New Inhibitors of Respiratory Syncytial Virus (RSV) Replication Based on Monoterpene-Substituted Arylcoumarins
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.3. Molecular Modeling Study
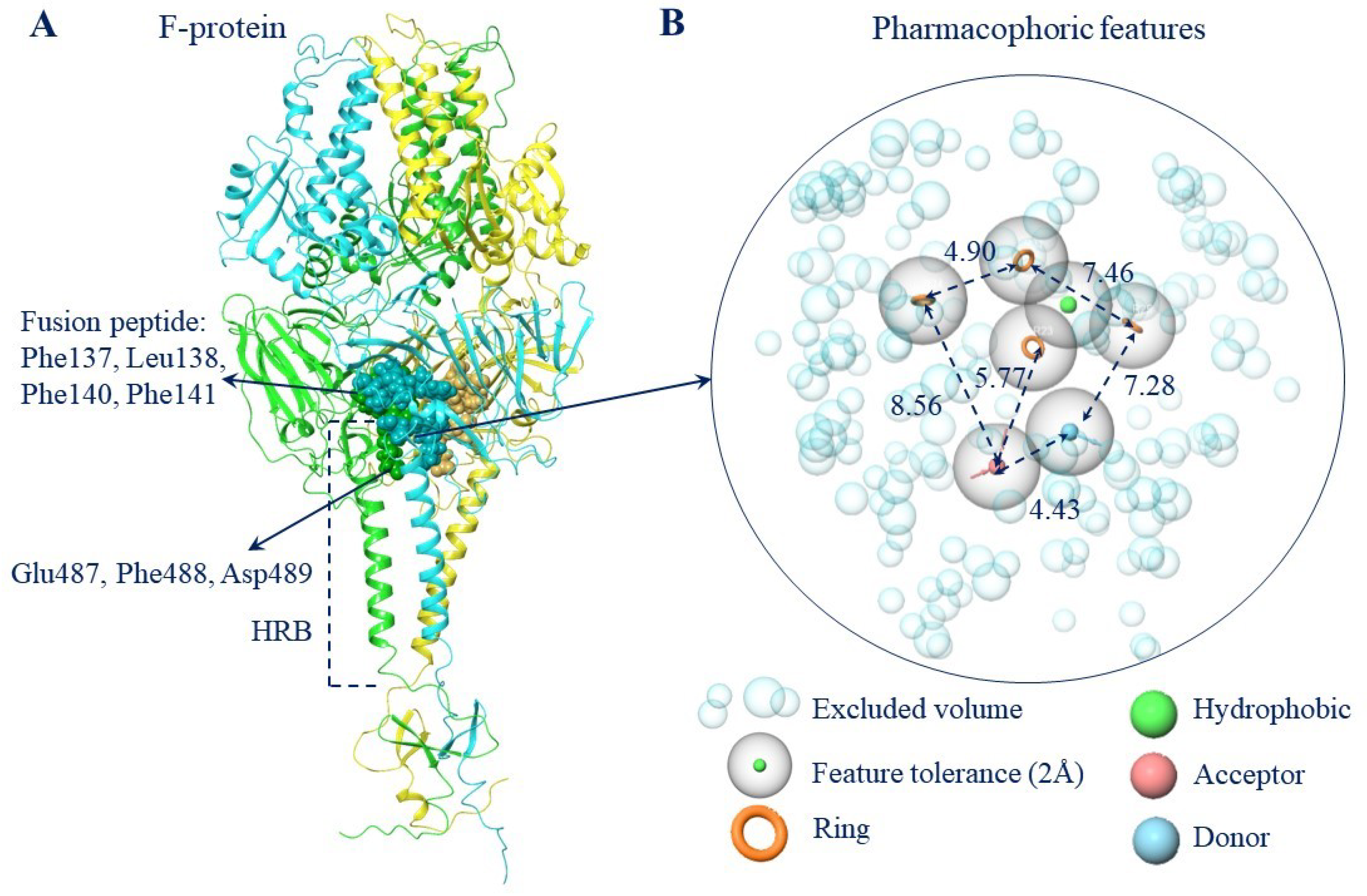
2.3.1. The Pharmacophoric Profile of the Binding Site to F Protein Inhibitor
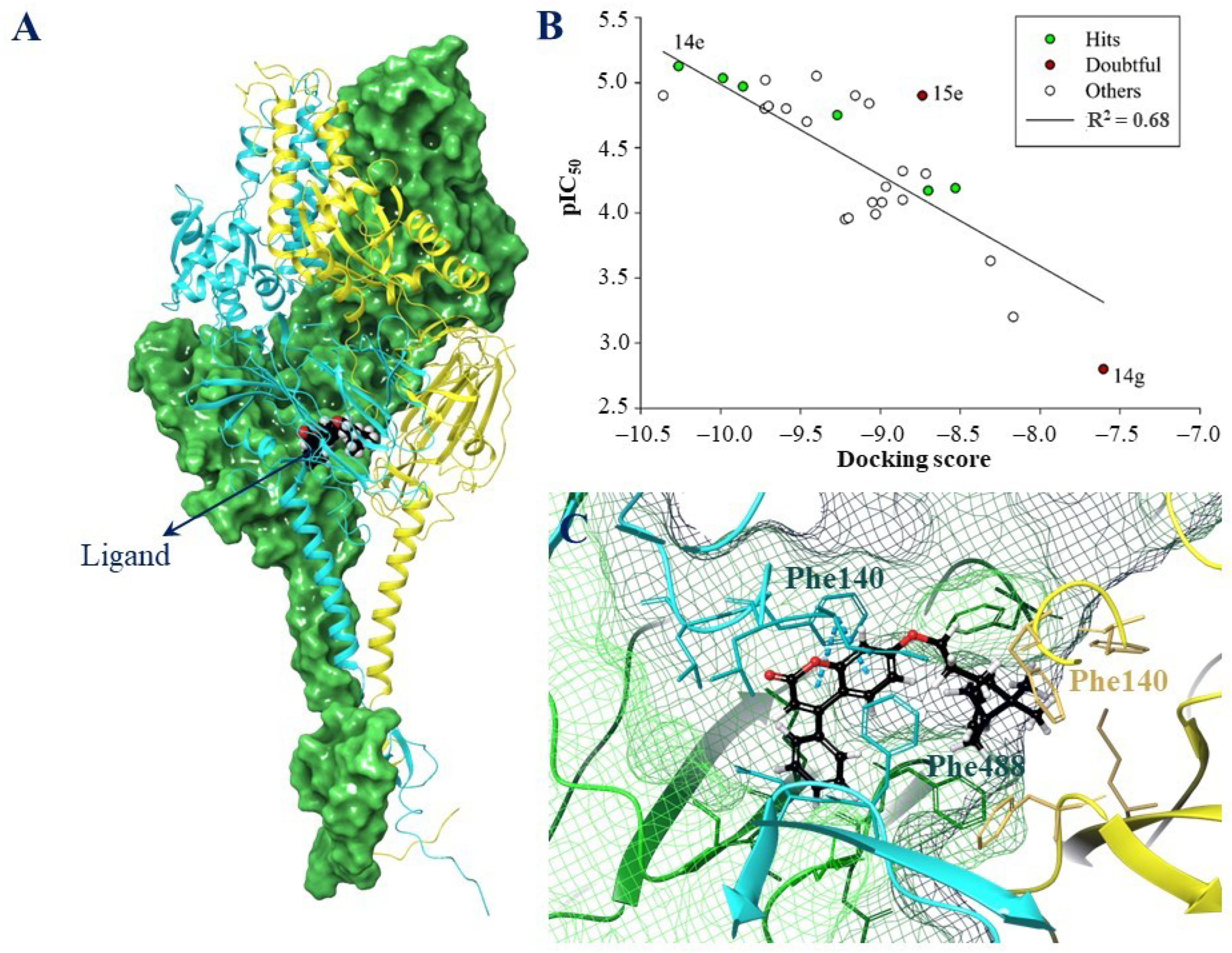
2.3.2. The Molecular Docking Data
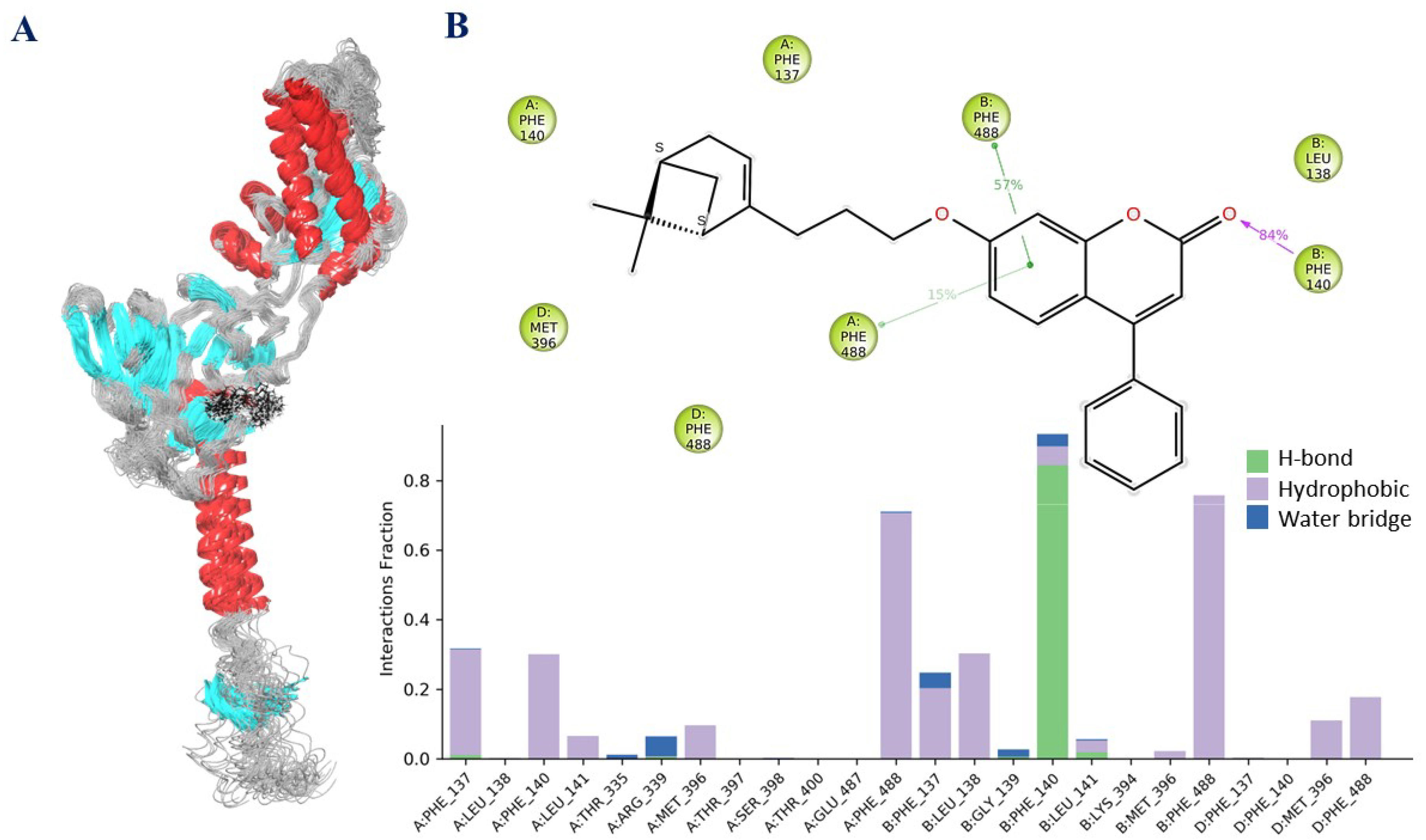
2.3.3. The Results of Molecular Dynamics Simulations
3. Materials and Methods
3.1. Chemistry
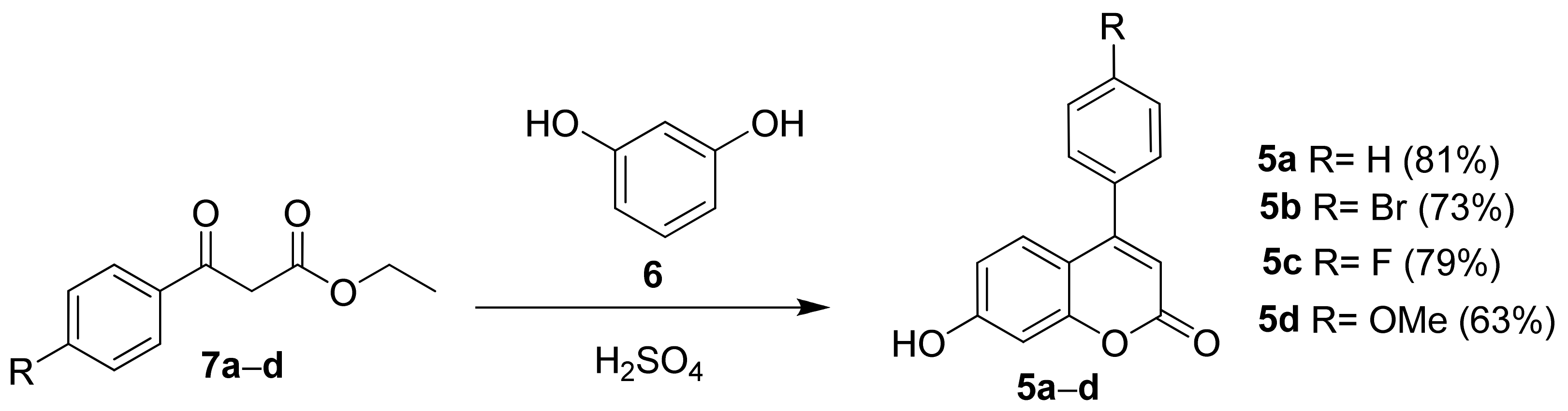
3.1.1. Synthesis of Coumarins 5a–d
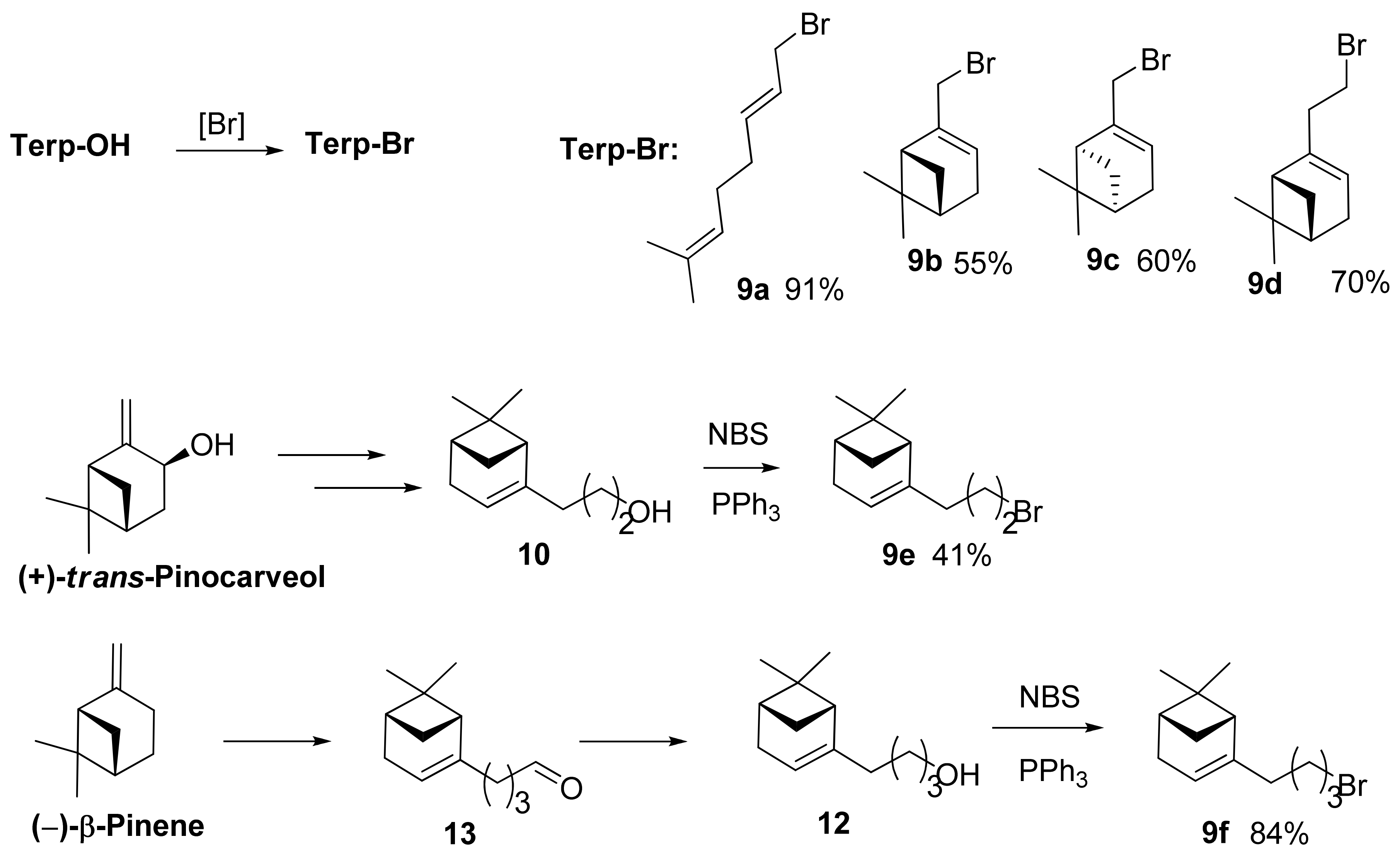
3.1.2. Synthesis of Bromides 9a–c
3.1.3. Synthesis of Bromides 9d–f
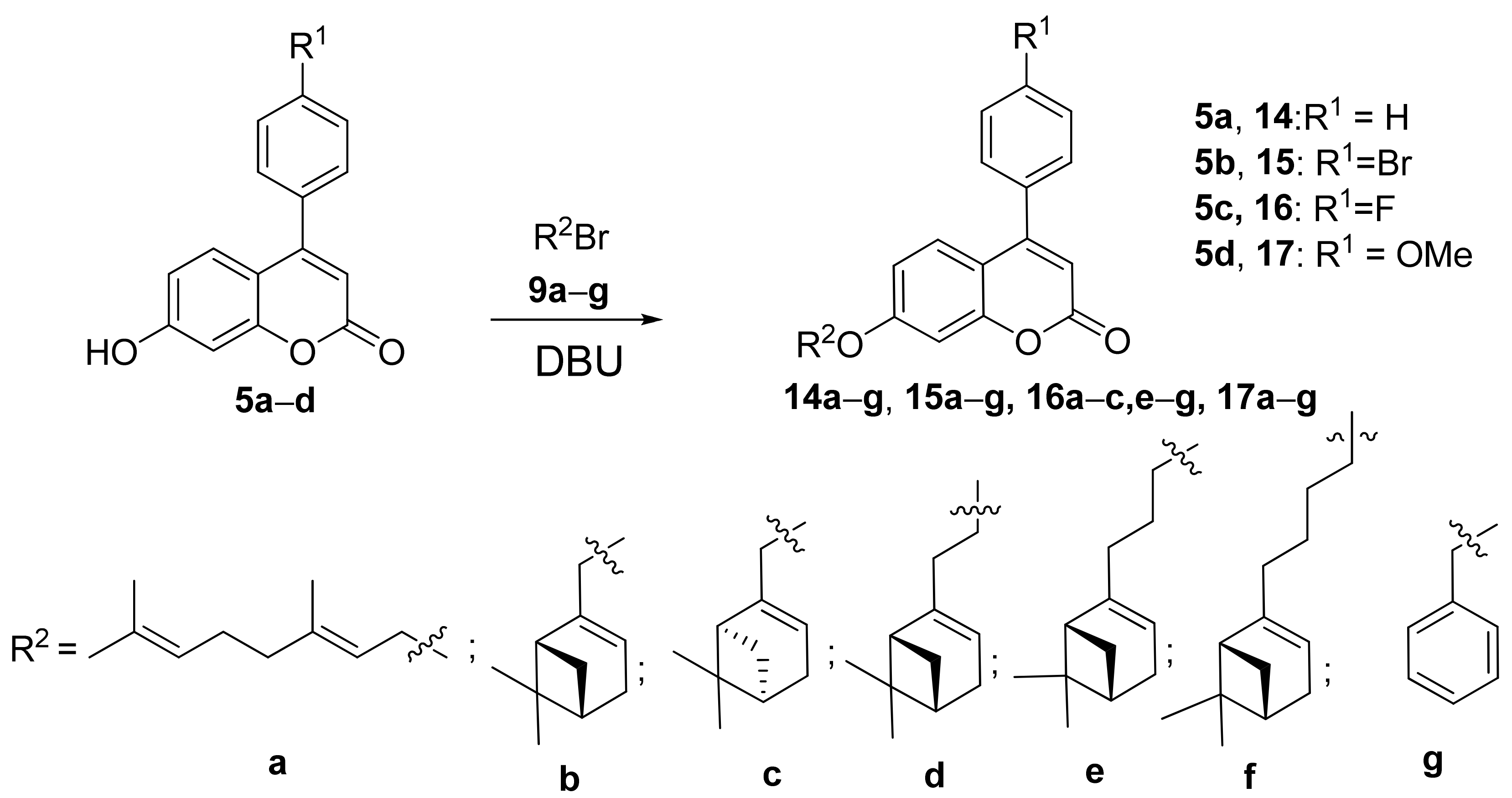
