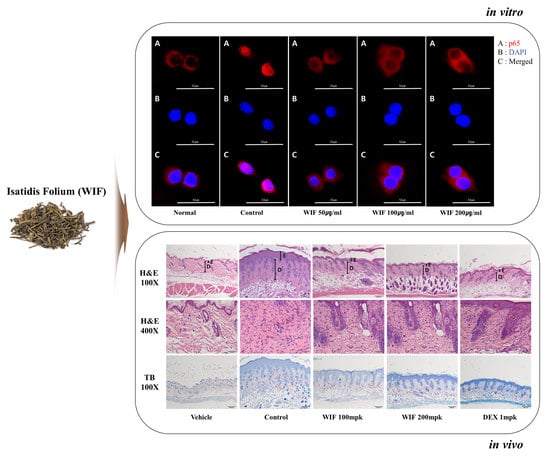
Anti-Atopic Effect of Isatidis Folium Water Extract in TNF-α/IFN-γ-Induced HaCaT Cells and DNCB-Induced Atopic Dermatitis Mouse Model
Abstract
1. Introduction
2. Results
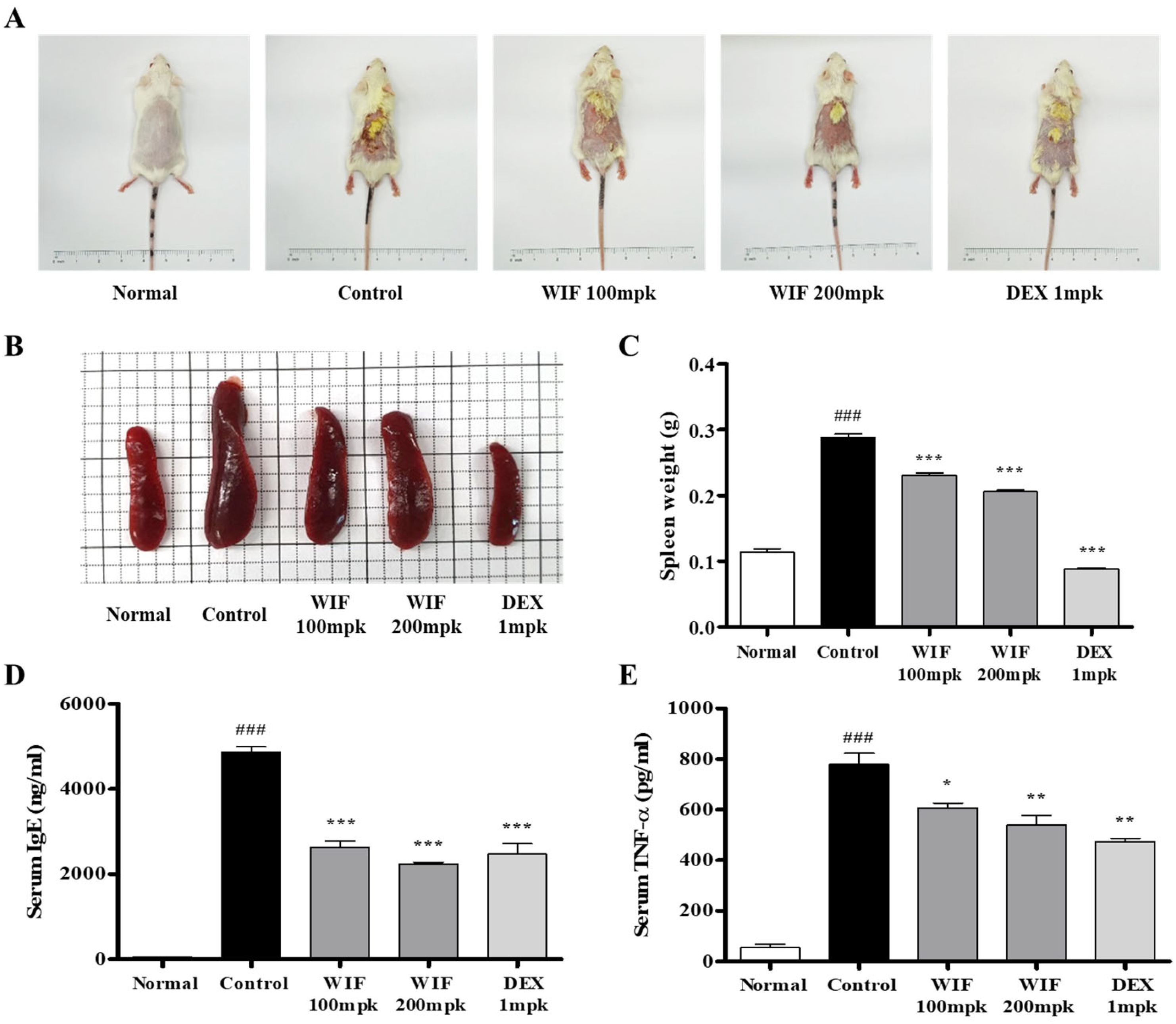
2.1. Effects of Isatidis Folium Water Extract (WIF) on AD-like Symptoms in Mice
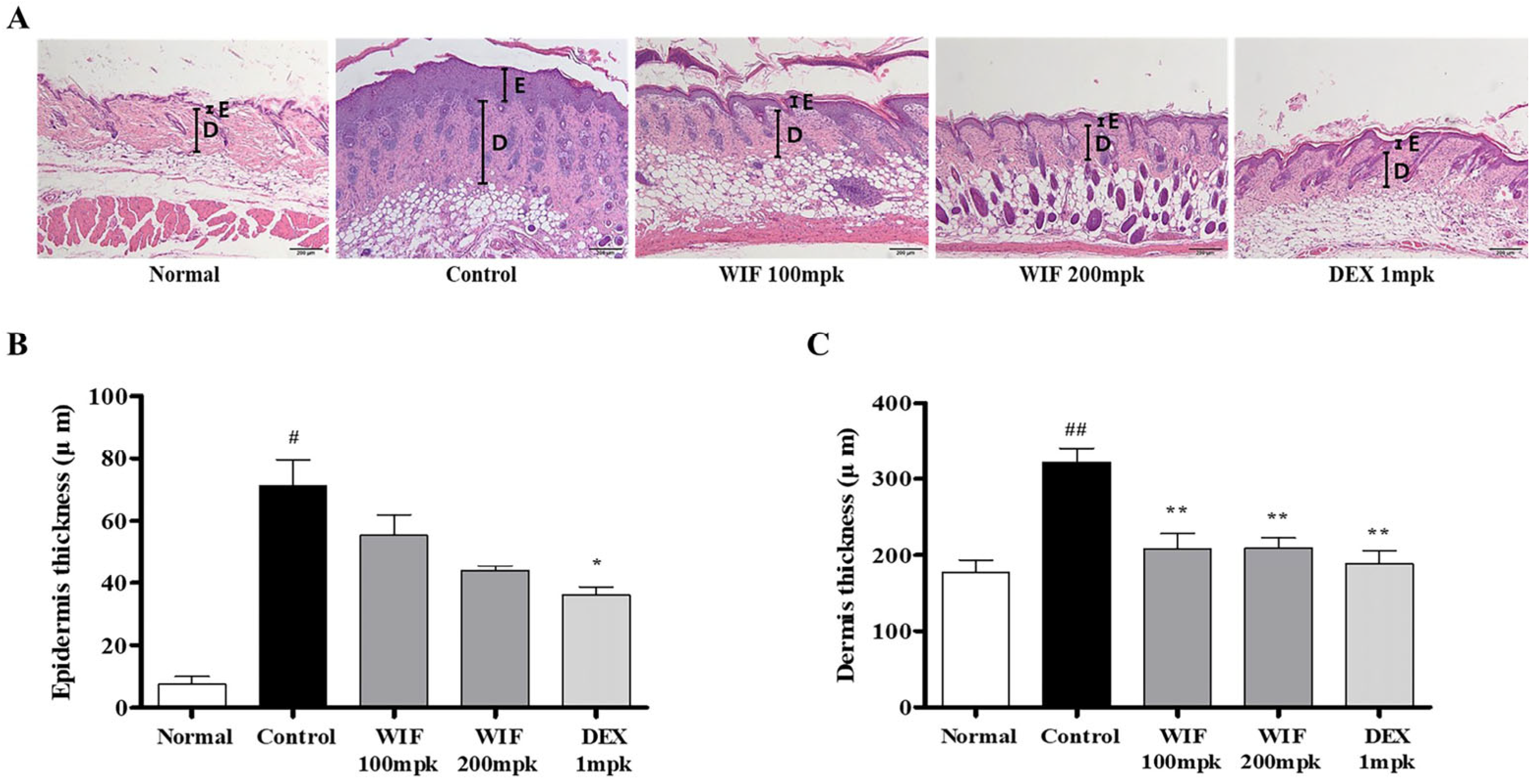
2.2. Effects of WIF on Epidermal and Dermal Thicknesses in AD-like Mouse Model
2.3. Effects of WIF on Immune Cell Infiltration in AD-like Mouse Model
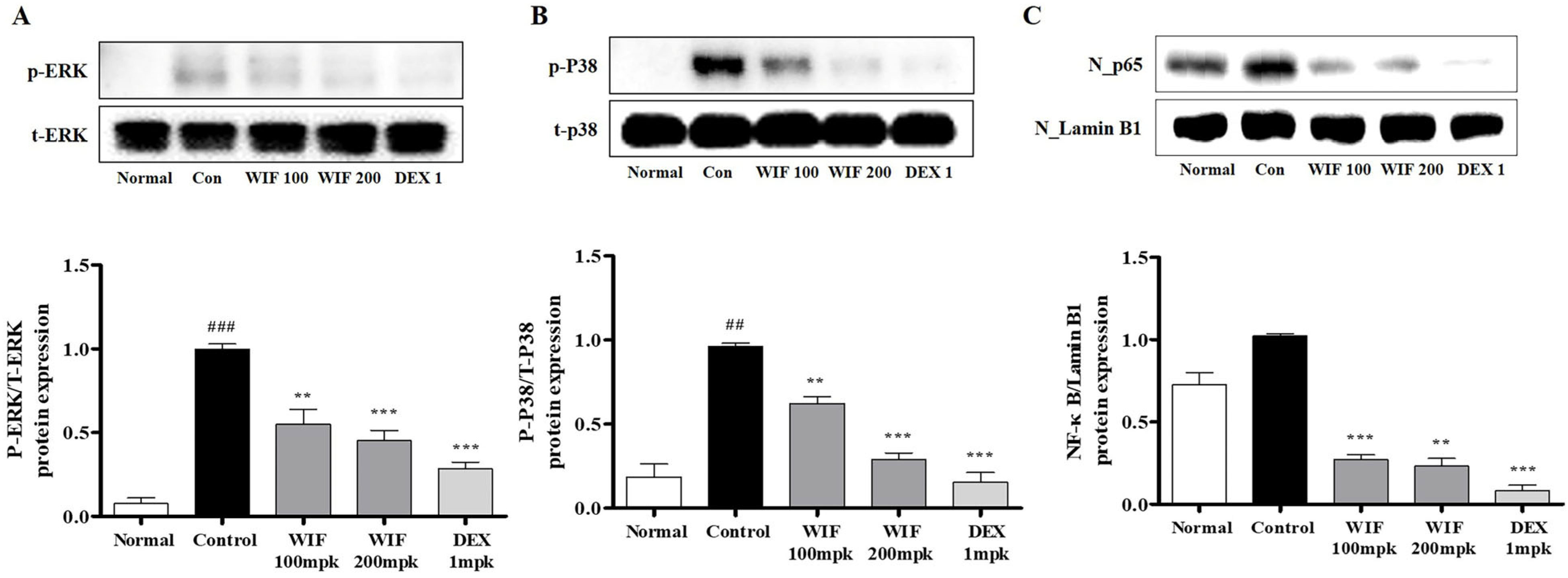
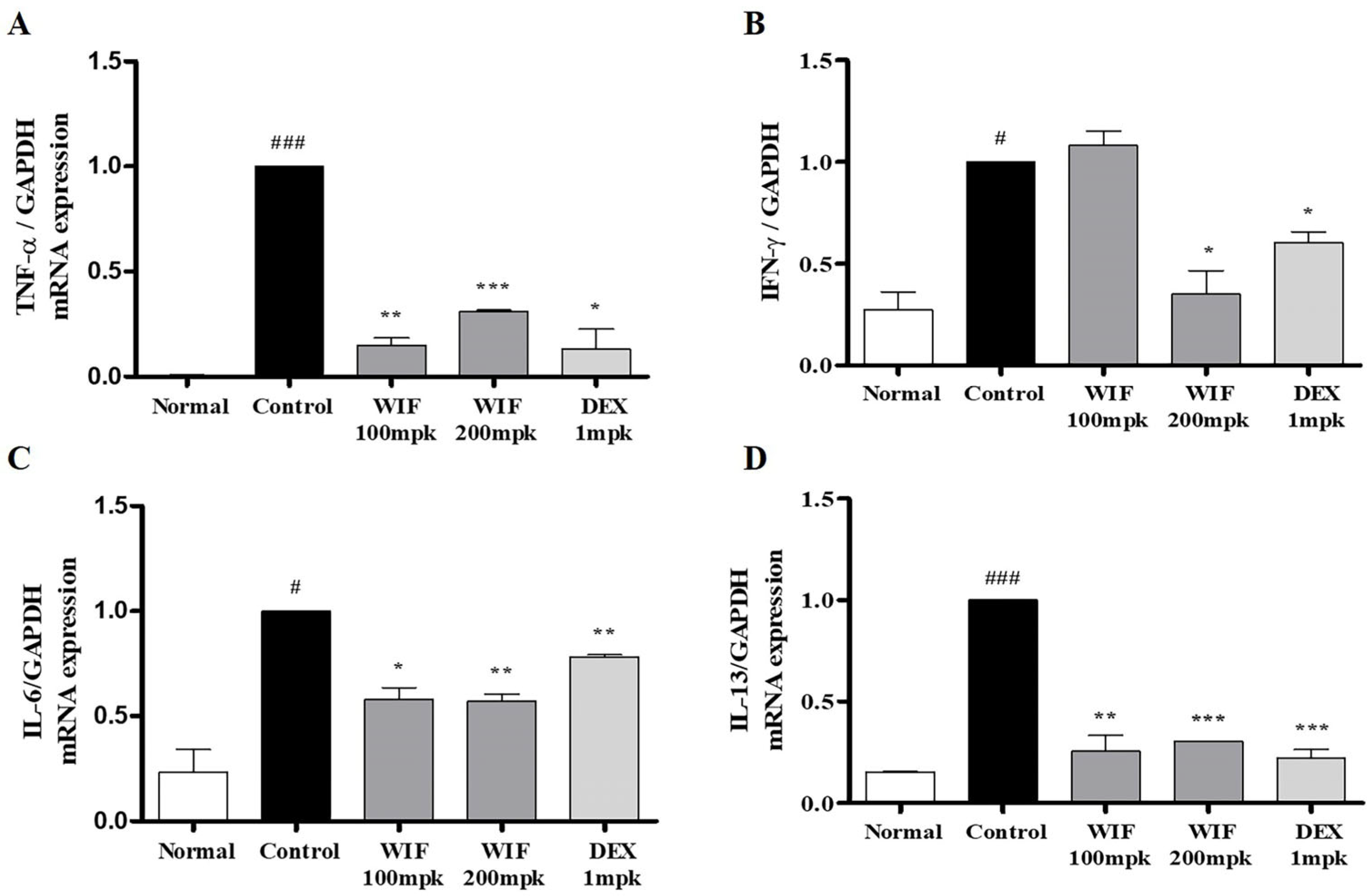
2.4. Effects of WIF on Inflammation-Related mRNA and Protein Expression in AD-like Mouse Model
2.5. Effects of WIF on Inflammation-Related mRNA in AD-like Mouse Model
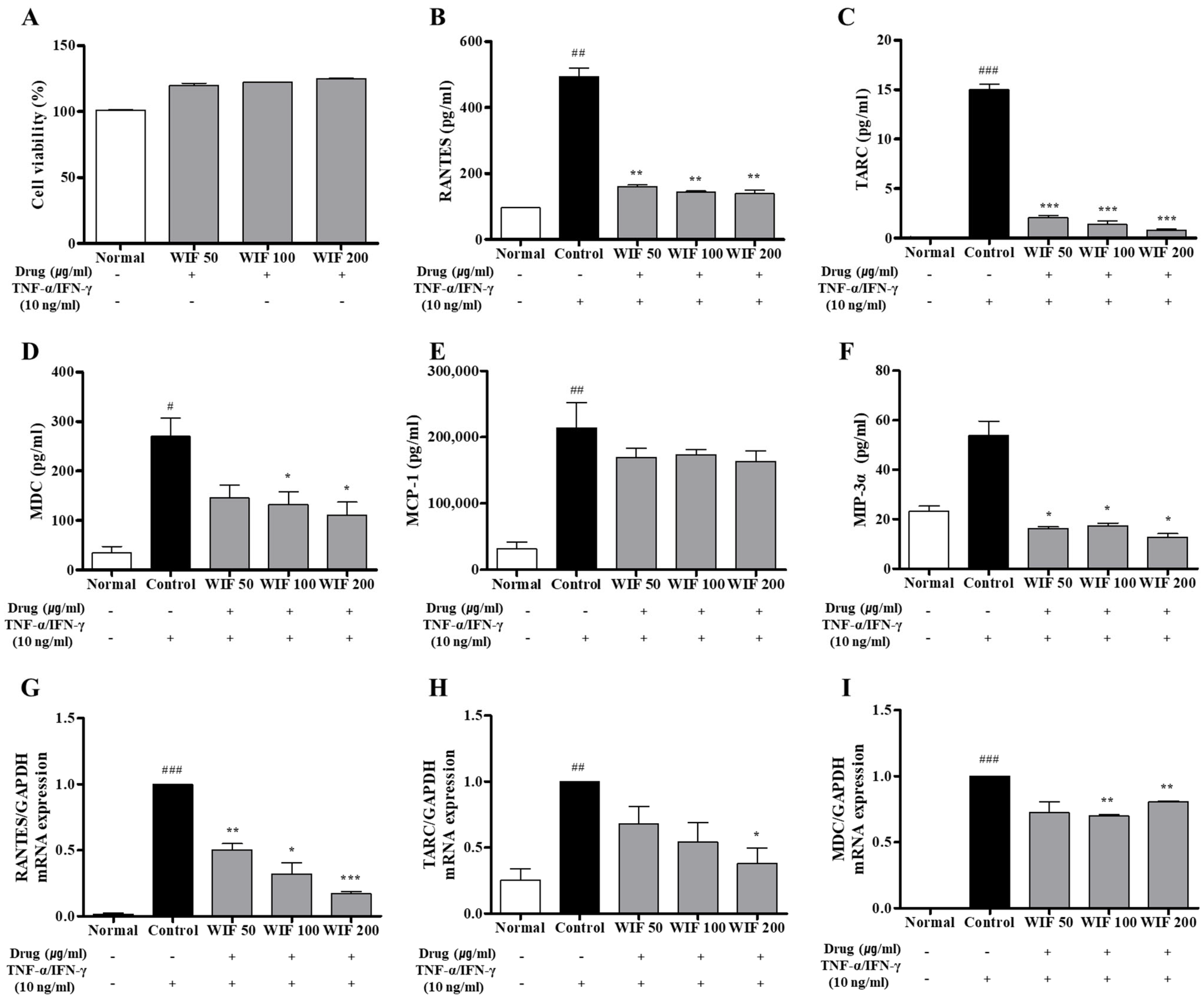
2.6. Effects of WIF on TNF-α/IFN-γ–Induced Pro-Inflammatory Chemokines Release and mRNA Expression in Human Keratinocytes
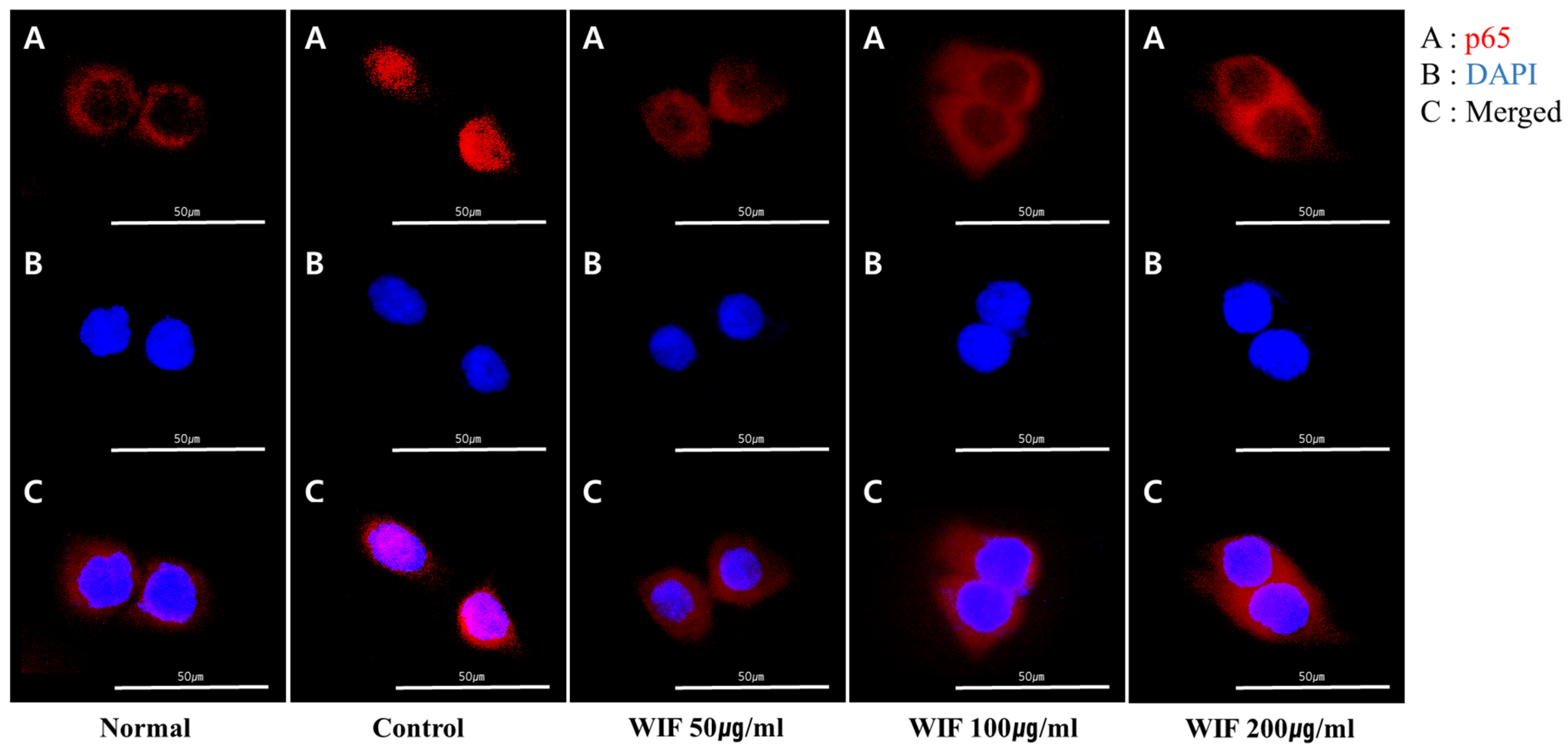
2.7. Effect of WIF on TNF-α/IFN-γ-Induced NF-κB p65 Translocation in HaCaT Cells
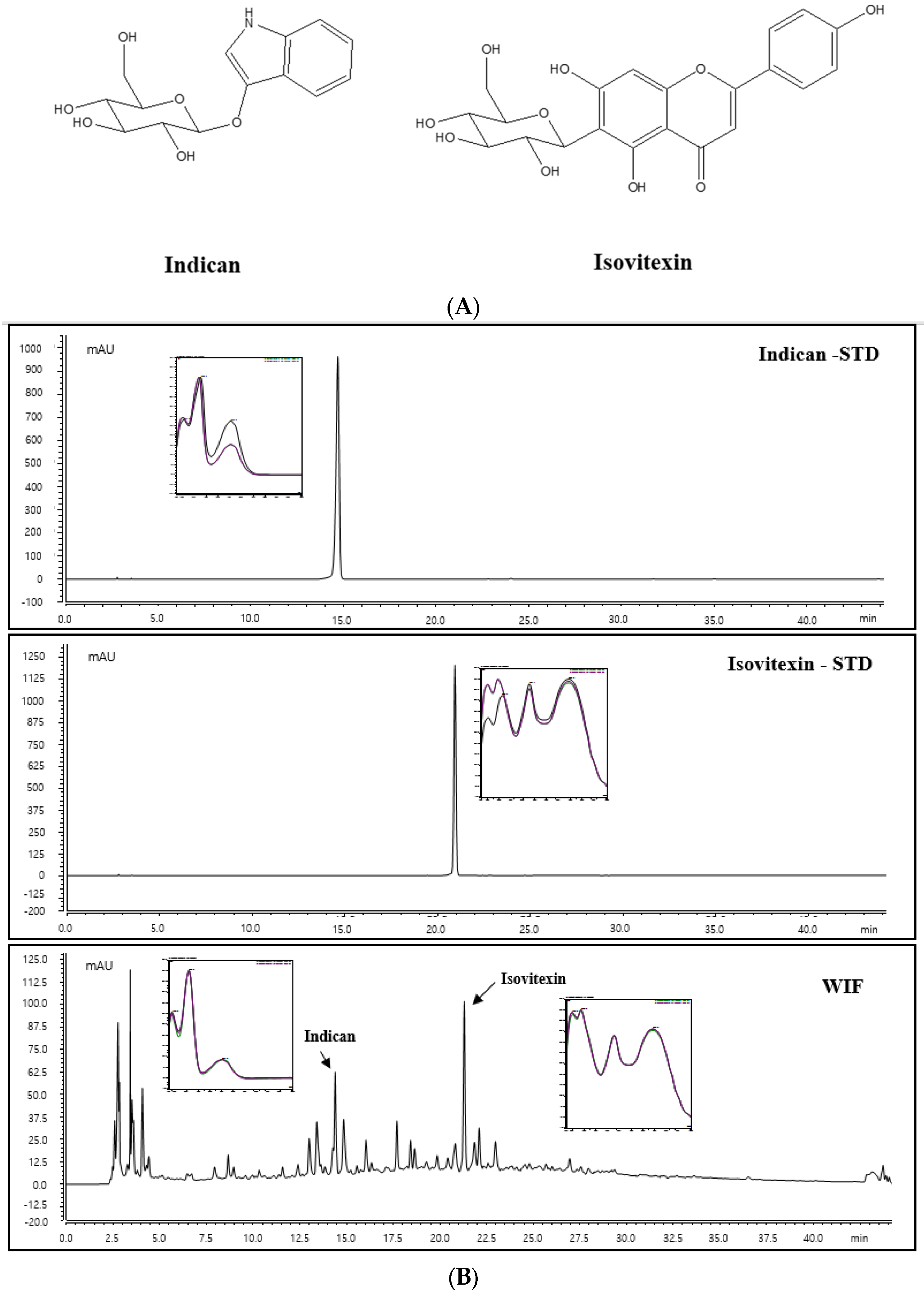
