Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics
Abstract
1. Introduction
2. Epidemiology of Chronic Liver Diseases
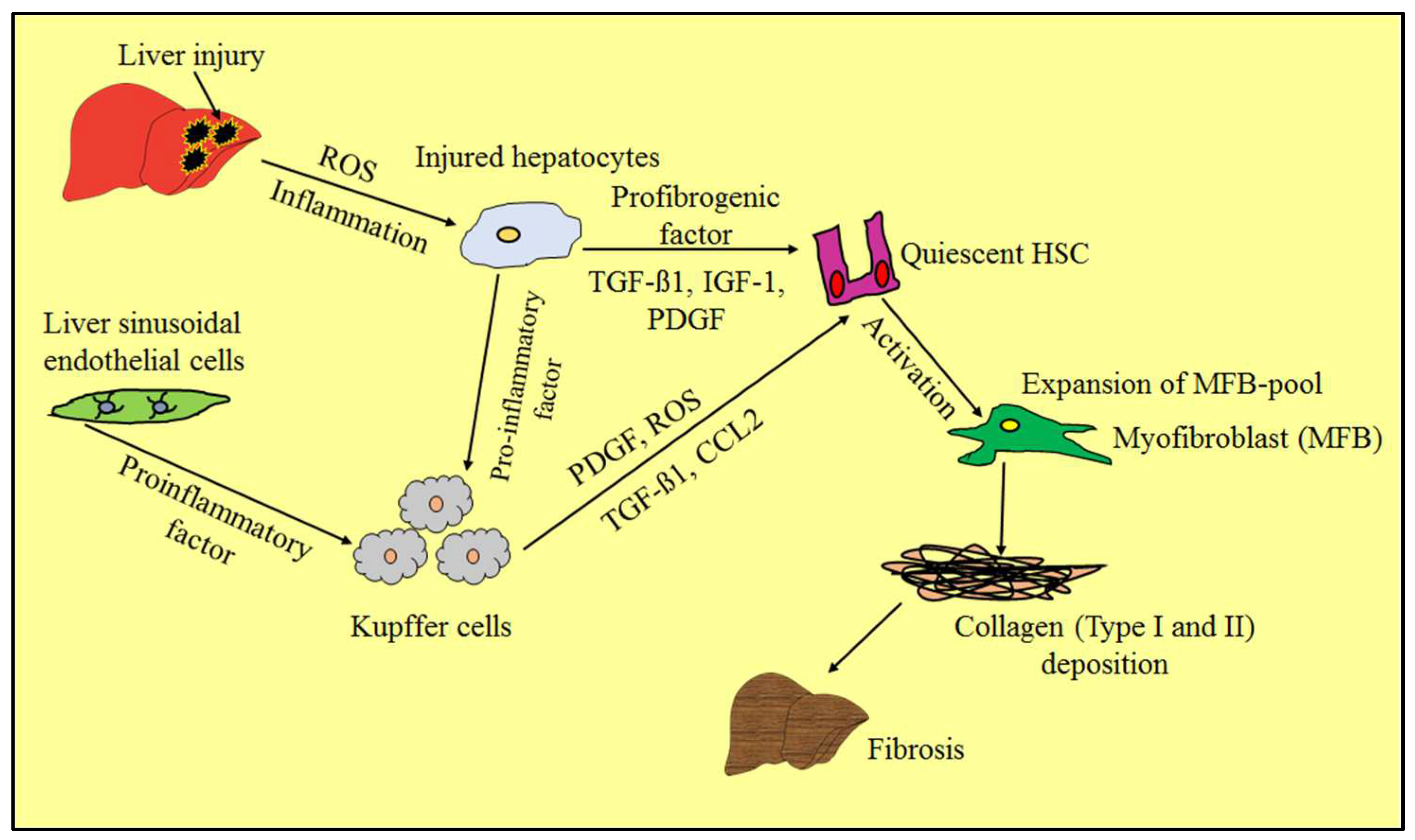
3. Pathogenesis and Pathophysiology of Liver Fibrosis
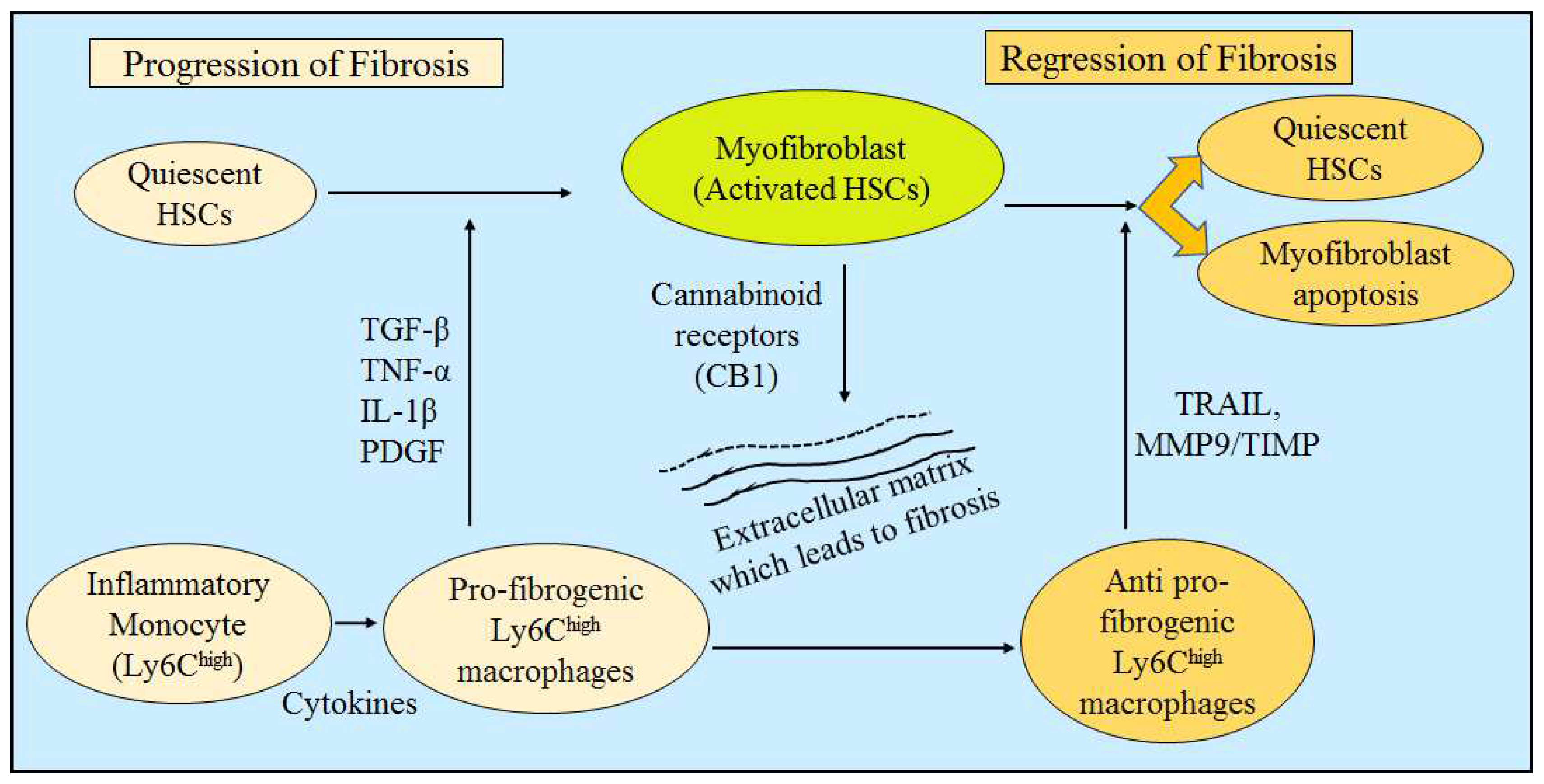
4. Evidence of Liver Fibrosis Progression and Regression
4.1. Liver Fibrosis Progression: Upregulation in Fibrolytic Activity
4.2. Liver Fibrosis Regression: Cell Apoptosis or Downregulation of Hepatic Stellate Cells
5. Clinical Evaluation of Liver Fibrosis
5.1. Pharmacological Elements of Liver
5.2. Paramedical Evaluation of Liver Fibrosis
5.3. Nanotechnology-Based Diagnosis of Liver Fibrosis
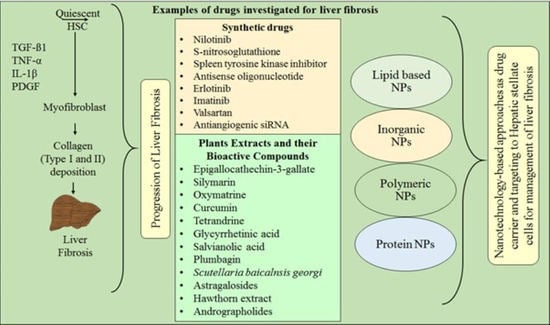
6. Synthetic Therapeutic Agents for Liver Fibrosis Management
6.1. Hepatic Stellate Cells-Targeting Potential Substances and Extracellular Matrix-Interfering Modulators
6.2. Hepatoprotective Agents
6.3. Anti-Inflammatory and Antioxidant Therapies
7. Role of Nanotechnology in Liver Fibrosis Therapy Using Synthetic Drugs
8. Emerging Perspectives of Herbal Compounds in Liver Fibrosis Therapy
8.1. Epigallocathechin-3-gallate (EGCG)
8.2. Silymarin
8.3. Oxymatrine
8.4. Curcumin
8.5. Tetrandrine
8.6. Glycyrrhetinic Acid
8.7. Salvianolic Acid
8.8. Plumbagin
8.9. Scutellara Baicalnsis Georgi
8.10. Astragalosides
8.11. Hawthorn Extract
8.12. Andrographolides
9. Role of Nanotechnology in Liver Fibrosis Therapy Using Herbal Compounds
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-smooth muscle actin |
| ACE | Angiotensin-converting enzyme |
| AFP | Alpha-fetoprotein |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| API | Active pharmaceutical ingredient |
| ASK-1 | Apoptosis-signal regulating kinase-1 |
| AST | Aspartate aminotransferase |
| CAT | Catalase |
| CB1 | Cannabinoid receptor type 1 |
| CCL2 | Chemokine |
| CCl4 | Carbon tetrachloride |
| CLDs | Chronic liver diseases |
| COX | Cyclooxygenase |
| CRMs | Cis-acting regulatory modules |
| CTGF | Connective tissue growth factor |
| DCs | Dendritic cells |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
| ECM | Extracellular matrix |
| EGCG | Epigallocathechin-3-gallate |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| GM-CT-01 | Galactomannan |
| GR-MD-02 | Galactoarabino-rhamnogalaturonan |
| GSH | Glutathione |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HSCs | Hepatic stellate cells |
| IL-1 | Interleukins-1 |
| IL-2 | Interleukins-2 |
| IL-13 | Interleukin-13 |
| JNK | c-Jun N-terminal kinases |
| LOXL2 | Lysyl oxidase-like 2 |
| LPO | Lipid peroxidation |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malonaldehyde |
| MFBs | Myofibroblasts |
| MMP-1 | Matrix metalloproteinase-1 |
| MRI | Magnetic resonance imaging |
| NASH | Non-alcoholic steatohepatitis |
| NFkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLCs | Nanostructured lipid carriers |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| OM | Oxymatrine |
| PPARs | Peroxisome proliferator active receptors |
| PDGF | Platelet-derived growth factor |
| PDGFR | Platelet derived growth factor receptor |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| SAB@MSNs-RhB | Rhodamine B covalently grafted salvianolic acid B-loaded mesoporous silica nanoparticles |
| TGF-β | Transforming growth factor-β |
| TIMP-1 | Tissue inhibitor metalloproteinase-1 |
| TLRs | Toll-like receptors |
| TNF-ɑ | Tumor necrosis factor-ɑ |
| TRAIL | Tumor necrosis (TNF)-related apoptosis-inducing ligands |
| VEGF | Vascular endothelial growth factor |
References
- Latief, U.; Ahmad, R. Herbal Remedies for Liver Fibrosis: A Review on the Mode of Action of Fifty Herbs. J. Tradit. Complement. Med. 2018, 8, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, G.; Li, Y.; Li, X. Flavonoids from Aurantii Fructus Immaturus and Aurantii Fructus: Promising Phytomedicines for the Treatment of Liver Diseases. Chin. Med. 2020, 15, 89. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, G.; Luo, S.; Yin, W.; Song, H. The Value of Platelet Count in Evaluating the Degree of Liver Fibrosis in Patients with Chronic Hepatitis B. J. Clin. Lab. Anal. 2020, 34, e23270. [Google Scholar] [CrossRef]
- Zhang, F.; Kong, D.; Lu, Y.; Zheng, S. Peroxisome Proliferator-Activated Receptor-γ as a Therapeutic Target for Hepatic Fibrosis: From Bench to Bedside. Cell. Mol. Life Sci. 2013, 70, 259–276. [Google Scholar]
- Garcia-Tsao, G.; Friedman, S.; Iredale, J.; Pinzani, M. Now There Are Many (Stages) Where before There Was One: In Search of a Pathophysiological Classification of Cirrhosis. Hepatology 2010, 51, 1445. [Google Scholar]
- Parola, M.; Pinzani, M. Liver Fibrosis: Pathophysiology, Pathogenetic Targets and Clinical Issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Ponzetto, A.; Figura, N. Thrombosis of the Portal Venous System in Cirrhotic Patients. Ann. Hepatol. 2019, 17, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Huizer-Pajkos, A.; Cogger, V.C.; McLachlan, A.J.; Le Couteur, D.G.; Jones, B.; de Cabo, R.; Hilmer, S.N. Age-Related Pseudocapillarization of the Liver Sinusoidal Endothelium Impairs the Hepatic Clearance of Acetaminophen in Rats. J. Gerontol. Ser. A 2011, 66, 400–408. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Lin, H.-C. Alteration of Intrahepatic Microcirculation in Cirrhotic Livers. J. Chin. Med. Assoc. 2015, 78, 430–437. [Google Scholar] [PubMed]
- Pinzani, M.; Rombouts, K. Liver Fibrosis: From the Bench to Clinical Targets. Dig. Liver Dis. 2004, 36, 231–242. [Google Scholar] [CrossRef]
- Marcellin, P.; Kutala, B.K. Liver Diseases: A Major, Neglected Global Public Health Problem Requiring Urgent Actions and Large-scale Screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Byass, P. The Global Burden of Liver Disease: A Challenge for Methods and for Public Health. BMC Med. 2014, 12, 159. [Google Scholar] [CrossRef]
- Scaglione, S.; Kliethermes, S.; Cao, G.; Shoham, D.; Durazo, R.; Luke, A.; Volk, M.L. The Epidemiology of Cirrhosis in the United States. J. Clin. Gastroenterol. 2015, 49, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.-C.; Roudot-Thoraval, F. The Burden of Liver Disease in Europe: A Review of Available Epidemiological Data. J. Hepatol. 2013, 58, 593–608. [Google Scholar]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic Steatohepatitis Is the Most Rapidly Growing Indication for Liver Transplantation in Patients with Hepatocellular Carcinoma in the US. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Bugianesi, E. Should We Undertake Surveillance for HCC in Patients with NAFLD? J. Hepatol. 2018, 68, 326–334. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, I. Emerging Insights into Transforming Growth Factor β Smad Signal in Hepatic Fibrogenesis. Gut 2007, 56, 284–292. [Google Scholar] [CrossRef]
- Yoshida, K.; Matsuzaki, K. Differential Regulation of TGF-β/Smad Signaling in Hepatic Stellate Cells between Acute and Chronic Liver Injuries. Front. Physiol. 2012, 3, 53. [Google Scholar] [CrossRef]
- Ramadori, P.; Klag, T.; Malek, N.P.; Heikenwalder, M. Platelets in Chronic Liver Disease, from Bench to Bedside. JHEP Rep. 2019, 1, 448–459. [Google Scholar] [CrossRef]
- Cho, J.-J.; Hocher, B.; Herbst, H.; Jia, J.-D.; Ruehl, M.; Hahn, E.G.; Riecken, E.O.; Schuppan, D. An Oral Endothelin-A Receptor Antagonist Blocks Collagen Synthesis and Deposition in Advanced Rat Liver Fibrosis. Gastroenterology 2000, 118, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Gabele, E.; Schoonhoven, R.; Morris, T.; Lehnert, M.; Yang, L.; Brenner, D.A.; Rippe, R.A. Prolonged Infusion of Angiotensin II into Normal Rats Induces Stellate Cell Activation and Proinflammatory Events in Liver. Am. J. Physiol. Liver Physiol. 2003, 285, G642–G651. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Bertolani, C. Adipokines in Liver Diseases. Hepatology 2009, 50, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Korah, T.E.; El-Sayed, S.; ElShafie, M.K.; Hammoda, G.E.; Safan, M.A. Significance of Serum Leptin and Adiponectin Levels in Egyptian Patients with Chronic Hepatitis C Virus Associated Hepatic Steatosis and Fibrosis. World J. Hepatol. 2013, 5, 74. [Google Scholar] [CrossRef]
