A Novel Drug with Potential to Treat Hyperbilirubinemia and Prevent Liver Damage Induced by Hyperbilirubinemia: Carbon Dots Derived from Platycodon grandiflorum
Abstract
1. Introduction
2. Results
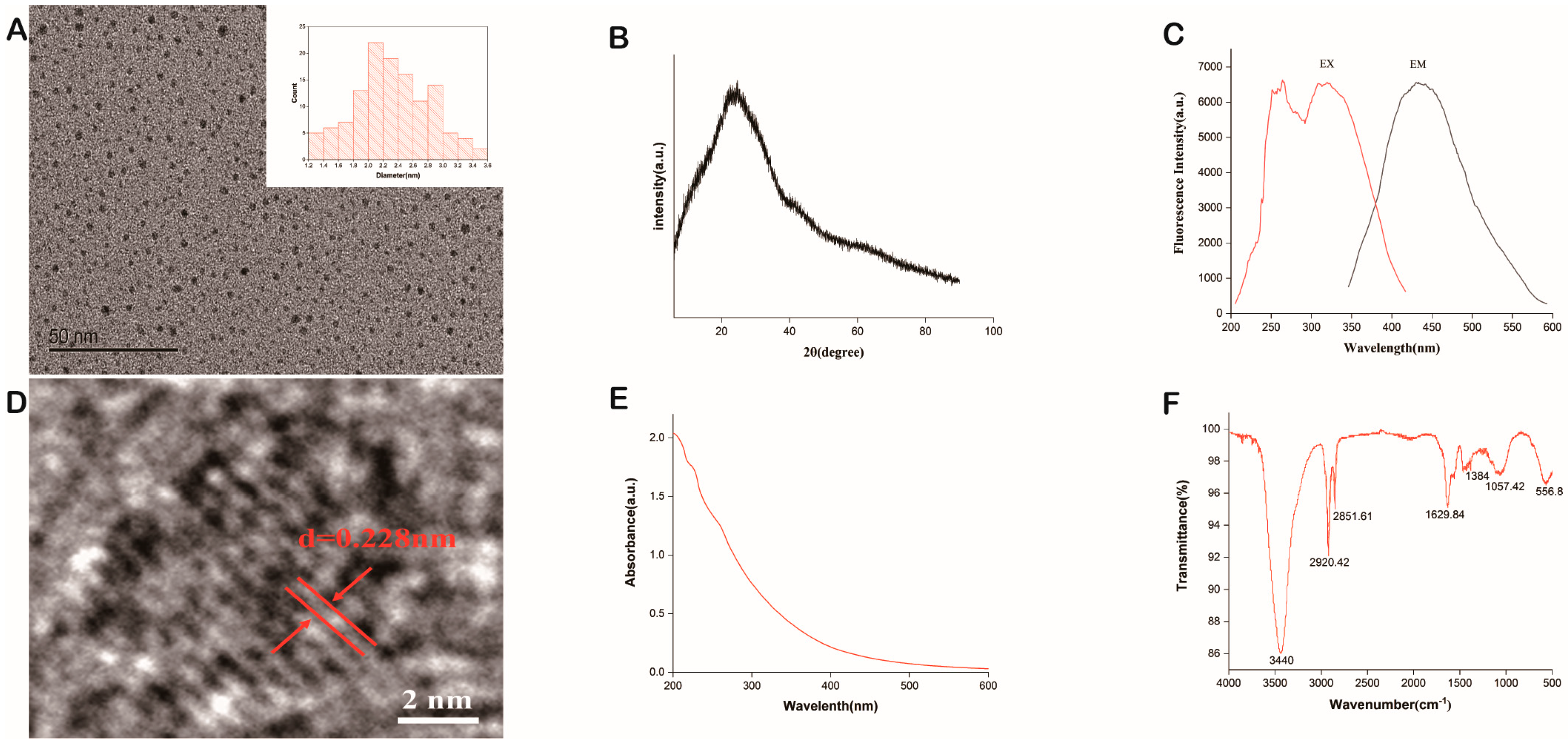
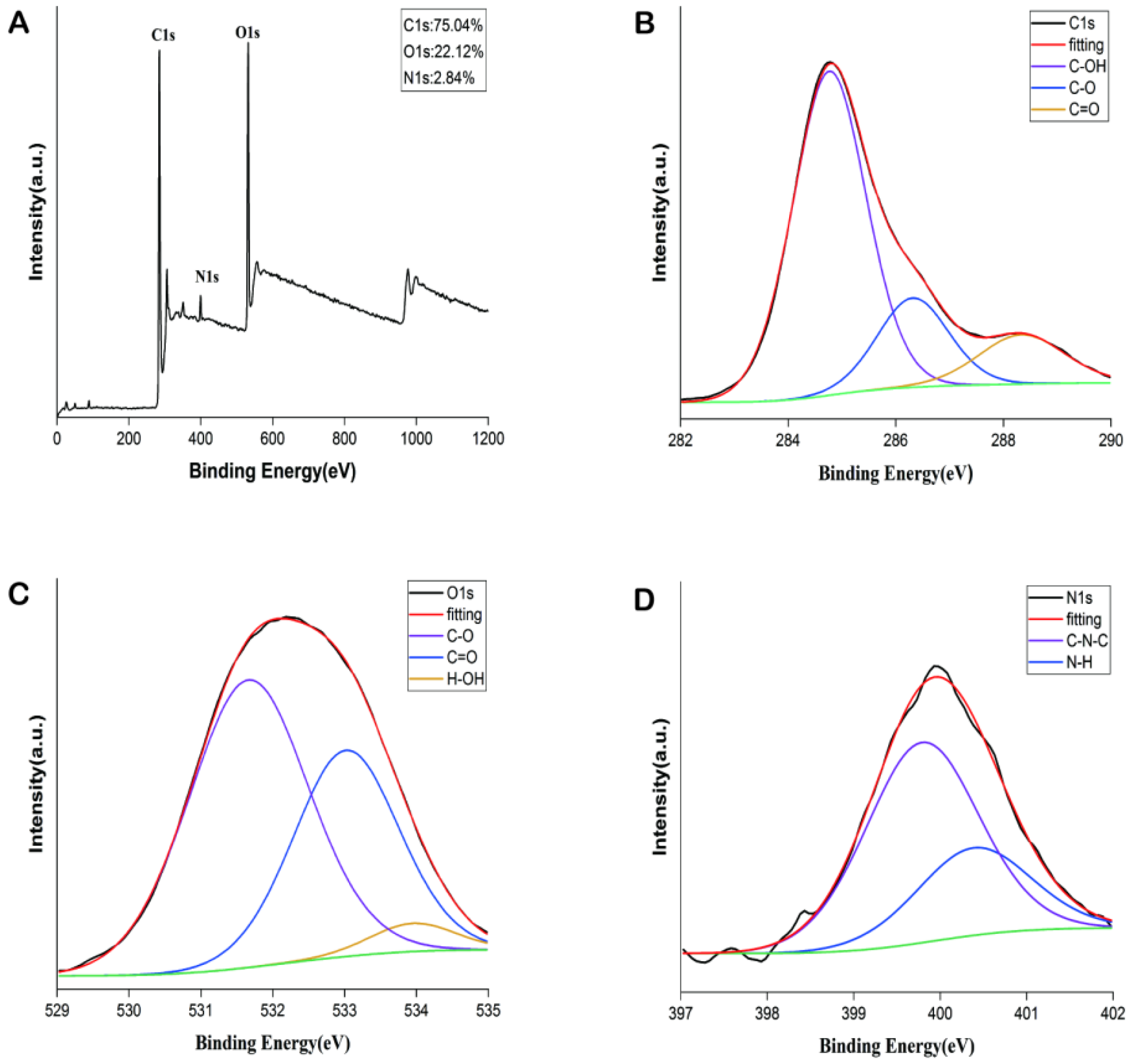
2.1. Characterization of PGC-CDs
2.2. Cellular Toxicity
2.3. Effect of PGC-CDs on H2O2-Induced RAW264.7 Cells
2.4. Effects of PGC-CDs on General Condition
2.5. Effects of PGC-CDs on Neurological Function
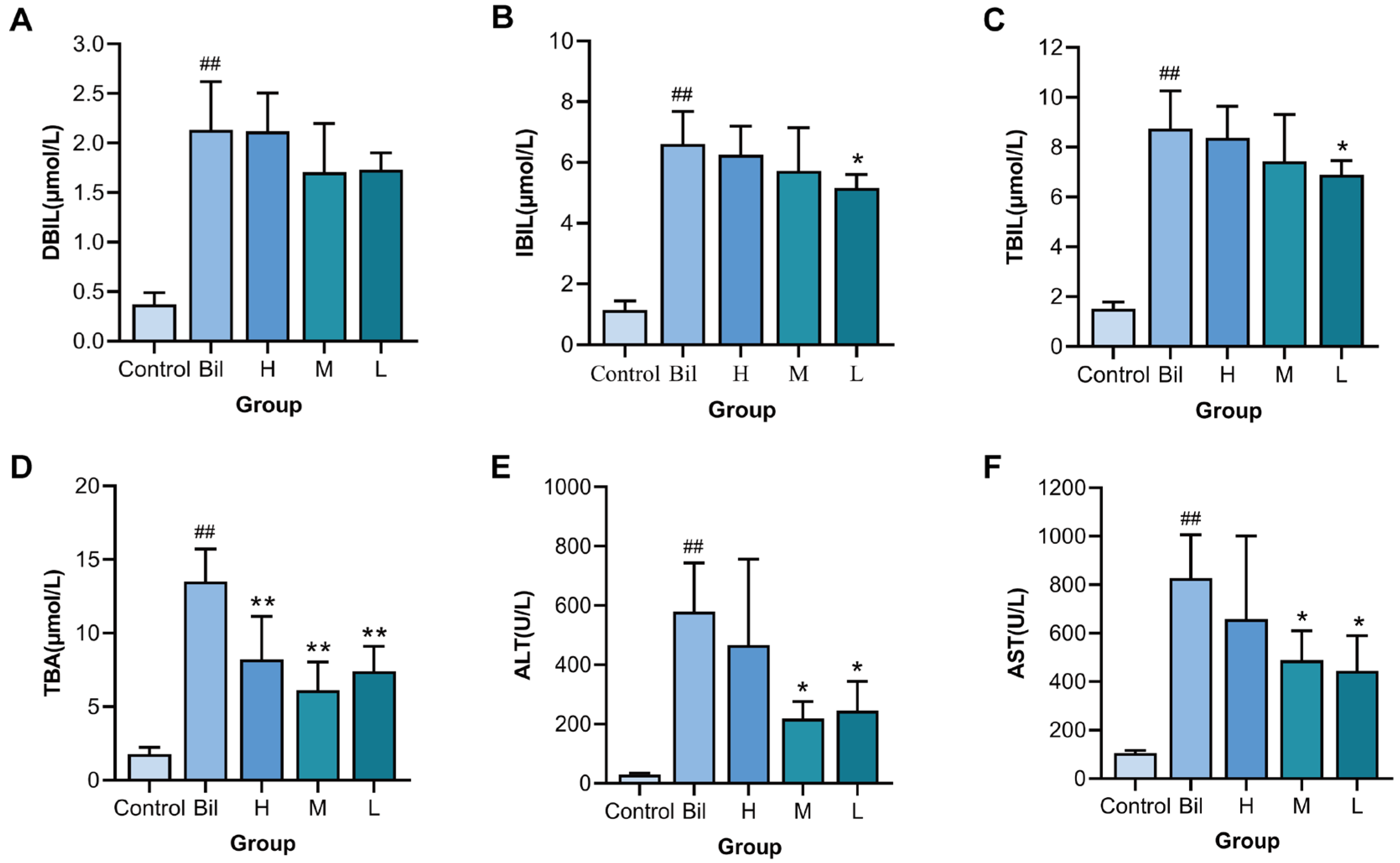
2.6. Effects of PGC-CDs on Biochemical Levels
2.7. Effects of PGC-CDs on Inflammatory Factors
2.8. Effects of PGC-CDs on SOD, MDA, GSH and CAT Levels in Liver Tissue
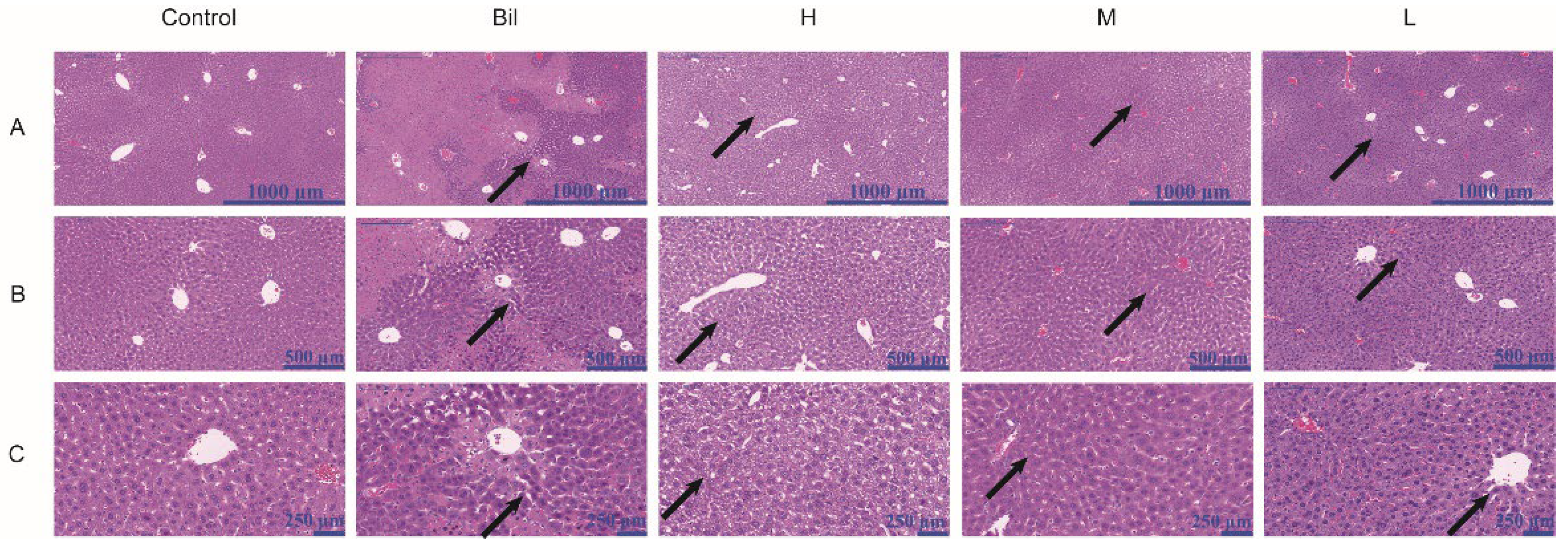
2.9. Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
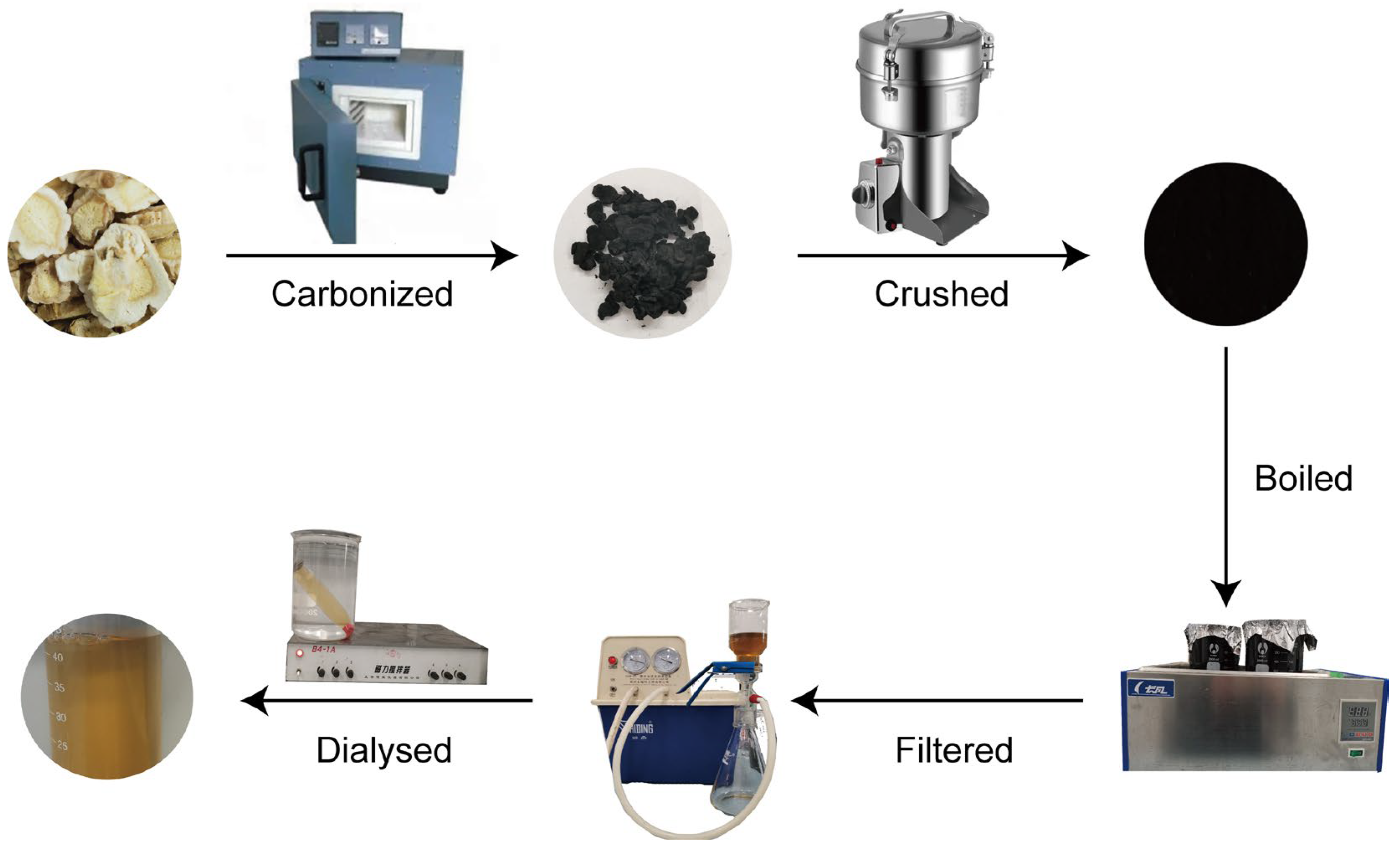
4.3. Preparation of PGC-CDs
4.4. Characterization of PGC-CDs
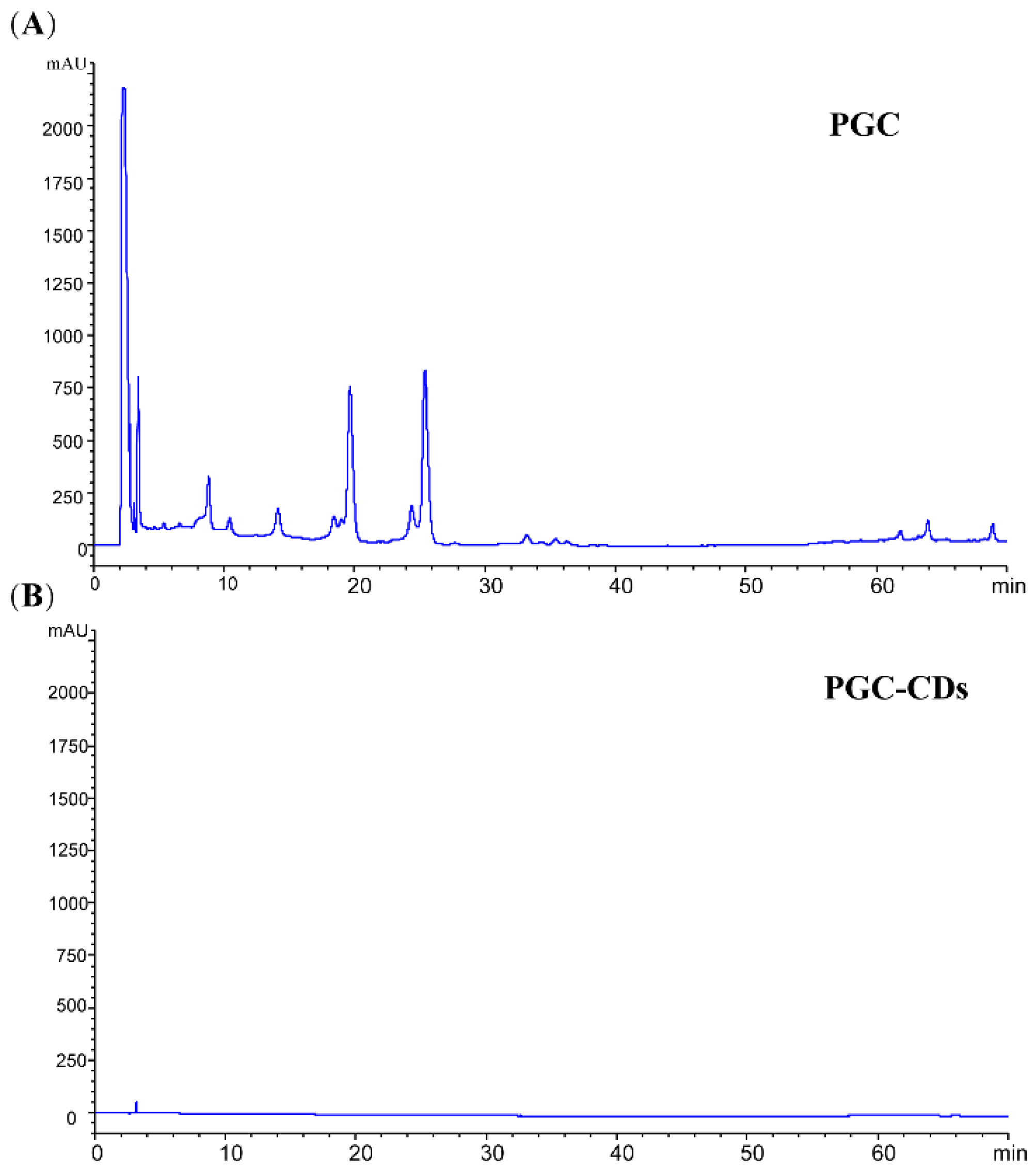
4.5. Fingerprint Analysis of PGC and PGC-CDs by High-Performance Liquid Chromatography
4.6. Cytotoxicity Assessment: CCK-8 Assay
4.7. Evaluation of the Antioxidant Activity of PGC-CDs in Cells
4.8. Bilirubin-Induced Hyperbilirubinemia Model and Drug Treatment
4.9. General Condition Observation
4.10. Neurological Function Observation
4.11. Biochemical Analysis
4.12. Detection of Inflammatory Factors and Markers of Oxidative Stress
4.13. Histopathological Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| PG | Platycodon grandiflorum |
| CDs | Carbon dots |
| PGC | Platycodon grandiflorum carbonisata |
| PGC-CDs | Platycodon grandiflorum-based carbon dots |
| TEM | Transmission electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| XRD | X-ray diffraction |
| UV-Vis | Ultraviolet-visible |
| FTIR | Fourier transform infrared |
| XPS | X-ray photoelectron spectroscopy |
| HPLC | High-performance liquid chromatogram |
| Bil group | Bilirubin group |
| DBIL | Direct bilirubin |
| IBIL | Indirect bilirubin |
| TBIL | Total bilirubin |
| TBA | Total bile acid |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| CAT | Catalase |
| H&E | Hematoxylin and eosin |
| DW | Deionized water |
| DMEM | Dulbecco’s modified Eagle’s medium |
| CCK-8 | Cell counting kit-8 |
| PBS | Phosphate-buffered saline |
References
