Induction, Flavonoids Contents, and Bioactivities Analysis of Hairy Roots and True Roots of Tetrastigma hemsleyanum Diels et Gilg
Abstract
1. Introduction
2. Results
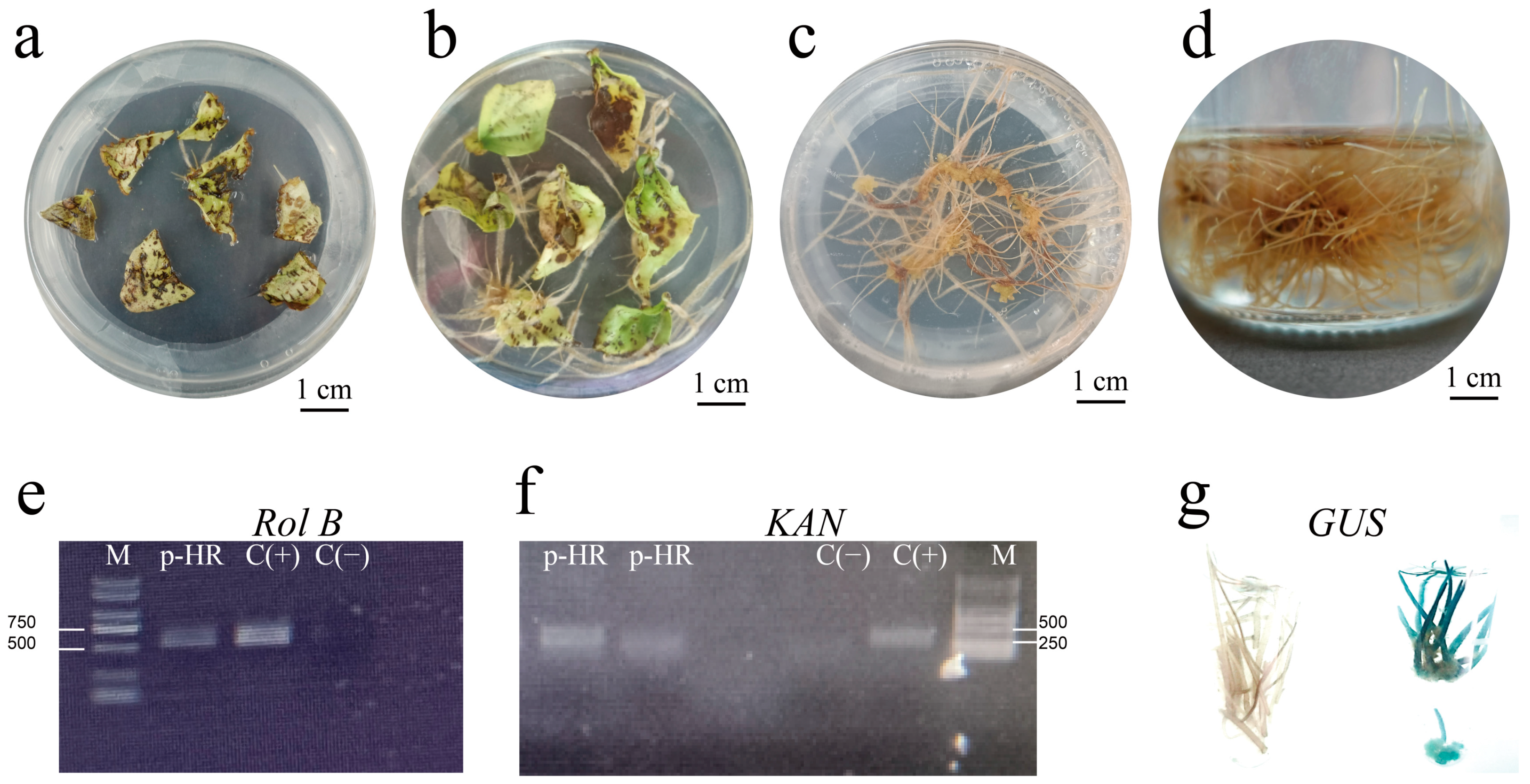
2.1. Induction of Hairy Roots
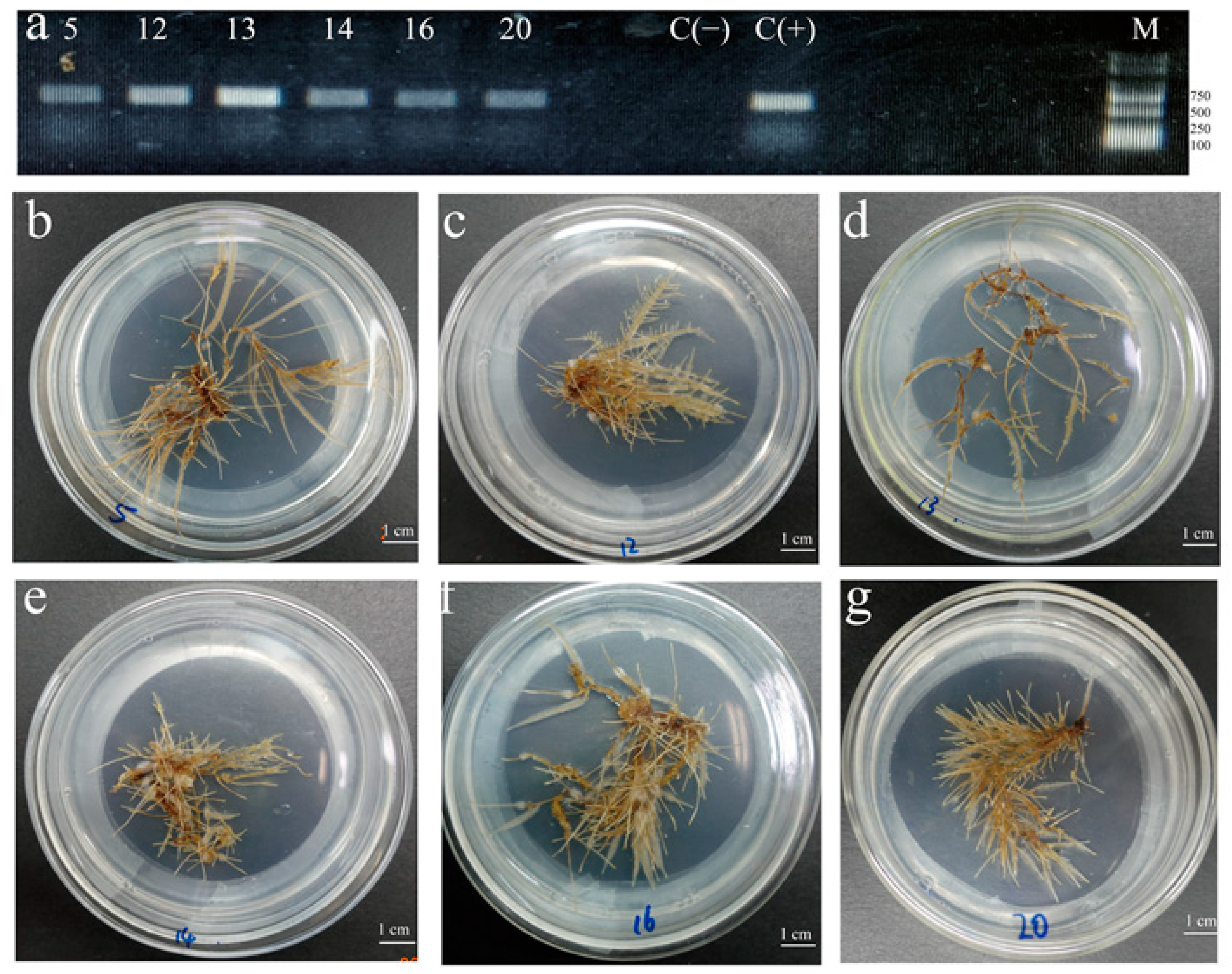
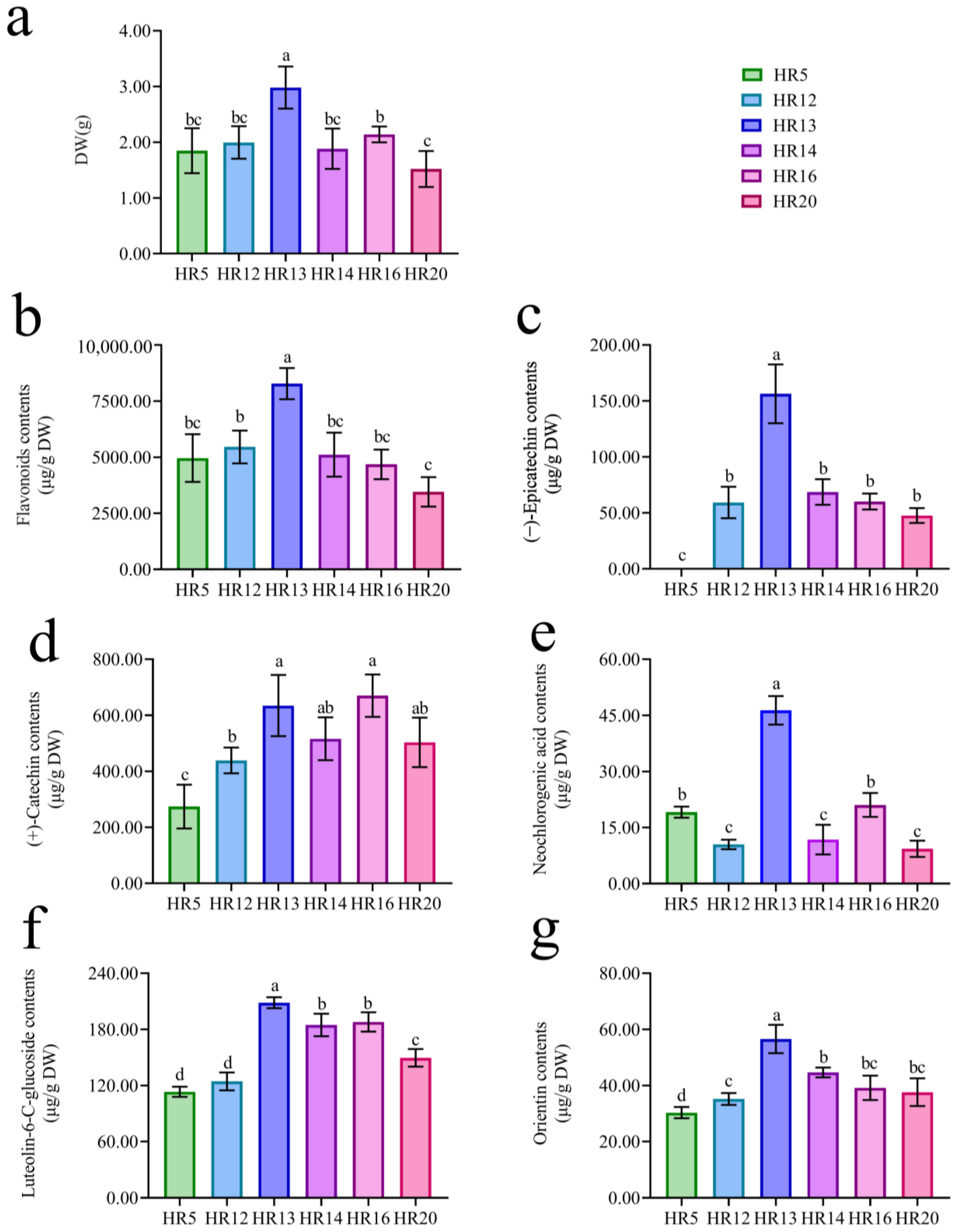
2.2. Selection of Hairy Root Lines with High Biomass and High Flavonoids Contents
2.3. Comparison of Flavonoids Content of Hairy Roots and True Roots
2.4. Comparison of Antioxidant Activity of Hairy Roots and True Roots
3. Discussion
3.1. Establishment of a Highly Efficient Hairy Roots Induction System
3.2. High (+)-Catechin, (−)-Epicatechin, Luteolin-6-C-Glucoside, and Orientin Contents of T. Hemsleyanum Hairy Roots Induced by Agropine Type A. Rhizogenes Ar Qual
3.3. Higher Flavonoids Contents and Antioxidant Activity of T. hemsleyanum Hairy Roots Than True Roots
4. Materials and Methods
4.1. Plant Materials and Chemicals
4.2. Induction of Hairy Roots
4.3. Identification of Hairy Roots
4.4. Selection of Hairy Root Lines with High Biomass and High Flavonoids Production
4.5. Comparison of Flavonoids in Hairy Roots and True Roots
4.6. Measurement of 1,1-Diphenyl-2-picrylhyrdrazyl Free-Radical Scavenging Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T. hemsleyanum | Tetrastigma hemsleyanum Diels et Gilg |
| A. rhizogenes | Agrobacterium rhizogenes |
| HPLC | high-performance liquid chromatography |
| DW | dry weight |
| 6-BA | N6-benzyl adenine |
| AS | acetosyringone |
| NAA | 1-naphthylacetic acid |
| IBA | 3-indolebutyric acid |
| KT | 6-Furfurylamino-purine |
| ORF | open reading frame |
| KAN | kanamycin resistance |
| PCR | polymerase chain reaction |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| DPPH | 1,1-diphenyl-2-picrylhyrdrazyl |
| HR | hairy root |
| TR | true root |
| UHLPC-MS | ultra-high-performance liquid chromatography tandem mass spectrometry |
References
