Thermodynamics Investigation and Artificial Neural Network Prediction of Energy, Exergy, and Hydrogen Production from a Solar Thermochemical Plant Using a Polymer Membrane Electrolyzer
Abstract
:1. Introduction
- At a local low current density, when the effects of ohmic losses can be ignored, the activation voltage drop obtained from the Volmer–Butler equation is the dominant drop.
- In the middle of the diagram, it is linear, and ohmic drops cause the voltage drop.
- The dominant drop is the concentration voltage drop at a high current density.
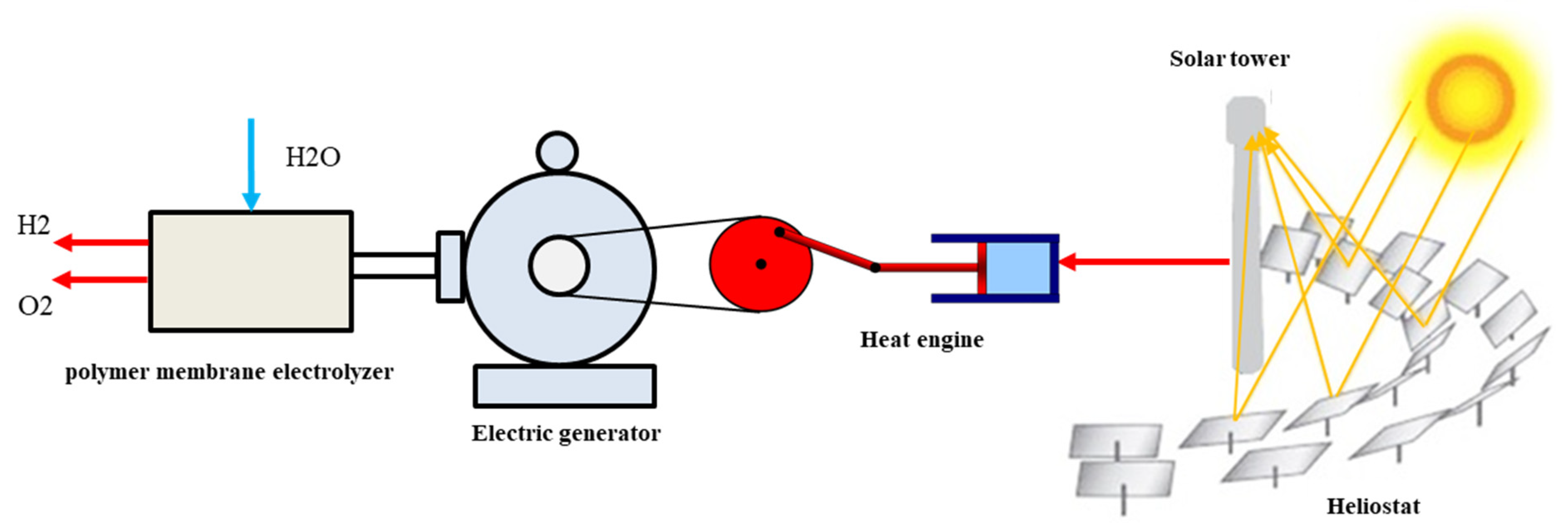
2. System Description
| The anode | |
| The cathode | |
| The whole reaction |
3. Thermodynamic Analysis
3.1. Solar Tower
3.2. Heat Engine
3.3. Electric Generator
3.4. Polymer Membrane Electrolyzer
3.4.1. Activation Voltage Drop
3.4.2. Activation Voltage Drop
3.5. Overall Efficiency
4. Results and Discussion
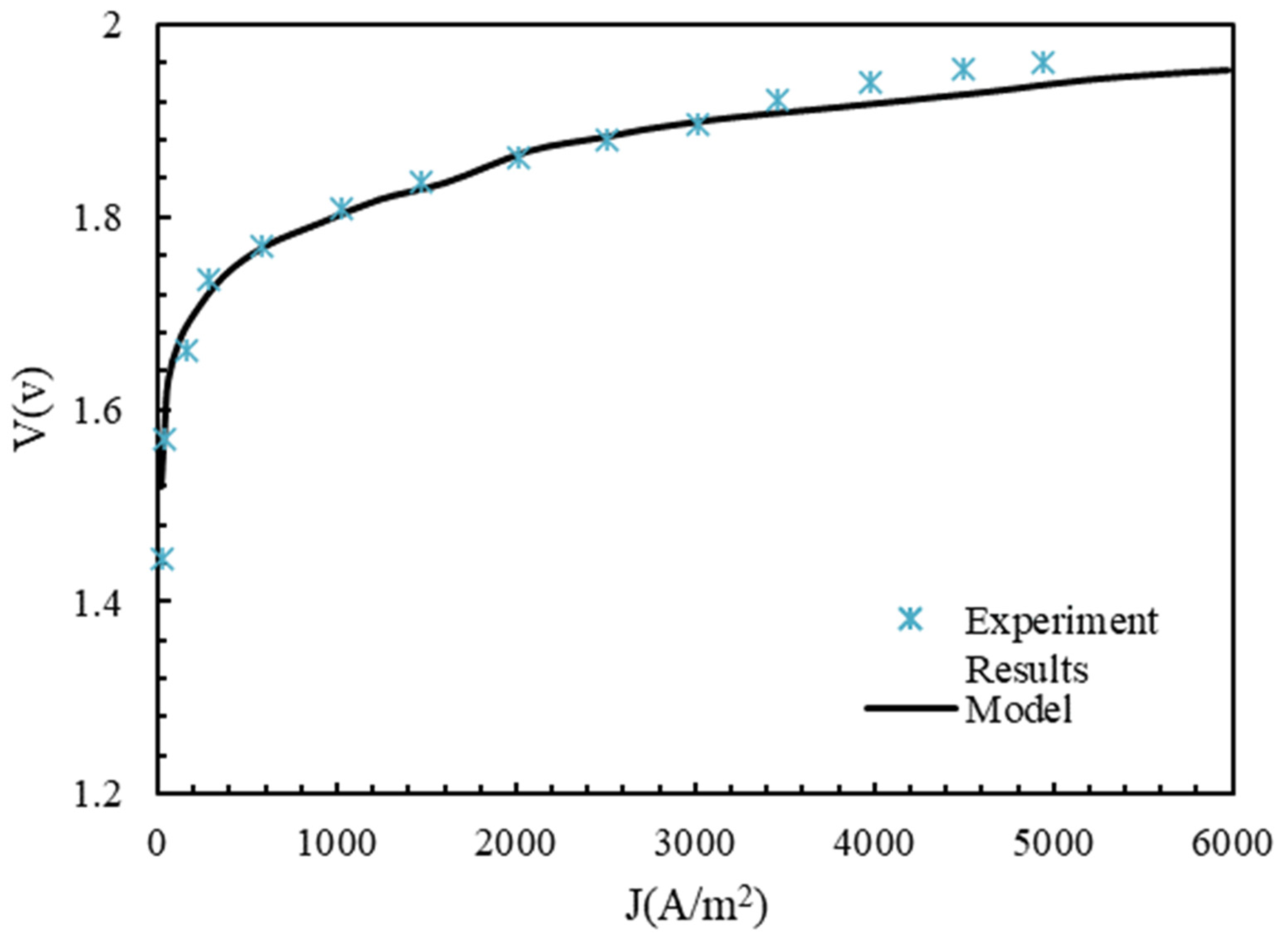
4.1. Electrolyzer Validation
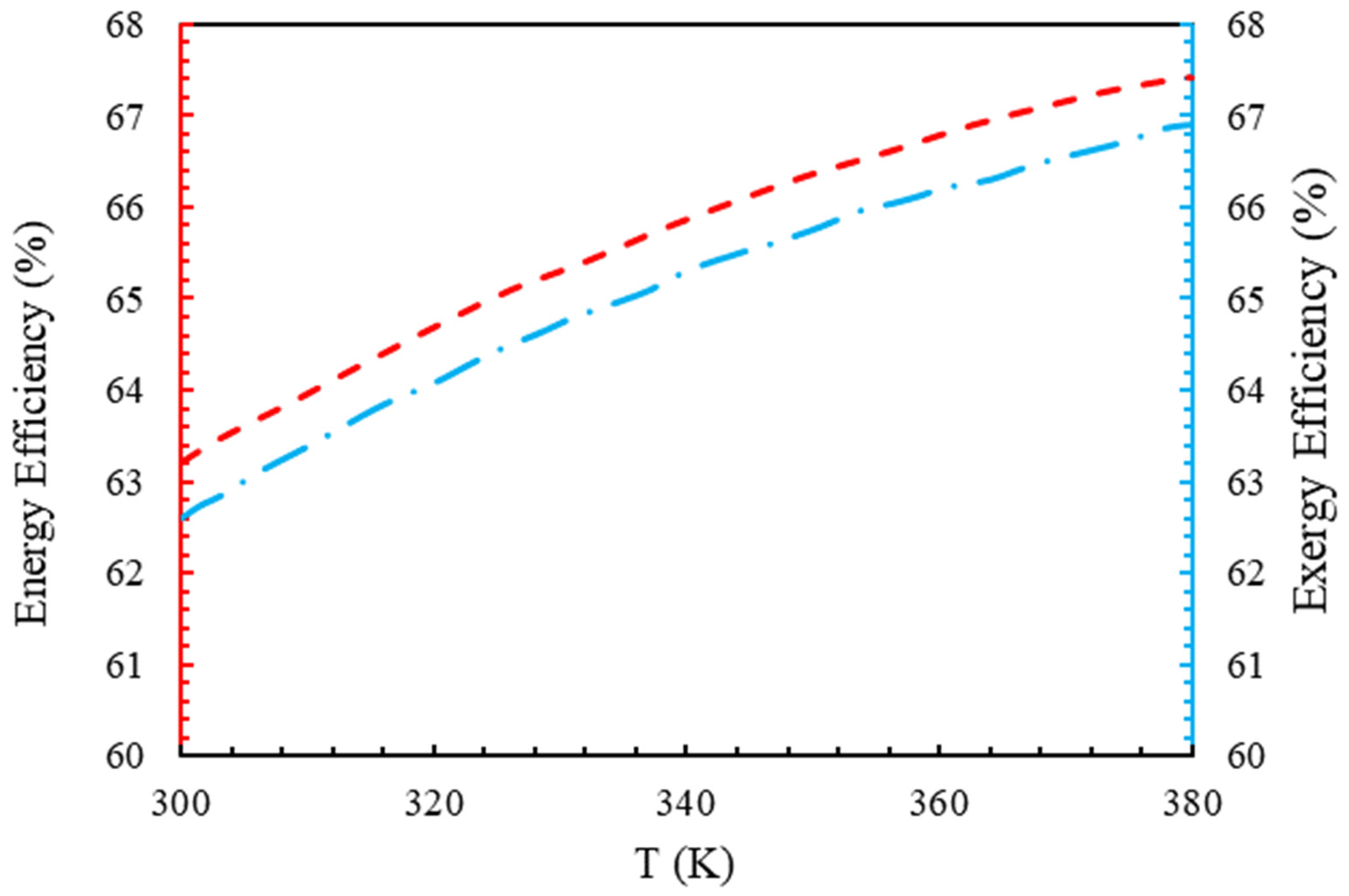
4.2. Energy Efficiency and Exergy
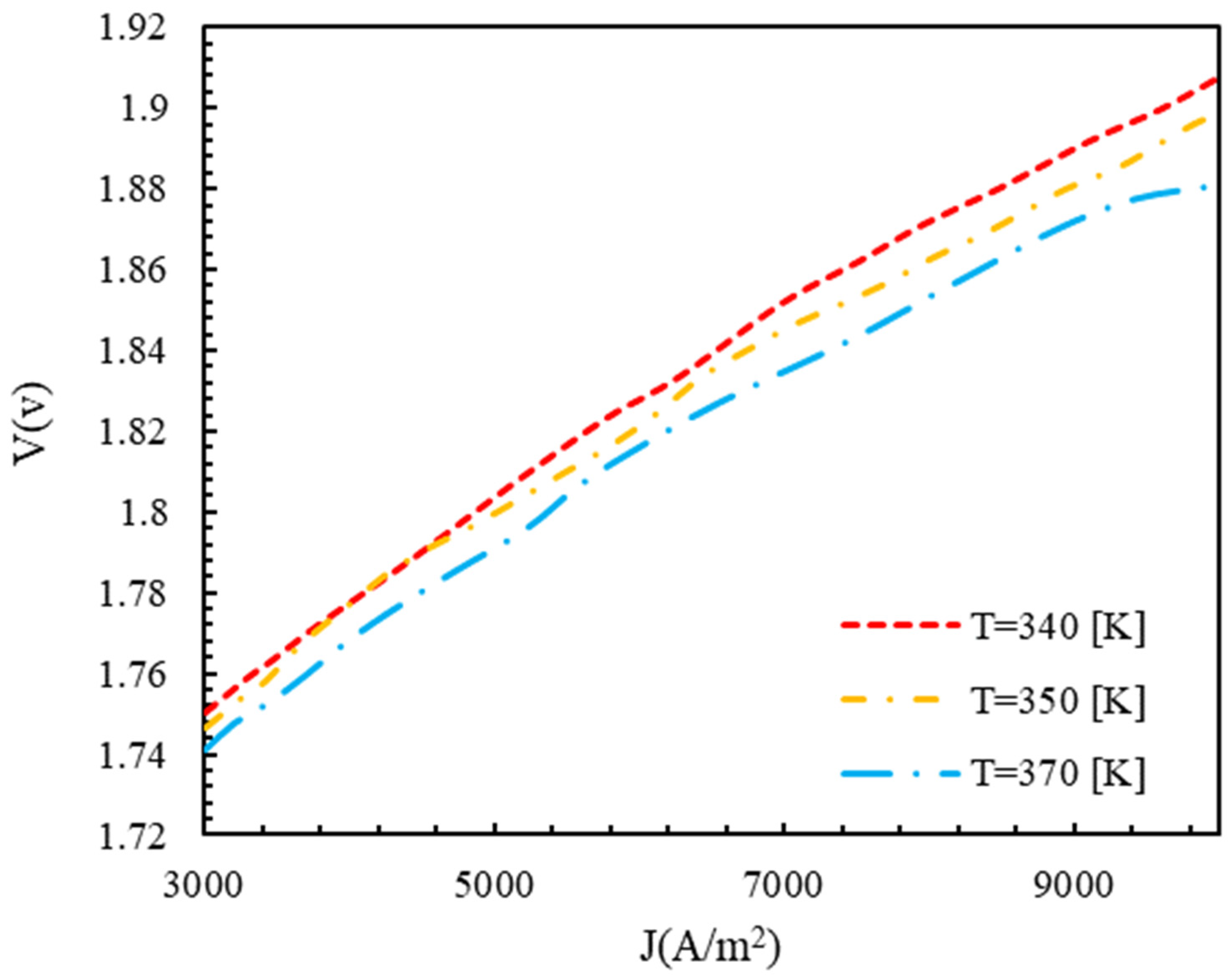
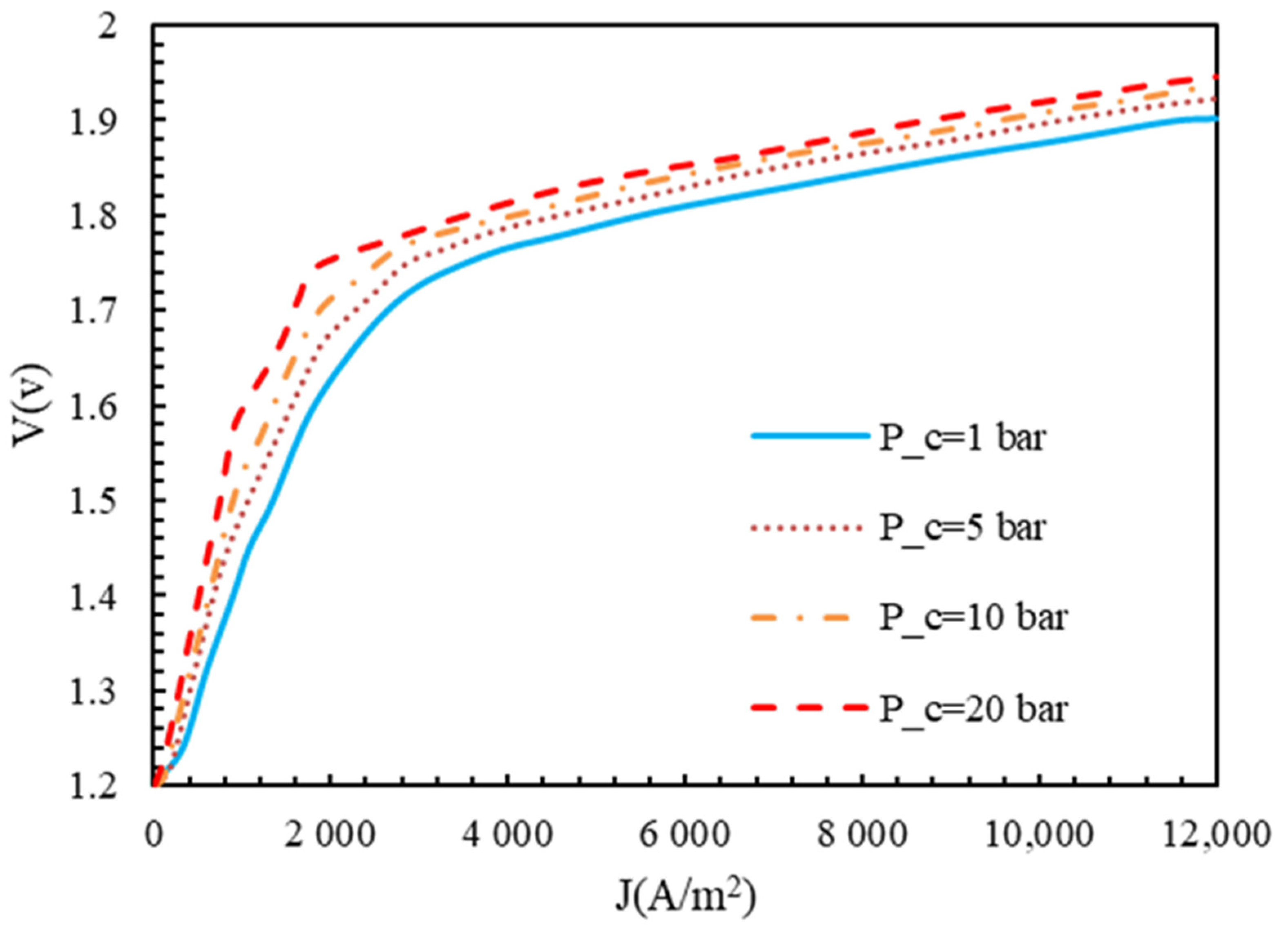
4.3. Effect of Functional Parameters
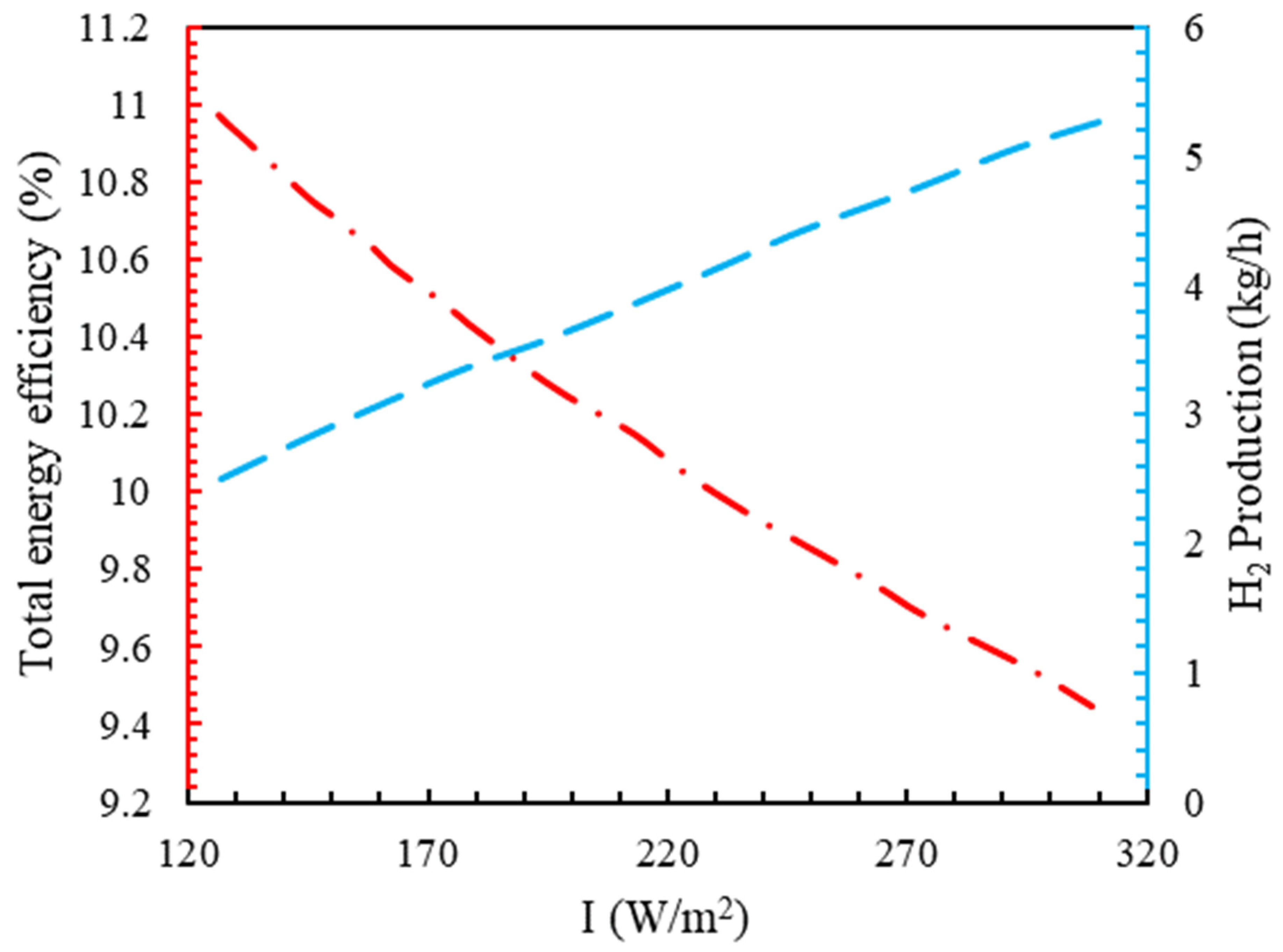
4.4. The Effect of the Intensity of Solar Radiation
5. Artificial Neural Network
5.1. ANN Model for Energy Efficiency
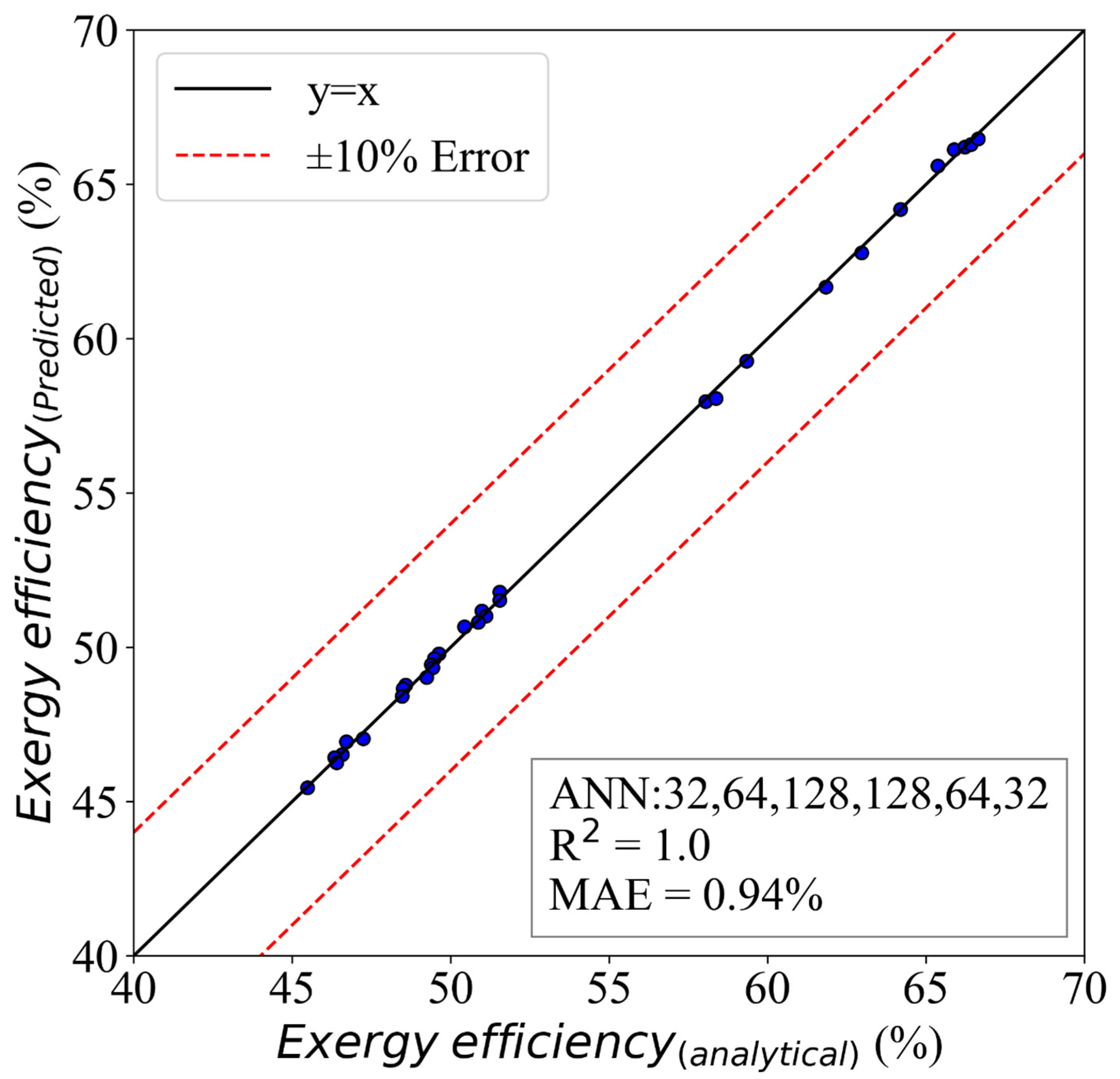
5.2. ANN Model for Exergy Efficiency
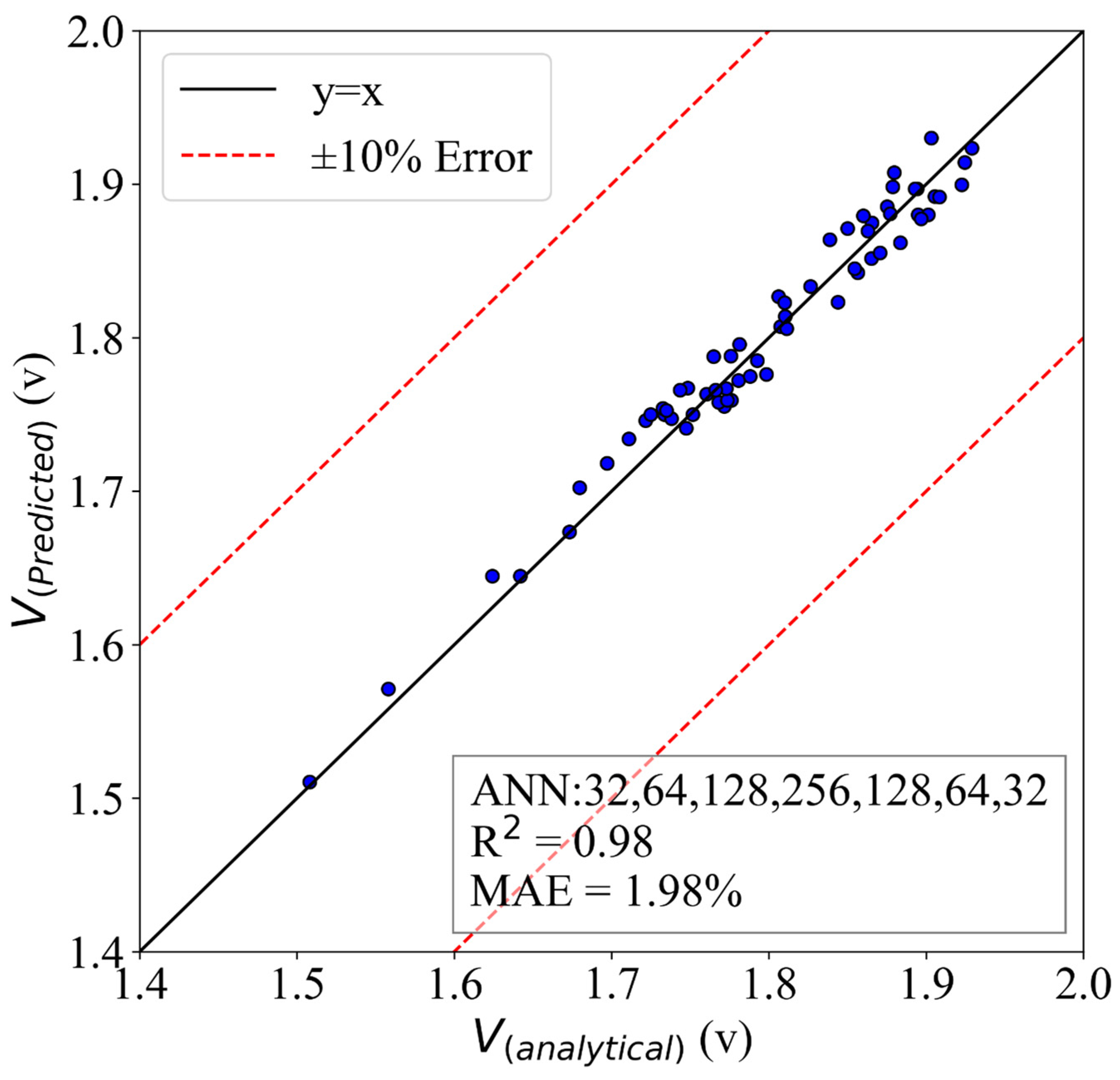
5.3. ANN Model for Voltage
5.4. ANN Model for Production
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Bozorgnezhad, A.; Shams, M.; Ahmadi, G.; Kanani, H.; Hasheminasab, M. The experimental study of water accumulation in PEMFC cathode channel. In Fluids Engineering Division Summer Meeting; American Society of Mechanical Engineers: New York, NY, USA, 2015; Volume 57212, p. V001T22A004. [Google Scholar]
- Liu, W.; Zhao, C.; Zhou, Y.; Xu, X. Modeling of Vapor-Liquid Equilibrium for Electrolyte Solutions Based on COSMO-RS Interaction. J. Chem. 2022, 2022, 9070055. [Google Scholar]