- Kwon, J.; Lee, H.; Kim, N.; Lee, J.H.; Woo, M.H.; Kim, J.; Kim, Y.S.; Lee, D. Effect of processing method on platycodin D content in Platycodon grandiflorum roots. Arch. Pharmacal Res. 2017, 40, 1087–1093. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable Carbon Dots with Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 26936–26946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, K.; Zheng, X.; Luo, Q.; Zhang, Q.; Ji, X.; Wei, Y. Synthesis of carbon dots with antiphage activity using caffeic acid. Anal. Methods Adv. Methods Appl. 2021, 13, 5165–5172. [Google Scholar] [CrossRef]
- Xia, J.; Kawamura, Y.; Suehiro, T.; Chen, Y.; Sato, K. Carbon dots have antitumor action as monotherapy or combination therapy. Drug Discov. Ther. 2019, 13, 114–117. [Google Scholar] [CrossRef]
- Christensen, I.L.; Sun, Y.P.; Juzenas, P. Carbon dots as antioxidants and prooxidants. J. Biomed. Nanotechnol. 2011, 7, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhao, Y.; Zhu, Y.; Xiong, W.; Luo, J.; Cheng, J.; Zhang, Y.; Zhang, M.; Qu, H.; Zhao, Y. Carbon dots from Artemisiae Argyi Folium Carbonisata: Strengthening the anti-frostbite ability. Artif. Cells Nanomed. Biotechnol. 2021, 49, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Alas, M.O.; Genc, R. Differential Immunomodulatory Effect of Carbon Dots Influenced by the Type of Surface Passivation Agent. Inflammation 2020, 43, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lu, F.; Cheng, J.; Zhang, M.; Zhu, Y.; Zhang, Y.; Kong, H.; Qu, H.; Zhao, Y. Hypoglycemic Bioactivity of Novel Eco-Friendly Carbon Dots Derived from Traditional Chinese Medicine. J. Biomed. Nanotechnol. 2018, 14, 2146–2155. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, J.; Sun, Z.; Kong, H.; Zhang, Y.; Wang, S.; Wang, X.; Zhao, Y.; Qu, H. Protective Effects of Carbon Dots Derived from Phellodendri Chinensis Cortex Carbonisata against Deinagkistrodon acutus Venom-Induced Acute Kidney Injury. Nanoscale Res. Lett. 2019, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, L.; Li, X.; Zheng, J.; Chen, Q.; Yang, Z.; Cheng, M.; Chen, Y.; Wu, Y.; Zhang, W.; et al. Green functional carbon dots derived from herbal medicine ameliorate blood—Brain barrier permeability following traumatic brain injury. Nano Res. 2022, 15, 9274–9285. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, M.; Cheng, J.; Zhang, Y.; Luo, J.; Liu, Y.; Kong, H.; Qu, H.; Zhao, Y. Effect of Lonicerae japonicae Flos Carbonisata-Derived Carbon Dots on Rat Models of Fever and Hypothermia Induced by Lipopolysaccharide. Int. J. Nanomed. 2020, 15, 4139–4149. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Kong, H.; Cheng, J.; Zhang, M.; Sun, Z.; Wang, S.; Liu, J.; Qu, H.; Zhao, Y. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif. Cells Nanomed. Biotechnol. 2020, 48, 68–76. [Google Scholar] [CrossRef]
- Kong, H.; Zhao, Y.; Cao, P.; Luo, J.; Liu, Y.; Qu, H.; Zhang, Y.; Zhao, Y. The Bioactivity of Scutellariae Radix Carbonisata-Derived Carbon Dots: Antiallergic Effect. J. Biomed. Nanotechnol. 2021, 17, 2485–2494. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, F.; Zhang, Y.; Zhang, M.; Zhao, Y.; Luo, J.; Kong, H.; Qu, H. Water-Soluble Carbon Dots in Cigarette Mainstream Smoke: Their Properties and the Behavioural, Neuroendocrinological, and Neurotransmitter Changes They Induce in Mice. Int. J. Nanomed. 2021, 16, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Vodret, S.; Bortolussi, G.; Schreuder, A.B.; Jašprová, J.; Vitek, L.; Verkade, H.J.; Muro, A.F. Albumin administration prevents neurological damage and death in a mouse model of severe neonatal hyperbilirubinemia. Sci. Rep. 2015, 5, 16203. [Google Scholar] [CrossRef] [PubMed]
- Bortolussi, G.; Muro, A.F. Experimental models assessing bilirubin neurotoxicity. Pediatr. Res. 2020, 87, 17–25. [Google Scholar] [CrossRef]
- Kato, S.; Iwata, O.; Yamada, Y.; Kakita, H.; Yamada, T.; Nakashima, H.; Sugiura, T.; Suzuki, S.; Togari, H. Standardization of phototherapy for neonatal hyperbilirubinemia using multiple-wavelength irradiance integration. Pediatr. Neonatol. 2020, 61, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kanmaz, H.G.; Okur, N.; Dilli, D.; Yeşilyurt, A.; Oğuz, Ş.S. The effect of phototherapy on sister chromatid exchange with different light density in newborn hyperbilirubinemia. Turk Pediatri Ars. 2017, 52, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Smith, T.; Timler, G. Developmental influence of unconjugated hyperbilirubinemia and neurobehavioral disorders. Pediatr. Res. 2019, 85, 191–197. [Google Scholar] [CrossRef]
