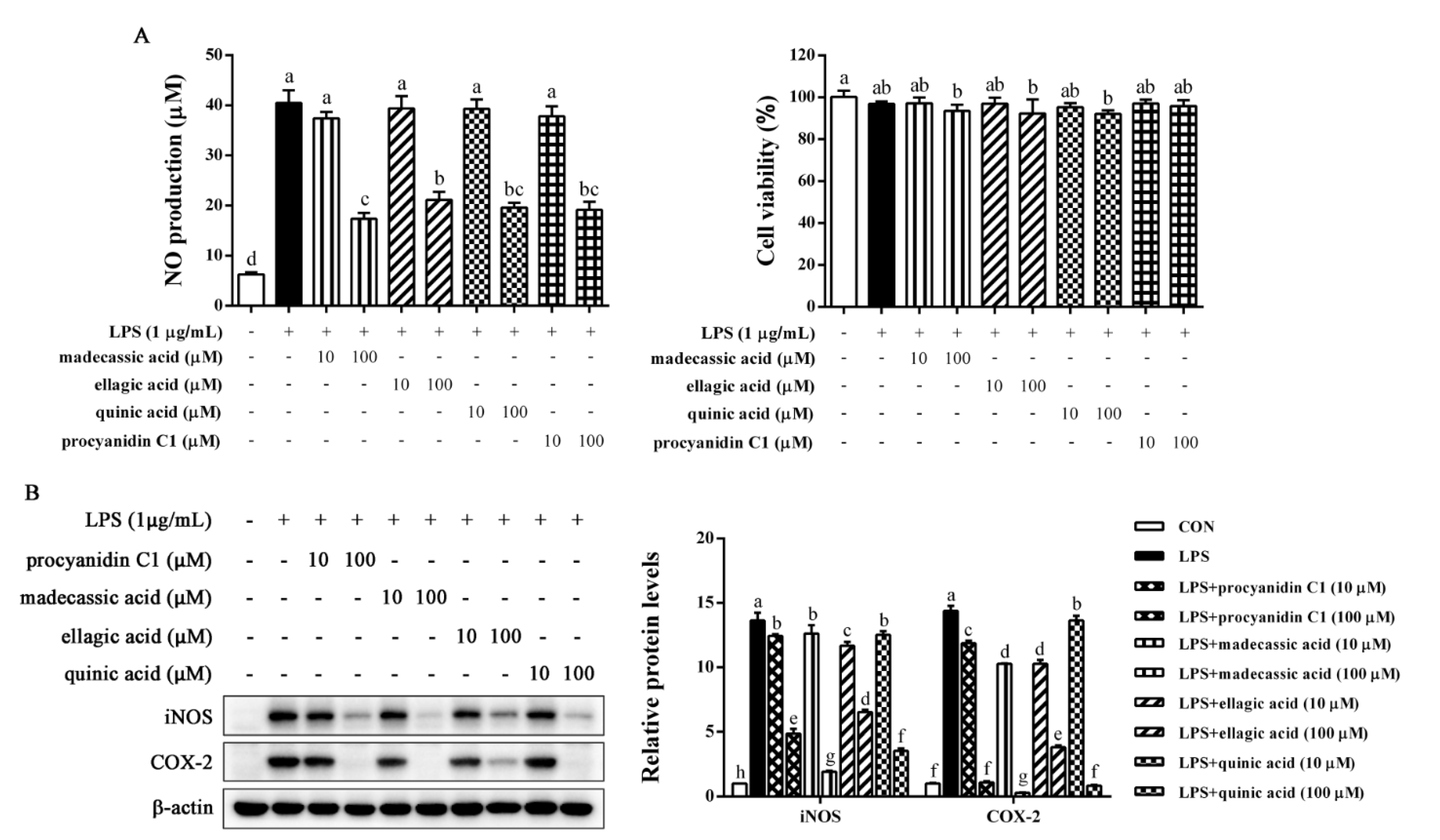
2.7. Identification of Anti-Inflammatory Compounds in EFR
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction Preparation
4.2. Chemicals and Reagents
4.3. Cell Culture
4.4. MTT Assay
4.5. Griess Assay
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Measurement of Cellular ADP: ATP Ratio
4.8. Preparation of Nuclear and Cytosolic Extracts
4.9. Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR Analysis
4.10. Immunofluorescence Assay
4.11. Western Blot Analysis
4.12. LC–MS Detection
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Baker, B.; Maitra, U.; Geng, S.; Li, L. Molecular and cellular mechanisms responsible for cellular stress and low-grade inflammation induced by a super-low dose of endotoxin. J. Biol. Chem. 2014, 289, 16262–16269. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, J.H.; Jeong, D.; Hong, Y.H.; Park, S.H.; Yang, Y.; Jang, Y.J.; Kim, J.H.; Cho, J.Y. 3-Deazaadenosine, an S-adenosylhomocysteine hydrolase inhibitor, attenuates lipopolysaccharide-induced inflammatory responses via inhibition of AP-1 and NF-kappaB signaling. Biochem. Pharmacol. 2020, 182, 114264. [Google Scholar] [CrossRef]
- Guo, T.; Lin, Q.; Li, X.; Nie, Y.; Wang, L.; Shi, L.; Xu, W.; Hu, T.; Guo, T.; Luo, F. Octacosanol Attenuates Inflammation in Both RAW264.7 Macrophages and a Mouse Model of Colitis. J. Agric. Food Chem. 2017, 65, 3647–3658. [Google Scholar] [CrossRef]
- Huang, S.H.; Lee, C.H.; Wang, H.M.; Chang, Y.W.; Lin, C.Y.; Chen, C.Y.; Chen, Y.H. 6-Dehydrogingerdione restrains lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages. J. Agric. Food Chem. 2014, 62, 9171–9179. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Chen, Z.; Hagler, J.; Palombella, V.J.; Melandri, F.; Scherer, D.; Ballard, D.; Maniatis, T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995, 9, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Plaisance, S.; Boone, E.; De Bosscher, K.; Schmitz, M.L.; Fiers, W.; Haegeman, G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998, 273, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- von Knethen, A.; Callsen, D.; Brune, B. NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol. Biol. Cell 1999, 10, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.; Gao, Y.; Cai, N.; Jiang, Z.; Xu, X. Anti-inflammatory activity of guluronate oligosaccharides obtained by oxidative degradation from alginate in lipopolysaccharide-activated murine macrophage RAW 264.7 cells. J. Agric. Food Chem. 2015, 63, 160–168. [Google Scholar] [CrossRef]
- Kim, N.; Lertnimitphun, P.; Jiang, Y.; Tan, H.; Zhou, H.; Lu, Y.; Xu, H. Andrographolide inhibits inflammatory responses in LPS-stimulated macrophages and murine acute colitis through activating AMPK. Biochem. Pharmacol. 2019, 170, 113646. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Kim, J.I.; Kim, H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, L.; Dong, D.; Xu, L.; Yin, L.; Qi, Y.; Han, X.; Lin, Y.; Liu, K.; Peng, J. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013, 141, 2108–2116. [Google Scholar] [CrossRef]
