One-Step Synthesis of Self-Stratification Core-Shell Latex for Antimicrobial Coating
Abstract
1. Introduction
2. Results
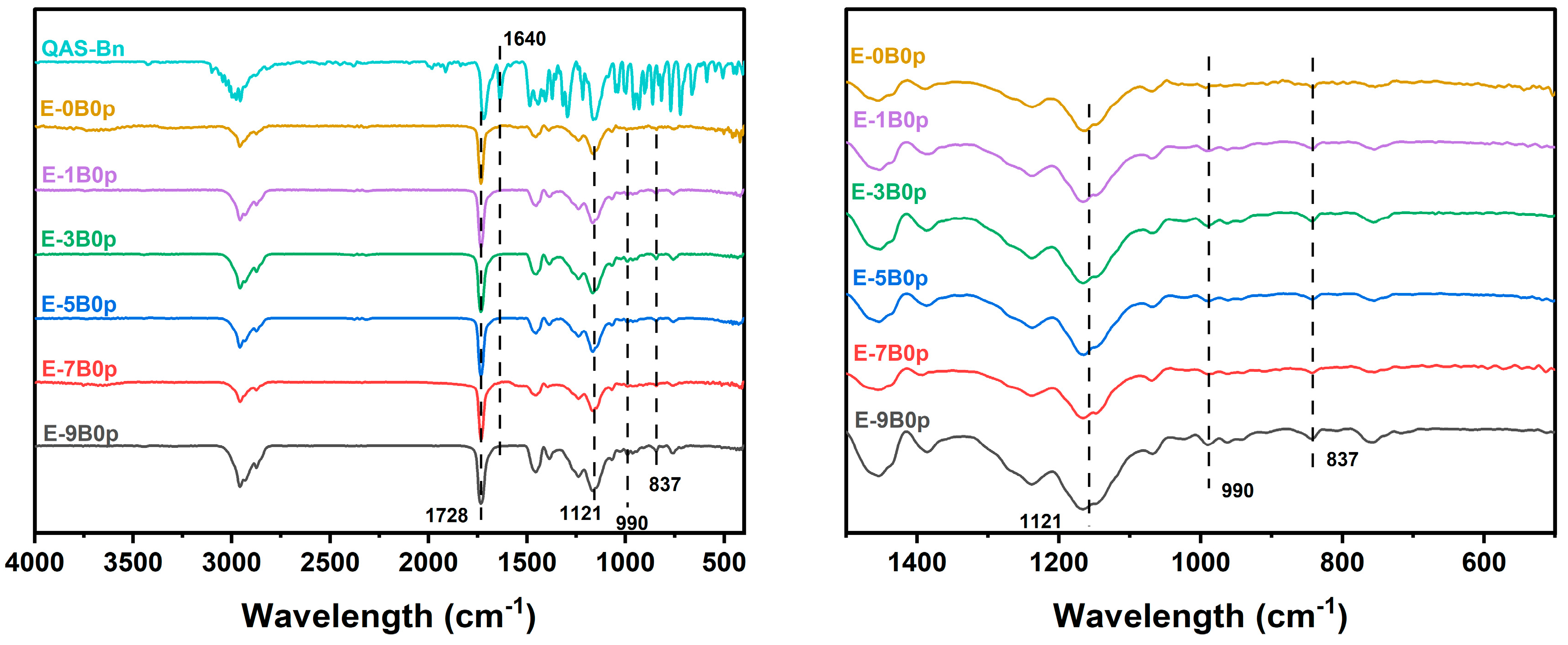
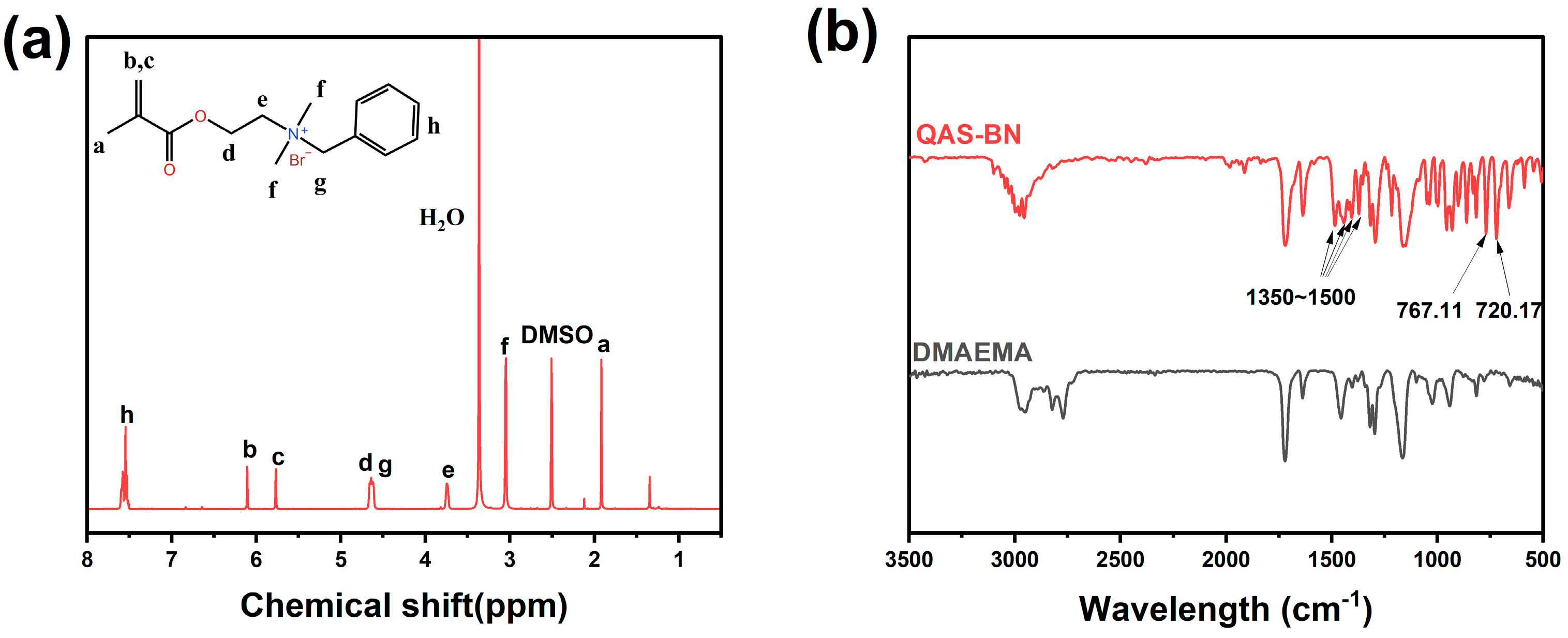
2.1. Copolymer Verification
2.2. The Effect of QAS-BN Dosage on the Emulsion Performance
2.2.1. Size and Zeta Potential of Latex Particles
2.2.2. Morphology of Latex Particles
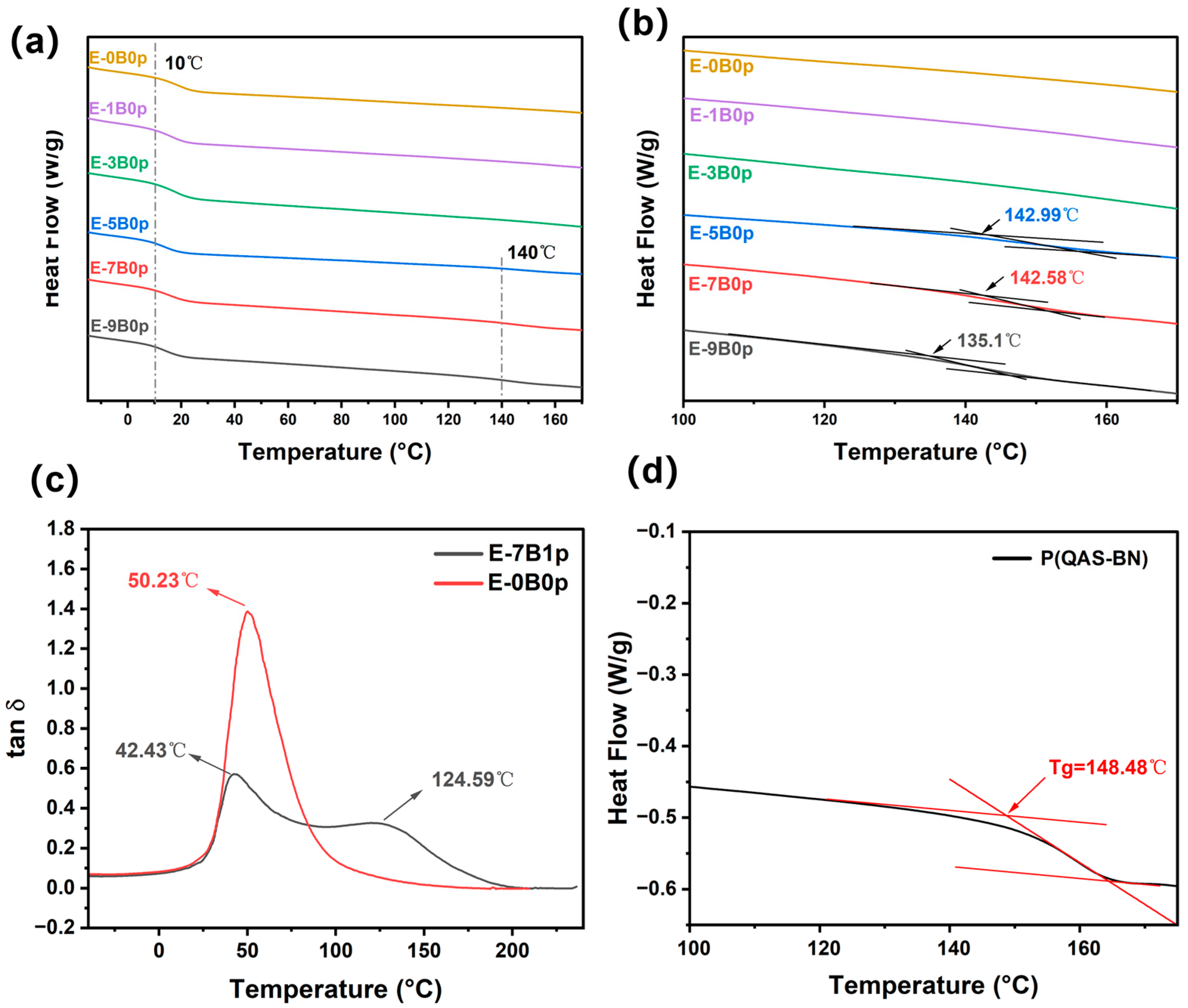
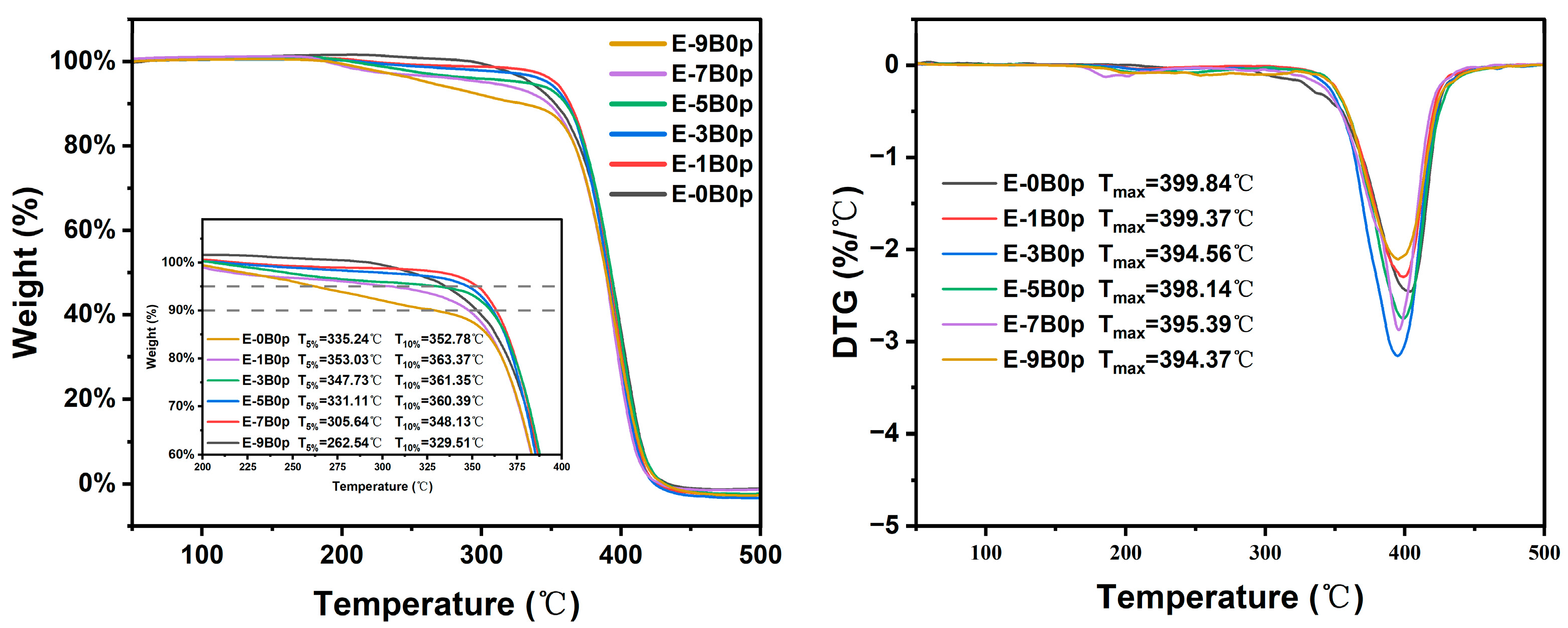
2.2.3. Thermal Properties of Latex Films
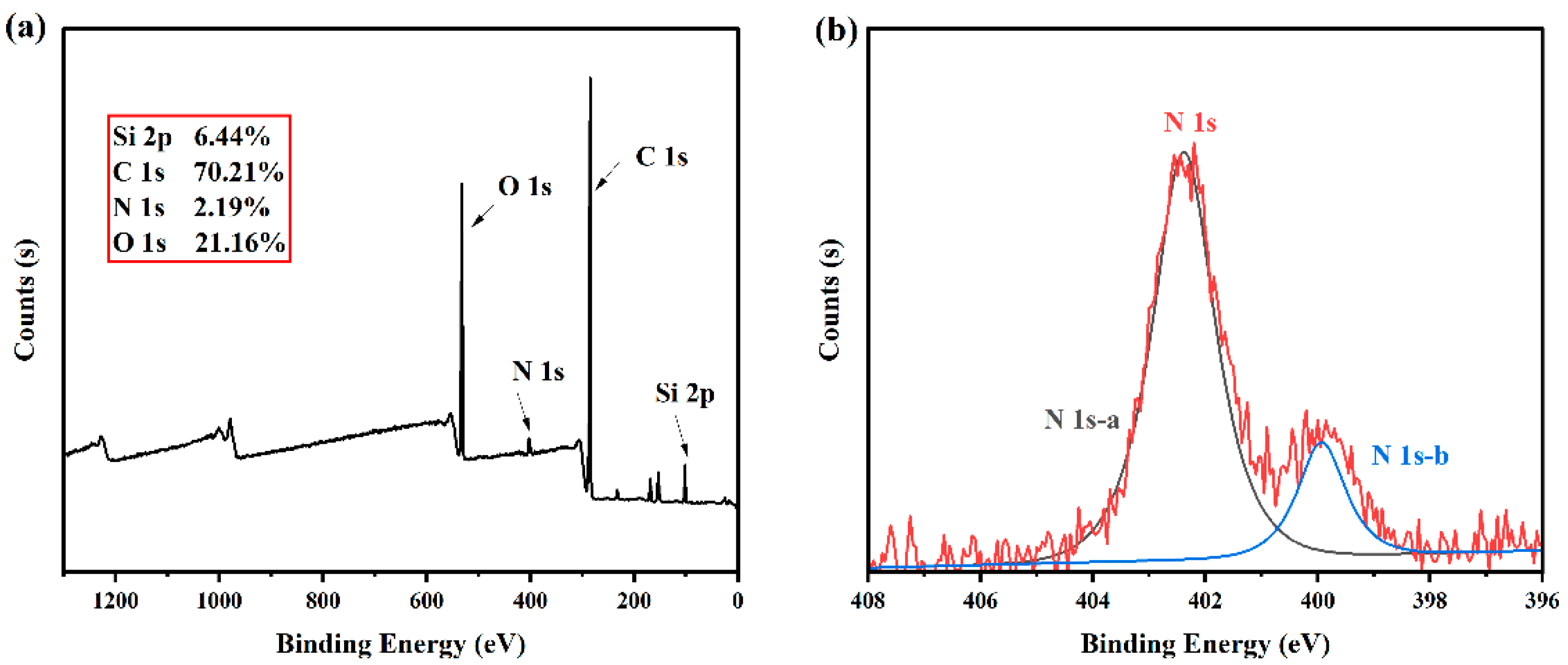
2.2.4. XPS of Latex Film
2.2.5. Surface Morphology of Latex Film
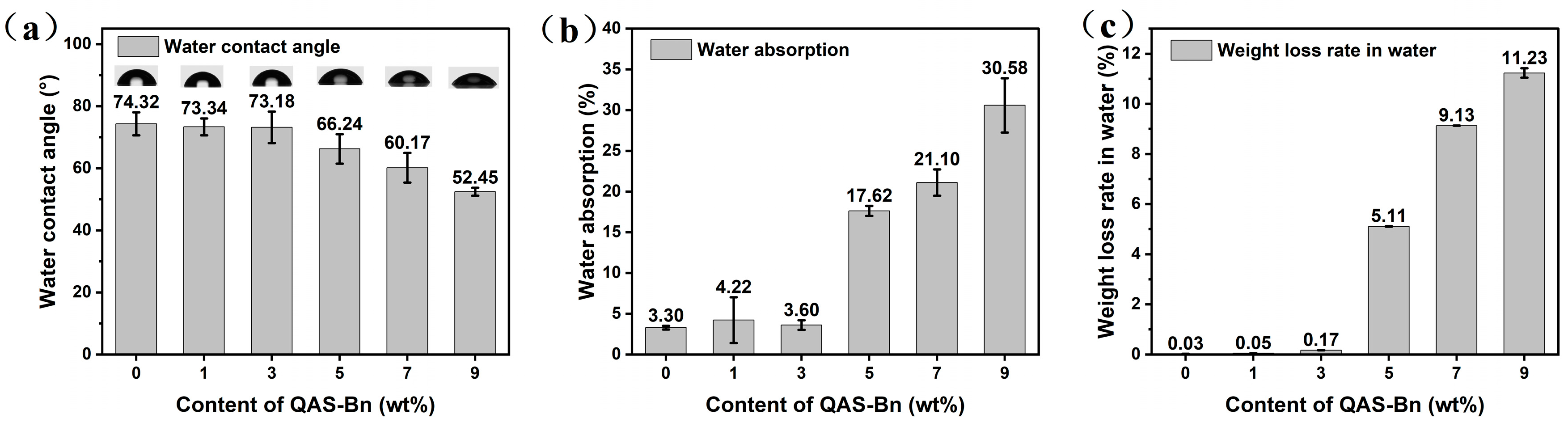
2.2.6. Hydrophobicity of Latex Film
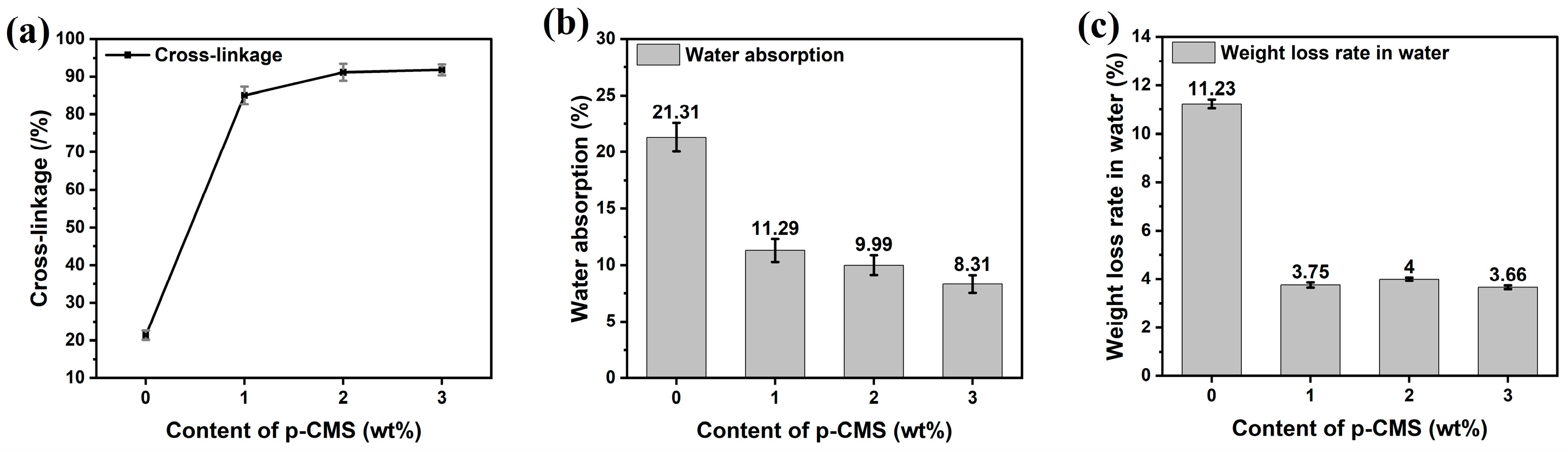
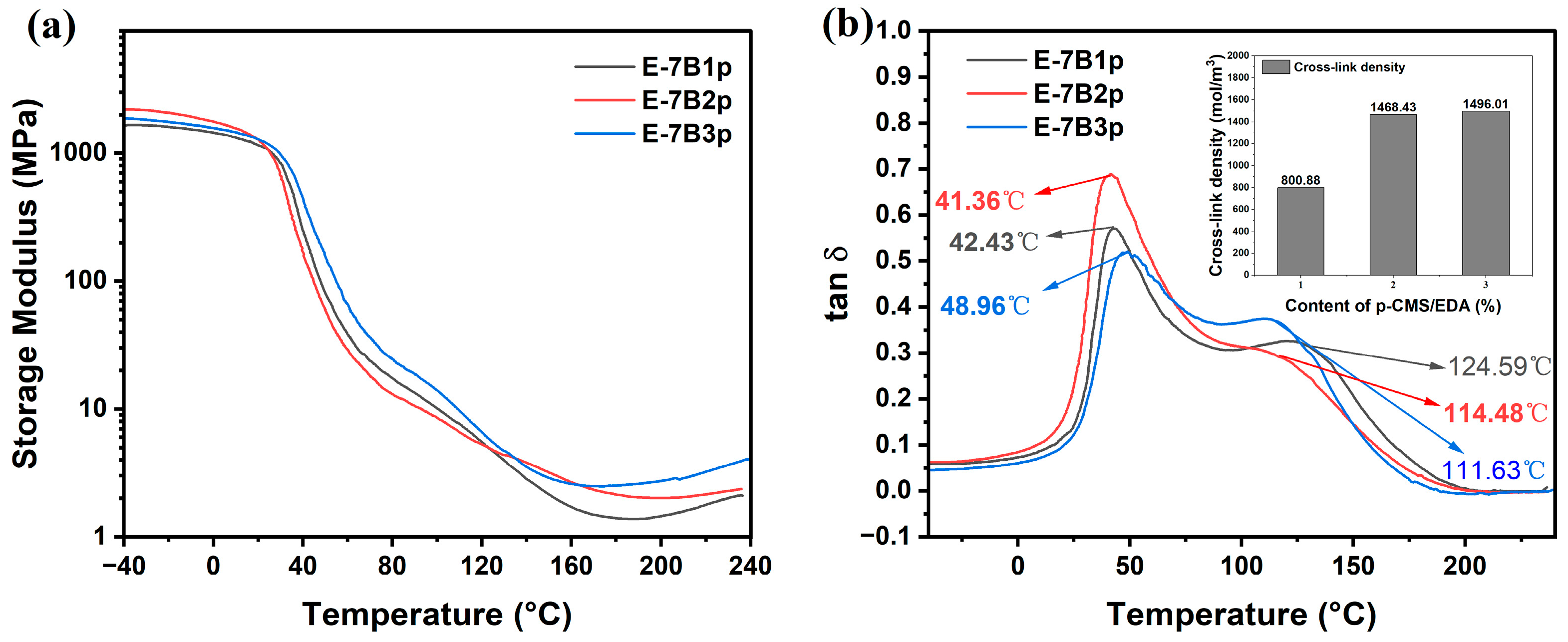
2.3. Effect of Crosslinking Agent
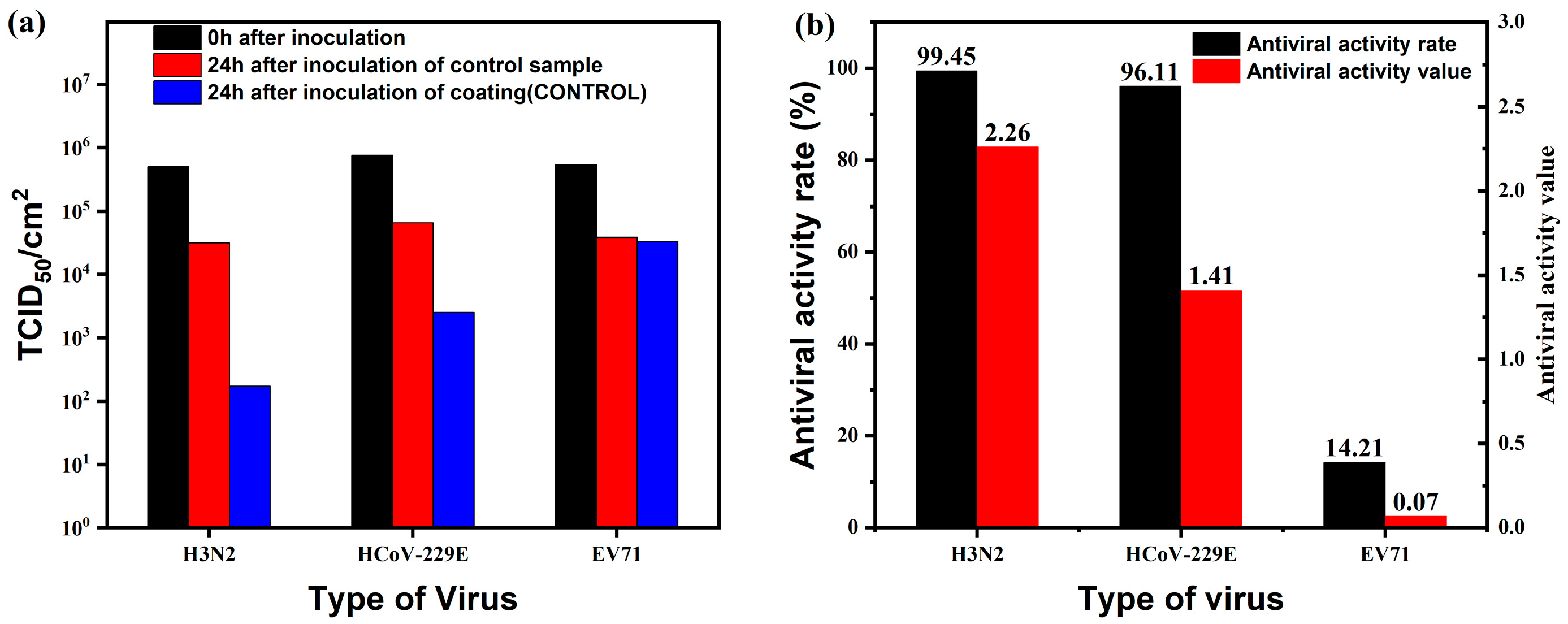
2.4. Antimicrobial Properties of Coatings
3. Materials and Methods
3.1. Materials
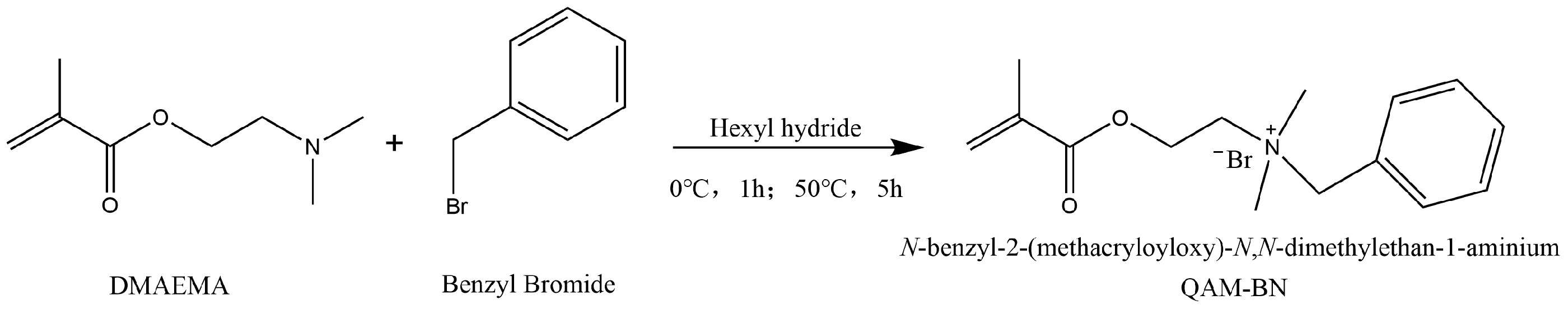
3.2. Synthesis of Antimicrobial Functional Monomer
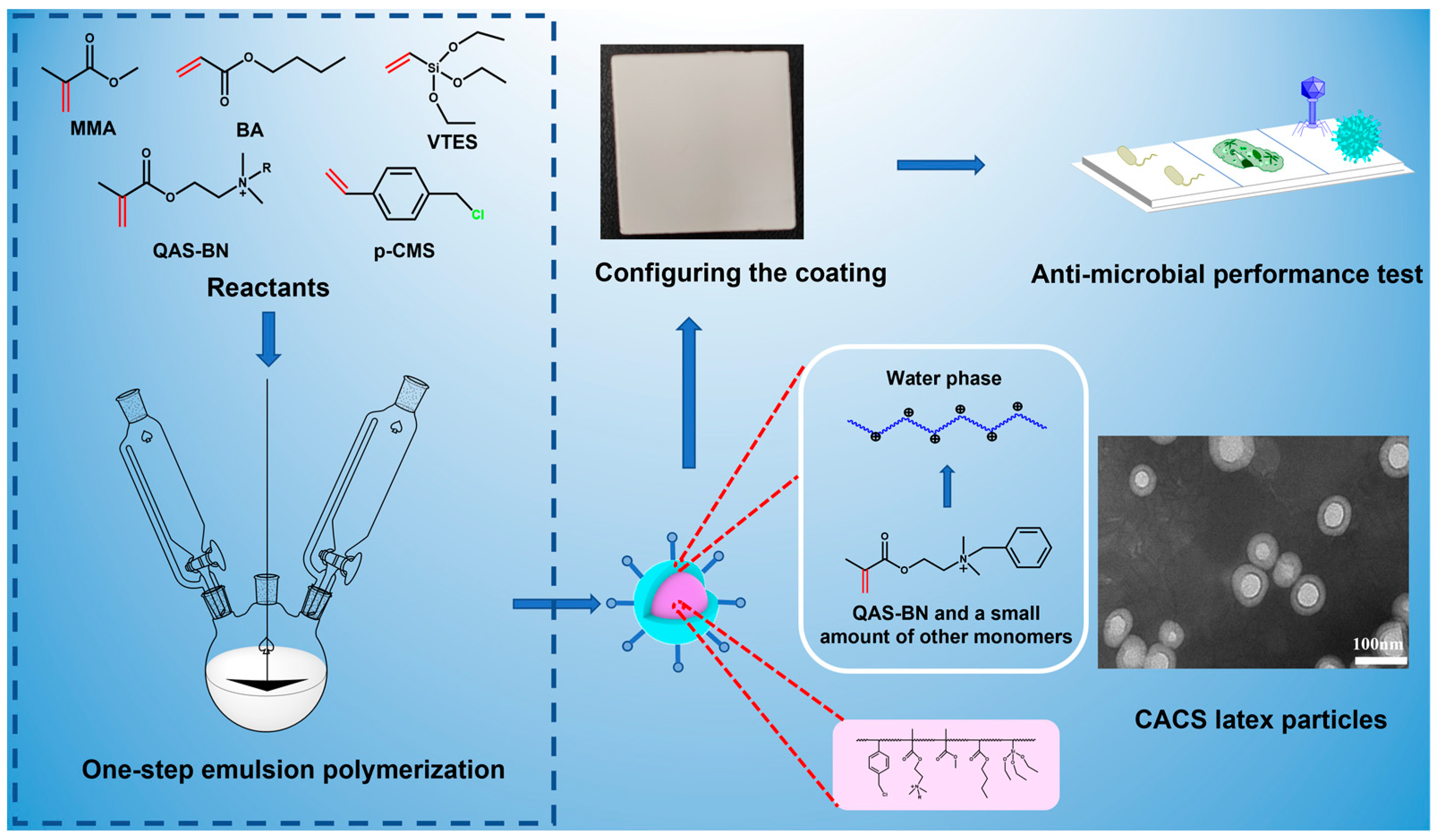
3.3. Synthesis of CACS Emulsion
3.4. Coating Preparation
3.5. Characterization
3.5.1. Emulsion Performance
3.5.2. Film Performance
3.5.3. Antimicrobial Properties of Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saud, Z.; Richards, C.A.J.; Williams, G.; Stanton, R.J. Anti-Viral Organic Coatings for High Touch Surfaces Based on Smart-Release, Cu2+ Containing Pigments. Prog. Org. Coat. 2022, 172, 107135. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Whitten, D.G.; Kell, A. Fluorescent Cellulose Wipe as a New and Sustainable Light-Activated Antibacterial and Antiviral Agent. ACS Mater. Lett. 2022, 4, 356–362. [Google Scholar] [CrossRef]
- Kalli, S.; Araya-Cloutier, C.; Hageman, J.; Vincken, J.-P. Insights into the Molecular Properties Underlying Antibacterial Activity of Prenylated (Iso)Flavonoids against MRSA. Sci. Rep. 2021, 11, 14180. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, B.; Liu, X.; Li, Z.; Zhu, S.; Liang, Y.; Cui, Z.; Wu, S. Recent Progress in Photocatalytic Antibacterial. ACS Appl. Bio Mater. 