3.1.4. Synthesis of Compounds 14a–g, 15a–g, 16a–c, 16e–g, and 17a–g
- 7-(3-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)propoxy)-4-phenyl-2H-chromen-2-one 14e Yield 56%, Method b. = −14.9 (c = 0.83, CHCl3). HRMS: 399.1959 [M − H]+; calcd. 399.1955 (C27H27O3). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.82 (s, 3H-C(27)); 1.13 (d, 1H, 2J = 8.5, H-C(25a)); 1.25 (s, 3H-C(26)); 1.79–1.91 (m, 2H-C(17)); 2.02 (ddd, 1H, J(24,22) = J(24,25s)= 5.6, J(24,20) = 1.4, H-C(24)); 2.05–2.13 (m, 3H, 2H-C(18), H-C(22)); 2.17 (dm, 1H, 2J = 17.5, H-C(21)) 2.23 (dm, 1H, 2J = 17.5, H’-C(21)); 2.35 (ddd. 1H, 2J = 8.5, J(25s,22) = J(25s,24) = 5.6, H-C(25s)); 3.99 (t, 2H, J(16,17) = 6.5, 2H-C(16)); 5.20–5.23 (m, H-C(20)); 6.18 (s, H-C(3)); 6.76 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.85 (d, 1H, J(9,7) = 2.5, H-C(9)); 7.35 (d, 1H, J(6,7) = 8.9, H-C(6)); 7.39–7.43 (m, 2H, H-(C-11), H-C(15)); 7.47–7.51 (m, 3H, H-C(12), H-C(13), H-C(14)). 13C-NMR (CDCl3, δC): 155.88 (s, C(1)); 161.16 (s, C(2)); 111.56 (d, C(3)); 155.72 (s, C(4)); 112.20 (s, C(5)); 127.77 (d, C(6)); 112.59 (d, C(7)); 162.23 (s, C(8)); 101.41 (d, C(9)); 135.49 (s, C(10)); 128.24 (d, C(11)); 128.67 (d, C(12); 129.41 (d, C(13)); 128.67 (d, C(14); 128.24 (d, C(15)); 68.15 (t, C(16)); 26.44 (t, C(17)); 32.87 (t, C(18)); 146.93 (s, C(19)); 116.67 (d, C(20)); 31.13 (t, C(21)); 40.68 (d, C(22)); 37.82 (s, C(23)); 45.58 (d, C(24)); 31.57 (t, C(25)); 26.17 (q, C(26)); 21.06 (q, C(27)).