- Salehmin, M.N.I.; Husaini, T.; Goh, J.; Sulong, A.B. High-pressure PEM water electrolyser: A review on challenges and mitigation strategies towards green and low-cost hydrogen production. Energy Convers. Manag. 2022, 268, 115985. [Google Scholar]
- Shi, M.; Wang, R.; Li, L.; Chen, N.; Xiao, P.; Yan, C.; Yan, X. Redox-active polymer integrated with MXene for ultra-stable and fast aqueous proton storage. Adv. Funct. Mater. 2023, 33, 2209777. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sánchez-Sánchez, M.C.; Alvarez-Galvan, M.C.; del Valle, F.; Fierro, J.L.G. Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Wu, L.; Ma, D.; Wang, Z.; Zhang, J.; Zhang, B.; Li, J.; Liao, J.; Tong, J. A deep CNN-based constitutive model for describing of statics characteristics of rock materials. Eng. Fract. Mech. 2023, 279, 109054. [Google Scholar]
- Steinfeld, A. Solar thermochemical production of hydrogen––A review. Sol. Energy 2005, 78, 603–615. [Google Scholar]
- Wu, L.Y.; Ma, D.; Wang, Z.; Zhang, J.W. Prediction and prevention of mining-induced water inrush from rock strata separation space by 3D similarity simulation testing: A case study of Yuan Zigou coal mine, China. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 202. [Google Scholar] [CrossRef]
- Hasheminasab, M.; Bozorgnezhad, A.; Shams, M.; Ahmadi, G.; Kanani, H. Simultaneous investigation of PEMFC performance and water content at different flow rates and relative humidities. In International Conference on Nanochannels, Microchannels, and Minichannels; American Society of Mechanical Engineers: New York, NY, USA, 2014; Volume 46278, p. V001T07A002. [Google Scholar]
- Zaboli, S.; Alimoradi, H.; Shams, M. Numerical investigation on improvement in pool boiling heat transfer characteristics using different nanofluid concentrations. J. Therm. Anal. Calorim. 2022, 147, 10659–10676. [Google Scholar]
- He, Y.; Wang, F.; Du, G.; Pan, L.; Wang, K.; Gerhard, R.; Plath, R.; Rozga, P.; Trnka, P. Revisiting the thermal ageing on the metallised polypropylene film capacitor: From device to dielectric film. High Volt. 2022, 1–10. [Google Scholar] [CrossRef]
