Effects of Pretreated Phosphogypsum and Granulated Blast-Furnace Slag on the Rheological Properties of the Paste Excited by NaOH
Abstract
:1. Introduction
2. Results and Discussion
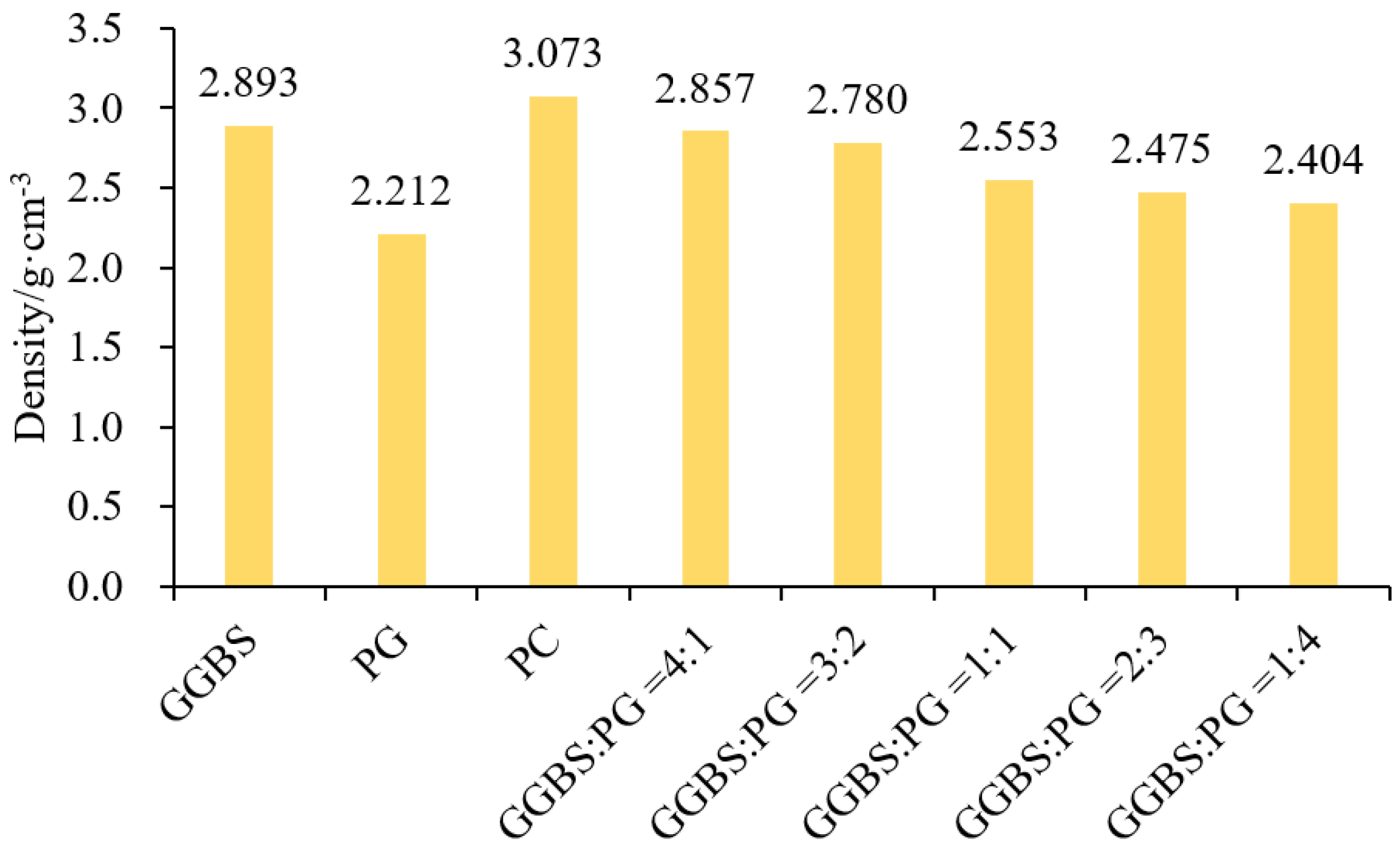
2.1. Density of Composite Material
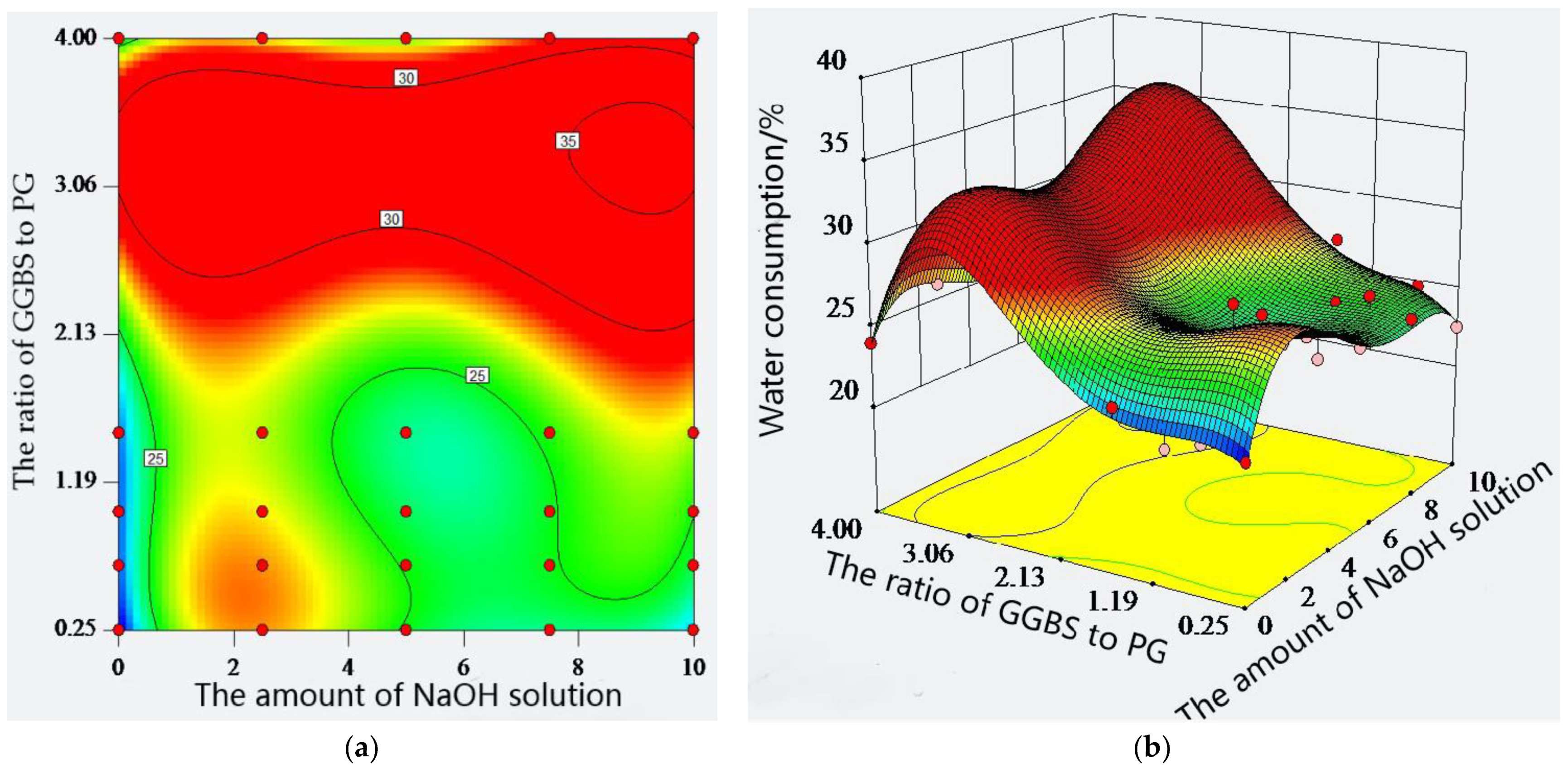
2.2. Standard Consistency Water Consumption of Composite Material
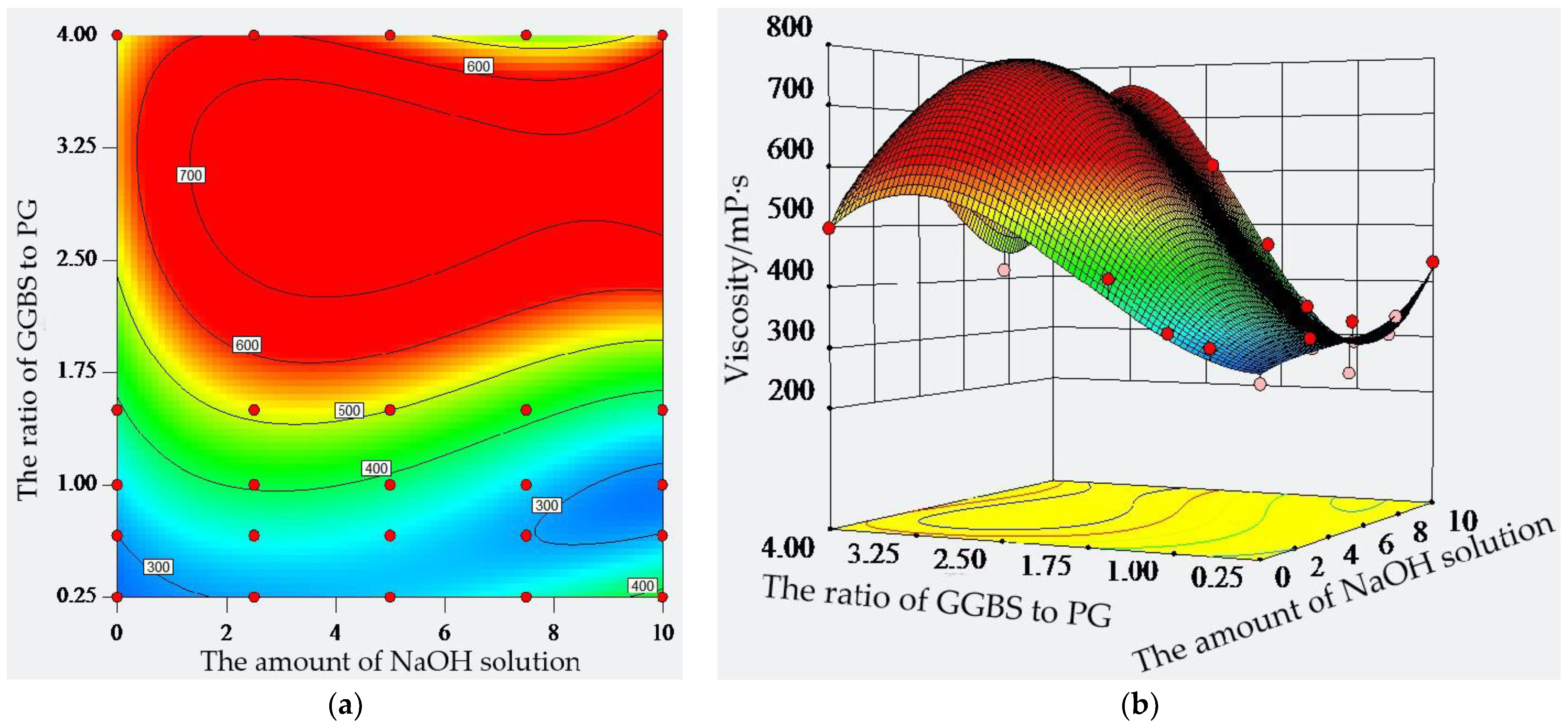
2.3. Viscosity of Composite Material
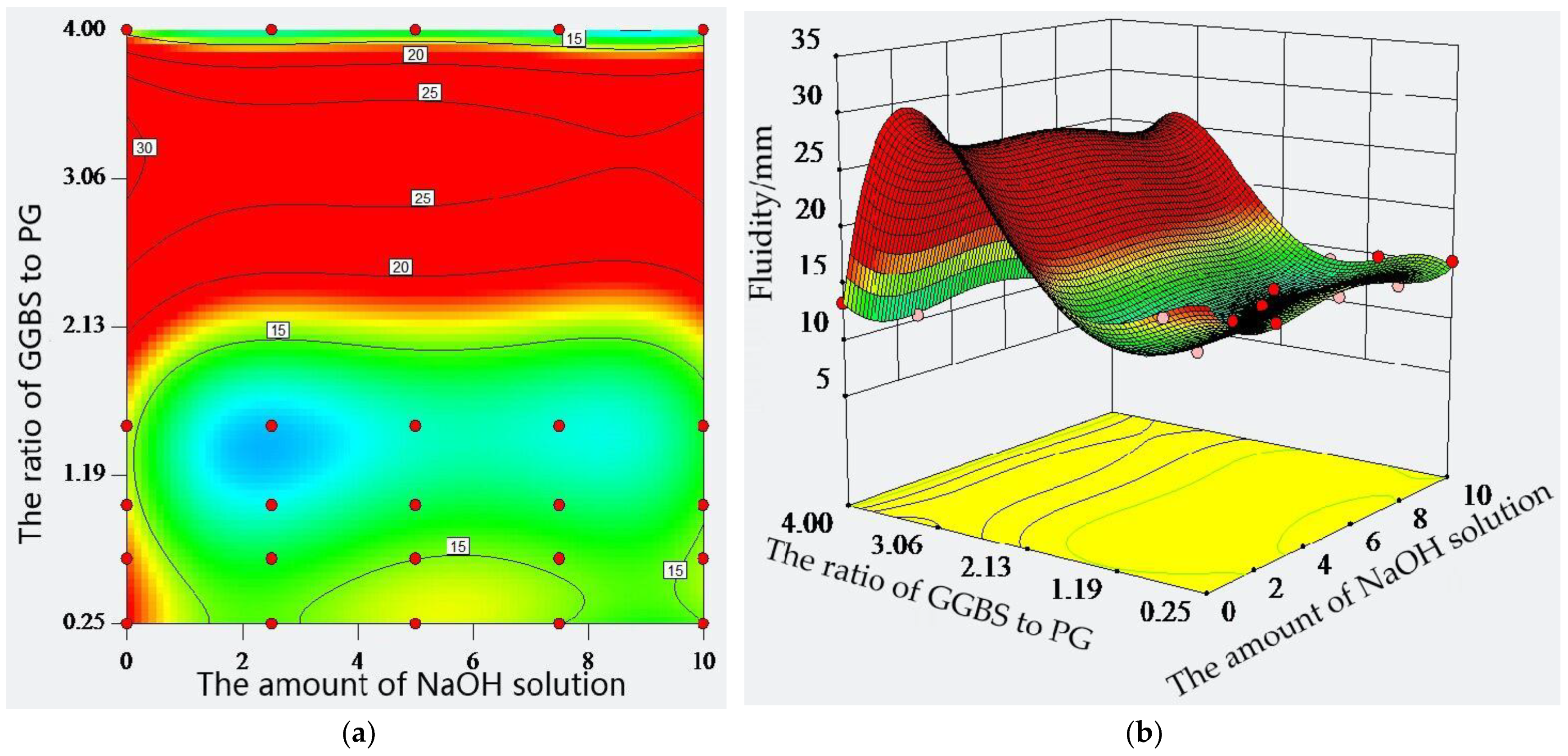
2.4. Fluidity of Composite Material
2.5. Water Release Ratio of Composite Material
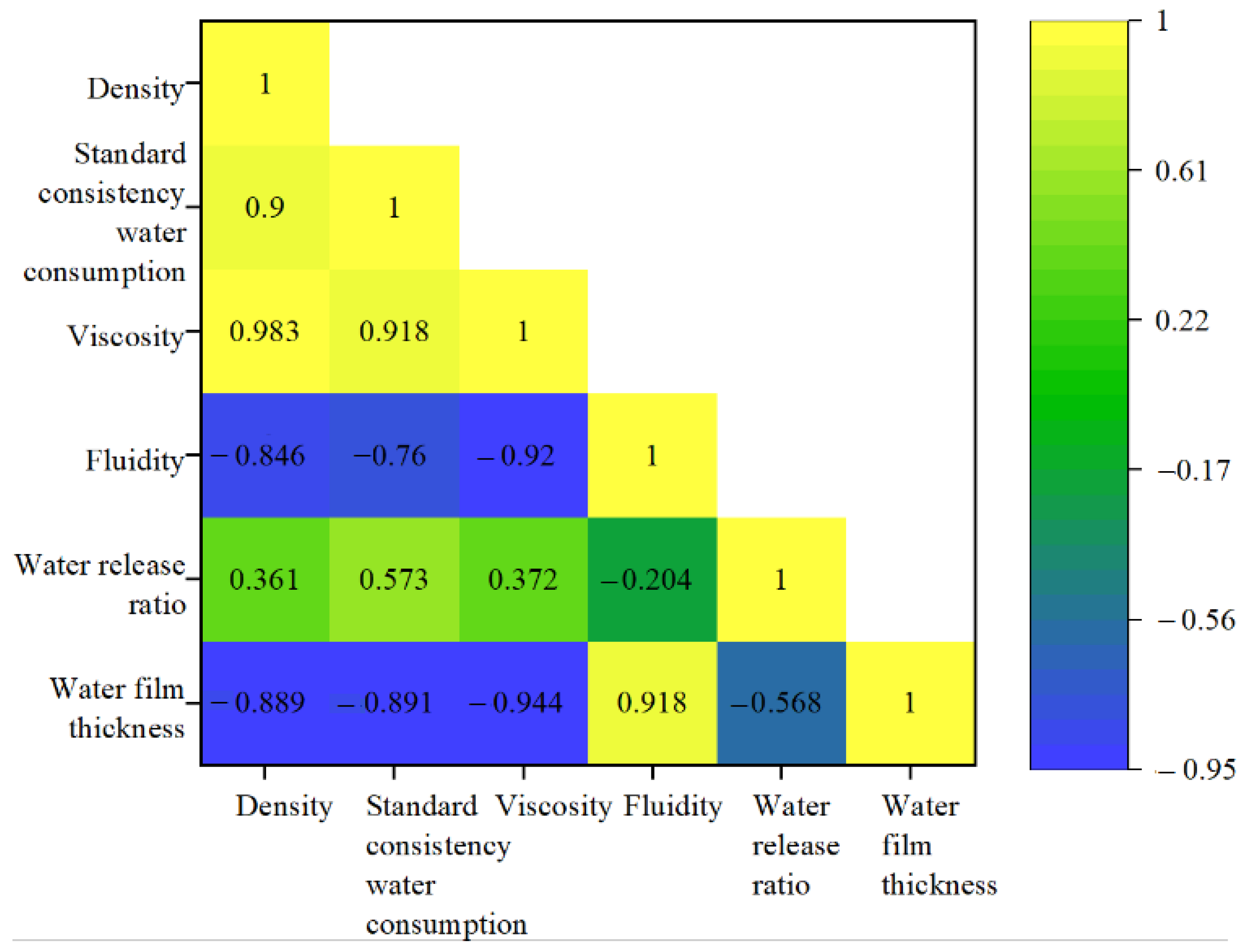
2.6. Water Film Thickness of Composite Material and Influencing Factor Analysis
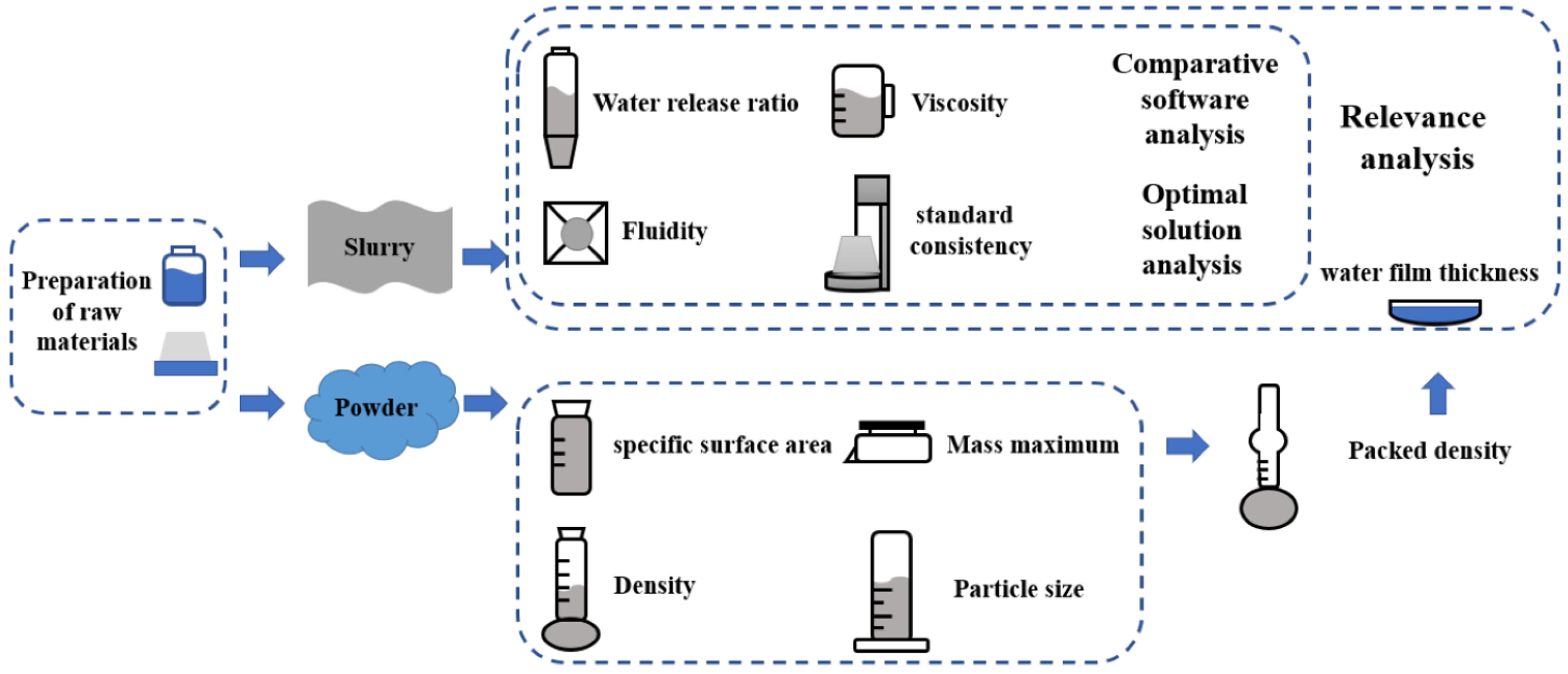
3. Materials and Methods
3.1. Experimental Materials
3.2. Experimental Design
3.3. Test Methodology
3.3.1. Density
3.3.2. Standard Consistency Water Consumption
3.3.3. Viscosity
3.3.4. Fluidity
3.3.5. Water Release ratio
3.3.6. Calculation of Filling Density and Water Film Thickness
4. Conclusions
- (1)
- The density of the mixture with GGBS and PG gradually decreases with the decrease in GGBS content and the increase in PG content. Compared with the density at 4:1, the density of the mixture at the ratios of 3:2, 1:1, 2:3, and 