- 7-(4-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)butoxy)-4-phenyl-2H-chromen-2-one 14f Yield 40%, Method a. M.p. 40 °C. = −12.8 (c = 1.85, CHCl3) HRMS: 413.2114 [M − H]+; calcd. 413.2111 (C28H29O3). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.82 (s, 3H-C(28)); 1.12 (d, 1H, 2J = 8.5, H-C(26a)); 1.25 (s, 3H-C(27)); 1.44–1.58 (m, 2H-C(18)); 1.75–1.83 (m, 2H-C(17)); 1.96–2.02 (m, 3H, 2H-C(19), H-C(25)); 2.04–2.09 (m, H-C(23)); 2.17 (dm, 1H, 2J = 17.5, H-C(22)); 2.23 (dm, 1H, 2J = 17.5, H’-C(22)); 2.34 (ddd, 1H, 2J = 8.5, J(26s,23) = J(26s,25) = 5.6, H-C(26s)); 4.01 (t, 2H, J(16,17) = 6.5, 2H-C(16)); 5.17–5.21 (m, H-C(21)); 6.18 (s, H-C(3)); 6.76 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.85 (d, J(9,7) = 2.5, H-C(9)); 7.34 (d, 1H, J(6,7) = 8.9, H-C(6)); 7.39–7.43 (m, 2H, H-(C-11), H-C(15)); 7.46–7.51 (m, 3H, H-C(12), H-C(13), H-C(14)). 13C-NMR (CDCl3, δC): 155.90 (s, C(1)); 161.13 (s, C(2)); 111.58 (d, C(3)); 155.70 (s, C(4)); 112.21 (s, C(5)); 127.76 (d, C(6)); 112.58 (d, C(7)); 162.25 (s, C(8)); 101.47 (d, C(9)); 135.52 (c, C(10)); 128.24 (d, C(11)); 128.67 (d, C(12); 129.40 (d, C(13)); 128.67 (d, C(14); 128.24 (d, C(15)); 68.42 (t, C(16)); 28.59 (t, C(17)); 23.35 (t, C(18)); 36.34 (t, C(19)); 147.71 (c, C(20)); 116.16 (d, C(21)); 31.14 (t, C(22)); 40.76 (d, C(23)); 37.81 (c, C(24)); 45.63 (d, C(25)); 31.55 (t, C(26)); 26.22 (q, C(27)); 21.07 (q, C(28)).
- 4-(4-Bromophenyl)-7-(3-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)propoxy)-2H-chromen-2-one 15e Yield 68%, Method b. = −13.1 (c = 0.55, CHCl3). HRMS: 477.1066 [M − H]+; calcd. 477.1060 (C27H26O379Br1). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.82 (s, 3H-C(27)); 1.13 (d, 1H, 2J = 8.5, H-C(25a)); 1.26 (s, 3H-C(26)); 1.79–1.91 (2H-C(17)); 2.02 (ddd, 1H, J(24,22) = J(24,25s)= 5.6, J(24,20) = 1.4, H-C(24)); 2.05–2.13 (m, 2H-C(18), H-C(22)); 2.17 (dm, 1H, 2J = 17.5, H-C(21)); 2.23 (dm, 1H, J = 17.5, H’-C(17)); 2.35 (ddd, 1H, 2J = 8.5, J(25s,22) = J(25s,24) = 5.6, H-C(25s)); 3.99 (t, 2H, J(16,17) = 6.5, 2H-C(16)); 5.20–5.23 (m, 1H, H-C(20)); 6.16 (s, H-C(3)); 6.77 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.85 (d, J(9,7) = 2.5, H-C(9)); 7.27–7.31 (m, 3H, H-C(6), H-(C-11), H-C(15)); 7.63 (d, 2H, J(12,11) = J(14,15) = 8.5, H-C(12), H-C(14)). 13C-NMR (CDCl3, δC): 155.92 (s, C(1)); 160.88 (s, C(2)); 111.67 (d, C(3)); 154.51 (s, C(4)); 111.84 (s, C(5)); 127.45 (d, C(6)); 112.78 (d, C(7)); 162.44 (s, C(8)); 101.55 (d, C(9)); 134.36 (c, C(10)); 129.85 (d, C(11)); 132.00 (d, C(12); 123.88 (c, C(13)); 132.00 (d, C(14); 129.85 (d, C(15)); 68.23 (t, C(16)); 26.45 (t, C(17)); 32.88 (t, C(18)); 146.93 (c, C(19)); 116.72 (d, C(20)); 31.15 (t, C(21)); 40.72 (d, C(22)); 37.85 (c, C(23)); 45.63 (d, C(24)); 31.59 (t, C(25)); 26.20 (q, C(26)); 21.08 (q, C(27)).
- 4-(4-Bromophenyl)-7-(4-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)butoxy)-2H-chromen-2-one 15f Yield 38%, Method a. M.p. 44 °C. = −8.2 (c = 0.95, CHCl3). HRMS: 491.1214 [M − H]+; calcd. 491.1216 (C28H29O379Br1). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.81 (s, 3H-C(28)); 1.12 (d, 1H, 2J = 8.5, H-C(26a)); 1.25 (s, 3H-C(27)); 1.43–1.57 (m, 2H-C(18)); 1.75–1.83 (m, 2H-C(17)); 1.96–2.02 (m, 3H, 2H-C(19), H-C(25)); 2.03–2.09 (m, H-C(23)); 2.16 (dm, 2J = 17.5, H-C(22)); 2.23 (dm, 1H, 2J = 17.5, H’-C(22)); 2.34 (ddd, 1H, 2J = 8.5, J(26s,23) = J(26s,25) = 5.6, H-C(26s)); 4.00 (t, 2H, J(16,17) = 6.5, 2H-C(16)); 5.17–5.21 (m, 1H, H-C(21)); 6.15 (s, 1H, H-C(3)); 6.76 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.85 (d, 1H, J = 2.5, H-C(9)); 7.26–7.31 (m, 3H, H-C(6), H-(C-11), H-C(15)); 7.63 (m, 2H, H-C(12), H-C(14)). 13C-NMR (CDCl3, δC): 155.92 (s, C(1)); 160.85 (s, C(2)); 111.67 (d, C(3)); 154.48 (s, C(4)); 111.81 (s, C(5)); 127.43 (d, C(6)); 112.75 (d, C(7)); 162.44 (s, C(8)); 101.59 (d, C(9)); 134.37 (c, C(10)); 129.84 (d, C(11)); 131.99 (d, C(12); 123.87 (c, C(13)); 131.99 (d, C(14); 129.84 (d, C(15)); 68.49 (t, C(16)); 28.58 (t, C(17)); 23.35 (t, C(18)); 36.34 (t, C(19)); 147.70 (c, C(20)); 116.19 (d, C(21)); 31.15 (t, C(22)); 40.76 (d, C(23)); 37.82 (c, C(24)); 45.64 (d, C(25)); 31.56 (t, C(26)); 26.23 (q, C(27)); 21.08 (q, C(28)).
- 7-(3-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)propoxy)-4-(4-fluorophenyl)-2H-chromen-2-one 16e Yield 72%, Method b. = −16.6 (c = 0.70, CHCl3). HRMS: 417.1856 [M − H]+; calcd. 417.1861 (C27H26O379F1).∙1H-NMR (CDCl3, δ ppm, J, Hz): 0.81 (s, 3H-C(27)); 1.13 (d, 1H, 2J = 8.5, H-C(25a)); 1.25 (s, 3H-C(26)); 1.79–1.91 (m, 2H-C(17)); 2.02 (ddd, J(24,22) = J(24,25s) = 5.6, J(24,20) = 1.3, H-C(24)); 2.05–2.13 (m, 2H-C(18), H-C(22)); 2.17 (dm, 2J = 17.5, H-C(21)); 2.23 (dm, 1H, 2J = 17.5, H’-C(21)); 2.35 (ddd, 1H, 2J = 8.5, J(25s,22) = J(25s,24) = 5.6, H-C(25s)); 3.99 (t, 2H, J = 6.5, J(16,17) = 6.5, 2H-C(16)); 5.20–5.23 (m, H-C(20)); 6.16 (s, H-C(3)); 6.77 (dd, J = 8.8, 2.5, H-C(7)); 6.85 (d, J = 2.5, H-C(9)); 7.16–7.21 (m, 2H, H-(C-12), H-C(14)); 7.31 (d, J = 8.8, H-C(6)); 7.38–7.43 (m, 2H, H-C(11), H-C(15)). 13C-NMR (CDCl3, δC): 155.88 (s, C(1)); 160.98 (s, C(2)); 111.71 (d, C(3)); 154.65 (s, C(4)); 112.08 (s, C(5)); 127.52 (d, C(6)); 112.71 (d, C(7)); 162.35 (s, C(8)); 101.49 (d, C(9)); 131.48 (d, J = 3.4, C(10)); 130.18 (d, J = 8.3, C(11) and C(15)); 115.89 (d, J = 21.7, C(12) and C(14)); 163.32 (d, 1J = 250.1, C(13)); 68.19 (t, C(16)); 26.43 (t, C(17)); 32.86 (t, C(18)); 146.92 (c, C(19)); 116.70 (d, C(20)); 31.13 (t, C(21)); 40.68 (d, C(22)); 37.83 (c, C(23)); 45.59 (d, C(24)); 31.58 (t, C(25)); 26.18 (q, C(26)); 21.07 (q, C(27)).