- Jia, Y.; Ji, L.; Zhang, S.; Xu, L.; Yin, L.; Li, L.; Zhao, Y.; Peng, J. Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 2012, 50, 3133–3141. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, B.; Han, X.; Xu, L.; Qi, Y.; Yin, L.; Xu, Y.; Zhao, Y.; Liu, K.; Peng, J. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 55, 60–69. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Qi, Y.; Peng, J. Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation. Molecules 2016, 21, 952. [Google Scholar] [CrossRef]
- Tao, X.; Sun, X.; Xu, L.; Yin, L.; Han, X.; Qi, Y.; Xu, Y.; Zhao, Y.; Wang, C.; Peng, J. Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats. Nutrients 2016, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Gao, C.Y.; Guo, C.; Zhou, M.M.; Luo, J.; Kong, L.Y. The Anti-inflammatory Activities of Two Major Withanolides from Physalis minima Via Acting on NF-kappaB, STAT3, and HO-1 in LPS-Stimulated RAW264.7 Cells. Inflammation 2017, 40, 401–413. [Google Scholar] [CrossRef]
- Gao, H.; Kang, N.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 2020, 69, 153197. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Dong, D.; Xu, L.; Han, X.; Qi, Y.; Xu, Y.; Yin, L.; Liu, K.; Peng, J. Effects of the total saponins from Rosa laevigata Michx fruit against acetaminophen-induced liver damage in mice via induction of autophagy and suppression of inflammation and apoptosis. Molecules 2014, 19, 7189–7206. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, S.; Yin, L.; Tang, X.; Xu, Y.; Han, X.; Qi, Y.; Peng, J. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 62, 120–130. [Google Scholar] [CrossRef]
- Billack, B. Macrophage activation: Role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hazzalin, C.A.; Mahadevan, L.C. MAPK-regulated transcription: A continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 2002, 3, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Kemp, B.E.; Stapleton, D.; Campbell, D.J.; Chen, Z.P.; Murthy, S.; Walter, M.; Gupta, A.; Adams, J.J.; Katsis, F.; van Denderen, B.; et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003, 31, 162–168. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1188. [Google Scholar] [CrossRef]
- Chang, M.Y.; Ho, F.M.; Wang, J.S.; Kang, H.C.; Chang, Y.; Ye, Z.X.; Lin, W.W. AICAR induces cyclooxygenase-2 expression through AMP-activated protein kinase-transforming growth factor-beta-activated kinase 1-p38 mitogen-activated protein kinase signaling pathway. Biochem. Pharmacol. 2010, 80, 1210–1220. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Liu, C.; Zhang, X.; Zhao, Z.; Xu, J.; Zhang, X.; Zhou, K.; Gao, P.; Li, D. Selenium-containing polysaccharides isolated from Rosa laevigata Michx fruits exhibit excellent anti-oxidant and neuroprotective activity in vitro. Int. J. Biol. Macromol. 2022, 209, 1222–1233. [Google Scholar] [CrossRef]
- Gao, P.; Han, T.; Jin, M.; Li, D.; Jiang, F.; Zhang, L.; Liu, X. Extraction and isolation of polyhydroxy triterpenoids from Rosa laevigata Michx. fruit with anti-acetylcholinesterase and neuroprotection properties. RSC Adv. 2018, 8, 38131–38139. [Google Scholar] [CrossRef]
- Won, J.H.; Shin, J.S.; Park, H.J.; Jung, H.J.; Koh, D.J.; Jo, B.G.; Lee, J.Y.; Yun, K.; Lee, K.T. Anti-inflammatory effects of madecassic acid via the suppression of NF-kappaB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med. 2010, 76, 251–257. [Google Scholar] [CrossRef]