- Vodret, S.; Bortolussi, G.; Iaconcig, A.; Martinelli, E.; Tiribelli, C.; Muro, A.F. Attenuation of neuro-inflammation improves survival and neurodegeneration in a mouse model of severe neonatal hyperbilirubinemia. Brain Behav. Immun. 2018, 70, 166–178. [Google Scholar] [CrossRef]
- Cohen, R.S.; Wong, R.J.; Stevenson, D.K. Understanding neonatal jaundice: A perspective on causation. Pediatr. Neonatol. 2010, 51, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ma, X.; Shen, X.; Bao, Y.; Chen, L.; Bhutani, V.K. Neonatal hyperbilirubinemia management: Clinical assessment of bilirubin production. Semin. Perinatol. 2021, 45, 151351. [Google Scholar] [CrossRef]
- He, C.H.; Qu, Y. Research advances in neonatal hyperbilirubinemia and gene polymorphisms. Zhongguo Dang Dai Er Ke Za Zhi = Chin. J. Contemp. Pediatr. 2020, 22, 280–284. [Google Scholar]
- Maisels, M.J.; Kring, E. The contribution of hemolysis to early jaundice in normal newborns. Pediatrics 2006, 118, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xiao, K.; Cui, J.; Ye, X.H.; Zhang, Y.C.; Mao, L.; Selvaggi, G.; Yen, J.; Stebbing, J. Successful Treatment with Ensartinib After Alectinib-induced Hyperbilirubinemia in ALK-Positive NSCLC. OncoTargets Ther. 2021, 14, 3409–3415. [Google Scholar] [CrossRef]
- Anjana, R.R.; Anjali Devi, J.S.; Jayasree, M.; Aparna, R.S.; Aswathy, B.; Praveen, G.L.; Lekha, G.M.; Sony, G. S,N-doped carbon dots as a fluorescent probe for bilirubin. Mikrochim. Acta 2017, 185, 11. [Google Scholar] [CrossRef]
- Tabatabaee, R.S.; Golmohammadi, H.; Ahmadi, S.H. Easy Diagnosis of Jaundice: A Smartphone-Based Nanosensor Bioplatform Using Photoluminescent Bacterial Nanopaper for Point-of-Care Diagnosis of Hyperbilirubinemia. ACS Sens. 2019, 4, 1063–1071. [Google Scholar] [CrossRef]
- Hayashi, H.; Mizuno, T.; Horikawa, R.; Nagasaka, H.; Yabuki, T.; Takikawa, H.; Sugiyama, Y. 4-Phenylbutyrate modulates ubiquitination of hepatocanalicular MRP2 and reduces serum total bilirubin concentration. J. Hepatol. 2012, 56, 1136–1144. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, J.; Zhang, Y.; Kong, H.; Wang, S.; Luo, J.; Qu, H.; Zhao, Y. Green synthesis of Zingiberis rhizoma-based carbon dots attenuates chemical and thermal stimulus pain in mice. Nanomedicine 2020, 15, 851–869. [Google Scholar] [CrossRef]
- Zhang, J.H.; Niu, A.; Li, J.; Fu, J.W.; Xu, Q.; Pei, D.S. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci. Rep. 2016, 6, 37860. [Google Scholar] [CrossRef] [PubMed]