2021, 4, 3909–3936. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Llorente, O.; Barquero, A.; Paulis, M.; Leiza, J.R. Challenges to Incorporate High Contents of Bio-Based Isobornyl Methacrylate (IBOMA) into Waterborne Coatings. Prog. Org. Coat. 2022, 172, 107137. [Google Scholar] [CrossRef]
- Gao, D.; Li, Y.; Lyu, B.; Jin, D.; Ma, J. Silicone Quaternary Ammonium Salt Based Nanocomposite: A Long-Acting Antibacterial Cotton Fabric Finishing Agent with Good Softness and Air Permeability. Cellulose 2020, 27, 1055–1069. [Google Scholar] [CrossRef]
- Wang, L.-S.; Xu, S.; Gopal, S.; Kim, E.; Kim, D.; Brier, M.; Solanki, K.; Dordick, J.S. Facile Fabrication of Antibacterial and Antiviral Perhydrolase-Polydopamine Composite Coatings. Sci. Rep. 2021, 11, 12410. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Lin, Z.; Ding, L.; Zhang, X.; Dai, R.; Yan, Q.; Wang, X. Highly Sensitive Dissolved Oxygen Sensor with a Sustainable Antifouling, Antiabrasion, and Self-Cleaning Superhydrophobic Surface. ACS Omega 2019, 4, 1715–1721. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, B.; Yu, B.; Ma, B.; Hu, C.; Ulbricht, M.; Qu, J. Maintaining Antibacterial Activity against Biofouling Using a Quaternary Ammonium Membrane Coupling with Electrorepulsion. Environ. Sci. Technol. 2023, 57, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Heo, J.W.; Chen, J.; Kim, Y.S. Water-Soluble Lignin Quaternary Ammonium Salt for Electrospun Morphology-Controllable Antibacterial Polyvinyl Alcohol/ Lignin Quaternary Ammonium Salt Nanofibers. J. Clean. Prod. 2022, 368, 133219. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Zhang, Y.; Tan, L. Improvement of Antibacterial and Antifouling Properties of a Cellulose Acetate Membrane by Surface Grafting Quaternary Ammonium Salt. ACS Appl. Mater. Interfaces 2022, 14, 38358–38369. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Matinlinna, J.P.; Pichika, M.R.; Mak, K.