1:4 decreases by 2.70%, 10.64%, 13.37%, and 15.86%, respectively. The standard consistency water consumption of the paste shows ups and downs after adding a sodium hydroxide solution, GGBS, and PG, and they have similar influence laws on the standard consistency water consumption of the paste;
- (2)
- The viscosity of the paste with GGBS and PG at ratios of 4:1, 3:2, 1:1, 2:3, and 1:4 gradually decreases by 15.5%, 32.1%, 36.1%, and 46.8%, respectively, compared with the GGBS and PG ratio of 4:1. The ratio of GGBS to PG has a greater effect on the viscosity than the amount of sodium hydroxide solution;
- (3)
- The compounds of PG and GGBS and the amount of sodium hydroxide solution have a significant effect on the fluidity of the paste, and the ratio of GGBS to PG has a greater effect on the fluidity of the paste than the amount of sodium hydroxide solution. Therefore, the larger the amount of sodium hydroxide solution is, the more unfavorable conditions are to the fluidity and viscosity of the paste;
- (4)
- The effect of the amount of sodium hydroxide solution on the water release ratio of the paste is greater than that of the ratio of GGBS to PG. Overall, the higher the content of GGBS, the greater the water release ratio of the composite paste, and the higher the content of PG, the smaller the water release ratio of the composite paste;