- Jiang, Y.L.; Xu, Z.J.; Cao, Y.F.; Wang, F.; Chu, C.; Zhang, C.; Tao, Y.; Wang, P. HPLC fingerprinting-based multivariate analysis of chemicalcomponents in Tetrastigma Hemsleyanum Diels et Gilg: Correlation to their antioxidant and neuraminidase inhibition activities. J. Pharm. Biomed. Anal. 2021, 205, 114314. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, L.; Ru, Y.; Weng, S.H.; Chen, Z.; Wang, J.; Guo, L.H.; Lin, Z.Y.; Pan, W.; Qiu, B. Antibacterial mechanism of Tetrastigma hemsleyanum Diels et Gilg’s polysaccharides by metabolomics based on HPLC/MS. Int. J. Biol. Macromol. 2019, 140, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, J.; Du, R.; Yu, Q.; Gong, L.; Jiang, H.; Rong, R. Qualitative and Quantitative Analysis for the Chemical Constituents of Tetrastigma hemsleyanum Diels et Gilg Using Ultra-High Performance Liquid Chromatography/Hybrid Quadrupole-Orbitrap Mass Spectrometry and Preliminary Screening for Anti-Influenza Virus Components. Evid.-Based Complement. Altern. Med. 2019, 2019, 9414926. [Google Scholar] [CrossRef]
- Zhan, L.H.; Pu, J.B.; Zheng, J.R.; Hang, S.N.; Pang, L.S.; Dai, M.H.; Ji, C.L. Tetrastigma hemsleyanum Diels et Gilg ameliorates lipopolysaccharide induced sepsis via repairing the intestinal mucosal barrier. Biomed. Pharmacother. 2022, 148. [Google Scholar] [CrossRef]
- Zhuang, B.; Bi, Z.M.; Wang, Z.Y.; Duan, L.; Lai, C.J.S.; Liu, E.H. Chemical profiling and quantitation of bioactive compounds in Platycladi Cacumen by UPLC-Q-TOF-MS/MS and UPLC-DAD. J. Pharm. Biomed. Anal. 2018, 154, 207–215. [Google Scholar] [CrossRef]
- Sun, Y.; Hui, Q.; Chen, R.; Li, H.; Peng, H.; Chen, F.; Deng, Z. Apoptosis in human hepatoma HepG2 cells induced by the phenolics of Tetrastigma hemsleyanum leaves and their antitumor effects in H22 tumor-bearing mice. J. Funct. Foods 2018, 40, 349–364. [Google Scholar] [CrossRef]
- Du, S.; Xiang, T.; Song, Y.; Huang, L.; Sun, Y.; Han, Y. Transgenic hairy roots of Tetrastigma hemsleyanum: Induction, propagation, genetic characteristics and medicinal components. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 373–382. [Google Scholar] [CrossRef]