-K.; Nagendrababu, V.; Fawzy, A.S. A Quaternary Ammonium Silane Antimicrobial Triggers Bacterial Membrane and Biofilm Destruction. Sci. Rep. 2020, 10, 10970. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Z.; Xiang, J.; Fan, H.; Chen, Y.; Yan, J. Preparation and Characteristics of Waterborne Polyurethane with Various Lengths of Fluorinated Side Chains. Appl. Surf. Sci. 2019, 494, 610–618. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, J.; Zhou, J.; Wang, Y.; Zhang, J. Bio-Based Core-Shell Casein-Based Silica Nano-Composite Latex by Double-in Situ Polymerization: Synthesis, Characterization and Mechanism. Chem. Eng. J. 2013, 228, 281–289. [Google Scholar] [CrossRef]
- Zhi, L.; Shi, X.; Zhang, E.; Gao, C.; Gai, H.; Wang, H.; Liu, Z.; Zhang, T. Synthesis and Performance of Double-Chain Quaternary Ammonium Salt Glucosamide Surfactants. Molecules 2022, 27, 2149. [Google Scholar] [CrossRef]
- Zeng, M.; Xu, J.; Luo, Q.; Hou, C.; Qiao, S.; Fu, S.; Fan, X.; Liu, J. Constructing Antibacterial Polymer Nanocapsules Based on Pyridine Quaternary Ammonium Salt. Mater. Sci. Eng. C 2020, 108, 110383. [Google Scholar] [CrossRef]
- Kawabata, N.; Yamazaki, K.; Otake, T.; Oishi, I.; Minekawa, Y. Removal of Pathogenic Human Viruses by Insoluble Pyridinium-Type Resin. Epidemiol. Infect. 1990, 105, 633–642. [Google Scholar] [CrossRef]
- Wood, A.; Payne, D. The Action of Three Antiseptics/Disinfectants against Enveloped and Non-Enveloped Viruses. J. Hosp. Infect. 1998, 38, 283–295. [Google Scholar] [CrossRef]
- Sundberg, D. Structured, Composite Nanoparticles from Emulsion Polymerization—Morphological Possibilities. Biomacromolecules 2020, 21, 4388–4395. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kumar, D. Core-Shell Quantum Dots: A Review on Classification, Materials, Application, and Theoretical Modeling. J. Alloys Compd. 2022, 924, 166508. [Google Scholar] [CrossRef]
- Zou, H.; Luo, Z.; Yang, X.; Xie, Q.; Zhou, Y. Toward Emerging Applications Using Core-Shell Nanostructured Materials: A Review. J. Mater. Sci. 2022, 57, 10912–10942. [Google Scholar] [CrossRef]