- (5)
- The variation in the ratio of GGBS to PG has a significant effect on the water film thickness of the paste, showing that the greater the PG mixture, the greater the water film thickness of the paste, reaching 1.122 μm, which is 2.31 times the minimum water film thickness of the composite material. At the same time, the water film thickness of the paste is negatively correlated with the standard consistency water consumption, viscosity, and water release ratio, and positively correlated with the fluidity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Dong, S.K.; Yu, S.H.; Chen, L.L.; Zhuo, Q.; Wu, F.F.; Xie, L.L.; Liu, L.Y. Comparative study on the effects of five nano-metallic oxide particles on the long-term mechanical property and durability of cement mortar. Buildings 2023, 13, 619. [Google Scholar] [CrossRef]
- Xie, L.L.; Deng, M. Effects of modulus on mineral composition and microstructures of phosphorus-containing Portland cement clinker. J. Nanjing Tech Univ. (Nat. Sci. Ed.) 2021, 43, 473–479. [Google Scholar]
- Cui, K.; Liang, K.K.; Chang, J.; Lau, D. Investigation of the macro performance, mechanism, and durability of multiscale steel fiber reinforced low-carbon ecological UHPC. Constr. Build. Mater. 2022, 327, 126921. [Google Scholar] [CrossRef]
- Tuan, N.V.; Phung, Q.T.; Seetharam, S.; Thang, N.C. Synergistic effects of ground granulated blast furnace slag and silica fume on the hydration and compressive strength of extremely low w/b ratio cement pastes. Mater. Today Commun. 2022, 33, 104531. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Dong, S.K.; Liu, L.Y.; Wu, F.F. A sustainable composite cementitious material manufactured by phosphogypsum waste. Appl. Sci. 2022, 12, 12718. [Google Scholar] [CrossRef]
- Xie, L.L.; Deng, M.; Tang, J.H.; Liu, K.W. The effect of fluorapatite in limestones on the mineral compositions of portland cement clinkers. Constr. Build. Mater. 2021, 273, 122042. [Google Scholar] [CrossRef]
- Wang, Y.D.; Zhang, N.; Zhou, Y.; Li, C.; Zhao, J.H.; Zhang, Y.H. Photocatalysis in alkali activated cementitious materials. J. Build. Eng. 2022, 46, 103749. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.K.; Yao, G.; Wang, Z.M.; Lyu, X.J. Preparation and characterization of an alkali-activated cementitious material with blast-furnace slag, soda sludge, and industrial gypsum. Constr. Build. Mater. 2022, 340, 127735. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Romdhane, C.B.; Massoudi, C. Phosphogypsum: Potential uses and problems–A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Alaa, M. Rashad. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar]
- Gu, S.Y.; Miao, J.Y.; Hou, C.H.; Wang, Y.Y.; Wang, H.B.; Xu, X.C. Comprehensive utilization of phosphogypsum and research suggestions on key common technology innovation. Conserv. Util. Miner. Resour. 2020, 40, 115–120. [Google Scholar]
- Gao, Y.; Sun, D.; Han, C.L.; Huang, J.J. Comperhensive utilization of phosphogypsum: Adsorption of methylene blue and its application in bricks. Surf. Rev. Lett. 2021, 28, 2150075. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Deng, Z.B. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef]
- Neto, J.S.A.; Bersch, J.D.; Silv, T.S.M.; Rodriguez, E.D.; Suzuki, S.; Kirchheim, A.P. Influence of phosphogypsum purification with lime on the properties of cementitious matrices with and without plasticizer. Constr. Build. Mater. 2021, 299, 123935. [Google Scholar] [CrossRef]
- Rosales, J.; Pérez, S.M.; Cabrera, M.J.; Bolivar, J.P.; Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Altun, I.A.; Sert, Y. Utilization of weathered phosphogypsum as set retarder in Portland cement. Cem. Concr. Res. 2004, 34, 677–680. [Google Scholar] [CrossRef]