- Janssen, H.; Bringmann, J.; Emonts, B.; Schroeder, V. Safety-related studies on hydrogen production in high-pressure electrolysers. Int. J. Hydrogen Energy 2004, 29, 759–770. [Google Scholar] [CrossRef]
- Godula-Jopek, A. Hydrogen Production: By Electrolysis; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kokoh, K.; Mayousse, E.; Napporn, T.; Servat, K.; Guillet, N.; Soyez, E.; Grosjean, A.; Rakotondrainibé, A.; Paul-Joseph, J. Efficient multi-metallic anode catalysts in a PEM water electrolyzer. Int. J. Hydrogen Energy 2014, 39, 1924–1931. [Google Scholar] [CrossRef]
- Harrison, K.W.; Hernández-Pacheco, E.; Mann, M.; Salehfar, H. Semiempirical model for determining PEM electrolyzer stack characteristic. J. Fuel Cell Sci. Technol. 2006, 3, 220–223. [Google Scholar] [CrossRef]
- Dale, N.; Mann, M.; Salehfar, H. Semiempirical model based on thermodynamic principles for determining 6kW proton exchange membrane electrolyzer stack characteristics. J. Power Sources 2008, 185, 1348–1353. [Google Scholar] [CrossRef]
- Santarelli, M.; Medina, P.; Calì, M. Fitting regression model and experimental validation for a high-pressure PEM electrolyzer. Int. J. Hydrogen Energy 2009, 34, 2519–2530. [Google Scholar] [CrossRef]
- Chandesris, M.; Médeau, V.; Guillet, N.; Chelghoum, S.; Thoby, D.; Fouda-Onana, F. Membrane degradation in PEM water electrolyzer: Numerical modeling and experimental evidence of the influence of temperature and current density. Int. J. Hydrogen Energy 2015, 40, 1353–1366. [Google Scholar] [CrossRef]
- Alimoradi, H.; Soltani, M.; Shahali, P.; Moradi Kashkooli, F.; Larizadeh, R.; Raahemifar, K.; Adibi, M.; Ghasemi, B. Experimental investigation on improvement of wet cooling tower efficiency with diverse packing compaction using ANN-PSO algorithm. Energies 2021, 14, 167. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Wang, Q.; Ouyang, L.; Chen, X.; Li, J. Numerical Modeling of Branching-Streamer Propagation in Ester-Based Insulating Oil Under Positive Lightning Impulse Voltage: Effects from Needle Curvature Radius. IEEE Trans. Dielectr. Electr. Insul. 2022, 30, 139–147. [Google Scholar] [CrossRef]