- Duan, S.; Wu, R.; Xiong, Y.-H.; Ren, H.-M.; Lei, C.; Zhao, Y.-Q.; Zhang, X.-Y.; Xu, F.-J. Multifunctional Antimicrobial Materials: From Rational Design to Biomedical Applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Zhang, X.; Wang, F.; Song, J.; Zhang, W.; Wang, Z.; Li, M. Nonmetal (S or Se)-Bridged Core Shell Electrodes for Promoting Electrochemical Performance. Adv. Energy Mater. 2022, 12, 2200777. [Google Scholar] [CrossRef]
- Yang, J.; Mu, Y.; Li, X. Morphology of Multilayer Core-Shell Emulsion: Influence of Crosslinking Agent. Mater. Lett. 2022, 322, 132493. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Ozkan, C.K.; Yilmaz, O. Synthesis and Application of Reactive Acrylic Latexes: Effect of Particle Morphology. Polymers 2022, 14, 2187. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, W.; Xu, P.; Cai, X.; Dong, W.; Chen, M.; Du, M.; Liu, T.; Jan Lemstra, P.; Ma, P. The Bonding Strength, Water Resistance and Flame Retardancy of Soy Protein-Based Adhesive by Incorporating Tailor-Made Core-Shell Nanohybrid Compounds. Chem. Eng. J. 2022, 428, 132390. [Google Scholar] [CrossRef]
- Chen, D.; Ding, M.; Huang, Z.; Wang, Y. Styrene–Acrylic Emulsion with “Transition Layer” for Damping Coating: Synthesis and Characterization. Polymers 2021, 13, 1406. [Google Scholar] [CrossRef]
- Mu, Y.; Han, L.; Du, D.; Yang, Y.; Li, X. Reversible Thermochromic and Thermal Insulation Performances of K2O·nSiO2 Based Fire-Resistant Glass via in-Situ Preparation. Constr. Build. Mater. 2022, 344, 128168. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Bao, Y.; Liu, J.; Zhang, J. Research Advances in Polymer Emulsion Based on “Core-Shell” Structure Particle Design. Adv. Colloid Interface Sci. 2013, 197–198, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yang, Y.; Xu, L.; Zhang, Y.; Hu, Y.; Xu, Z. In-Situ Preparation and Performance of Cold Resistant K2O·5SiO2 Based Anti-Fire Glass. Constr. Build. Mater. 2021, 308, 125067. [Google Scholar] [CrossRef]