- Dong, S.K.; Zhuo, Q.; Chen, L.L.; Wu, F.F.; Xie, L.L. Reuse of pretreated red mud and phosphogypsum as supplementary cementitious material. Sustainability 2023, 15, 2856. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.S. Investigation on phosphogypsum–steel slag–granulated blast-furnace slag–limestone cement. Constr. Build. Mater. 2010, 24, 1296–1301. [Google Scholar] [CrossRef]
- Li, B.X.; Li, L.; Chen, X.; Ma, Y.; Zhou, M.K. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder. Constr. Build. Mater. 2022, 338, 127630. [Google Scholar] [CrossRef]
- Mkadmini Hammi, K.; Hammi, H.; Hamzaoui, A.H. Use of mixture design approach for the optimization and performance of cost-effective cementitious quaternary system: Portland cement–fly ash–silica fume–phosphogypsum. Chem. Afr. 2021, 4, 835–848. [Google Scholar] [CrossRef]
- Harrou, A.; Gharibi, E.; Nasri, H.; Fagel, N.; Ouahabi, M.E. Physico-mechanical properties of phosphogypsum and black steel slag as aggregate for bentonite-lime based materials. Mater. Today Proc. 2020, 31 (Suppl. S1), 51–55. [Google Scholar] [CrossRef]