- Rong, G.; Cheng, M.; Sheng, Z.; Liu, X.; Zhang, Y.; Wang, J. Investigation of counter-rotating shock wave and wave direction control of hollow rotating detonation engine with Laval nozzle. Phys. Fluids 2022, 34, 056104. [Google Scholar] [CrossRef]
- Tributsch, H. Photovoltaic hydrogen generation. Int. J. Hydrogen Energy 2008, 33, 5911–5930. [Google Scholar] [CrossRef]
- Scamman, D.; Bustamante, H.; Hallett, S.; Newborough, M. Off-grid solar-hydrogen generation by passive electrolysis. Int. J. Hydrogen Energy 2014, 39, 19855–19868. [Google Scholar] [CrossRef]
- Gibson, T.L.; Kelly, N.A. Optimization of solar powered hydrogen production using photovoltaic electrolysis devices. Int. J. Hydrogen Energy 2008, 33, 5931–5940. [Google Scholar] [CrossRef]
- Paul, B.; Andrews, J. Optimal coupling of PV arrays to PEM electrolysers in solar–hydrogen systems for remote area power supply. Int. J. Hydrogen Energy 2008, 33, 490–498. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Wang, Z.; Ma, D.; Zhang, J.-W.; Wu, G.; Wen, S.; Zha, M.; Wu, L. A continuous damage statistical constitutive model for sandstone and mudstone based on triaxial compression tests. Rock Mech. Rock Eng. 2022, 55, 4963–4978. [Google Scholar] [CrossRef]
- Li, H.-M.; Li, G.-X.; Li, L. Comparative investigation on combustion characteristics of ADN-based liquid propellants in inert gas and oxidizing gas atmospheres with resistive ignition method. Fuel 2023, 334, 126742. [Google Scholar] [CrossRef]
- Chand, K.; Paladino, O. Recent developments of membranes and electrocatalysts for the hydrogen production by anion exchange membrane water electrolysers: A review. Arab. J. Chem. 2023, 16, 104451. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Zhao, Y. From classical thermodynamics to phase-field method. Prog. Mater. Sci. 2022, 124, 100868. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Improvement of Alcaligenes sp. TB performance by Fe-Pd/multi-walled carbon nanotubes: Enriched denitrification pathways and accelerated electron transport. Bioresour. Technol. 2021, 327, 124785. [Google Scholar] [CrossRef]