- Gil, T.Y.; Hong, C.H.; An, H.J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef]
- Jang, S.A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-alpha-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef]
- Byun, E.B.; Sung, N.Y.; Byun, E.H.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Byun, M.W.; et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-kappaB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2013, 15, 450–456. [Google Scholar] [CrossRef]

| Compounds | Formula | m/z | Retention Time (min) | Ion Mode |
|---|---|---|---|---|
| Myrianthic acid | C30H48O6 | 469.3293 | 6.4002 | pos |
| Glabrolide | C30H44O4 | 469.3313 | 4.7757 | pos |
| Madecassic acid | C30H48O6 | 503.3342 | 5.9403 | neg |
| 16b-16-Hydroxy-3-oxo-1,12-oleanadien-28-oic acid | C30H44O4 | 469.3311 | 4.1621 | pos |
| Ganolucidic acid B | C30H46O6 | 503.3361 | 6.2071 | pos |
| N6-(l-1,3-Dicarboxypropyl)-l-lysine | C11H20N2O6 | 276.1455 | 1.5622 | pos |
| PC(18:1(9E)/0:0)[U] | C26H52NO7P | 522.3557 | 8.3217 | pos |
| 1,2-Dimethoxy-13-methyl-[1,3]benzodioxolo [5,6-c]phenanthridine | C21H17NO4 | 348.1243 | 4.6405 | pos |
| Ellagic acid | C14H6O8 | 301.0002 | 3.4479 | neg |
| Ile Ser Arg Lys | C21H42N8O6 | 501.3214 | 6.0690 | neg |
| Tomentosolic acid | C30H46O3 | 437.3411 | 8.2216 | pos |
| PC(16:0/0:0)[U]/PC(16:0/0:0)[rac] | C24H50NO7P | 496.3406 | 8.0506 | pos |
| 4,4′-Methylenebis (2,6-di-tert-butylphenol) | C29H44O2 | 425.3429 | 5.0260 | pos |
| Cyanidin 3-O-rutinoside | C27H31O15 | 1189.2998 | 2.2124 | neg |
| Quinic acid | C7H12O6 | 191.0577 | 0.7015 | neg |
| Procyanidin C1 | C45H38O18 | 865.1970 | 2.6351 | neg |
| 5-Acetylamino-6-formylamino-3-methyluracil | C8H10N4O4 | 451.1260 | 2.4086 | neg |
| (−)-Catechin | C15H14O6 | 289.0696 | 2.7075 | neg |
| 1-Oleoyl-sn-glycero-3-phosphocholine | C26H52NO7P | 566.3457 | 8.3592 | neg |
| Neriifolin | C30H46O8 | 533.3122 | 5.2195 | neg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Lin, T.; Chen, Y.; Chen, F.; Zhang, S.; Pang, H.; Huang, L.; Yu, C.; Wang, G.; Wu, C. Ethanol Extract of Rosa laevigata Michx. Fruit Inhibits Inflammatory Responses through NF-κB/MAPK Signaling Pathways via AMPK Activation in RAW 264.7 Macrophages. Molecules 2023, 28, 2813. https://doi.org/10.3390/molecules28062813
Wu H, Lin T, Chen Y, Chen F, Zhang S, Pang H, Huang L, Yu C, Wang G, Wu C. Ethanol Extract of Rosa laevigata Michx. Fruit Inhibits Inflammatory Responses through NF-κB/MAPK Signaling Pathways via AMPK Activation in RAW 264.7 Macrophages. Molecules. 2023; 28(6):2813. https://doi.org/10.3390/molecules28062813
Chicago/Turabian StyleWu, Hongtan, Tingting Lin, Yupei Chen, Fangfang Chen, Shudi Zhang, Haiyue Pang, Lisen Huang, Chihli Yu, Gueyhorng Wang, and Chun Wu. 2023. "Ethanol Extract of Rosa laevigata Michx. Fruit Inhibits Inflammatory Responses through NF-κB/MAPK Signaling Pathways via AMPK Activation in RAW 264.7 Macrophages" Molecules 28, no. 6: 2813. https://doi.org/10.3390/molecules28062813
APA StyleWu, H., Lin, T., Chen, Y., Chen, F., Zhang, S., Pang, H., Huang, L., Yu, C., Wang, G., & Wu, C. (2023). Ethanol Extract of Rosa laevigata Michx. Fruit Inhibits Inflammatory Responses through NF-κB/MAPK Signaling Pathways via AMPK Activation in RAW 264.7 Macrophages. Molecules, 28(6), 2813. https://doi.org/10.3390/molecules28062813