2.8. Identification of Indican and Isovitexin of WIF via High-Performance Liquid Chromatography-Diode Array Detection Analysis
2.9. Indican and Isovitexin Contents in WIF
3. Discussion
4. Materials and Methods
4.1. Preparation of WIF
4.2. HPLC Instrument and Condition
4.3. AD-Like Mouse Model and Histological Analysis
4.4. Cell Culture and Cytotoxicity Assay
4.5. Biochemical Parameters
4.6. Real-Time RT-PCR Analysis
4.7. Western Blot Analysis
4.8. Immunocytochemistry Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Vakharia, P.P.; Cella, D.; Silverberg, J.I. Patient-reported outcomes and quality of life measures in atopic dermatitis. Clin. Dermatol. 2018, 36, 616–630. [Google Scholar] [CrossRef]
- Das, P.; Mounika, P.; Yellurkar, M.L.; Prasanna, V.S.; Sarkar, S.; Velayutham, R.; Arumugam, S. Keratinocytes: An Enigmatic Factor in Atopic Dermatitis. Cells 2022, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Di Altobrando, A.; La Placa, M.; Neri, I.; Piraccini, B.M.; Vincenzi, C. Contact dermatitis due to masks and respirators during COVID-19 pandemic: What we should know and what we should do. Dermatol. Ther. 2020, 33, e14528. [Google Scholar] [CrossRef] [PubMed]