- Ikejima, K.; Takei, Y.; Honda, H.; Hirose, M.; Yoshikawa, M.; Zhang, Y.-J.; Lang, T.; Fukuda, T.; Yamashina, S.; Kitamura, T. Leptin Receptor–Mediated Signaling Regulates Hepatic Fibrogenesis and Remodeling of Extracellular Matrix in the Rat. Gastroenterology 2002, 122, 1399–1410. [Google Scholar] [CrossRef]
- Moreno, M.; Chaves, J.F.; Sancho-Bru, P.; Ramalho, F.; Ramalho, L.N.; Mansego, M.L.; Ivorra, C.; Dominguez, M.; Conde, L.; Millán, C. Ghrelin Attenuates Hepatocellular Injury and Liver Fibrogenesis in Rodents and Influences Fibrosis Progression in Humans. Hepatology 2010, 51, 974–985. [Google Scholar] [CrossRef]
- Lee, U.E.; Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Efsen, E.; Romanelli, R.G.; Caligiuri, A.; Pastacaldi, S.; Batignani, G.; Bonacchi, A.; Caporale, R.; Laffi, G.; Pinzani, M. Ligands of Peroxisome Proliferator-Activated Receptor γ Modulate Profibrogenic and Proinflammatory Actions in Hepatic Stellate Cells. Gastroenterology 2000, 119, 466–478. [Google Scholar] [CrossRef]
- Galli, A.; Crabb, D.W.; Ceni, E.; Salzano, R.; Mello, T.; Svegliati–Baroni, G.; Ridolfi, F.; Trozzi, L.; Surrenti, C.; Casini, A. Antidiabetic Thiazolidinediones Inhibit Collagen Synthesis and Hepatic Stellate Cell Activation in Vivo and in Vitro. Gastroenterology 2002, 122, 1924–1940. [Google Scholar] [CrossRef]
- Seki, E.; De Minicis, S.; Österreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-β Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, R. Resveratrol Mitigate Structural Changes and Hepatic Stellate Cell Activation in N′-Nitrosodimethylamine-Induced Liver Fibrosis via Restraining Oxidative Damage. Chem. Biol. Interact. 2014, 221, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmed, S.; Khan, N.U.; Hasnain, A. Operculina Turpethum Attenuates N-Nitrosodimethylamine Induced Toxic Liver Injury and Clastogenicity in Rats. Chem. Biol. Interact. 2009, 181, 145–153. [Google Scholar] [CrossRef]
- Efsen, E.; Bonacchi, A.; Pastacaldi, S.; Valente, A.J.; Wenzel, U.O.; Tosti-Guerra, C.; Pinzani, M.; Laffi, G.; Abboud, H.E.; Gentilini, P. Agonist-specific Regulation of Monocyte Chemoattractant Protein-1 Expression by Cyclooxygenase Metabolites in Hepatic Stellate Cells. Hepatology 2001, 33, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.A.; Mizuno, M.; Kendall, T.J.; Constandinou, C.M.; Benyon, R.C.; Duffield, J.S.; Iredale, J.P. Scar-Associated Macrophages Are a Major Source of Hepatic Matrix Metalloproteinase-13 and Facilitate the Resolution of Murine Hepatic Fibrosis. J. Immunol. 2007, 178, 5288–5295. [Google Scholar] [CrossRef]
- Iredale, J. Defining Therapeutic Targets for Liver Fibrosis: Exploiting the Biology of Inflammation and Repair. Pharmacol. Res. 2008, 58, 129–136. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Yanase, K.; Namisaki, T.; Imazu, H. Tissue Inhibitor of Metalloproteinases-1 Attenuates Spontaneous Liver Fibrosis Resolution in the Transgenic Mouse. Hepatology 2002, 36, 850–860. [Google Scholar]
- Issa, R.; Zhou, X.; Constandinou, C.M.; Fallowfield, J.; Millward-Sadler, H.; Gaca, M.D.A.; Sands, E.; Suliman, I.; Trim, N.; Knorr, A. Spontaneous Recovery from Micronodular Cirrhosis: Evidence for Incomplete Resolution Associated with Matrix Cross-Linking. Gastroenterology 2004, 126, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.J.; Bradford, B.U.; Pan, C.Q.; Cheung, E.; Schauer, M.; Knorr, A.; Krebs, B.; Kraft, S.; Zahn, S.; Brocks, B. Antifibrotic Effects of a Tissue Inhibitor of Metalloproteinase-1 Antibody on Established Liver Fibrosis in Rats. Hepatology 2004, 40, 1106–1115. [Google Scholar] [CrossRef]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective Depletion of Macrophages Reveals Distinct, Opposing Roles during Liver Injury and Repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef]
- Barnes, M.A.; McMullen, M.R.; Roychowdhury, S.; Madhun, N.Z.; Niese, K.; Olman, M.A.; Stavitsky, A.B.; Bucala, R.; Nagy, L.E. Macrophage Migration Inhibitory Factor Is Required for Recruitment of Scar-associated Macrophages during Liver Fibrosis. J. Leukoc. Biol. 2015, 97, 161–169. [Google Scholar] [CrossRef]
- Pellicoro, A.; Aucott, R.L.; Ramachandran, P.; Robson, A.J.; Fallowfield, J.A.; Snowdon, V.K.; Hartland, S.N.; Vernon, M.; Duffield, J.S.; Benyon, R.C. Elastin Accumulation Is Regulated at the Level of Degradation by Macrophage Metalloelastase (MMP-12) during Experimental Liver Fibrosis. Hepatology 2012, 55, 1965–1975. [Google Scholar] [CrossRef]
- Tacke, F.; Zimmermann, H.W. Macrophage Heterogeneity in Liver Injury and Fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [PubMed]
- Jiao, J.; Sastre, D.; Fiel, M.I.; Lee, U.E.; Ghiassi-Nejad, Z.; Ginhoux, F.; Vivier, E.; Friedman, S.L.; Merad, M.; Aloman, C. Dendritic Cell Regulation of Carbon Tetrachloride–Induced Murine Liver Fibrosis Regression. Hepatology 2012, 55, 244–255. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Reversion of Hepatic Stellate Cell to a Quiescent Phenotype: From Myth to Reality? J. Hepatol. 2013, 59, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Troeger, J.S.; Mederacke, I.; Gwak, G.; Dapito, D.H.; Mu, X.; Hsu, C.C.; Pradere, J.; Friedman, R.A.; Schwabe, R.F. Deactivation of Hepatic Stellate Cells during Liver Fibrosis Resolution in Mice. Gastroenterology 2012, 143, 1073–1083. [Google Scholar] [PubMed]
- Kisseleva, T.; Cong, M.; Paik, Y.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H. Myofibroblasts Revert to an Inactive Phenotype during Regression of Liver Fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef] [PubMed]
- Spycher, C.; Zimmermann, A.; Reichen, J. The Diagnostic Value of Liver Biopsy. BMC Gastroenterol. 2001, 1, 12. [Google Scholar] [CrossRef]
- Rosselli, M.; MacNaughtan, J.; Jalan, R.; Pinzani, M. Beyond Scoring: A Modern Interpretation of Disease Progression in Chronic Liver Disease. Gut 2013, 62, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic Fibrosis: Concept to Treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of Liver Biopsy and Non-Invasive Diagnostic Tests for the Diagnosis of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2014, 20, 475. [Google Scholar] [CrossRef]
- Dulai, P.S.; Sirlin, C.B.; Loomba, R. MRI and MRE for Non-Invasive Quantitative Assessment of Hepatic Steatosis and Fibrosis in NAFLD and NASH: Clinical Trials to Clinical Practice. J. Hepatol. 2016, 65, 1006–1016. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, A.; Nazeer, S.S.; Nimi, N.; Arumugam, S.; Shenoy, S.J.; Jayasree, R.S. Synthesis and Characterization of Dextran Stabilized Superparamagnetic Iron Oxide Nanoparticles for in Vivo MR Imaging of Liver Fibrosis. Carbohydr. Polym. 2014, 101, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, A.; Nazeer, S.S.; Jeevan, M.; Nimi, N.; Arumugam, S.; Harikrishnan, V.S.; Varma, P.R.H.; Jayasree, R.S. Citrate Coated Iron Oxide Nanoparticles with Enhanced Relaxivity for in Vivo Magnetic Resonance Imaging of Liver Fibrosis. Colloids Surf. B Biointerfaces 2014, 117, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Teixeira-Clerc, F.; Lotersztajn, S. Cannabinoid Signaling and Liver Therapeutics. J. Hepatol. 2013, 59, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Iredale, J.P. Models of Liver Fibrosis: Exploring the Dynamic Nature of Inflammation and Repair in a Solid Organ. J. Clin. Investig. 2007, 117, 539–548. [Google Scholar] [CrossRef]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C. Allosteric Inhibition of Lysyl Oxidase–like-2 Impedes the Development of a Pathologic Microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef]