- 7-(4-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)butoxy)-4-(4-fluorophenyl)-2H-chromen-2-one 16f Yield 55%, Method a. M.p. 40 °C. = −8.0 (c = 1.28, CHCl3). HRMS: 431.2023 [M − H]+; calcd. 431.2017 (C28H28O379F1).∙1H-NMR (CDCl3, δ ppm, J, Hz): 0.81 (s, 3H-C(28)); 1.12 (d, 1H, 2J = 8.5, H-C(26a)); 1.25 (s, 3H-C(27)); 1.43–1.57 (m, 2H-C(18)); 1.76–1.82 (m, 2H-C(17)); 1.96–2.02 (m, 3H, 2H-C(19), H-C(25)); 2.04–2.08 (m, H-C(23)); 2.17 (dm, 1H, 2J = 17.4, H-C(22)); 2.23 (dm, 1H, 2J = 17.4, H’-C(22)); 2.33 (ddd, 1H, 2J = 8.5, J(26s,23) = J(26s,25) = 5.6, H-C(26s)); 4.00 (t, 2H, J(16,17) = 6.5, 2H-C(16)); 5.17–5.20 (m, H-C(21)); 6.15 (s, H-C(3)); 6.77 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.85 (d, 1H, J = 2.5, H-C(9)); 7.16–7.21 (m, 2H, J(12,11)= J(14,15) = 8.7, J(12(14),F) = 9.0, H-C(12), H-C(14)); 7.30 (d, 1H, J(6,7) = 8.9, H-C(6)); 7.38–7.43 (m. 2H, J(11,12)= J(15,14) =8.7, J(11(15),F)= 5.2, H-(C-11), H-C(15)). 13C-NMR (CDCl3, δC): 155.90 (s, C(1)); 161.00 (s, C(2)); 111.73 (d, C(3)); 154.65 (s, C(4)); 112.08 (s, C(5)); 127.52 (d, C(6)); 112.72 (d, C(7)); 162.36 (s, C(8)); 101.54 (d, C(9)); 131.50 (d, 4J = 3.5, C(10)); 130.21 (d, C(11)); 115.89 (d, 2J = 21.7, C(12) and C(14)); 163.34 (d, 1J = 250.1, C(13)); 130.16 (d, C(15)); 68.47 (t, C(16)); 28.58 (t, C(17)); 23.35 (t, C(18)); 36.35 (t, C(19)); 147.71 (c, C(20)); 116.19 (d, C(21)); 31.15 (t, C(22)); 40.75 (d, C(23)); 37.83 (c, C(24)); 45.62 (d, C(25)); 31.56 (t, C(26)); 26.22 (q, C(27)); 21.08 (q, C(28)).
- 7-(Benzyloxy)-4-(4-fluorophenyl)-2H-chromen-2-one 16g Yield 50%, Method b. HRMS: 346.1006 [M]+; calcd. 346.1000 (C22H15O3F1). 1H-NMR (CDCl3, δ ppm, J, Hz): 5.12 (s, 2H, 2H-C(16)); 6.17 (s, 1H, H-C(3)); 6.86 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.94 (d, 1H, J(9,7) = 2.5, H-C(9)); 7.16–7.21 (m, 2H, J(12,11) = J(14,15) = 8.6, J(12(14),F) = 8.6, H-C(12), H-C(14)); 7.32 (d, 1H, J(6,7) = 8.9, H-C(6)); 7.30–7.35 (m, 1H, H-C(20)); 7.35–7.44 (m, 6H, H-C(11), H-C(15), H-C(18), H-C(19), H-C(21), H-C(22)). 13C-NMR (CDCl3, δC): 155.81 (s, C(1)); 160.83 (s, C(2)); 112.02 (d, C(3)); 154.56 (s, C(4)); 112.50 (s, C(5)); 127.64 (d, C(6)); 112.93 (d, C(7)); 161.83 (s, C(8)); 102.17 (d, C(9)); 131.43 (d, 4J = 3.4, C(10)); 130.18 (d, 3J = 8.4, C(11) and C(15)); 115.90 (d, 2J = 21.8, C(12) and C(14)); 163.36 (d, 1J = 250.1, C(13)); 70.43 (t, C(16)); 135.62 (s, C(17)); 127.37 and 128.65 (2d, C(18), C(22) and C(19), C(21)); 128.28 (d, C(20)).
- 7-(3-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)propoxy)-4-(4-methoxyphenyl)-2H-chromen-2-one 17e Yield 40 %, Method b. HRMS: 429.2055 [M − H]+; calcd. 429.2060 (C28H29O4). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.81 (s, 3H-C(28)); 1.12 (d, 1H, 2J = 8.5, H-C(26a)); 1.25 (s, 3H-C(27)); 1.78–1.91 (m, 2H-C(18)); 2.01 (ddd, 1H, J(25,26s) = J(25,23) = 5.6, J(25,21) = 1.4, H-C(25)); 2.04–2.12 (m, 3H, 2H-C(19), H-C(23)); 2.16 (dm, 1H, 2J = 17.5, H-C(22)); 2.23 (dm, 1H, 2J = 17.5, H’-C(22)); 2.35 (ddd, 1H, 2J = 8.5, J(26s,23) = J(26s,25) = 5.6, H-C(26s)); 3.85 (c, 3H-C(16)); 3.98 (t, 2H, J(17,18) = 6.5, 2H-C(17)); 5.20–5.23 (m, H-C(21)); 6.14 (s, H-C(3)); 6.76 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.83 (d, 1H, J(9,7) = 2.5, H-C(9)); 7.00 (br.d, 2H, J(12,11) = J(14,15) = 8.6, H-C(12), H-C(14)); 7.36 (br.d, 2H, J(11,12) = J(15,14) = 8.8, H-(C-11), H-C(15)); 7.40 (d, 1H, J(6,7) = 8.9, H-C(6). 13C-NMR (CDCl3, δC): 155.91 (s, C(1)); 160.61 (s, C(2)); 111.05 (d, C(3)); 155.37 (s, C(4)); 112.34 (s, C(5)); 127.78 (d, C(6)); 112.48 (d, C(7)); 162.14 (s, C(8)); 101.43 (d, C(9)); 127.78 (c, C(10)); 129.71 (d, C(11)); 114.12 (d, C(12); 160.61 (c, C(13)); 114.12 (d, C(14); 129.71 (d, C(15)); 55.27 (k, C(16)); 68.13 (t, C(17)); 26.46 (t, C(18)); 32.87 (t, C(19)); 146.94 (c, C(20)); 116.65 (d, C(21)); 31.12 (t, C(22)); 40.70 (d, C(23)); 37.81 (c, C(24)); 45.62 (d, C(25)); 31.56 (t, C(26)); 26.17 (q, C(27)); 21.05 (q, C(28)).
- 7-(4-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)butoxy)-4-(4-methoxyphenyl)-2H-chromen-2-one 17f Yield 44%, Method b. = −7.2 (c = 1.03, CHCl3) HRMS: 443.2222 [M − H]+; calcd. 443.2217 (C29H31O4). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.81 (s, 3H-C(29)); 1.12 (d, 1H, 2J = 8.5, H-C(27a)); 1.25 (s, 3H-C(28)); 1.44–1.56 (m, 2H-C(19)); 1.75–1.82 (m, 2H-C(18)); 1.96–2.01 (m, 3H, 2H-C(20), H-C(26)); 2.03–2.07 (m, H-C(24)); 2.16 (dm, 1H, 2J = 17.4, H-C(23)); 2.23 (dm, 1H, 2J = 17.4, H’-C(23)); 2.33 (ddd, 1H, 2J = 8.5, J(27s,24) = J(27s,26) = 5.6, H-C(27s)); 3.86 (c, 3H, C(16)); 4.00 (t, 2H, J(17,18) = 6.5, H-C(17)); 5.17–5.20 (m, 1H, H-C(22)); 6.15 (s, 1H, H-C(3)); 6.76 (dd, 1H, J(7,6) = 8.9, J(7,9) = 2.5, H-C(7)); 6.84 (d, J(9,7) = 2.5, H-C(9)); 7.00 (d, J(12,11) = J(14,15) = 8.8, 2H, H-C(12), H-C(14)); 7.36 (d, J(11,12) = J(15,14) = 8.8, 2H, H-(C-11), H-C(15)); 7.41 (d, J(6,7) = 8.9, H-C(6)). 13C-NMR (CDCl3, δC): 155.89 (s, C(1)); 161.31 (s, C(2)); 111.03 (d, C(3)); 155.37 (s, C(4)); 112.48 (s, C(5)); 127.77 (d, C(6)); 112.48 (d, C(7)); 162.12 (s, C(8)); 101.44 (d, C(9)); 127.73 (c, C(10)); 129.71 (d, C(11)); 114.11 (d, C(12); 160.59 (c, C(13)); 114.11 (d, C(14); 129.71 (d, C(15)); 55.27 (q, C(16)); 68.38 (t, C(17)); 28.58 (t, C(18)); 23.33 (t, C(19)); 36.33 (t, C(20)); 147.69 (c, C(21)); 116.13 (d, C(22)); 31.12 (t, C(23)); 40.72 (d, C(24)); 37.79 (c, C(25)); 45.59 (d, C(26)); 31.53 (t, C(27)); 26.20 (q, C(28)); 21.06 (q, C(29)).