- Bazarah, A.; Majlan, E.H.; Husaini, T.; Zainoodin, A.; Alshami, I.; Goh, J.; Masdar, M.S. Factors influencing the performance and durability of polymer electrolyte membrane water electrolyzer: A review. Int. J. Hydrogen Energy 2022, 47, 35976–35989. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- AlZahrani, A.A.; Dincer, I. Design and analysis of a solar tower based integrated system using high temperature electrolyzer for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 8042–8056. [Google Scholar] [CrossRef]
- Joshi, A.S.; Dincer, I.; Reddy, B.V. Solar hydrogen production: A comparative performance assessment. Int. J. Hydrogen Energy 2011, 36, 11246–11257. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y. Energy and exergy analysis of hydrogen production by a proton exchange membrane (PEM) electrolyzer plant. Energy Convers. Manag. 2008, 49, 2748–2756. [Google Scholar] [CrossRef]
- Biaku, C.; Dale, N.; Mann, M.; Salehfar, H.; Peters, A.; Han, T. A semiempirical study of the temperature dependence of the anode charge transfer coefficient of a 6kW PEM electrolyzer. Int. J. Hydrogen Energy 2008, 33, 4247–4254. [Google Scholar] [CrossRef]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Simple PEM water electrolyser model and experimental validation. Int. J. Hydrogen Energy 2012, 37, 1927–1938. [Google Scholar] [CrossRef]
- Rivero, R.; Garfias, M. Standard chemical exergy of elements updated. Energy 2006, 31, 3310–3326. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Calì, M. Theoretical model and experimental analysis of a high pressure PEM water electrolyser for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- Ioroi, T.; Yasuda, K.; Siroma, Z.; Fujiwara, N.; Miyazaki, Y. Thin film electrocatalyst layer for unitized regenerative polymer electrolyte fuel cells. J. Power Sources 2002, 112, 583–587. [Google Scholar] [CrossRef]