- Zhu, M.X.; Yan, L.H. [Resource investigation on rare and endangered She medicine Tetrastigma hemsleyanum in Zhejiang Province]. Zhong Yao Cai= Zhongyaocai= J. Chin. Med. Mater. 2014, 37, 766–770. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, N.; Zhang, Y.C.; Fu, C.X. Development and characterization of microsatellite markers for the Chinese endangered medicinal plant Tetrastigma hemsleyanum. Genet. Mol. Res. 2014, 13, 9062–9067. [Google Scholar] [CrossRef]
- Hu, W.; Zheng, Y.; Xia, P.; Liang, Z. The research progresses and future prospects of Tetrastigma hemsleyanum Diels et Gilg: A valuable Chinese herbal medicine. J. Ethnopharmacol. 2021, 271, 113836. [Google Scholar] [CrossRef]
- Aggarwal, P.R.; Nag, P.; Choudhary, P.; Chakraborty, N.; Chakraborty, S. Genotype-independent Agrobacterium rhizogenes-mediated root transformation of chickpea: A rapid and efficient method for reverse genetics studies. Plant Methods 2018, 14, 55. [Google Scholar] [CrossRef]
- Shi, M.; Liao, P.; Nile, S.H.; Georgiev, M.I.; Kai, G. Biotechnological Exploration of Transformed Root Culture for Value-Added Products. Trends Biotechnol. 2021, 39, 137–149. [Google Scholar] [CrossRef]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S.H.; Wang, Y.; Zhou, W.; et al. Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Yakupova, A.B.; Musin, K.G.; Kuluev, B.R. A comparative analysis of the content of sugars and flavonoids in the hairy roots of sunflower Heliantus annuus L. Probl. Biol. Med. Pharm. Chem. 2020, 23, 9–18. [Google Scholar] [CrossRef]
- Cao, D.M.; Vu, P.T.B.; Hoang, M.T.T.; Bui, A.L.; Quach, P.N.D. Developing a Sufficient Protocol for the Enhancement of alpha-Glucosidase Inhibitory Activity by Urena lobata L. Aeroponic Hairy Roots Using Exogenous Factors, a Precursor, and an Elicitor. Plants 2020, 9, 548. [Google Scholar] [CrossRef]
- Baek, S.; Han, J.E.; Ho, T.T.; Park, S.Y. Development of Hairy Root Cultures for Biomass and Triterpenoid Production in Centella asiatica. Plants 2022, 11, 148. [Google Scholar] [CrossRef]