- Mewada, A.; Pandey, S.; Shinde, S.; Mishra, N.; Oza, G.; Thakur, M.; Sharon, M.; Sharon, M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Kong, H.; Zhang, M.; Cheng, J.; Wu, J.; Qu, H.; Zhao, Y. Carbon Dots from Paeoniae Radix Alba Carbonisata: Hepatoprotective Effect. Int. J. Nanomed. 2020, 15, 9049–9059. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, M.; Xin, T. Mst1 contributes to nasal epithelium inflammation via augmenting oxidative stress and mitochondrial dysfunction in a manner dependent on Nrf2 inhibition. J. Cell. Physiol. 2019, 234, 23774–23784. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Zeng, J.; Lu, C.; Huang, H.; Huang, J. Effect of Recombinant Netrin-1 Protein Combined with Peripheral Blood Mesenchymal Stem Cells on Angiogenesis in Rats with Arteriosclerosis Obliterans. BioMed Res. Int. 2022, 2022, 3361605. [Google Scholar] [CrossRef]
- Safi, H.J.; Miller, C.C., 3rd; Azizzadeh, A.; Iliopoulos, D.C. Observations on delayed neurologic deficit after thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 1997, 26, 616–622. [Google Scholar] [CrossRef]
- Luo, W.K.; Zhang, L.L.; Yang, Z.Y.; Guo, X.H.; Wu, Y.; Zhang, W.; Luo, J.K.; Tang, T.; Wang, Y. Herbal medicine derived carbon dots: Synthesis and applications in therapeutics, bioimaging and sensing. J. Nanobiotechnology 2021, 19, 320. [Google Scholar] [CrossRef]
- Gudimella, K.K.; Gedda, G.; Kumar, P.S.; Babu, B.K.; Yamajala, B.; Rao, B.V.; Singh, P.P.; Kumar, D.; Sharma, A. Novel synthesis of fluorescent carbon dots from bio-based Carica Papaya Leaves: Optical and structural properties with antioxidant and anti-inflammatory activities. Environ. Res. 2022, 204, 111854. [Google Scholar] [CrossRef]
- Zhao, W.-B.; Wang, R.-T.; Liu, K.-K.; Du, M.-R.; Wang, Y.; Wang, Y.-Q.; Zhou, R.; Liang, Y.-C.; Ma, R.-N.; Sui, L.-Z.; et al. Near-infrared carbon nanodots for effective identification and inactivation of Gram-positive bacteria. Nano Res. 2022, 15, 1699–1708. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Lu, F.; Zhang, M.; Kong, H.; Cheng, J.; Luo, J.; Zhao, Y.; Qu, H. The neuroprotective effect of pretreatment with carbon dots from Crinis Carbonisatus (carbonized human hair) against cerebral ischemia reperfusion injury. J. Nanobiotechnology 2021, 19, 257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Hu, B.; Zhuang, Q.F.; Wang, Y.; Luo, X.B.; Xie, Y.; Zhou, D. Green Synthesis of Fluorescent Nitrogen-Sulfur Co-Doped Carbon Dots from Scallion Leaves for Hemin Sensing. Anal. Lett. 2020, 53, 1704–1718. [Google Scholar] [CrossRef]