- Eskandari, E.; Alimoradi, H.; Pourbagian, M.; Shams, M. Numerical investigation and deep learning-based prediction of heat transfer characteristics and bubble dynamics of subcooled flow boiling in a vertical tube. Korean J. Chem. Eng. 2022, 39, 3227–3245. [Google Scholar] [CrossRef]
- Alimoradi, H.; Eskandari, E.; Pourbagian, M.; Shams, M. A parametric study of subcooled flow boiling of Al2O3/water nanofluid using numerical simulation and artificial neural networks. Nanoscale Microscale Thermophys. Eng. 2022, 26, 129–159. [Google Scholar] [CrossRef]
- Zhao, D.; He, Q.; Yu, J.; Guo, M.; Fu, J.; Li, X.; Ni, M. A data-driven digital-twin model and control of high temperature proton exchange membrane electrolyzer cells. Int. J. Hydrogen Energy 2022, 47, 8687–8699. [Google Scholar]
- Alimoradi, H.; Zaboli, S.; Shams, M. Numerical simulation of surface vibration effects on improvement of pool boiling heat transfer characteristics of nanofluid. Korean J. Chem. Eng. 2022, 39, 69–85. [Google Scholar] [CrossRef]
- Zhao, D.; Xia, Z.; Guo, M.; He, Q.; Xu, Q.; Li, X.; Ni, M. Dynamic hierarchical modeling and control strategy of high temperature proton exchange electrolyzer cell system. Int. J. Hydrogen Energy 2022, 47, 22302–22315. [Google Scholar]
- Nejadian, M.M.; Ahmadi, P.; Houshfar, E. Comparative optimization study of three novel integrated hydrogen production systems with SOEC, PEM, and alkaline electrolyzer. Fuel 2023, 336, 126835. [Google Scholar]
- Salari, A.; Hakkaki-Fard, A.; Jalalidil, A. Hydrogen production performance of a photovoltaic thermal system coupled with a proton exchange membrane electrolysis cell. Int. J. Hydrogen Energy 2022, 47, 4472–4488. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Enhancement in the pool boiling heat transfer of copper surface by applying electrophoretic deposited graphene oxide coatings. Int. J. Multiph. Flow 2023, 159, 104350. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Bubble behavior and nucleation site density in subcooled flow boiling using a novel method for simulating the microstructure of surface roughness. Korean J. Chem. Eng. 2022, 39, 2945–2958. [Google Scholar]