- Syklowska-Baranek, K.; Rymaszewski, W.; Gawel, M.; Rokicki, P.; Pilarek, M.; Grech-Baran, M.; Hennig, J.; Pietrosiuk, A. Comparison of elicitor-based effects on metabolic responses of Taxus x media hairy roots in perfluorodecalin-supported two-phase culture system. Plant Cell Rep. 2019, 38, 85–99. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, M.M.; Pu, S.; Chen, L.; Zhang, X.; Zhang, J.; Zhu, C.S. Identification of secondary metabolites in Tripterygium wilfordii hairy roots and culture optimization for enhancing wilforgine and wilforine production. Ind. Crop. Prod. 2020, 148, 112276. [Google Scholar] [CrossRef]
- Naliwajski, M.R.; Wilenska, B.; Misicka, A.; Pietrosiuk, A.; Syklowska-Baranek, K. HPLC-PDA-ESI-HRMS-Based Profiling of Secondary Metabolites of Rindera graeca Anatomical and Hairy Roots Treated with Drought and Cold Stress. Cells 2022, 11, 931. [Google Scholar] [CrossRef]
- Campos, G.; Chialva, C.; Miras, S.; Lijavetzky, D. New Technologies and Strategies for Grapevine Breeding Through Genetic Transformation. Front. Plant Sci. 2021, 12, 767522. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Li, B.; Lu, X.; Kang, J.; Cao, X. Establishment of in vitro culture system for Codonopsis pilosula transgenic hairy roots. 3 Biotech 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Rekha, K.; Chung, I.-M. Induction of hairy roots by Agrobacterium rhizogenes-mediated transformation of spine gourd (Momordica dioica Roxb. ex. willd) for the assessment of phenolic compounds and biological activities. Sci. Hortic. 2016, 198, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Isca, V.M.S.; Picot, L.; Wielanek, M.; Sliwinski, T.; Sitarek, P. Preliminary Phytochemical Analysis and Evaluation of the Biological Activity of Leonotis nepetifolia (L.) R. Br Transformed Roots Extracts Obtained through Rhizobium rhizogenes-Mediated Transformation. Cells 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Tashackori, H.; Sharifi, M.; Ahmadian Chashmi, N.; Behmanesh, M.; Safaie, N.; Sagharyan, M. Physiological, biochemical, and molecular responses of Linum album to digested cell wall of Piriformospora indica. Physiol. Mol. Biol. Plants 2021, 27, 2695–2708. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Park, N.I.; Park, S.U. Effect of Light and Dark on the Phenolic Compound Accumulation in Tartary Buckwheat Hairy Roots Overexpressing ZmLC. Int. J. Mol. Sci. 2021, 22, 4702. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; He, S.; Gao, C.; Wang, C.; Shao, Y. Effects of Natural Flavonoid Isoorientin on Growth Performance and Gut Microbiota of Mice. J. Agric. Food Chem. 2018, 66, 9777–9784. [Google Scholar] [CrossRef]
- Li, F.; Liao, X.; Jiang, L.; Zhao, J.; Wu, S.; Ming, J. Orientin Attenuated D-GalN/LPS-Induced Liver Injury through the Inhibition of Oxidative Stress via Nrf2/Keap1 Pathway. J. Agric. Food Chem. 2022, 70, 7953–7967. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, X.; Liu, J.; Lu, Y.; Wang, Z.-Y.; Xu, X.-J.; Fu, Y.-J. Effective Production of Phenolic Compounds with Health Benefits in Pigeon Pea Cajanus cajan (L.) Millsp. Hairy Root Cultures. J. Agric. Food Chem. 2020, 68, 8350–8361. [Google Scholar] [CrossRef]
- Shafi, W.; Mansoor, S.; Jan, S.; Singh, D.B.; Kazi, M.; Raish, M.; Alwadei, M.; Mir, J.I.; Ahmad, P. Variability in Catechin and Rutin Contents and Their Antioxidant Potential in Diverse Apple Genotypes. Molecules 2019, 24, 943. [Google Scholar] [CrossRef]
- Rigling, M.; Liu, Z.; Hofele, M.; Prozmann, J.; Zhang, C.; Ni, L.; Fan, R.; Zhang, Y. Aroma and catechin profile and in vitro antioxidant activity of green tea infusion as affected by submerged fermentation with Wolfiporia cocos (Fu Ling). Food Chem. 2021, 361, 130065. [Google Scholar] [CrossRef]
- Naeini, M.S.; Naghavi, M.R.; Bihamta, M.R.; Sabokdast, M.; Salehi, M. Production of some benzylisoquinoline alkaloids in Papaver armeniacum L. hairy root cultures elicited with salicylic acid and methyl jasmonate. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 261–271. [Google Scholar] [CrossRef]