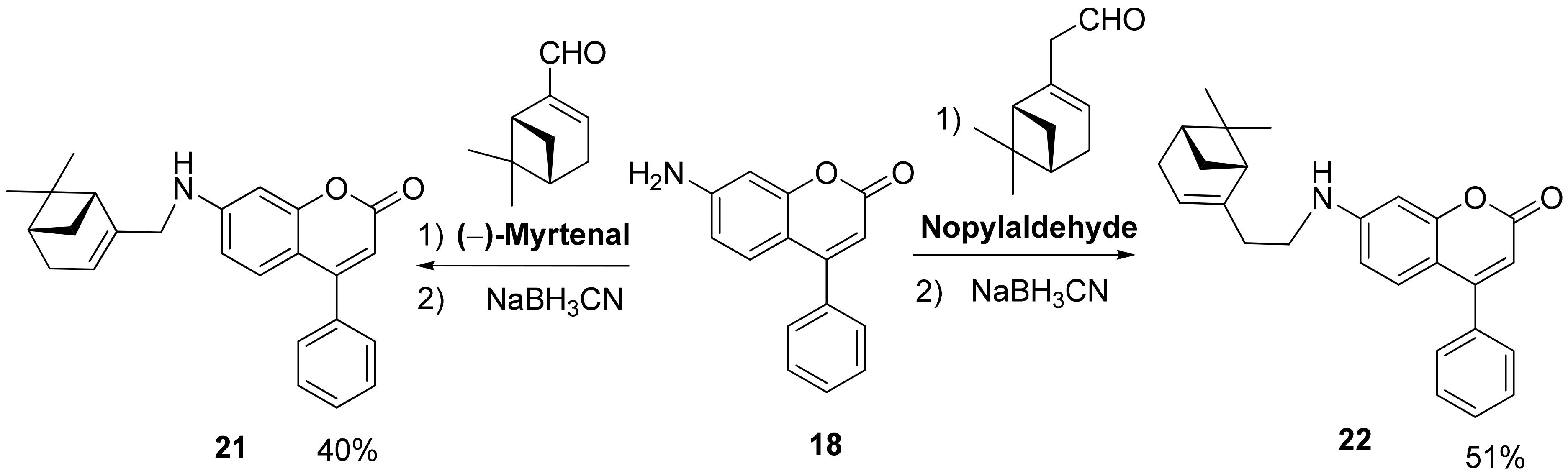
3.1.5. Synthesis of 7-Aminocoumarins 21 and 22
- 7-(((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methylamino)-4-phenyl-2H-chromen-2-one 21 Yield 33%, Method a. M.p. 141 °C. = −28.14 (c = 0.73, CHCl3). HRMS: 371.1877 [M]+; calcd. 371.1880 (C25H25O2N1). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.80 (s, 3H-C(26)); 1.14 (d, 1H, 2J = 8.6, H-C(24a)); 1.26 (s, 3H-C(25)); 2.05–2.12 (m, 2H, H-C(21), H-C(23)); 2.20 (dm, 1H, 2J = 17.7, H-C(20)); 2.27 (dm, 1H, 2J = 17.7, H’-C(20)); 2.37 (ddd, 1H, 2J = 8.6, J(24s,21) = J(24s,23) = 5.6, H-C(24s)); 3.64–3.71 (br.s, 2H-C(17)); 4.72 (br. s, 1H, H-N(16)), 5.42–5.46 (m, H-C(19)); 6.03 (s, H-C(3)); 6.44 (dd, 1H, J(7,6) = 8.8, J(7,9) = 2.1, H-C(7)); 6.53 (d, 1H, J(9,7) = 2.1, H-C(9)); 7.19 (d, 1H, J(6,7) = 8.7, H-C(6)); 7.38–7.42 (m, 2H, H-(C-11), H-C(15)); 7.44–7.49 (3H, H-C(12), H-C(13), H-C(14)). 13C-NMR (CDCl3, δC): 156.47 (s, C(1)); 161.86 (s, C(2)); 108.98 (d, C(3)); 156.10 (s, C(4)); 109.38 (s, C(5)); 127.63 (d, C(6)); 110.59 (d, C(7)); 151.41 (s, C(8)); 98.60 (d, C(9)); 135.97 (s, C(10)); 128.24 (d, C(11)); 128.52 (d, C(12); 129.14 (d, C(13)); 128.52 (d, C(14); 128.24 (d, C(15)); 48.30 (t, C(17)); 143.75 (s, C(18)); 118.82 (d, C(19)); 30.99 (t, C(20)); 40.67 (d, C(21)); 38.02 (s, C(22)); 43.81 (d, C(23)); 31.45 (t, C(24)); 25.99 (q, C(25)); 21.01 (q, C(26)).
- 7-(2-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethylamino)-4-phenyl-2H-chromen-2-one 22 Yield 51%, Method b. = −17.70 (c = 0.73, CHCl3) HRMS: 385.2033 [M]+; calcd. 385.2036 (C26H27O2N1). 1H-NMR (CDCl3, δ ppm, J, Hz): 0.80 (s, 3H-C(27)); 1.10 (d, 1H, 2J = 8.6, H-C(25a)); 1.26 (s, 3H-C(26)); 2.03 (ddd, 1H, H-C(24); 2.06–2.11 (m, 2H, H-C(22)); 2.20 (dm, 1H, 2J = 17.7, H-C(21)); 2.27 (dm, 1H, 2J = 17.7, H’-C(21)); 2.30–2.38 (m, 3H, 2H-C(18), H-C(25s)); 3.13–3.23 (m, 2H-C(17)); 5.32–5.35 (m, H-C(20)); 6.07 (s, H-C(3)); 6.54 (d, 1H, J(7,6) = 8.8, H-C(7)); 6.64 (d, 1H, J(9,7) = 2.1, H-C(9)); 7.23 (d, J(6,7) = 8.7, H-C(6)); 7.37–7.42 (m, 2H, H-C(11), H-C(15)); 7.44–7.50 (m, 3H, H-C(12), H-C(13), H-C(14)). 13C-NMR (CDCl3, δC): 156.35 (s, C(1)); 161.47 (s, C(2)); 109.82 (d, C(3)); 155.90 (s, C(4)); 110.58 (s, C(5)); 127.87 (d, C(6)); 111.50 (d, C(7)); 154.76 (s, C(8)); 115.69 (d, C(9)); 135.79 (s, C(10)); 128.23 (d, C(11)); 128.58 (d, C(12); 129.25 (d, C(13)); 128.58 (d, C(14); 128.23 (d, C(15)); 41.95 (t, C(17)); 35.42 (t, C(18)); 144.52 (s, C(19)); 119.31 (d, C(20)); 31.20 (t, C(21)); 40.56 (d, C(22)); 37.87 (s, C(23)); 45.15 (d, C(24)); 31.61 (t, C(25)); 26.06 (q, C(26)); 21.09 (q, C(27)).