- Ikenaga, N.; Peng, Z.-W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective Targeting of Lysyl Oxidase-like 2 (LOXL2) Suppresses Hepatic Fibrosis Progression and Accelerates Its Reversal. Gut 2017, 66, 1697–1708. [Google Scholar] [CrossRef]
- la Cour Poulsen, L.; Siersbæk, M.; Mandrup, S. PPARs: Fatty Acid Sensors Controlling Metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef]
- Lee, C.-H.; Olson, P.; Hevener, A.; Mehl, I.; Chong, L.-W.; Olefsky, J.M.; Gonzalez, F.J.; Ham, J.; Kang, H.; Peters, J.M. PPARδ Regulates Glucose Metabolism and Insulin Sensitivity. Proc. Natl. Acad. Sci. USA 2006, 103, 3444–3449. [Google Scholar] [CrossRef]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W. Hepatoprotective Effects of the Dual Peroxisome Proliferator-activated Receptor Alpha/Delta Agonist, GFT505, in Rodent Models of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Eagle, A.R.; Vats, D.; Morel, C.R.; Goforth, M.H.; Subramanian, V.; Mukundan, L.; Ferrante, A.W.; Chawla, A. Alternative M2 Activation of Kupffer Cells by PPARδ Ameliorates Obesity-Induced Insulin Resistance. Cell Metab. 2008, 7, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.-C.; Staels, B. Sorting out the Roles of PPARα in Energy Metabolism and Vascular Homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- Bataller, R.; Ginès, P.; Nicolás, J.M.; Görbig, M.N.; Garcia–Ramallo, E.; Gasull, X.; Bosch, J.; Arroyo, V.; Rodés, J. Angiotensin II Induces Contraction and Proliferation of Human Hepatic Stellate Cells. Gastroenterology 2000, 118, 1149–1156. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Fukui, H. Blockade of Renin–Angiotensin System in Antifibrotic Therapy. J. Gastroenterol. Hepatol. 2007, 22, S93–S95. [Google Scholar] [CrossRef]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell Death and Cell Death Responses in Liver Disease: Mechanisms and Clinical Relevance. Gastroenterology 2014, 147, 765–783. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A.; Karin, M. A Liver Full of JNK: Signaling in Regulation of Cell Function and Disease Pathogenesis, and Clinical Approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef]
- Noureddin, M.; Anstee, Q.M.; Loomba, R. Emerging Anti-fibrotic Therapies in the Treatment of Non-alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2016, 43, 1109–1123. [Google Scholar] [CrossRef]
- Rotman, Y.; Sanyal, A.J. Current and Upcoming Pharmacotherapy for Non-Alcoholic Fatty Liver Disease. Gut 2017, 66, 180–190. [Google Scholar] [CrossRef]
- Barreyro, F.J.; Holod, S.; Finocchietto, P.V.; Camino, A.M.; Aquino, J.B.; Avagnina, A.; Carreras, M.C.; Poderoso, J.J.; Gores, G.J. The Pan-caspase Inhibitor Emricasan (IDN-6556) Decreases Liver Injury and Fibrosis in a Murine Model of Non-alcoholic Steatohepatitis. Liver Int. 2015, 35, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Canbay, A.; Feldstein, A.; Baskin-Bey, E.; Bronk, S.F.; Gores, G.J. caspase Inhib. IDN-6556 attenuates hepatic Inj. Fibros. bile duct ligated mouse. J. Pharmacol. Exp. Ther. 2004, 308, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Vircheva, S.; Alexandrova, A.; Georgieva, A.; Mateeva, P.; Zamfirova, R.; Kubera, M.; Kirkova, M. In Vivo Effects of Pentoxifylline on Enzyme and Non-enzyme Antioxidant Levels in Rat Liver after Carrageenan-induced Paw Inflammation. Cell Biochem. Funct. 2010, 28, 668–672. [Google Scholar] [CrossRef]
- Zein, C.O.; Yerian, L.M.; Gogate, P.; Lopez, R.; Kirwan, J.P.; Feldstein, A.E.; McCullough, A.J. Pentoxifylline Improves Nonalcoholic Steatohepatitis: A Randomized Placebo-controlled Trial. Hepatology 2011, 54, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, S.; Raurell, I.; Torres-Arauz, M.; García-Lezana, T.; Genescà, J.; Martell, M. A Nitric Oxide-Donating Statin Decreases Portal Pressure with a Better Toxicity Profile than Conventional Statins in Cirrhotic Rats. Sci. Rep. 2017, 7, 40461. [Google Scholar] [CrossRef] [PubMed]
- Meireles, C.Z.; Pasarin, M.; Lozano, J.J.; García-Calderó, H.; Gracia-Sancho, J.; García-Pagán, J.C.; Bosch, J.; Abraldes, J.G. Simvastatin Attenuates Liver Injury in Rodents with Biliary Cirrhosis Submitted to Hemorrhage/Resuscitation. Shock 2017, 47, 370–377. [Google Scholar] [CrossRef]
- Trebicka, J.; Hennenberg, M.; Laleman, W.; Shelest, N.; Biecker, E.; Schepke, M.; Nevens, F.; Sauerbruch, T.; Heller, J. Atorvastatin Lowers Portal Pressure in Cirrhotic Rats by Inhibition of RhoA/Rho-kinase and Activation of Endothelial Nitric Oxide Synthase. Hepatology 2007, 46, 242–253. [Google Scholar] [CrossRef]
- Abraldes, J.G.; Rodríguez-Vilarrupla, A.; Graupera, M.; Zafra, C.; García-Calderó, H.; García-Pagán, J.C.; Bosch, J. Simvastatin Treatment Improves Liver Sinusoidal Endothelial Dysfunction in CCl4 Cirrhotic Rats. J. Hepatol. 2007, 46, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Maeso-Díaz, R.; García-Cardena, G.; Abraldes, J.G.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. KLF2 Exerts Antifibrotic and Vasoprotective Effects in Cirrhotic Rat Livers: Behind the Molecular Mechanisms of Statins. Gut 2015, 64, 1434–1443. [Google Scholar] [CrossRef]
- La Mura, V.; Pasarín, M.; Meireles, C.Z.; Miquel, R.; Rodríguez-Vilarrupla, A.; Hide, D.; Gracia-Sancho, J.; García-Pagán, J.C.; Bosch, J.; Abraldes, J.G. Effects of Simvastatin Administration on Rodents with Lipopolysaccharide-induced Liver Microvascular Dysfunction. Hepatology 2013, 57, 1172–1181. [Google Scholar] [CrossRef]
- Trebicka, J.; Hennenberg, M.; Odenthal, M.; Shir, K.; Klein, S.; Granzow, M.; Vogt, A.; Dienes, H.-P.; Lammert, F.; Reichen, J. Atorvastatin Attenuates Hepatic Fibrosis in Rats after Bile Duct Ligation via Decreased Turnover of Hepatic Stellate Cells. J. Hepatol. 2010, 53, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Tacke, F. Liver Macrophages in Tissue Homeostasis and Disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef]
- Lefebvre, E.; Moyle, G.; Reshef, R.; Richman, L.P.; Thompson, M.; Hong, F.; Chou, H.; Hashiguchi, T.; Plato, C.; Poulin, D. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS ONE 2016, 11, e0158156. [Google Scholar] [CrossRef] [PubMed]
- Mossanen, J.C.; Krenkel, O.; Ergen, C.; Govaere, O.; Liepelt, A.; Puengel, T.; Heymann, F.; Kalthoff, S.; Lefebvre, E.; Eulberg, D. Chemokine (C-C Motif) Receptor 2–Positive Monocytes Aggravate the Early Phase of Acetaminophen-induced Acute Liver Injury. Hepatology 2016, 64, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Singh, S.; Sharma, N.; Zahoor, I.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules 2022, 27, 3705. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N.; Sachdeva, M.; Behl, T.; Zahoor, I.; Fuloria, N.K.; Sekar, M.; Fuloria, S.; Subramaniyan, V.; Alsubayiel, A.M. Focusing the Pivotal Role of Nanotechnology in Huntington’s Disease: An Insight into the Recent Advancements. Environ. Sci. Pollut. Res. 2022, 29, 73809–73827. [Google Scholar] [CrossRef]
- Sharma, N.; Zahoor, I.; Sachdeva, M.; Subramaniyan, V.; Fuloria, S.; Fuloria, N.K.; Naved, T.; Bhatia, S.; Al-Harrasi, A.; Aleya, L. Deciphering the Role of Nanoparticles for Management of Bacterial Meningitis: An Update on Recent Studies. Environ. Sci. Pollut. Res. 2021, 28, 60459–60476. [Google Scholar] [CrossRef]