- Szepietowski, J.C.; Matusiak, L.; Szepietowska, M.; Krajewski, P.K.; Bialynicki-Birula, R. Face Mask-induced Itch: A Self-questionnaire Study of 2315 Responders During the COVID-19 Pandemic. Acta Derm. Venereol. 2020, 100, adv00152. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sözener, Z.; Cevhertas, L.; Nadeau, K.; Akdis, M.; Akdis, C.A. Environmental factors in epithelial barrier dysfunction. J. Allergy Clin. Immunol. 2020, 145, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Huang, J.; He, H.; Zhou, L.; He, Y.; Yan, J.; Tao, A. Succinate and mitochondrial DNA trigger atopic march from atopic dermatitis to intestinal inflammation. J. Allergy Clin. Immunol. 2022, 151, 1050–1066.e7. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.; Guttman-Yassky, E. Biomarkers in atopic dermatitis—A review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1171. [Google Scholar] [CrossRef] [PubMed]
- Parnia, S.; Frew, A.J. Chemokines and atopic dermatitis. J. Allergy Clin. Immunol. 2000, 105, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.; Streib, J.E.; Kisich, K.; Boguniewicz, M.; Hamid, Q.A.; Howell, M.D. Macrophage inflammatory protein 3alpha deficiency in atopic dermatitis skin and role in innate immune response to vaccinia virus. J. Allergy Clin. Immunol. 2007, 119, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Hajar, T.; Leshem, Y.A.; Hanifin, J.M.; Nedorost, S.T.; Lio, P.A.; Paller, A.S.; Block, J.; Simpson, E.L. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J. Am. Acad. Dermatol. 2015, 72, 541–549.e2. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, Z.; Zhang, X.; Shi, Y. Novel Targeted Biological Agents for the Treatment of Atopic Dermatitis. BioDrugs 2021, 35, 401–415. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Gao, T.; Chen, Y.; Yang, Q.; Fu, C.; Zhu, Y.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Speranza, J.; Miceli, N.; Taviano, M.F.; Ragusa, S.; Kwiecien, I.; Szopa, A.; Ekiert, H. Isatis tinctoria L. (Woad): A Review of its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies. Plants 2020, 9, 298. [Google Scholar] [CrossRef]
- Spataro, G.; Negri, V. Adaptability and variation in Isatis tinctoria L.: A new crop for Europe. Euphytica 2008, 163, 89–102. [Google Scholar] [CrossRef]