3.2. Biology
3.2.1. Cytotoxicity Test
3.2.2. Antiviral Activity
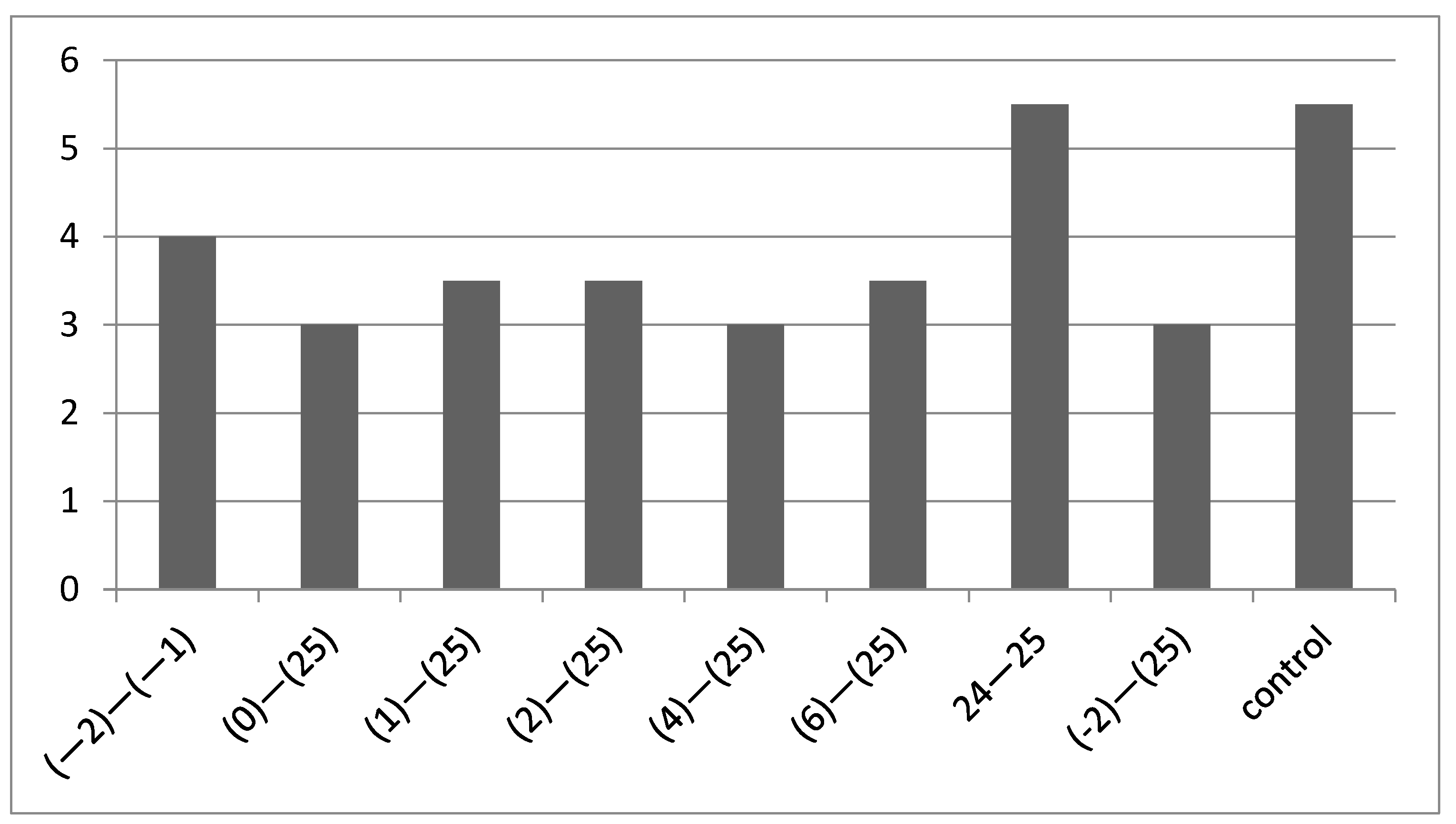
3.2.3. Time-of-addition Assay
3.3. Molecular Modeling Study
3.3.1. Protein and Ligands Preparations
3.3.2. Binging Site Analysis
3.3.3. Molecular Docking
3.3.4. Molecular Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, Y.; Reeves, R.M.; Wang, X.; Bassat, Q.; Brooks, W.A.; Cohen, C.; Moore, D.P.; Nunes, M.; Rath, B.; Campbell, H.; et al. RSV Global Epidemiology Network; RESCEU investigators. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: A systematic analysis. Lancet Glob. Health 2019, 7, e1031–e1045. [Google Scholar] [CrossRef] [PubMed]
- Krivitskaya, V.Z. Respiratory Syncytial Virus Infection. Pathogenesis Peculiarities, Prevention and Treatment Strategies. Curr. Pediatr. 2013, 12, 35–43. [Google Scholar] [CrossRef]
- Karron, R.A. Respiratory syncytial virus and parainfluenza virus vaccines. In Vaccines, 6th ed.; Plotkin, S.A., Orenstein, W., Offit, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1146–1153. [Google Scholar] [CrossRef]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Malley, R.; DeVincenzo, J.; Ramilo, O.; Dennehy, P.H.; Meissner, H.C.; Gruber, W.C.; Sanchez, P.J.; Jafri, H.; Balsley, J.; Carlin, D.; et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J. Infect. Dis. 1998, 178, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Vendeville, S.; Tahri, A.; Hu, L.; Demin, S.; Cooymans, L.; Vos, A.; Kwanten, L.; Van den Berg, J.; Battles, M.B.; McLellan, J.S.; et al. Discovery of 3-({5-Chloro-1-[3-(methylsulfonyl)propyl]-1H-indol-2-yl}methyl)-1-(2,2,2-trifluoroethyl)-1,3-dihydro-2H-imidazo[4,5-c]pyridin-2-one (JNJ-53718678), a Potent and Orally Bioavailable Fusion Inhibitor of Respiratory Syncytial Virus. J. Med. Chem. 2020, 63, 8046–8058. [Google Scholar] [CrossRef]
- Cockerill, G.S.; Angell, R.M.; Bedernjak, A.; Chuckowree, I.; Fraser, I.; Gascon-Simorte, J.; Gilman, M.S.A.; Good, J.A.D.; Harland, R.; Johnson, S.M.; et al. Discovery of Sisunatovir (RV521), an Inhibitor of Respiratory Syncytial Virus Fusion. J. Med. Chem. 2021, 64, 3658–3676. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Kuzminykh, L.V.; Shtro, A.A.; Klabukov, A.M.; Galochkina, A.V.; Nikolaeva, Y.V.; Petukhova, G.D.; Borisevich, S.S.; Khamitov, E.M.; et al. Discovery of N-Containing (−)-Borneol Esters as Respiratory Syncytial Virus Fusion Inhibitors. Pharmaceuticals 2022, 15, 1390. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Antunes Fernandes, K.H.; Diedrich, D.; Gotardi, J.; Freire Franco, M.S.; Tomich de Paula da Silva, C.H.; Duarte de Souza, A.P.; Baggio Gnoatto, S.C. New triazole-substituted triterpene derivatives exhibiting anti-RSV activity: Synthesis, biological evaluation, and molecular modeling. Beilstein J. Org. Chem. 2022, 18, 1524–1531. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Shtro, A.A.; Galochkina, A.V.; Nikolaeva, Y.V.; Petukhova, G.D.; Borisevich, S.S.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. Monoterpene-Containing Substituted Coumarins as Inhibitorsof Respiratory Syncytial Virus (RSV) Replication. Molecules 2021, 26, 7493. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Korchagina, D.V.; Reynisson, J.; et al. New Promising Inhibitors of Tyrosyl-DNA Phosphodiesterase I (Tdp 1) Combining 4-Arylcoumarin and Monoterpenoid Moieties as Components of Complex Antitumor Therapy. Int. J. Mol. Sci. 2020, 21, 126. [Google Scholar] [CrossRef]
- Kiesgen de Richter, R.; Bonato, M.; Follet, M.; Kamenka, J.M. The (+)- and (−)-[2-(1,3-dithianyl0]myrtanylborane. Solid and stable monoalkylboranes for asymmetric hydroboration. J. Org. Chem. 1990, 55, 2855–2860. [Google Scholar] [CrossRef]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N.; et al. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1) combining 7-hydroxycoumarin and monoterpenoid moieties. Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Akgun, B.; Hall, D.G. Fast and Tight Boronate Formation for Click Bioorthogonal Conjugation. Angew. Chem. Int. Ed. 2016, 55, 3909–3913. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, I.V.; Volcho, K.P.; Korchagina, D.V.; Barkhash, V.A.; Salakhutdinov, N.F. Reactions of Allyl Alcohols of the Pinane Series and of Their Epoxides in the Presence of Montmorillonite Clay. Helv. Chim. Acta 2007, 90, 353–368. [Google Scholar] [CrossRef]
- Chapuis, C.R.; Brauchli, R. Preparation of Campholenal Analogues: Chirons for the Lipophilic Moiety of Sandalwood-Like Odorant Alcohols. Helv. Chim. Acta 1992, 75, 1527–1546. [Google Scholar] [CrossRef]
- Cointeaux, L.; Berrien, J.-F.; Mayrargue, J. Synthesis of cardamom peroxide analogues by radical cyclization of hydroperoxyalkenes. Tetrahedron Lett. 2002, 43, 6275–6277. [Google Scholar] [CrossRef]
- Pozdnev, V.F. Improved method for synthesis of 7-amino-4-methylcoumarin. Chem. Heterocycl. Compd. 1990, 26, 264–265. [Google Scholar] [CrossRef]