- Hou, Y.-K.; Zhang, Z.-J.; Li, R.-T.; Peng, J.; Chen, S.-Y.; Yue, Y.-R.; Zhang, W.-H.; Sun, B.; Chen, J.-X.; Zhou, Q. Remodeling the Tumor Microenvironment with Core-Shell Nanosensitizer Featuring Dual-Modal Imaging and Multimodal Therapy for Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 2602–2616. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Shi, X.; Zuo, C.; Ma, M.; Zhang, L.; Zhang, H.; Li, X.; Yang, G.-Y.; Tang, Y.; Wu, R. Engineering of SPECT/Photoacoustic Imaging/Antioxidative Stress Triple-Function Nanoprobe for Advanced Mesenchymal Stem Cell Therapy of Cerebral Ischemia. ACS Appl. Mater. Interfaces 2020, 12, 37885–37895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, P.; Wu, X.; Lin, X.; Zhao, L.; Huang, H.; Wu, J.; Cai, H.; Xu, M.; Zhou, H.; et al. Multifunctional Biosensor Constructed by Ag-Coating Magnetic-Assisted Unique Urchin Core Porous Shell Structure for Dual SERS Enhancement, Enrichment, and Quantitative Detection of Multi-Components Inflammatory Markers. Biosens. Bioelectron. 2022, 210, 114257. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cui, Y.; Sun, Z.; Cao, M.; Liu, Y.; Fu, K.; Zhou, Y. Sodium Alginate Coupled with Organosilane Quaternary Ammonium Salt for the Antibacterial Application. Cellulose 2023, 30, 449–462. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Chen, Y.; Chen, Q.; Bai, L.; Gu, J.; Li, Z. Engineered Latex Particles Using Core-Shell Emulsion Polymerization: From a Strawberry-like Surface Pattern to a Shape-Memory Film. ACS Appl. Polym. Mater. 2022, 4, 1276–1285. [Google Scholar] [CrossRef]
- Mu, Y.; Qiu, T.; Li, X.; Guan, Y.; Zhang, S.; Li, X. Layer-by-Layer Synthesis of Multilayer Core−Shell Latex and the Film Formation Properties. Langmuir 2011, 27, 4968–4978. [Google Scholar] [CrossRef]
- Lingxiao, W.; Guilong, X.; Min, T.; Yun, L. Preparation and Properties of Quaternary Phosphonium Salt Containing Poly-Acrylate Emulsion. Prog. Org. Coat. 2023, 175, 107337. [Google Scholar] [CrossRef]