- Lei, J.; Liu, X.; Chen, X.; Guan, P.; Feng, W.; Zhang, J.; Luo, H.; Liu, F.; Zhang, Y. Multi-length-scale heterogeneous structured ion exchange membranes for cost-effective electrolysis and hydrogen production. Chem. Eng. J. 2021, 431, 133994. [Google Scholar]







| Solar Tower Parameter | Value |
|---|---|
| Heliostat efficiency | 70% |
| Receiver’s efficiency | 88% |
| Heliostat area | 6100 |
| Parameter | Value |
|---|---|
| Water pressure () | 1 bar |
| Hydrogen pressure () | 1 bar |
| Oxygen pressure () | 1 bar |
| Membrane thickness () | 183 |
| Electrolyzer temperature () | 353 K |
| Lower heating value of hydrogen () | 224 |
| The cathode activation energy () | 18 |
| The anode activation energy () | 76 |
| Parameter | Value |
|---|---|
| Hydrogen pressure () | |
| Oxygen pressure () | |
| Water pressure () | |
| Electrolyzer temperature () | |
| Membrane thickness () | 50 |
| Model | Layer Structure | Mean Absolute Error (%) | |
|---|---|---|---|
| 1 | (32) | 3.14% | 0.96 |
| 2 | (32,64) | 2.98% | 0.97 |
| 3 | (32,64,32) | 2.86% | 0.97 |
| 4 | (32,64,64,32) | 2.61% | 0.98 |
| 5 | (32,64,128,64,32) | 2.43% | 0.98 |
| 6 | (32,64,128,128,64,32) | 2.21% | 0.98 |
| 7 * | (32,64,128,256,128,64,32) | 2.05% | 0.98 |
| 8 | (32,64,128,256,256,128,64,32) | 2.09% | 0.98 |
| 9 | (32,64,128,256,512,256,128,64,32) | 2.13% | 0.98 |
| Model | Activation Function | Mean Absolute Error (%) | |
|---|---|---|---|
| 1 | Linear | 2.87% | 0.97 |
| 2 * | ReLU | 2.05% | 0.98 |
| 3 | Sigmoid | 2.46% | 0.98 |
| Model | Batch Size | Mean Absolute Error (%) | |
|---|---|---|---|
| 1 | 2 | 2.98% | 0.97 |
| 2 | 4 | 2.47% | 0.98 |
| 3 | 8 | 2.36% | 0.98 |
| 4 | 16 | 2.39% | 0.98 |
| 5 * | 32 | 2.05% | 0.98 |
| 6 | 64 | 2.86% | 0.97 |
| Model | Epochs | Mean Absolute Error (%) | |