- Heneweer, C.; Gendy, S.E.; Peñate-Medina, O. Liposomes and Inorganic Nanoparticles for Drug Delivery and Cancer Imaging. Ther. Deliv. 2012, 3, 645–656. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Guo, X.; Luo, S.; Zhuang, H.; Zhou, J.; Xu, N.; Yan, Z. Chemically Modified Liposomes Carrying TRAIL Target Activated Hepatic Stellate Cells and Ameliorate Hepatic Fibrosis in Vitro and in Vivo. J. Cell. Mol. Med. 2019, 23, 1951–1962. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chan, K.-M.; Chiang, T.; Liu, J.-Y.; Chern, G.-G.; Hsu, F.-F.; Wu, Y.-H.; Liu, Y.-C.; Chen, Y. Dual-Functional Nanoparticles Targeting CXCR4 and Delivering Antiangiogenic SiRNA Ameliorate Liver Fibrosis. Mol. Pharm. 2016, 13, 2253–2262. [Google Scholar] [CrossRef]
- Lebre, F.; Bento, D.; Jesus, S.; Borges, O. Chitosan-Based Nanoparticles as a Hepatitis B Antigen Delivery System. Methods Enzymol. 2012, 509, 127–142. [Google Scholar]
- Craparo, E.F.; Teresi, G.; Licciardi, M.; Bondí, M.L.; Cavallaro, G. Novel Composed Galactosylated Nanodevices Containing a Ribavirin Prodrug as Hepatic Cell-Targeted Carriers for HCV Treatment. J. Biomed. Nanotechnol. 2013, 9, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of Polymeric Nanoparticles for Biomedical Delivery Applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-B.; Fan, Q.-Q.; Xing, L.; Cui, P.-F.; He, Y.-J.; Zhu, J.-C.; Wang, L.; Pang, T.; Oh, Y.-K.; Zhang, C. Vitamin A-Decorated Biocompatible Micelles for Chemogene Therapy of Liver Fibrosis. J. Control. Release 2018, 283, 113–125. [Google Scholar] [CrossRef]
- Fan, Q.-Q.; Zhang, C.-L.; Qiao, J.-B.; Cui, P.-F.; Xing, L.; Oh, Y.-K.; Jiang, H.-L. Extracellular Matrix-Penetrating Nanodrill Micelles for Liver Fibrosis Therapy. Biomaterials 2020, 230, 119616. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.T.; Dong, Z.; Su, L.; Boyer, C.; George, J.; Davis, T.P.; Wang, J. The Use of Nanoparticles to Deliver Nitric Oxide to Hepatic Stellate Cells for Treating Liver Fibrosis and Portal Hypertension. Small 2015, 11, 2291–2304. [Google Scholar] [CrossRef]
- Kurniawan, D.W.; Jajoriya, A.K.; Dhawan, G.; Mishra, D.; Argemi, J.; Bataller, R.; Storm, G.; Mishra, D.P.; Prakash, J.; Bansal, R. Therapeutic Inhibition of Spleen Tyrosine Kinase in Inflammatory Macrophages Using PLGA Nanoparticles for the Treatment of Non-Alcoholic Steatohepatitis. J. Control. Release 2018, 288, 227–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Zha, Y.; Hu, W.; Gao, Z.; Zang, Y.; Chen, J.; Zhang, J.; Dong, L. Corona-Directed Nucleic Acid Delivery into Hepatic Stellate Cells for Liver Fibrosis Therapy. ACS Nano 2015, 9, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Tammam, S.N.; El Safy, S.; Abdel-Halim, M.; Asimakopoulou, A.; Weiskirchen, R.; Mansour, S. Prevention of Hepatic Stellate Cell Activation Using JQ1-and Atorvastatin-Loaded Chitosan Nanoparticles as a Promising Approach in Therapy of Liver Fibrosis. Eur. J. Pharm. Biopharm. 2019, 134, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Nakagawa, S.; Higashi, T.; Vincek, A.; Venkatesh, A.; De Galarreta, M.R.; Koh, A.P.; Goossens, N.; Hirschfield, H.; Bian, C.B. Cell Type-Specific Pharmacological Kinase Inhibition for Cancer Chemoprevention. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 317–325. [Google Scholar] [CrossRef] [PubMed]
- El-Mezayen, N.S.; El-Hadidy, W.F.; El-Refaie, W.M.; Shalaby, T.I.; Khattab, M.M.; El-Khatib, A.S. Hepatic Stellate Cell-Targeted Imatinib Nanomedicine versus Conventional Imatinib: A Novel Strategy with Potent Efficacy in Experimental Liver Fibrosis. J. Control. Release 2017, 266, 226–237. [Google Scholar] [CrossRef]
- El-Mezayen, N.S.; El-Hadidy, W.F.; El-Refaie, W.M.; Shalaby, T.I.; Khattab, M.M.; El-Khatib, A.S. Oral Vitamin-A-Coupled Valsartan Nanomedicine: High Hepatic Stellate Cell Receptors Accessibility and Prolonged Enterohepatic Residence. J. Control. Release 2018, 283, 32–44. [Google Scholar] [CrossRef]
- Tee, J.K.; Peng, F.; Ho, H.K. Effects of Inorganic Nanoparticles on Liver Fibrosis: Optimizing a Double-Edged Sword for Therapeutics. Biochem. Pharmacol. 2019, 160, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic Nanoparticles as Carriers for Efficient Cellular Delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Peng, F.; Tee, J.K.; Setyawati, M.I.; Ding, X.; Yeo, H.L.A.; Tan, Y.L.; Leong, D.T.; Ho, H.K. Inorganic Nanomaterials as Highly Efficient Inhibitors of Cellular Hepatic Fibrosis. ACS Appl. Mater. Interfaces 2018, 10, 31938–31946. [Google Scholar] [CrossRef]
- Adhikari, A.; Polley, N.; Darbar, S.; Bagchi, D.; Pal, S.K. Citrate Functionalized Mn3O4 in Nanotherapy of Hepatic Fibrosis by Oral Administration. Future Sci. OA 2016, 2, FSO146. [Google Scholar] [CrossRef]
- Rani, V.; Verma, Y.; Rana, K.; Rana, S.V.S. Zinc Oxide Nanoparticles Inhibit Dimethylnitrosamine Induced Liver Injury in Rat. Chem. Biol. Interact. 2018, 295, 84–92. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, T.G.; Garcia, V.B.; de Araújo, A.A.; da Silva Gasparotto, L.H.; Silva, H.; Guerra, G.C.B.; de Castro Miguel, E.; de Carvalho Leitão, R.F.; da Silva Costa, D.V.; Cruz, L.J. Spherical Neutral Gold Nanoparticles Improve Anti-Inflammatory Response, Oxidative Stress and Fibrosis in Alcohol-Methamphetamine-Induced Liver Injury in Rats. Int. J. Pharm. 2018, 548, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; EL-Megharbel, S.M.; Altalhi, T.; Gobouri, A.A.; Alrogi, A.A. Hypolipidemic and Hepatoprotective Synergistic Effects of Selenium Nanoparticles and Vitamin. E against Acrylamide-induced Hepatic Alterations in Male Albino Mice. Appl. Organomet. Chem. 2020, 34, e5458. [Google Scholar] [CrossRef]
- Böttger, R.; Pauli, G.; Chao, P.-H.; Fayez, N.A.L.; Hohenwarter, L.; Li, S.-D. Lipid-Based Nanoparticle Technologies for Liver Targeting. Adv. Drug Deliv. Rev. 2020, 154, 79–101. [Google Scholar] [CrossRef]
- Reebye, V.; Huang, K.-W.; Lin, V.; Jarvis, S.; Cutilas, P.; Dorman, S.; Ciriello, S.; Andrikakou, P.; Voutila, J.; Saetrom, P. Gene Activation of CEBPA Using SaRNA: Preclinical Studies of the First in Human SaRNA Drug Candidate for Liver Cancer. Oncogene 2018, 37, 3216–3228. [Google Scholar] [CrossRef]
- Jiménez Calvente, C.; Sehgal, A.; Popov, Y.; Kim, Y.O.; Zevallos, V.; Sahin, U.; Diken, M.; Schuppan, D. Specific Hepatic Delivery of Procollagen A1 (I) Small Interfering RNA in Lipid-like Nanoparticles Resolves Liver Fibrosis. Hepatology 2015, 62, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Leber, N.; Kaps, L.; Aslam, M.; Schupp, J.; Brose, A.; Schäffel, D.; Fischer, K.; Diken, M.; Strand, D.; Koynov, K. SiRNA-Mediated in Vivo Gene Knockdown by Acid-Degradable Cationic Nanohydrogel Particles. J. Control. Release 2017, 248, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Scheyda, K.M.; Warzecha, K.T.; Rizzo, L.Y.; Hittatiya, K.; Luedde, T.; Storm, G.; Trautwein, C.; Lammers, T.; Tacke, F. Fluorescent Cell-Traceable Dexamethasone-Loaded Liposomes for the Treatment of Inflammatory Liver Diseases. Biomaterials 2015, 37, 367–382. [Google Scholar] [CrossRef]
- Lin, T.-T.; Gao, D.-Y.; Liu, Y.-C.; Sung, Y.-C.; Wan, D.; Liu, J.-Y.; Chiang, T.; Wang, L.; Chen, Y. Development and Characterization of Sorafenib-Loaded PLGA Nanoparticles for the Systemic Treatment of Liver Fibrosis. J. Control. Release 2016, 221, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, M.; Fu, L.; Lin, J.; Zhou, X.; Zhou, P.; Huang, P.; Hu, H.; Han, Y. Liver-Targeted Delivery of TSG-6 by Calcium Phosphate Nanoparticles for the Management of Liver Fibrosis. Theranostics 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Nuhn, L.; Aslam, M.; Brose, A.; Foerster, F.; Rosigkeit, S.; Renz, P.; Heck, R.; Kim, Y.O.; Lieberwirth, I. Nanomedicine: In Vivo Gene-Silencing in Fibrotic Liver by SiRNA-Loaded Cationic Nanohydrogel Particles (Adv. Healthcare Mater. 18/2015). Adv. Healthc. Mater. 2015, 4, 2737. [Google Scholar] [CrossRef]