- Wu, C.; Dai, D.; Zhao, X.; Wang, H.; Li, T. One-Step Rapid Fabrication of MOF@polymer Core-Shell Particles through Non-Solvent Induced Surface Deposition. J. Mater. Chem. A 2022, 10, 24676–24684. [Google Scholar] [CrossRef]
- Bilgin, S.; Tomovska, R.; Asua, J.M. Fundamentals of Chemical Incorporation of Ionic Monomers onto Polymer Colloids: Paving the Way for Surfactant-Free Waterborne Dispersions. RSC Adv. 2016, 6, 63754–63760. [Google Scholar] [CrossRef]
- Buback, M.; Hutchinson, R.A.; Lacík, I. Radical Polymerization Kinetics of Water-Soluble Monomers. Prog. Polym. Sci. 2023, 138, 101645. [Google Scholar] [CrossRef]
- Shen, J.; Lin, X.; Liu, J.; Li, X. Effects of Cross-Link Density and Distribution on Static and Dynamic Properties of Chemically Cross-Linked Polymers. Macromolecules 2019, 52, 121–134. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Renewable Epoxy Networks Derived from Lignin-Based Monomers: Effect of Cross-Linking Density. ACS Sustain. Chem. Eng. 2016, 4, 6082–6089. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, S.; Duan, H. An Ionic Liquid Based on a Cyclic Guanidinium Cation Is an Efficient Medium for the Selective Oxidation of Benzyl Alcohols. Tetrahedron Lett. 2004, 45, 2013–2015. [Google Scholar] [CrossRef]
- Meek, K.M.; Elabd, Y.A. Alkaline Chemical Stability of Polymerized Ionic Liquids with Various Cations. Macromolecules 2015, 48, 7071–7084. [Google Scholar] [CrossRef]
- Strehmel, V. Application of Ionic Liquids in Synthesis of Polymeric Binders for Coatings. Prog. Org. Coat. 2015, 89, 297–313. [Google Scholar] [CrossRef]
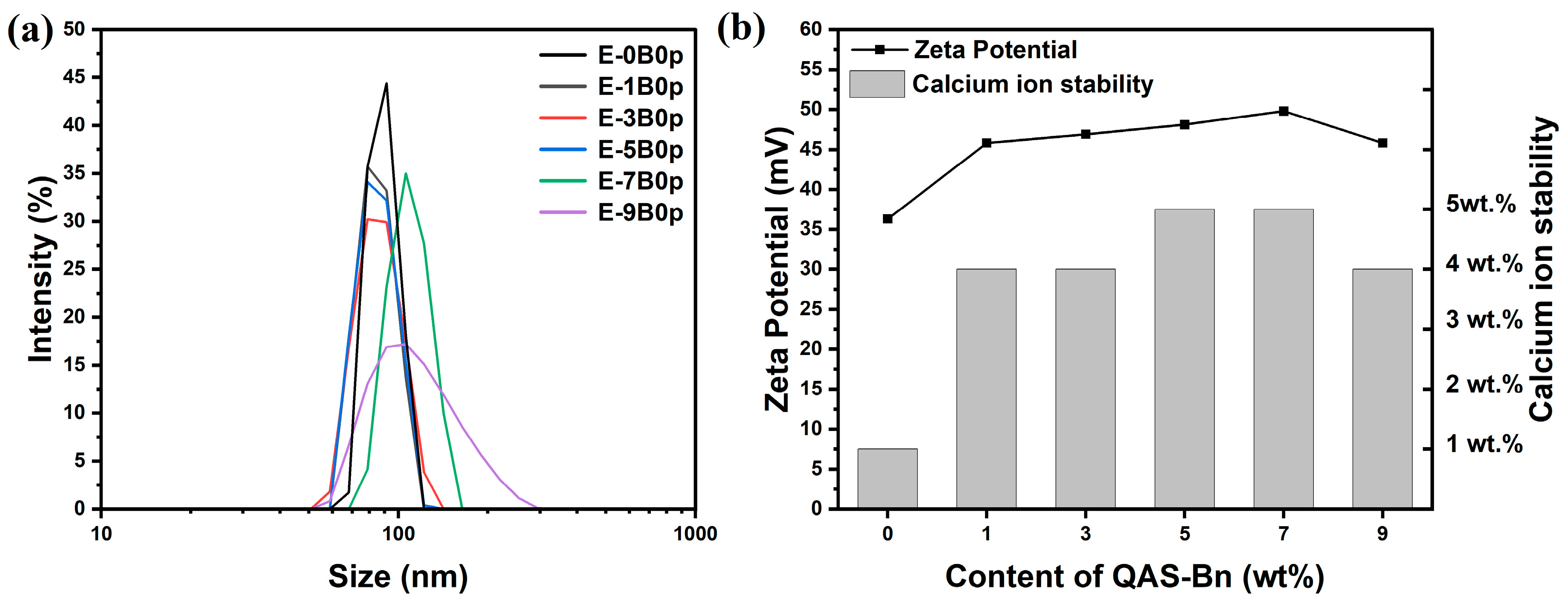




| Sample | Monomer Conversion (%) | Gelation Rate (wt.%) | Particle Size (nm) | PDI | Zeta Potential (mv) | Stability of Calcium Ions |
|---|---|---|---|---|---|---|
| E-0B0p | 99.93 | / | 82.61 | 0.017 | +36.3 | 1 wt.% |
| E-1B0p | 99.98 | / | 82.97 | 0.033 | +45.8 | 4 wt.% |
| E-3B0p | 99.88 | / | 83.95 | 0.040 | +46.9 | 4 wt.% |
| E-5B0p | 98.60 | / | 88.06 | 0.066 | +48.1 | 5 wt.% |
| E-7B0p | 96.45 | 1.21 | 111.2 | 0.118 | +49.8 | 5 wt.% |