- Hamburger, M. Isatis tinctoria—From the rediscovery of an ancient medicinal plant towards a novel anti-inflammatory phytopharmaceutical. Phytochem. Rev. 2002, 1, 333–344. [Google Scholar] [CrossRef]
- Lee, C.L.; Wang, C.M.; Kuo, Y.H.; Yen, H.R.; Song, Y.C.; Chou, Y.L.; Chen, C.J. IL-17A inhibitions of indole alkaloids from traditional Chinese medicine Qing Dai. J. Ethnopharmacol. 2020, 255, 112772. [Google Scholar] [CrossRef]
- Wong, L.W.; Goh, C.B.S.; Tan, J.B.L. A Systemic Review for Ethnopharmacological Studies on Isatis indigotica Fortune: Bioactive Compounds and their Therapeutic Insights. Am. J. Chin. Med. 2022, 50, 161–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Z.Q.; Xiao, H. Antiviral activity of the effective monomers from Folium Isatidis against influenza virus in vivo. Virol. Sin. 2010, 25, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.P.; Liu, Y.Y.; Liu, Z.; Li, J.; Zhao, L.M.; Xiao, H.; Ding, X.H.; Yang, Z.Q. Antiviral activity of Folium isatidis derived extracts in vitro and in vivo. Am. J. Chin. Med. 2013, 41, 957–969. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Jin, J.; Dong, L.; Xu, F.; Chen, S.; Wang, Z.; Liang, G.; Shan, X. n-Butanol extract from Folium isatidis inhibits lipopolysaccharide-induced inflammatory cytokine production in macrophages and protects mice against lipopolysaccharide-induced endotoxic shock. Drug Des. Dev Ther. 2015, 9, 5601–5609. [Google Scholar] [CrossRef]
- Lotts, T.; Kabrodt, K.; Hummel, J.; Binder, D.; Schellenberg, I.; Stander, S.; Agelopoulos, K. Isatis tinctoria L.-derived Petroleum Ether Extract Mediates Anti-inflammatory Effects via Inhibition of Interleukin-6, Interleukin-33 and Mast Cell Degranulation. Acta Derm. Venereol. 2020, 100, adv00131. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Cho, C.W.; Kim, C.T.; Jeong, W.S.; Kang, J.S. Evaluation of the Antiwrinkle Activity of Enriched Isatidis Folium Extract and an HPLC-UV Method for the Quality Control of Its Cream Products. Plants 2020, 9, 1586. [Google Scholar] [CrossRef]
- Wang, C.C.; Hsiao, C.Y.; Hsu, Y.J.; Ko, H.H.; Chang, D.C.; Hung, C.F. Anti-Inflammatory Effects of Cycloheterophyllin on Dinitrochlorobenzene-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Molecules 2022, 27, 2610. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Schmuth, M.; Neyer, S.; Rainer, C.; Grassegger, A.; Fritsch, P.; Romani, N.; Heufler, C. Expression of the C-C chemokine MIP-3 alpha/CCL20 in human epidermis with impaired permeability barrier function. Exp. Dermatol. 2002, 11, 135–142. [Google Scholar] [CrossRef]
- Nakayama, T.; Fujisawa, R.; Yamada, H.; Horikawa, T.; Kawasaki, H.; Hieshima, K.; Izawa, D.; Fujiie, S.; Tezuka, T.; Yoshie, O. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int. Immunol. 2001, 13, 95–103. [Google Scholar] [CrossRef]
- Min, G.Y.; Kim, T.I.; Kim, J.H.; Cho, W.K.; Yang, J.H.; Ma, J.Y. Inhibitory effect of Isatis tinctoria L. water extract on DNCB-induced atopic dermatitis in BALB/c mice and HaCaT cells. Chin. Med. 2022, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Min, G.Y.; Kim, J.H.; Kim, T.I.; Cho, W.K.; Yang, J.H.; Ma, J.Y. Indigo Pulverata Levis (Chung-Dae, Persicaria tinctoria) Alleviates Atopic Dermatitis-like Inflammatory Responses In Vivo and In Vitro. Int. J. Mol. Sci. 2022, 23, 553. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, G.