|---|---|---|---|
| 1 | 1500 | 5.21% | 0.89 |
| 2 | 2500 | 4.76% | 0.92 |
| 3 | 6000 | 3.15% | 0.96 |
| 4 | 15,000 | 2.55% | 0.98 |
| 5 * | 25,000 | 1.98% | 0.98 |
| 6 | 35,000 | 2.05% | 0.98 |
| 7 | 45,000 | 2.08% | 0.98 |
| 8 | 55,000 | 2.09% | 0.98 |
| Hyperparameter | Energy Efficiency | Exergy Efficiency | Voltage | Production |
|---|---|---|---|---|
| Layer structure | (32,64,64,32) | (32,64,128,128,64,32) | (32,64,128,256,128,64,32) | (32,64,128,64,32) |
| Batch size | 32 | 8 | 32 | 16 |
| Epochs | 45,000 | 35,000 | 25,000 | 35,000 |
| Activation function | ReLU | Sigmoid | ReLU | ReLU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Jery, A.; Salman, H.M.; Al-Khafaji, R.M.; Nassar, M.F.; Sillanpää, M. Thermodynamics Investigation and Artificial Neural Network Prediction of Energy, Exergy, and Hydrogen Production from a Solar Thermochemical Plant Using a Polymer Membrane Electrolyzer. Molecules 2023, 28, 2649. https://doi.org/10.3390/molecules28062649
El Jery A, Salman HM, Al-Khafaji RM, Nassar MF, Sillanpää M. Thermodynamics Investigation and Artificial Neural Network Prediction of Energy, Exergy, and Hydrogen Production from a Solar Thermochemical Plant Using a Polymer Membrane Electrolyzer. Molecules. 2023; 28(6):2649. https://doi.org/10.3390/molecules28062649
Chicago/Turabian StyleEl Jery, Atef, Hayder Mahmood Salman, Rusul Mohammed Al-Khafaji, Maadh Fawzi Nassar, and Mika Sillanpää. 2023. "Thermodynamics Investigation and Artificial Neural Network Prediction of Energy, Exergy, and Hydrogen Production from a Solar Thermochemical Plant Using a Polymer Membrane Electrolyzer" Molecules 28, no. 6: 2649. https://doi.org/10.3390/molecules28062649
APA StyleEl Jery, A., Salman, H. M., Al-Khafaji, R. M., Nassar, M. F., & Sillanpää, M. (2023). Thermodynamics Investigation and Artificial Neural Network Prediction of Energy, Exergy, and Hydrogen Production from a Solar Thermochemical Plant Using a Polymer Membrane Electrolyzer. Molecules, 28(6), 2649. https://doi.org/10.3390/molecules28062649