- Violatto, M.B.; Casarin, E.; Talamini, L.; Russo, L.; Baldan, S.; Tondello, C.; Messmer, M.; Hintermann, E.; Rossi, A.; Passoni, A. Dexamethasone Conjugation to Biodegradable Avidin-Nucleic-Acid-Nano-Assemblies Promotes Selective Liver Targeting and Improves Therapeutic Efficacy in an Autoimmune Hepatitis Murine Model. ACS Nano 2019, 13, 4410–4423. [Google Scholar] [CrossRef]
- Melgert, B.N.; Olinga, P.; Jack, V.K.; Molema, G.; Meijer, D.K.F.; Poelstra, K. Dexamethasone Coupled to Albumin Is Selectively Taken up by Rat Nonparenchymal Liver Cells and Attenuates LPS-Induced Activation of Hepatic Cells. J. Hepatol. 2000, 32, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Scalera, A.; Iadevaia, M.D.; Miranda, A.; Zulli, C.; Gaeta, L.; Tuccillo, C.; Federico, A.; Loguercio, C. Herbal Products: Benefits, Limits, and Applications in Chronic Liver Disease. Evid.-Based Complement. Altern. Med. 2012, 2012, 837939. [Google Scholar] [CrossRef]
- Donà, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil Restraint by Green Tea: Inhibition of Inflammation, Associated Angiogenesis, and Pulmonary Fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef]
- El Bedoui, J.; Oak, M.-H.; Anglard, P.; Schini-Kerth, V.B. Catechins Prevent Vascular Smooth Muscle Cell Invasion by Inhibiting MT1-MMP Activity and MMP-2 Expression. Cardiovasc. Res. 2005, 67, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lu, J.; Subramanian, A.; Sonenshein, G.E. Microarray-Assisted Pathway Analysis Identifies Mitogen-Activated Protein Kinase Signaling as a Mediator of Resistance to the Green Tea Polyphenol Epigallocatechin 3-Gallate in Her-2/Neu–Overexpressing Breast Cancer Cells. Cancer Res. 2006, 66, 5322–5329. [Google Scholar] [CrossRef]
- Gaça, M.D.A.; Zhou, X.; Issa, R.; Kiriella, K.; Iredale, J.P.; Benyon, R.C. Basement Membrane-like Matrix Inhibits Proliferation and Collagen Synthesis by Activated Rat Hepatic Stellate Cells: Evidence for Matrix-Dependent Deactivation of Stellate Cells. Matrix Biol. 2003, 22, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhen, M.; Wang, Q.; Huang, X.; Cao, L.; Chen, X.; Sun, K.; Liu, Y.; Li, W.; Zhang, L. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits Oxidative Damage and Preventive Effects on Carbon Tetrachloride–Induced Hepatic Fibrosis. J. Nutr. Biochem. 2007, 18, 795–805. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-Gallate (EGCG) Reduces Liver Inflammation, Oxidative Stress and Fibrosis in Carbon Tetrachloride (CCl4)-Induced Liver Injury in Mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Stanca, E.; Lunetti, P.; Blonda, M.; Tamborra, R.; Siculella, L.; Vendemiale, G.; Capobianco, L.; Giudetti, A.M. Silybin Exerts Antioxidant Effects and Induces Mitochondrial Biogenesis in Liver of Rat with Secondary Biliary Cirrhosis. Free Radic. Biol. Med. 2014, 73, 117–126. [Google Scholar] [CrossRef]
- Stanca, E.; Serviddio, G.; Bellanti, F.; Vendemiale, G.; Siculella, L.; Giudetti, A.M. Down-Regulation of LPCAT Expression Increases Platelet-Activating Factor Level in Cirrhotic Rat Liver: Potential Antiinflammatory Effect of Silybin. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, J.Y.; Wu, T.T.; Ho, P.C.; Huang, C.Y.; Shyu, J.C.; Hsieh, Y.S.; Tsai, C.C.; Liu, Y.C. Effects of Silymarin on the Resolution of Liver Fibrosis Induced by Carbon Tetrachloride in Rats. J. Viral Hepat. 2008, 15, 508–514. [Google Scholar] [CrossRef]
- Kang, J.S.; Morimura, K.; Salim, E.I.; Wanibuchi, H.; Yamaguchi, S.; Fukushima, S. Persistence of Liver Cirrhosis in Association with Proliferation of Nonparenchymal Cells and Altered Location of α-Smooth Muscle Actin-Positive Cells. Toxicol. Pathol. 2005, 33, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Morini, S.; Corradini, S.G.; Franchitto, A.; Merli, M.; Siciliano, M.; Gentili, F.; Muda, A.O.; Berloco, P.; Rossi, M. Alpha-SMA Expression in Hepatic Stellate Cells and Quantitative Analysis of Hepatic Fibrosis in Cirrhosis and in Recurrent Chronic Hepatitis after Liver Transplantation. Dig. Liver Dis. 2005, 37, 349–356. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora Flavescens Ait.: Traditional Usage, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.E.; Xinjing, S.L.; Fan, L.; Vitarbo, J.B.; Arfuso, F.; Tan, C.H.; Narula, A.S.; Kumar, A.P.; Sethi, G.; Ahn, K.S. Anti-Cancer Effects of Oxymatrine Are Mediated through Multiple Molecular Mechanism (s) in Tumor Models. Pharmacol. Res. 2019, 147, 104327. [Google Scholar] [CrossRef]
- Lan, X.; Zhao, J.; Zhang, Y.; Chen, Y.; Liu, Y.; Xu, F. Oxymatrine Exerts Organ-and Tissue-Protective Effects by Regulating Inflammation, Oxidative Stress, Apoptosis, and Fibrosis: From Bench to Bedside. Pharmacol. Res. 2020, 151, 104541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Z.; Chai, X.; Li, G.; Cui, H.; Wang, H.; Meng, Y.; Liu, H.; Wang, J.; Li, R. Oxymatrine Attenuates CCl4-Induced Hepatic Fibrosis via Modulation of TLR4-Dependent Inflammatory and TGF-Β1 Signaling Pathways. Int. Immunopharmacol. 2016, 36, 249–255. [Google Scholar] [CrossRef]
- Wu, X.-L.; Zeng, W.-Z.; Jiang, M.-D.; Qin, J.-P.; Xu, H. Effect of Oxymatrine on the TGFbeta-Smad Signaling Pathway in Rats with CCl4-Induced Hepatic Fibrosis. World J. Gastroenterol. 2008, 14, 2100. [Google Scholar] [CrossRef]
- Du, M.; Zhang, J.; Xu, D.; Li, W.; Liu, J.; Liu, F. Inhibition of Pro-collagen I Expression by Oxymatrine in Hepatic Stellate Cells Is Mediated via Nuclear Translocation of Y-box Binding Protein 1. Mol. Med. Rep. 2015, 12, 8101–8106. [Google Scholar] [CrossRef]
- Shi, G.-F.; Li, Q. Effects of Oxymatrine on Experimental Hepatic Fibrosis and Its Mechanism in Vivo. World J. Gastroenterol. 2005, 11, 268. [Google Scholar] [CrossRef]
- Chai, N.-L.; Fu, Q.; Shi, H.; Cai, C.-H.; Wan, J.; Xu, S.-P.; Wu, B.-Y. Oxymatrine Liposome Attenuates Hepatic Fibrosis via Targeting Hepatic Stellate Cells. World J. Gastroenterol. 2012, 18, 4199. [Google Scholar] [CrossRef]
- Tu, C.; Han, B.; Liu, H.; Zhang, S. Curcumin Protects Mice against Concanavalin A-Induced Hepatitis by Inhibiting Intrahepatic Intercellular Adhesion Molecule-1 (ICAM-1) and CXCL10 Expression. Mol. Cell. Biochem. 2011, 358, 53–60. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Muriel, P. Pharmacological Actions of Curcumin in Liver Diseases or Damage. Liver Int. 2009, 29, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Corson, T.W.; Crews, C.M. Molecular Understanding and Modern Application of Traditional Medicines: Triumphs and Trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdalla, A.M.; Mahmoud, A.M.; Abdelwahab, S.A.; Mahmoud, M.E. Protective Effects of Curcumin, α-Lipoic Acid, and N-Acetylcysteine against Carbon Tetrachloride-Induced Liver Fibrosis in Rats. J. Physiol. Biochem. 2012, 68, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Vergara, P.; Moreno, M.G.; Muriel, P. Curcumin Prevents and Reverses Cirrhosis Induced by Bile Duct Obstruction or CCl4 in Rats: Role of TGF-β Modulation and Oxidative Stress. Fundam. Clin. Pharmacol. 2008, 22, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Zhang, F.; Zhang, Z.-L.; Ma, J.; Kong, D.-S.; Ni, G.-X.; Wang, A.-Y.; Chen, W.-X.; Lu, Y.; Zheng, S.-Z. Acupuncture Combined with Curcumin Disrupts Platelet-Derived Growth Factor β Receptor/Extracellular Signal-Regulated Kinase Signalling and Stimulates Extracellular Matrix Degradation in Carbon Tetrachloride-Induced Hepatic Fibrosis in Rats. Acupunct. Med. 2012, 30, 324–330. [Google Scholar] [CrossRef]