-Y.; Kim, T.I.; Kim, J.-H.; Cho, W.-K.; Yang, J.-H.; Ma, J.Y. Anti-Atopic Effect of Isatidis Folium Water Extract in TNF-α/IFN-γ-Induced HaCaT Cells and DNCB-Induced Atopic Dermatitis Mouse Model. Molecules 2023, 28, 3960. https://doi.org/10.3390/molecules28093960
Min G-Y, Kim TI, Kim J-H, Cho W-K, Yang J-H, Ma JY. Anti-Atopic Effect of Isatidis Folium Water Extract in TNF-α/IFN-γ-Induced HaCaT Cells and DNCB-Induced Atopic Dermatitis Mouse Model. Molecules. 2023; 28(9):3960. https://doi.org/10.3390/molecules28093960
Chicago/Turabian StyleMin, Ga-Yul, Tae In Kim, Ji-Hye Kim, Won-Kyung Cho, Ju-Hye Yang, and Jin Yeul Ma. 2023. "Anti-Atopic Effect of Isatidis Folium Water Extract in TNF-α/IFN-γ-Induced HaCaT Cells and DNCB-Induced Atopic Dermatitis Mouse Model" Molecules 28, no. 9: 3960. https://doi.org/10.3390/molecules28093960
APA StyleMin, G.-Y., Kim, T. I., Kim, J.-H., Cho, W.-K., Yang, J.-H., & Ma, J. Y. (2023). Anti-Atopic Effect of Isatidis Folium Water Extract in TNF-α/IFN-γ-Induced HaCaT Cells and DNCB-Induced Atopic Dermatitis Mouse Model. Molecules, 28(9), 3960. https://doi.org/10.3390/molecules28093960