- McLellan, J.S.; William, C.R.; Peeples, M.E. Structure and Function of Respiratory Syncytial Virus Surface Glycoproteins. In Challenges and Opportunities for Respiratory Syncytial Virus Vaccines; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 372, pp. 83–104. [Google Scholar] [CrossRef]
- Battles, M.B.; Langedijk, J.P.; Furmanova-Hollenstein, P.; Chaiwatpongsakorn, S.; Costello, H.M.; Kwanten, L.; Vranckx, L.; Vink, P.; Jaensch, S.; Jonckers, T.H.M.; et al. Molecular Mechanism of Respiratory Syncytial Virus Fusion Inhibitors. Nat. Chem. Biol. 2016, 12, 87–93. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal Chemical Properties of Successful Central Nervous System Drugs. NeuroRX 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Begoyan, V.V.; Weseliński, L.J.; Xia, S.; Fedie, J.; Kannan, S.; Ferrier, J.; Rao, S.; Tanasova, M. Multicolor GLUT5-permeable fluorescent probes for fructose transport analysis. Chem. Commun. 2018, 54, 3855–3858. [Google Scholar] [CrossRef]
- Ponomarev, K.; Pavlova, A.; Suslov, E.; Ardashov, O.; Korchagina, D.; Nefedov, A.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Synthesis and analgesic activity of new compounds combining azaadamantane and monoterpene moieties. Med. Chem. Res. 2015, 24, 4146–4156. [Google Scholar] [CrossRef]
- El-Haggar, R.; Al-Wabli, R.I. Anti-Inflammatory Screening and Molecular Modeling of Some Novel Coumarin Derivatives. Molecules 2015, 20, 5374–5391. [Google Scholar] [CrossRef] [PubMed]
- Rossey, I.; Hsieh, C.-L.; Sedeyn, K.; Ballegeer, M.; Schepens, B.; McLellan, J.S.; Saelens, X. A Vulnerable, Membrane-Proximal Site in Human Respiratory Syncytial Virus F Revealed by a Prefusion-Specific Single-Domain Antibody. J. Virol. 2021, 95, e02279-20. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Steven, D.L.; Smondyrev, A.M.; Knoll, E.H.; Rao, S.N.; Shaw, D.E.; Friesner, R.A. PHASE: A New Engine for Pharmacophore Perception, 3D QSAR Model Development, and 3D Database Screening: 1. Methodology and Preliminary Results. J. Comput.-Aided Mol. Des. 2006, 20, 647–671. [Google Scholar] [CrossRef]
- Aloia, R.C.; Jensen, F.C.; Curtain, C.C.; Mobley, P.W.; Gordon, L.M. Lipid Composition and Fluidity of the Human Immunodeficiency Virus. Proc. Natl. Acad. Sci. USA 1988, 85, 900–904. [Google Scholar] [CrossRef]
- Satoh, O.; Imai, H.; Yoneyama, T.; Miyamura, T.; Utsumi, H.; Inoue, K.; Umeda, M. Membrane Structure of the Hepatitis B Virus Surface Antigen Particle. J. Biochem. 2000, 127, 543–550. [Google Scholar] [CrossRef]



| N | CC50 *, µM | EC50 **, µM | SI *** | N | CC50, µM | EC50, µM | SI |
|---|---|---|---|---|---|---|---|
| 14a | 805 ± 97.6 | 67.3 ± 9.1 | 11.9 | 16a | 273 ± 16 | 47.4 ± 8 | 5.8 |
| 14b | 591 ± 85.2 | 18.0 ± 0.9 | 32.8 | 16b | 297 ± 8.5 | 102.9 ± 7.1 | 2.9 |
| 14c | 813 ± 32.4 | 233.6 ± 27.1 | 3.5 | 16c | 20.5 ± 4.9 | 14.3 ± 2.9 | 1.4 |
| 14d | 657 ± 20.4 | 82.5 ± 10.3 | 7.9 | 16e | 996 ± 8.2 | 109.9 ± 10.4 | 9 |
| 14e | 1443 ± 85.1 | 7.5 ± 0.4 | 192.6 | 16f | 109 ± 10.5 | 9.2 ± 1.1 | 11.8 |
| 14f | 1267 ± 180 | 110.9 ± 10.5 | 11.4 | 16g | 655 ± 29.7 | >690 | <0.9 |
| 14g | 1544 ± 73.7 | >1580 | <1 | 17a | 339 ± 26.3 | 58.3 ± 8.5 | 5.8 |
| 15a | 8.8 ± 0.5 | 8.8 ± 0.7 | 1 | 17b | 12.4 ± 2.8 | >12 | <1 |
| 15b | 46.6 ± 8.6 | 15.1 ± 1.1 | 3 | 17c | 14.7 ± 2.9 | >15 | <1 |
| 15c | 90.8 ± 11 | 48.7 ± 7.6 | 1.9 | 17d | 677 ± 22.9 | 64.1 ± 5.4 | 10.6 |
| 15d | 17.2 ± 2.6 | >20 | <0.8 | 17e | >100 | 35.9 ± 5.7 | 2.8 |
| 15e | 12.5 ± 1.4 | >12.5 | <1 | 17f | 117 ± 7 | 10.8 ± 1 | 10.8 |
| 15f | 91.3 ± 9.2 | 9.5 ± 0.8 | 9.6 | 17g | 16.8 ± 3.2 | >17 | <1 |
| 15g | 265 ± 29.5 | 84.0 ± 3 | 3.2 | 21 | 20.5 ± 1.4 | >13 | <1.5 |
| Ribavirin | >4000 | 80.1 ± 13.5 | 50 | 22 | 480 ± 18.4 | 79.9 ± 7.4 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khomenko, T.M.; Shtro, A.A.; Galochkina, A.V.; Nikolaeva, Y.V.; Garshinina, A.V.; Borisevich, S.S.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. New Inhibitors of Respiratory Syncytial Virus (RSV) Replication Based on Monoterpene-Substituted Arylcoumarins. Molecules 2023, 28, 2673. https://doi.org/10.3390/molecules28062673
Khomenko TM, Shtro AA, Galochkina AV, Nikolaeva YV, Garshinina AV, Borisevich SS, Korchagina DV, Volcho KP, Salakhutdinov NF. New Inhibitors of Respiratory Syncytial Virus (RSV) Replication Based on Monoterpene-Substituted Arylcoumarins. Molecules. 2023; 28(6):2673. https://doi.org/10.3390/molecules28062673
Chicago/Turabian StyleKhomenko, Tatyana M., Anna A. Shtro, Anastasia V. Galochkina, Yulia V. Nikolaeva, Anzhelika V. Garshinina, Sophia S. Borisevich, Dina V. Korchagina, Konstantin P. Volcho, and Nariman F. Salakhutdinov. 2023. "New Inhibitors of Respiratory Syncytial Virus (RSV) Replication Based on Monoterpene-Substituted Arylcoumarins" Molecules 28, no. 6: 2673. https://doi.org/10.3390/molecules28062673
APA StyleKhomenko, T. M., Shtro, A. A., Galochkina, A. V., Nikolaeva, Y. V., Garshinina, A. V., Borisevich, S. S., Korchagina, D. V., Volcho, K. P., & Salakhutdinov, N. F. (2023). New Inhibitors of Respiratory Syncytial Virus (RSV) Replication Based on Monoterpene-Substituted Arylcoumarins. Molecules, 28(6), 2673. https://doi.org/10.3390/molecules28062673