- Su, Y.; Lin, C.; Zhang, J.; Hu, B.; Wang, J.; Li, J.; Wang, S.; Liu, R.; Li, X.; Song, Z.; et al. One-Step Regeneration of Hairy Roots to Induce High Tanshinone Plants in Salvia miltiorrhiza. Front. Plant Sci. 2022, 13, 913985. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Wan, P.F.; Dong, C.L.; Li, Y.H. Optimization of total flavonoids content extracted from Flos Populi using response surface methodology. Ind. Crop. Prod. 2013, 43, 778–786. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative Properties of Vanillic Acid Esters in Multiple Antioxidant Assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef]

| A. rhizogenes | Explant Type | Induction Rate 1 (%) | Induction Rate 2 (%) | Positive Rate (%) | Positive Acquisition Rate (%) |
|---|---|---|---|---|---|
| C58C1 | stems | 90.80 ± 2.81 a | 337.90 ± 15.80 a | 5.43 ± 0.70 a | 18.14 ± 1.47 a |
| C58C1 | leaves | 36.73 ± 4.02 b | 101.23 ± 42.52 b | 39.40 ± 0.80 b | 40.00 ± 17.14 b |
| Ar Qual | stems | 90.48 ± 9.24 a | 342.86 ± 47.72 a | 88.15 ± 3.45 c | 25.71 ± 13.42 ab |
| Ar Qual | leaves | 75.17 ± 7.42 c | 426.67 ± 52.49 c | 96.57 ± 1.72 d | 317.27 ± 57.10 d |
| Infective Mode | Induction Rate 1 (%) | Induction Rate 2 (%) |
|---|---|---|
| No vacuum | 79.37 ± 9.64 a | 396.45 ± 74.65 a |
| Vacuum (0.5 min) | 38.33 ± 3.67 b | 90.32 ± 4.92 b |
| Vacuum (1.0 min) | 16.90 ± 5.97 c | 20.13 ± 18.67 c |
| Co-cultivation Times | Induction Rate 1 (%) | Induction Rate 2 (%) |
|---|---|---|
| 1 d | 5.30 ± 1.33 a | 5.30 ± 5.22 a |
| 2 d | 18.76 ± 5.59 b | 82.14 ± 21.71 b |
| 3 d | 65.14 ± 14.83 c | 326.65 ± 64.75 c |
| 4 d | 8.70 ± 3.83 a,b | 26.65 ± 10.90 d |
| Induction Medium Types | Induction Rate 1 (%) | Induction Rate 2 (%) |
|---|---|---|
| MS | 25.00 ± 8.33 a | 52.78 ± 31.55 a |
| 1/2 MS | 69.05 ± 27.04 b | 382.14 ± 93.55 b |
| 1/4 MS | 95.83 ± 7.22 b | 951.39 ± 231.85 c |
| B5 | 60.17 ± 12.85 a,b | 326.65 ± 90.89 b |
| N6 | 16.67 ± 5.56 a | 16.67 ± 5.56 a |
| NO. | Component Name | Linear | R2 | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| 1 | (+)-Catechin | y = 36,373x − 5711.7 | 0.9993 | 40.00–240.00 | 0.150 | 0.280 |
| 2 | (−)-Epicatechin | y = 25,331x + 1938.2 | 0.9990 | 0.30–24.00 | 0.150 | 0.300 |
| 3 | Neochlorogenic acid | y = 17,879x − 18,429 | 0.9992 | 1.23–66.67 | 0.025 | 0.063 |
| 4 | Luteolin-6-C-glucoside | y = 33,118x − 67,899 | 0.9997 | 1.23–66.67 | 0.029 | 0.060 |