- Wu, S.-J.; Tam, K.-W.; Tsai, Y.-H.; Chang, C.-C.; Chao, J.C.-J. Curcumin and Saikosaponin a Inhibit Chemical-Induced Liver Inflammation and Fibrosis in Rats. Am. J. Chin. Med. 2010, 38, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Yao, Q.; Xu, B.; Wang, J.; Zhou, C.; Zhang, S. Protective Effects of Curcumin against Hepatic Fibrosis Induced by Carbon Tetrachloride: Modulation of High-Mobility Group Box 1, Toll-like Receptor 4 and 2 Expression. Food Chem. Toxicol. 2012, 50, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, A.R.; Zaky, A.; Fawky, F.; Kandeel, K.M. Alteration of AP-Endonuclease1 Expression in Curcumin-Treated Fibrotic Rats. Ann. Hepatol. 2016, 10, 516–530. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Queisser, N.; Wolfson, M.L.; Fraga, C.G.; Adamo, A.M.; Oteiza, P.I. Curcumin Induces Cell-arrest and Apoptosis in Association with the Inhibition of Constitutively Active NF-κB and STAT3 Pathways in Hodgkin’s Lymphoma Cells. Int. J. Cancer 2008, 123, 56–65. [Google Scholar] [CrossRef]
- Shu, J.-C.; He, Y.-J.; Lv, X.; Ye, G.-R.; Wang, L.-X. Curcumin Prevents Liver Fibrosis by Inducing Apoptosis and Suppressing Activation of Hepatic Stellate Cells. J. Nat. Med. 2009, 63, 415–420. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, B.; Wang, J.; Liu, H.; Zhang, S.; Tu, C. Inhibition by Curcumin of Multiple Sites of the Transforming Growth Factor-Beta1 Signalling Pathway Ameliorates the Progression of Liver Fibrosis Induced by Carbon Tetrachloride in Rats. BMC Complement. Altern. Med. 2012, 12, 156. [Google Scholar] [CrossRef]
- Roberts, A.B.; Russo, A.; Felici, A.; Flanders, K.C. Smad3: A Key Player in Pathogenetic Mechanisms Dependent on TGF-β. Ann. N. Y. Acad. Sci. 2003, 995, 1–10. [Google Scholar] [CrossRef]
- Watanabe, S.; Yaginuma, R.; Ikejima, K.; Miyazaki, A. Liver Diseases and Metabolic Syndrome. J. Gastroenterol. 2008, 43, 509–518. [Google Scholar] [CrossRef]
- Stefanović, A.; Kotur-Stevuljević, J.; Spasić, S.; Bogavac-Stanojević, N.; Bujisić, N. The Influence of Obesity on the Oxidative Stress Status and the Concentration of Leptin in Type 2 Diabetes Mellitus Patients. Diabetes Res. Clin. Pract. 2008, 79, 156–163. [Google Scholar] [CrossRef]
- Friedman, J.M. Modern Science versus the Stigma of Obesity. Nat. Med. 2004, 10, 563–569. [Google Scholar] [CrossRef]
- Aleffi, S.; Petrai, I.; Bertolani, C.; Parola, M.; Colombatto, S.; Novo, E.; Vizzutti, F.; Anania, F.A.; Milani, S.; Rombouts, K. Upregulation of Proinflammatory and Proangiogenic Cytokines by Leptin in Human Hepatic Stellate Cells. Hepatology 2005, 42, 1339–1348. [Google Scholar] [CrossRef]
- Cayon, A.; Crespo, J.; Mayorga, M.; Guerra, A.; Pons-Romero, F. Increased Expression of Ob-Rb and Its Relationship with the Overexpression of TGF-β1 and the Stage of Fibrosis in Patients with Nonalcoholic Steatohepatitis. Liver Int. 2006, 26, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, S.; Chen, A. Curcumin Eliminates Leptin’s Effects on Hepatic Stellate Cell Activation via Interrupting Leptin Signaling. Endocrinology 2009, 150, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, A. Curcumin Protects Hepatic Stellate Cells against Leptin-Induced Activation in Vitro by Accumulating Intracellular Lipids. Endocrinology 2010, 151, 4168–4177. [Google Scholar] [CrossRef]
- Bhagya, N.; Chandrashekar, K.R. Tetrandrine and Cancer–An Overview on the Molecular Approach. Biomed. Pharmacother. 2018, 97, 624–632. [Google Scholar]
- Li, D.-G.; Wang, Z.-R.; Lu, H.-M. Pharmacology of Tetrandrine and Its Therapeutic Use in Digestive Diseases. World J. Gastroenterol. 2001, 7, 627. [Google Scholar] [CrossRef]
- Yin, M.-F.; Lian, L.-H.; Piao, D.-M.; Nan, J.-X. Tetrandrine Stimulates the Apoptosis of Hepatic Stellate Cells and Ameliorates Development of Fibrosis in a Thioacetamide Rat Model. World J. Gastroenterol. 2007, 13, 1214. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Wang, T.; Liu, B.; Yang, J.; Chen, X.; Wang, S.; Yin, C.; Ye, Z. Effects of Tetrandrine on Cytosolic Free Calcium Concentration in Corpus Cavernosum Smooth Muscle Cells of Rabbits. Asian J. Androl. 2006, 8, 405–409. [Google Scholar] [CrossRef]
- Bataller, R.; Gasull, X.; Ginès, P.; Hellemans, K.; Görbig, M.N.; Nicolás, J.M.; Sancho-Bru, P.; Heras, D.D.L.; Gual, A.; Geerts, A. In Vitro and in Vivo Activation of Rat Hepatic Stellate Cells Results in de Novo Expression of L-type Voltage-operated Calcium Channels. Hepatology 2001, 33, 956–962. [Google Scholar] [CrossRef]
- Park, P.; Nan, J.; Park, E.; Kang, H.; Kim, J.; Ko, G.; Sohn, D.H. Effect of Tetrandrine on Experimental Hepatic Fibrosis Induced by Bile Duct Ligation and Scission in Rats. Pharmacol. Toxicol. 2000, 87, 261–268. [Google Scholar] [CrossRef]
- Hsu, Y.; Chiu, Y.; Cheng, C.; Wu, C.; Lin, Y.; Huang, Y. Antifibrotic Effects of Tetrandrine on Hepatic Stellate Cells and Rats with Liver Fibrosis. J. Gastroenterol. Hepatol. 2007, 22, 99–111. [Google Scholar] [CrossRef]
- Li, X.; Jin, Q.; Wu, Y.-L.; Sun, P.; Jiang, S.; Zhang, Y.; Zhang, D.-Q.; Zhang, Y.-J.; Lian, L.-H.; Nan, J.-X. Tetrandrine Regulates Hepatic Stellate Cell Activation via TAK1 and NF-ΚB Signaling. Int. Immunopharmacol. 2016, 36, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wu, H. Advancement on Pharmacological Action of Glycyrrhetinic Acid. Med. Recapitul. 2009, 15, 1712–1715. [Google Scholar]
- Gong, X.L.; Luo, Y.; Tang, D.C.; Sun, X.F. Research Progress in Glycyrrhetic Acid and Its Derivatives. Strait Pharm. J. 2008, 20, 4–7. [Google Scholar]
- Luo, Y.; Zhu, M.L.; Sun, X.F.; Liang, Z.Q. Preparation of Water-Soluble Sodium Salt of Glycyrrhetinic Acid and 11-Deoxyglycyrrhetinic Acid and Research on Anti-Inflammatory Effect. Pract. Pharm. Clin. Remedies 2008, 11, 182–184. [Google Scholar]
- Veldt, B.J.; Hansen, B.E.; Ikeda, K.; Verhey, E.; Suzuki, H.; Schalm, S.W. Long-Term Clinical Outcome and Effect of Glycyrrhizin in 1093 Chronic Hepatitis C Patients with Non-Response or Relapse to Interferon. Scand. J. Gastroenterol. 2006, 41, 1087–1094. [Google Scholar] [CrossRef]
- Chen, J.; Pang, J.L.; Qin, Y.M.; Liang, N.C. Effects of 18b-Glycyrrhetinic Acid on the Proliferation, Adhesion and Invasion in HO-8910PM Cells. ShanDong Med. J. 2011, 51, 12–14. [Google Scholar]
- Chen, S.; Zou, L.; Li, L.; Wu, T. The Protective Effect of Glycyrrhetinic Acid on Carbon Tetrachloride-Induced Chronic Liver Fibrosis in Mice via Upregulation of Nrf2. PLoS ONE 2013, 8, e53662. [Google Scholar] [CrossRef]
- Taira, Z.; Yabe, K.; Hamaguchi, Y.; Hirayama, K.; Kishimoto, M.; Ishida, S.; Ueda, Y. Effects of Sho-Saiko-to Extract and Its Components, Baicalin, Baicalein, Glycyrrhizin and Glycyrrhetic Acid, on Pharmacokinetic Behavior of Salicylamide in Carbon Tetrachloride Intoxicated Rats. Food Chem. Toxicol. 2004, 42, 803–807. [Google Scholar] [CrossRef]
- Moro, T.; Shimoyama, Y.; Kushida, M.; Hong, Y.Y.; Nakao, S.; Higashiyama, R.; Sugioka, Y.; Inoue, H.; Okazaki, I.; Inagaki, Y. Glycyrrhizin and Its Metabolite Inhibit Smad3-Mediated Type I Collagen Gene Transcription and Suppress Experimental Murine Liver Fibrosis. Life Sci. 2008, 83, 531–539. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.-L.; Jin, J.; Ma, Y.-C.; Feng, Z.-Q. Glycyrrhizic Acid Inhibits Apoptosis and Fibrosis in Carbon-Tetrachloride-Induced Rat Liver Injury. World J. Gastroenterol. 2015, 21, 5271. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Liu, P. Salvia MiltiorrhizaBurge (Danshen): A Golden Herbal Medicine in Cardiovascular Therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Danshen: An Overview of Its Chemistry, Pharmacology, Pharmacokinetics, and Clinical Use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Zhang, X.; Xu, S.; He, S.; Huang, W.; Roberts, M.S. Compound Astragalus and Salvia Miltiorrhiza Extract Inhibits Cell Invasion by Modulating Transforming Growth Factor-β/Smad in HepG2 Cell. J. Gastroenterol. Hepatol. 2010, 25, 420–426. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, R.; Xu, T.; Zhang, S.; Zhao, Y.; Li, Z.; Wang, C.; Zhou, J.; Gao, D.; Hu, Y. Salvianolic Acid A Attenuates Endoplasmic Reticulum Stress and Protects against Cholestasis-Induced Liver Fibrosis via the SIRT1/HSF1 Pathway. Front. Pharmacol. 2018, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Liu, P.; Hu, Y.-Y.; Xu, L.-M.; Tan, Y.-Z.; Wang, Z.-N.; Liu, C. Effects of Salvianolic Acid-A on Rat Hepatic Stellate Cell Proliferation and Collagen Production in Culture. Acta Pharmacol. Sin. 2000, 21, 721–726. [Google Scholar] [PubMed]