| E-9B0p | 91.63 | 5.32 | 115.4 | 0.171 | +45.8 | 4 wt.% |
| Sample | QAS-BN (wt.%) | Core (nm) | Shell (nm) | Size (nm) | Shell (v/v.%) |
|---|---|---|---|---|---|
| E-0B0p | 0 | 87.44 | \ | 87.44 | \ |
| E-1B0p | 1 | 87.35 | \ | 87.35 | \ |
| E-3B0p | 3 | 87.28 | \ | 87.28 | \ |
| E-5B0p | 5 | 51.81 | 19.09 | 89.99 | 66.85 |
| E-7B0p | 7 | 58.44 | 20.89 | 100.22 | 66.00 |
| E-9B0p | 9 | 61.69 | 21.75 | 105.20 | 65.62 |
| Sample | T5% (°C) | T10% (°C) | Tmax (°C) |
|---|---|---|---|
| E-0B0p | 335.24 | 352.78 | 399.84 |
| E-1B0p | 353.03 | 363.37 | 399.37 |
| E-3B0p | 347.73 | 361.35 | 394.56 |
| E-5B0p | 331.11 | 360.39 | 398.14 |
| E-7B0p | 305.64 | 348.13 | 395.39 |
| E-9B0p | 262.54 | 329.51 | 394.37 |
| Sample | Conversion Rate /% | Size /nm | PDI | Crosslinkage /% | Water Absorption /% | Water Leaching Rate /% |
|---|---|---|---|---|---|---|
| E-7B0p | 96.45 | 111.2 | 0.118 | 21.36 ± 0.61 | 21.31 ± 0.63 | 11.23 ± 0.10 |
| E-7B1p | 97.98 | 120.5 | 0.122 | 85.06 ± 1.61 | 11.29 ± 0.50 | 3.75 ± 0.06 |
| E-7B2p | 99.13 | 124.3 | 0.134 | 91.11 ± 1.10 | 9.99 ± 0.43 | 4.00 ± 0.03 |
| E-7B3p | 99.05 | 126.5 | 0.144 | 91.76 ± 0.72 | 8.31 ± 0.40 | 3.66 ± 0.04 |
| Sample | Tg-Core/°C | Tg-Shell/°C | E/MPa | Ve/(mol/m3) |
|---|---|---|---|---|
| E-7B1p | 42.43 | 124.59 | 1.58902 | 800.88 |
| E-7B2p | 41.36 | 114.48 | 2.81856 | 1468.43 |
| E-7B3p | 48.96 | 111.63 | 2.89276 | 1496.01 |
| Durability Treatment | Testing Microorganism | The Average Number of Recovered Colonies after 24 h (cfu/Piece) | Antibacterial Rate (%) | |
|---|---|---|---|---|
| Blank Control Sample | Antibacterial Coating Sample | |||
| Before | E. coli (AS1.90) | 1.0 × 107 | <20 | >99.99 |
| S. aureus (AS1.89) | 2.9 × 106 | <20 | >99.99 | |
| After | E. coli (AS1.90) | 1.0 × 107 | <20 | >99.99 |
| S. aureus (AS1.89) | 2.9 × 106 | <20 | >99.99 | |
| Sample | Reaction Monomers | Initiator | Emulsifier | Solvent | |||||
|---|---|---|---|---|---|---|---|---|---|
| MMA (g) | BA (g) | QAS-BN (g) | p-CMS (g) | VTES (g) | AIBA (g) | CTAB (g) | LCN407 (g) | H2O (g) | |