- Zhang, L.C.; Zhang, A.L.; Li, K.; Wang, Q.; Han, Y.; Yao, B.; Gao, X.J.; Feng, L.Y. Research on the pretreatment and mechanical performance of undisturbed phosphogypsum. Case Stud. Constr. Mater. 2020, 13, 400. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Li, X.B.; Shi, Y.; Zhu, Q.Q.; Du, J. Reuse of phosphogypsum pretreated with water washing as aggregate for cemented backfill. Sci. Rep. 2022, 12, 16091. [Google Scholar] [CrossRef] [PubMed]
- Pliaka, M.; Gaidajis, G. Potential uses of phosphogypsum: A review. J. Environ. Sci. Health Part A 2022, 57, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.J.; Li, K.F.; Li, M.L.; Lin, S.Y.; Hao, T.Y.; Wang, T.Y.; Guo, Y.P.; Ling, Z. Investigations on the rehydration of recycled blended SCMs cement. Cem. Concr. Res. 2023, 163, 107036. [Google Scholar] [CrossRef]
- Kanavaris, F.; Soutsos, M.; Chen, J.F. Enabling sustainable rapid construction with high volume GGBS concrete through elevated temperature curing and maturity testing. J. Build. Eng. 2023, 63, 105434. [Google Scholar] [CrossRef]
- GB/T23456-2018; Phosphogypsum. China Standard Press: Beijing, China, 2018.
- GB/T 208-2014; Test Method for Determining Cement Density. China Standard Press: Beijing, China, 2014.
- GB/T1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Porland Cement. China Standard Press: Beijing, China, 2011.