- Qiang, G.; Yang, X.; Xuan, Q.; Shi, L.; Zhang, H.; Chen, B.; Li, X.; Zu, M.; Zhou, D.; Guo, J. Salvianolic Acid A Prevents the Pathological Progression of Hepatic Fibrosis in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Am. J. Chin. Med. 2014, 42, 1183–1198. [Google Scholar] [CrossRef]
- Sinha, S.; Pal, K.; Elkhanany, A.; Dutta, S.; Cao, Y.; Mondal, G.; Iyer, S.; Somasundaram, V.; Couch, F.J.; Shridhar, V. Plumbagin Inhibits Tumorigenesis and Angiogenesis of Ovarian Cancer Cells in Vivo. Int. J. Cancer 2013, 132, 1201–1212. [Google Scholar] [CrossRef]
- Xu, T.-P.; Shen, H.; Liu, L.-X.; Shu, Y.-Q. Plumbagin from Plumbago Zeylanica L Induces Apoptosis in Human Non-Small Cell Lung Cancer Cell Lines through NF-ΚB Inactivation. Asian Pac. J. Cancer Prev. 2013, 14, 2325–2331. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, T.; Zhang, Z.; Liu, X.; Huang, Z.; Zhang, Y.; Li, J. Plumbagin Inhibits Leptin-Induced Proliferation of Hepatic Stellate Cells via JAK2-STAT3 Pathway to Protect against Hepatic Fibrosis. Trop. J. Pharm. Res. 2013, 12, 691–698. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Chen, B.; Cai, Y.; Zou, Z.; Wang, J.; Lin, Z.; Wang, X.; Fu, L.; Hu, Y. Plumbagin Ameliorates CCl4-Induced Hepatic Fibrosis in Rats via the Epidermal Growth Factor Receptor Signaling Pathway. Evid.-Based Complement. Altern. Med. 2015, 2015, 645727. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, M.; Liu, X.; Yuan, Z.; Peng, Y.; Huang, Z.; Duan, X.; Zhao, T. Anti-Fibrotic Effect of Plumbagin on CCl4-Lesioned Rats. Cell. Physiol. Biochem. 2015, 35, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.-X.; Park, E.-J.; Kim, Y.-C.; Ko, G.; Sohn, D.H. Scutellaria baicalensis Inhibits Liver Fibrosis Induced by Bile Duct Ligation or Carbon Tetrachloride in Rats. J. Pharm. Pharmacol. 2002, 54, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-L.; Wang, P.-W.; Leu, Y.-L.; Wu, T.-H.; Wu, T.-S. Inhibitory Effects of Scutellaria baicalensis Extract on Hepatic Stellate Cells through Inducing G2/M Cell Cycle Arrest and Activating ERK-Dependent Apoptosis via Bax and Caspase Pathway. J. Ethnopharmacol. 2012, 139, 829–837. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhou, S.Z.; Cheng, X.Y.; Yi, B.; Shan, S.Z.; Wang, J.; Li, Q.F. Baicalein Attenuates Hypertrophic Scar Formation via Inhibition of the Transforming Growth Factor-β/Smad2/3 Signalling Pathway. Br. J. Dermatol. 2016, 174, 120–130. [Google Scholar] [CrossRef]
- Sun, H.; Che, Q.-M.; Zhao, X.; Pu, X.-P. Antifibrotic Effects of Chronic Baicalein Administration in a CCl4 Liver Fibrosis Model in Rats. Eur. J. Pharmacol. 2010, 631, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gong, Z.; Wang, B.; Guo, X.; Yang, L.; Li, D.; Zhang, Y. Astragaloside Inhibits Hepatic Fibrosis by Modulation of TGF-Β1/Smad Signaling Pathway. Evid.-Based Complement. Altern. Med. 2018, 2018, 3231647. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Z.; Zhao, Q.; Zhao, Z.; Liu, C. A Combination of Astragaloside I, Levistilide A and Calycosin Exerts Anti-Liver Fibrosis Effects in Vitro and in Vivo. Acta Pharmacol. Sin. 2018, 39, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Yongping, M.; Zhang, X.; Xuewei, L.; Fan, W.; Chen, J.; Zhang, H.; Chen, G.; Liu, C.; Liu, P. Astragaloside Prevents BDL-Induced Liver Fibrosis through Inhibition of Notch Signaling Activation. J. Ethnopharmacol. 2015, 169, 200–209. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, M.; Li, Q.; Zhang, F.; Wei, D.; Wen, Z.; Zhou, Y. Astragaloside Alleviates Hepatic Fibrosis Function via PAR2 Signaling Pathway in Diabetic Rats. Cell. Physiol. Biochem. 2017, 41, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Lashin, F.M.; Gamel, M.; Hassanin, S.O.; Abdalla, Y.; Amin, A. Hawthorn Herbal Preparation from Crataegus Oxyacantha Attenuates in Vivo Carbon Tetrachloride-Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants 2020, 9, 1173. [Google Scholar] [CrossRef]
- Lin, L.; Li, R.; Cai, M.; Huang, J.; Huang, W.; Guo, Y.; Yang, L.; Yang, G.; Lan, T.; Zhu, K. Andrographolide Ameliorates Liver Fibrosis in Mice: Involvement of TLR4/NF-ΚB and TGF-Β1/Smad2 Signaling Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 7808656. [Google Scholar] [CrossRef]
- Yan, H.; Huang, Z.; Bai, Q.; Sheng, Y.; Hao, Z.; Wang, Z.; Ji, L. Natural Product Andrographolide Alleviated APAP-Induced Liver Fibrosis by Activating Nrf2 Antioxidant Pathway. Toxicology 2018, 396, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, J.; Chen, F.; Guo, L.; Zhu, Z.; Shi, J. An Anti-ROS/Hepatic Fibrosis Drug Delivery System Based on Salvianolic Acid B Loaded Mesoporous Silica Nanoparticles. Biomaterials 2010, 31, 7785–7796. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, L.; Zhu, N.; Wang, K.; Xia, Y. Fabrication of Antimicrobial Curcumin Stabilized Platinum Nanoparticles and Their Anti-Liver Fibrosis Activity for Potential Use in Nursing Care. J. Photochem. Photobiol. B Biol. 2019, 195, 27–32. [Google Scholar] [CrossRef]
- Krithika, R.; Vhora, I.; Verma, R.J. Preparation, Toxicity Analysis and in Vivo Protective Effect of Phyllanthin-Loaded PLGA Nanoparticles against CCl4-Induced Hepatic Fibrosis. J. Drug Deliv. Sci. Technol. 2019, 51, 364–371. [Google Scholar] [CrossRef]
- Younis, N.; Shaheen, M.A.; Abdallah, M.H. Silymarin-Loaded Eudragit® RS100 Nanoparticles Improved the Ability of Silymarin to Resolve Hepatic Fibrosis in Bile Duct Ligated Rats. Biomed. Pharmacother. 2016, 81, 93–103. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Al-Sawahli, M.M.; Ahmed, O.A.A.; Fahmy, U.A.; Abdallah, H.M.; Hattori, M.; Ashour, O.M.; Abdel-Naim, A.B. Curcumin-Zein Nanospheres Improve Liver Targeting and Antifibrotic Activity of Curcumin in Carbon Tetrachloride-Induced Mice Liver Fibrosis. J. Biomed. Nanotechnol. 2016, 12, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.-L.; Kok, S.-L.; Gambari, R.; Kok, T.-W.; Leung, H.-Y.; Choi, K.-L.; Wong, C.-S.; Hau, D.-P.; Wong, W.-Y.; Lam, K.H. Evaluation of Berberine/Bovine Serum Albumin Nanoparticles for Liver Fibrosis Therapy. Green Chem. 2015, 17, 1640–1646. [Google Scholar] [CrossRef]
- Huang, S.; Chang, S.-J.; Yang, M.; Chen, J.J.-C.; Chang, W.H. Nanoscale Hepatoprotective Herbal Decoction Attenuates Hepatic Stellate Cell Activity and Chloroform-Induced Liver Damage in Mice. Int. J. Nanomed. 2011, 6, 1365. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin Loaded Multifunctional Nanoparticles to Enhance the Chemotherapeutic Efficacy in Hepatic Fibrosis. Biomed. Microdevices 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Negm, M.; Ahmed, N.; Barakat, L. The Role of Curcumin–Chitosan Nanoparticles in the Prevention and Treatment of Liver Fibrosis in Mice. Alfarama J. Basic Appl. Sci. 2022, 3, 8–28. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of Rat Liver Fibrosis by Selective Targeting of Hepatic Stellate Cells Using Hesperidin Carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, W.; Wang, Y.; Lei, W.; Feng, B.; Du, C.; Wang, X. Enhanced Efficacy of Curcumin with Phosphatidylserine-Decorated Nanoparticles in the Treatment of Hepatic Fibrosis. Drug Deliv. 2018, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticle Type | Nanoparticle Subtype | Drug | Targeted Ligands | Targeted Biological Structures | Ref. |