- Fan, Z.T.; Li, S.H.; Yuan, F.L.; Fan, L.Z. Fluorescent graphene quantum dots for biosensing and bioimaging. RSC Adv. 2015, 5, 19773–19789. [Google Scholar] [CrossRef]
- Li, D.; Xu, K.Y.; Zhao, W.P.; Liu, M.F.; Feng, R.; Li, D.Q.; Bai, J.; Du, W.L. Chinese Medicinal Herb-Derived Carbon Dots for Common Diseases: Efficacies and Potential Mechanisms. Front. Pharmacol. 2022, 13, 815479. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Jenkins, J.G.; Lynn, A.M.; Wood, A.E.; Trusler, G.A.; Barker, G.A. Acute hepatic failure following cardiac operation in children. J. Thorac. Cardiovasc. Surg. 1982, 84, 865–871. [Google Scholar] [CrossRef]
- Hou, W.C.; Chowdhury, I.; Goodwin, D.G., Jr.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical transformation of graphene oxide in sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, M.; Zhou, Q. Ambient water and visible-light irradiation drive changes in graphene morphology, structure, surface chemistry, aggregation, and toxicity. Environ. Sci. Technol. 2015, 49, 3410–3418. [Google Scholar] [CrossRef]
- Amini, N.; Bakhshayesh Eghbali, B.; Ramezani, S.; Hosseinpour Sarmadi, V.; Brouki Milan, P.; Ashraf, S.S.; Larijani, G.; Naderi Gharahgheshlagh, S.; Derakhshanmehr, B.; Mohebbi, S.L.; et al. Animal Kernicterus Models: Progress and Challenges. Brain Res. 2021, 1770, 147624. [Google Scholar] [CrossRef]
- Björnsson, E.; Olsson, R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005, 42, 481–489. [Google Scholar] [CrossRef]
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef]
- Makino, I.; Hashimoto, H.; Shinozaki, K.; Yoshino, K.; Nakagawa, S. Sulfated and nonsulfated bile acids in urine, serum, and bile of patients with hepatobiliary diseases. Gastroenterology 1975, 68, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Basiri, H.; Abouei Mehrizi, A.; Ghaee, A.; Farokhi, M.; Chekini, M.; Kumacheva, E. Carbon Dots Conjugated with Vascular Endothelial Growth Factor for Protein Tracking in Angiogenic Therapy. Langmuir ACS J. Surf. Colloids 2020, 36, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. American Chemical Society meeting. Nanomaterials show signs of toxicity. Science 2003, 300, 243. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- Wusthoff, C.J.; Loe, I.M. Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin. Fetal Neonatal Med. 2015, 20, 52–57. [Google Scholar] [CrossRef]
- Yang, W.C.; Zhao, L.L.; Li, Y.C.; Chen, C.H.; Chang, Y.J.; Fu, Y.C.; Wu, H.P. Bodyweight loss in predicting neonatal hyperbilirubinemia 72 hours after birth in term newborn infants. BMC Pediatr. 2013, 13, 145. [Google Scholar] [CrossRef]
- Hassan, B.; Zakerihamidi, M. The correlation between frequency and duration of breastfeeding and the severity of neonatal hyperbilirubinemia. J. Matern.-Fetal Neonatal Med. 2018, 31, 457–463. [Google Scholar] [CrossRef]
- Farouk, Z.L.; Slusher, T.M.; Danzomo, A.A.; Slusher, I.L. Knowledge, Observation and Practices Related to Neonatal Jaundice in a Rural Community in Kano, Nigeria. J. Trop. Pediatr. 2021, 67, fmaa134. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar]
- Trauner, M.; Fickert, P.; Stauber, R.E. Inflammation-induced cholestasis. J. Gastroenterol. Hepatol. 1999, 14, 946–959. [Google Scholar] [CrossRef]
- Moseley, R.H. Sepsis and cholestasis. Clin. Liver Dis. 1999, 3, 465–475. [Google Scholar] [CrossRef]
- Giraudi, P.J.; Bellarosa, C.; Coda-Zabetta, C.D.; Peruzzo, P.; Tiribelli, C. Functional induction of the cystine-glutamate exchanger system Xc(-) activity in SH-SY5Y cells by unconjugated bilirubin. PLoS ONE 2011, 6, e29078. [Google Scholar] [CrossRef]
- Tell, G.; Gustincich, S. Redox state, oxidative stress, and molecular mechanisms of protective and toxic effects of bilirubin on cells. Curr. Pharm. Des. 2009, 15, 2908–2914. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hu, C.; Xue, Y. In vitro evaluation of chitosan-coated liposome containing both coenzyme Q10 and alpha-lipoic acid: Cytotoxicity, antioxidant activity, and antimicrobial activity. J. Cosmet. Dermatol. 2018, 17, 258–262. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, Y.; Yang, Z.; Jin, H. Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H(2)O(2)-Induced Injury in RAW264.7 Cells. Mar. Drugs 2020, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hu, Y.; Gu, X.; Si, F.; Hua, Z. A novel newborn rat kernicterus model created by injecting a bilirubin solution into the cisterna magna. PLoS ONE 2014, 9, e96171. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, T.; Lv, S.; Gao, Y.; Masters, J.; Weng, H. Dexmedetomidine attenuates spinal cord ischemia-reperfusion injury through both anti-inflammation and anti-apoptosis mechanisms in rabbits. J. Transl. Med. 2018, 16, 209. [Google Scholar] [CrossRef]



| Group | Tarlov Score | ||||
|---|---|---|---|---|---|
| 4 h | 8 h | 12 h | 24 h | 48 h | |
| Control | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 |
| Bil | 1.33 ± 0.52 ## | 1.17 ± 0.41 ## | 1.50 ± 0.84 ## | 1 ± 0 ## | 1 ± 0 ## |
| H | 2.67 ± 0.52 * | 2.33 ± 1.03 | 3.17 ± 0.75 * | 2.33 ± 1.21 | 3.33 ± 1.21 ** |
| M | 3.67 ± 0.52 ** | 3.83 ± 0.41 ** | 3.67 ± 0.52 ** | 3.83 ± 0.41 ** | 3.83 ± 0.41 ** |
| L | 3.33 ± 1.21 | 3.67 ± 0.82 ** | 3.67 ± 0.82 * | 3.17 ± 1.17 | 3.80 ± 0.45 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Ma, H.; Li, X.; Wang, M.; Yang, Y.; Wu, T.; Zhang, Y.; Kong, H.; Qu, H.; Zhao, Y. A Novel Drug with Potential to Treat Hyperbilirubinemia and Prevent Liver Damage Induced by Hyperbilirubinemia: Carbon Dots Derived from Platycodon grandiflorum. Molecules 2023, 28, 2720. https://doi.org/10.3390/molecules28062720
Chen R, Ma H, Li X, Wang M, Yang Y, Wu T, Zhang Y, Kong H, Qu H, Zhao Y. A Novel Drug with Potential to Treat Hyperbilirubinemia and Prevent Liver Damage Induced by Hyperbilirubinemia: Carbon Dots Derived from Platycodon grandiflorum. Molecules. 2023; 28(6):2720. https://doi.org/10.3390/molecules28062720
Chicago/Turabian StyleChen, Rui, Huagen Ma, Xiaopeng Li, Meijun Wang, Yunbo Yang, Tong Wu, Yue Zhang, Hui Kong, Huihua Qu, and Yan Zhao. 2023. "A Novel Drug with Potential to Treat Hyperbilirubinemia and Prevent Liver Damage Induced by Hyperbilirubinemia: Carbon Dots Derived from Platycodon grandiflorum" Molecules 28, no. 6: 2720. https://doi.org/10.3390/molecules28062720
APA StyleChen, R., Ma, H., Li, X., Wang, M., Yang, Y., Wu, T., Zhang, Y., Kong, H., Qu, H., & Zhao, Y. (2023). A Novel Drug with Potential to Treat Hyperbilirubinemia and Prevent Liver Damage Induced by Hyperbilirubinemia: Carbon Dots Derived from Platycodon grandiflorum. Molecules, 28(6), 2720. https://doi.org/10.3390/molecules28062720