| E-0B0p | 104.63 | 104.63 | 0.00 | 0 | 15.75 | 0.45 | 2.8 | 4 | 275 |
| E-1B0p | 103.50 | 103.50 | 2.25 | 0 | |||||
| E-3B0p | 101.25 | 101.25 | 6.75 | 0 | |||||
| E-5B0p | 99.00 | 99.00 | 11.25 | 0 | |||||
| E-7B0p | 96.75 | 96.75 | 15.75 | 0 | |||||
| E-9B0p | 94.50 | 94.50 | 20.25 | 0 | |||||
| E-7B1p | 95.63 | 95.63 | 15.75 | 2.25 | |||||
| E-7B2p | 94.50 | 94.50 | 15.75 | 4.50 | |||||
| E-7B3p | 93.38 | 93.38 | 15.75 | 6.75 | |||||
| E-7B4p | 92.25 | 92.25 | 15.75 | 9.00 | |||||
| Material | Model | Weight/g |
|---|---|---|
| water | / | 274.82 |
| emulsifier | LCN407 | 1.64 |
| dispersant | 6208 | 18.02 |
| defoamer | 6393 | 1.31 |
| antifreeze | propylene glycol | 19.66 |
| titanium dioxide | R-706 | 221.20 |
| 800-mesh dicalcium | / | 294.94 |
| water-washed kaolin | / | 90.12 |
| calcined kaolin | / | 147.47 |
| Material | Model | Weight/g |
|---|---|---|
| CACS emulsion | / | 100 |
| film-forming additives | DPMA | 1 |
| defoamer | 2410 | 0.5 |
| dispersion | / | 20–100 |
| leveling agent | BYK-333 | 0.6 |
| thickening agent | RM-8W | appropriate content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, G.; Mu, Y.; Yuan, P.; Li, Y.; Li, X. One-Step Synthesis of Self-Stratification Core-Shell Latex for Antimicrobial Coating. Molecules 2023, 28, 2795. https://doi.org/10.3390/molecules28062795
Zhen G, Mu Y, Yuan P, Li Y, Li X. One-Step Synthesis of Self-Stratification Core-Shell Latex for Antimicrobial Coating. Molecules. 2023; 28(6):2795. https://doi.org/10.3390/molecules28062795
Chicago/Turabian StyleZhen, Guanzhou, Yuanchun Mu, Peichen Yuan, Yankun Li, and Xiaoyu Li. 2023. "One-Step Synthesis of Self-Stratification Core-Shell Latex for Antimicrobial Coating" Molecules 28, no. 6: 2795. https://doi.org/10.3390/molecules28062795
APA StyleZhen, G., Mu, Y., Yuan, P., Li, Y., & Li, X. (2023). One-Step Synthesis of Self-Stratification Core-Shell Latex for Antimicrobial Coating. Molecules, 28(6), 2795. https://doi.org/10.3390/molecules28062795