|---|---|---|---|---|---|
| Polymeric NPs | Poly lactic co glycolic b poly(ethylene glycol) maleimide micelle | Nilotinib | Collagenase I and retinol | Hepatic stellate cell | [97] |
| Diblock copolymers (POEGMA-b-VDM) | S-nitrosoglutathione | Vitamin A | Hepatic stellate cell | [98] | |
| Poly (lactic-co-glycolic acid) NPs | Spleen tyrosine kinase inhibitor (R406) | R406 | Macrophages | [99] | |
| Retinol conjugated polyetherimine nanoparticle | Antisense oligonucleotide (DNA oligomers) | Retinol conjugated polyetherimine | Hepatic stellate cell | [100] | |
| Retinol-chitosan NPs | Atorvastatin and JQ1 (which is thienotriazolodiazepine and acts as selective inhibitor of Bromodomain-containing Protein 4 signaling pathway) | Vitamin A | Hepatic stellate cell | [101] | |
| Inorganic NPs | Erlotinib-loaded myofibroblast-targeting nanoparticles | Erlotinib | Epidermal growth factor receptor | Hepatic sinusoids | [102] |
| Lipid based NPs | Liposomes | Imatinib | Vitamin A | Hepatic stellate cell | [103] |
| Liposomes | Valsartan | Vitamin A | Hepatic stellate cell | [104] | |
| Liposomes | Antiangiogenic siRNA | Vascular endothelial growth factor siRNA (Small interfering RNA) | Hepatic stellate cell | [91] |
| Type of Nanoparticles (NPs) | NPs as Drug Carrier | Drug | Ref. |
|---|---|---|---|
| Lipid based NPs | RNA oligonucleotide liposomal | MTL-CEBPA (saRNA) | [114] |
| Liposomes | Dexamethasone | [118] | |
| Cationic lipid NPs | Small interfering ribose nucleic acid to the procallogen 1 (I) gene | [115] | |
| Inorganic NPs | Poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) | Sorafenib | [119] |
| Iron oxide nanoparticles | Citrate | [54] | |
| Calcium phosphate NPs | Tumor necrosis factor-stimulated gene 6 | [120] | |
| Polymeric NPs | Ketal cross-linked cationic nanohydrogel | Cy5-labeled anti-col1α1 siRNA | [117] |
| Cationic nanohydrogel particles | Anti-col1α1 siRNA | [121] | |
| Protein NPs | Polyavidin-based NPs | Dexamethasone | [122] |
| Albumin NPs | Dexamethasone | [123] |
| Phytoconstituent | Inference | Ref. |
|---|---|---|
| Salvianolic acid B | According to the study, SAB@MSNs-RhB had significantly greater sustained-release capability than SAB@MSNs and displayed higher release rates and concentrations in the sustained release pattern beyond 96 h. These nanoparticles also improved cellular drug uptake, bioaccessibility, higher efficacy in suppressing reactive oxygen species levels, and management of hepatic fibrosis. | [204] |
| Curcumin | The results of the study showed that curcumin platinum nanoparticles had greater activity at concentrations of 1.5 and 1.25 g/mL, causing the viability of NIH3T3 cells to significantly decline to 44.06% and 48.96%, respectively, for decreasing collagen production by NIH3T3 fibroblast cell line, making them a suitable candidate for hepatic fibrosis. | [205] |
| Phyllanthin | The results of the study demonstrated that Phyllanthin nanoparticles could reverse the biochemical and histological alterations brought on by the hepatotoxin and produced a therapeutic effect at a dose of 5 mg/kg body weight, which was half the dose of conventional medication in carbon tetrachloride (CCl4)-induced fibrotic model. | [206] |
| Silymarin | The study looked at the effects of silymarin-loaded Eudragit RS100 nanoparticles for hepatoprotective and anti-fibrotic benefits of silymarin in cholestatic liver fibrosis, which acts by restoring liver function through antioxidant activity, which eventually leads to enhancement in anti-fibrotic effect in bile duct ligation induced fibrotic model. | [207] |
| Curcumin | According to the study, the bioavailability of the drug in curcumin-loaded zein nanospheres increased by 3.24 times when compared to the free solution, making them an ideal carrier for enhanced liver targeting and anti-fibrotic effectiveness in CCl4-induced fibrotic models. | [208] |
| Berberine | The study demonstrated that berberine-loaded bovine serum albumin nanoparticles decreased LX-2 cell growth and showed stronger caspase 3 activations at a lower dosage in comparison to free drugs. These nanoparticles exhibited drug release of 80% in 72 h, demonstrating sustained drug release. Berberine-loaded NPs provided hepatoprotection in CCl4-induced hepatotoxicity. | [209] |
| San-Huang-Xie-Xin-Tang (SHXXT) decoction | The research results showed that nanoformulation of SHXXT decoction had remarkable healing potential, as evident through a reduction in AST and ALT levels in liver injury in the chloroform-induced liver fibrotic model. | [210] |
| Naringenin | According to the study, naringenin-loaded solid lipid nanoparticles significantly decreased CCl4-induced liver fibrosis through a decrease in biochemical markers, including serum ALP, AST, and total bilirubin, as well as pro-inflammatory markers such as TNF-, IL1β, and IL-6. In addition, pro-MMP-2 and MMP-2 activation in the HSCs were enhanced by naringenin-SLN. | [211] |
| Curcumin | The results of the study showed that nano-curcumin nano-chitosan mixtures had considerable hepatoprotective action through regulation of the liver enzymes ALT, AST, and ALP; AFP; caspase-3; oxidative stress biomarker such as malondialdehyde; antioxidant biomarkers such as glutathione and catalase in CCl4-induced liver fibrosis in mice model. | [212] |
| Hesperidin | The study’s findings demonstrated that, in contrast to hesperidin alone, DSPE-SPE-sebacic acid conjugated liposomes loaded with hesperidin provided selective targeting of HSCs in CCL4-induced rat liver fibrosis. This was demonstrated by a significant reduction in serum ALT, AST, and ALP activities as well as albumin levels. | [213] |
| Curcumin | In CCl4-induced liver fibrosis in rats, phosphatidylserine-modified-NLCs loaded with curcumin selectively targeted hepatic macrophages as demonstrated by their highest concentration in the liver. These nanoparticles upregulated the levels of hepatocyte growth factors and matrix metalloprotease while downregulating the amounts of collagen fibers and alpha-smooth muscle actin. They also enhanced the levels of liver enzymes and pro-inflammatory cytokines in blood circulation. | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sharma, N.; Shukla, S.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Bungau, S.G.; Brisc, C. Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics. Molecules 2023, 28, 2811. https://doi.org/10.3390/molecules28062811
Singh S, Sharma N, Shukla S, Behl T, Gupta S, Anwer MK, Vargas-De-La-Cruz C, Bungau SG, Brisc C. Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics. Molecules. 2023; 28(6):2811. https://doi.org/10.3390/molecules28062811
Chicago/Turabian StyleSingh, Sukhbir, Neelam Sharma, Saurabh Shukla, Tapan Behl, Sumeet Gupta, Md. Khalid Anwer, Celia Vargas-De-La-Cruz, Simona Gabriela Bungau, and Cristina Brisc. 2023. "Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics" Molecules 28, no. 6: 2811. https://doi.org/10.3390/molecules28062811
APA StyleSingh, S., Sharma, N., Shukla, S., Behl, T., Gupta, S., Anwer, M. K., Vargas-De-La-Cruz, C., Bungau, S. G., & Brisc, C. (2023). Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics. Molecules, 28(6), 2811. https://doi.org/10.3390/molecules28062811