| 5 | Orientin | y = 15,053x – 36,659 | 0.9992 | 1.23–66.67 | 0.079 | 0.200 |
| Samples | The Content of Each Compound (μg/g DW) * | |||||
|---|---|---|---|---|---|---|
| Total Flavonoids | (+)-Catechin | (−)-Epicatechin | Neochlorogenic Acid | Luteolin-6-C-Glucoside | Orientin | |
| TR | 19,851.63 ± 575.36 a | 622.52 ± 97.53 a | 70.21 ± 25.12 b | 15.23 ± 0.38 b | 185.29 ± 1.19 b | 44.06 ± 0.79 b |
| HR | 8919.48 ± 740.97 b | 692.63 ± 127.24 a | 163.34 ± 31.86 a | 45.95 ± 3.46 a | 209.68 ± 6.03 a | 56.82 ± 4.75 a |
| No. | Name | IC50 (μg/mL) * |
|---|---|---|
| 1 | HR | 1.64 ± 0.16 f |
| 2 | TR | 2.34 ± 0.15 e |
| 3 | Rutin | 3.29 ± 0.54 d |
| 4 | (+)-Catechin | 1.41 ± 0.24 f |
| 5 | Orientin | 23.10 ± 6.67 b |
| 6 | Luteolin-6-C-glucoside | 20.32 ± 0.32 b |
| 7 | Neochlorogenic acid | 50.39 ± 4.4 a |
| 8 | (−)-Epicatechin | 10.46 ± 2.49 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, A.; Pu, H.; Yang, Y.; Ling, Z.; Xu, H.; Xu, J.; Yu, H.; Wu, X. Induction, Flavonoids Contents, and Bioactivities Analysis of Hairy Roots and True Roots of Tetrastigma hemsleyanum Diels et Gilg. Molecules 2023, 28, 2686. https://doi.org/10.3390/molecules28062686
Wang H, Wang A, Pu H, Yang Y, Ling Z, Xu H, Xu J, Yu H, Wu X. Induction, Flavonoids Contents, and Bioactivities Analysis of Hairy Roots and True Roots of Tetrastigma hemsleyanum Diels et Gilg. Molecules. 2023; 28(6):2686. https://doi.org/10.3390/molecules28062686
Chicago/Turabian StyleWang, Hongzhen, Anran Wang, Hanying Pu, Yuxin Yang, Zeyuan Ling, Haishun Xu, Juan Xu, Haizheng Yu, and Xueqian Wu. 2023. "Induction, Flavonoids Contents, and Bioactivities Analysis of Hairy Roots and True Roots of Tetrastigma hemsleyanum Diels et Gilg" Molecules 28, no. 6: 2686. https://doi.org/10.3390/molecules28062686
APA StyleWang, H., Wang, A., Pu, H., Yang, Y., Ling, Z., Xu, H., Xu, J., Yu, H., & Wu, X. (2023). Induction, Flavonoids Contents, and Bioactivities Analysis of Hairy Roots and True Roots of Tetrastigma hemsleyanum Diels et Gilg. Molecules, 28(6), 2686. https://doi.org/10.3390/molecules28062686