| Number | Vw | ||||||
|---|---|---|---|---|---|---|---|
| GGBS | 185.96 | 0.45 | 0.555 | 0.0477 | 32.038 | 71.703 | 0.447 |
| PG | 171.33 | 0.30 | 0.660 | 0.0343 | 23.822 | 16.950 | 1.405 |
| PC | 206.92 | 0.40 | 0.576 | 0.0430 | 29.494 | 67.406 | 0.438 |
| GGBS:PG = 4:1 | 186.46 | 0.40 | 0.609 | 0.0380 | 29.499 | 61.357 | 0.481 |
| GGBS:PG = 3:2 | 186.04 | 0.50 | 0.603 | 0.0395 | 34.420 | 48.809 | 0.709 |
| GGBS:PG = 1:1 | 178.98 | 0.55 | 0.580 | 0.0417 | 36.641 | 40.911 | 0.787 |
| GGBS:PG = 2:3 | 177.66 | 0.40 | 0.618 | 0.0379 | 29.499 | 37.475 | 0.896 |
| GGBS:PG = 1:4 | 173.66 | 0.40 | 0.624 | 0.0376 | 29.499 | 26.519 | 1.112 |
| Name | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| PG/wt.% | 1.52 | 8.64 | 0.12 | 38.92 | 0.32 | 0.22 | 0.02 | 50.24 | / |
| GGBS/wt.% | 15.75 | 32.9 | 0.3 | 39.78 | 8.86 | 0.29 | 0.43 | 0.37 | 1.32 |
| PC/wt.% | 7.67 | 26.1 | 4.69 | 54.87 | 2.91 | 0.46 | 0.34 | 2.96 | / |
| Number | GGBS/g | PG/g | Water/g | Sodium Hydroxide Solution/g | Sodium Hydroxide Solution Ratio/% |
|---|---|---|---|---|---|
| 1 | 800 | 200 | 450 | - | 0 |
| 2 | 600 | 400 | 450 | - | |
| 3 | 500 | 500 | 450 | - | |
| 4 | 400 | 600 | 450 | - | |
| 5 | 200 | 800 | 450 | - | |
| 6 | 800 | 200 | 438.75 | 11.25 | 2.5 |
| 7 | 600 | 400 | 438.75 | 11.25 | |
| 8 | 500 | 500 | 438.75 | 11.25 | |
| 9 | 400 | 600 | 438.75 | 11.25 | |
| 10 | 200 | 800 | 438.75 | 11.25 | |
| 11 | 800 | 200 | 427.5 | 22.5 | 5 |
| 12 | 600 | 400 | 427.5 | 22.5 | |
| 13 | 500 | 500 | 427.5 | 22.5 | |
| 14 | 400 | 600 | 427.5 | 22.5 | |
| 15 | 200 | 800 | 427.5 | 22.5 | |
| 16 | 800 | 200 | 416.25 | 33.75 | 7.5 |
| 17 | 600 | 400 | 416.25 | 33.75 | |
| 18 | 500 | 500 | 416.25 | 33.75 | |
| 19 | 400 | 600 | 416.25 | 33.75 | |
| 20 | 200 | 800 | 416.25 | 33.75 | |
| 21 | 800 | 200 | 405 | 45 | 10 |
| 22 | 600 | 400 | 405 | 45 | |
| 23 | 500 | 500 | 405 | 45 | |
| 24 | 400 | 600 | 405 | 45 | |
| 25 | 200 | 800 | 405 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Yu, S.; Chen, L.; Zhuo, Q.; Wu, F.; Xie, L.; Liu, L. Effects of Pretreated Phosphogypsum and Granulated Blast-Furnace Slag on the Rheological Properties of the Paste Excited by NaOH. Molecules 2023, 28, 2662. https://doi.org/10.3390/molecules28062662
Dong S, Yu S, Chen L, Zhuo Q, Wu F, Xie L, Liu L. Effects of Pretreated Phosphogypsum and Granulated Blast-Furnace Slag on the Rheological Properties of the Paste Excited by NaOH. Molecules. 2023; 28(6):2662. https://doi.org/10.3390/molecules28062662
Chicago/Turabian StyleDong, Shuangkuai, Songhan Yu, Liangliang Chen, Qi Zhuo, Fufei Wu, Lilan Xie, and Liuyan Liu. 2023. "Effects of Pretreated Phosphogypsum and Granulated Blast-Furnace Slag on the Rheological Properties of the Paste Excited by NaOH" Molecules 28, no. 6: 2662. https://doi.org/10.3390/molecules28062662
APA StyleDong, S., Yu, S., Chen, L., Zhuo, Q., Wu, F., Xie, L., & Liu, L. (2023). Effects of Pretreated Phosphogypsum and Granulated Blast-Furnace Slag on the Rheological Properties of the Paste Excited by NaOH. Molecules, 28(6), 2662. https://doi.org/10.3390/molecules28062662







