Antioxidant Activity of Natural Phenols and Derived Hydroxylated Biphenyls
Abstract
1. Introduction
1.1. Oxidative Stress and Natural Phenols as Antioxidants
- -
- -
- -
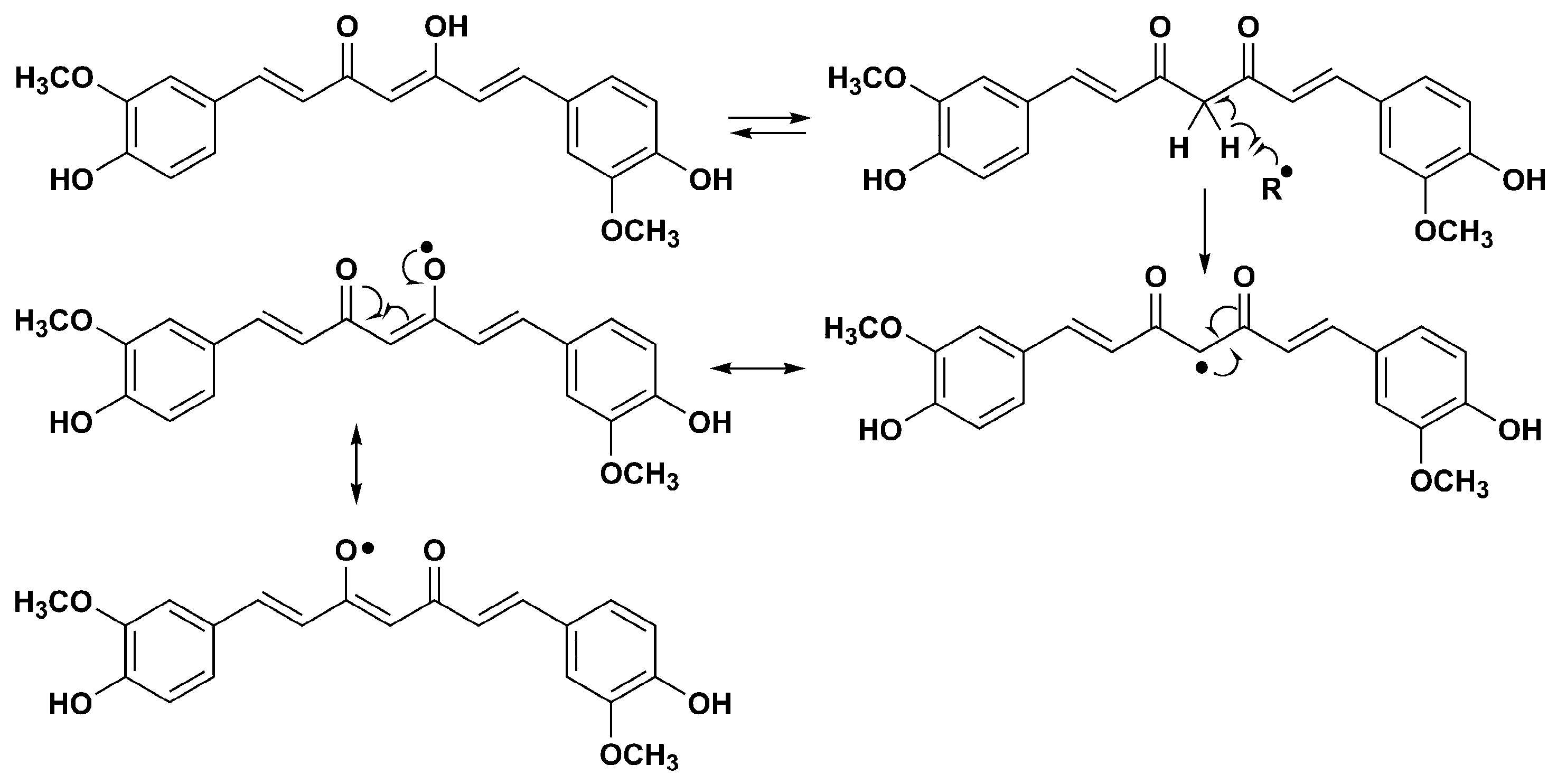
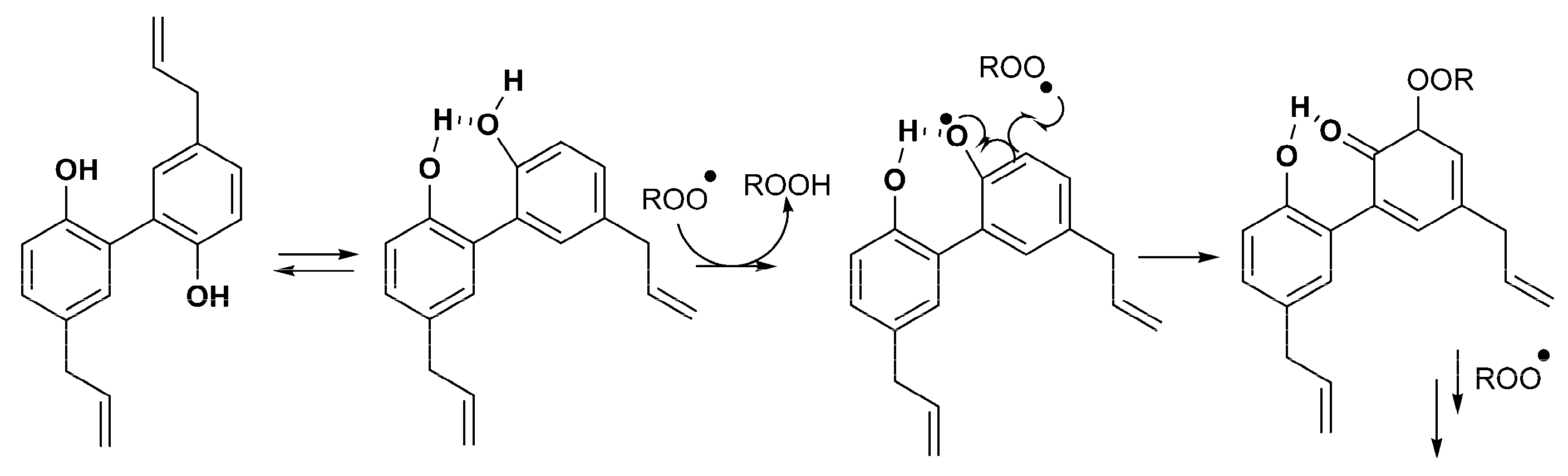
1.2. Redox Properties of Hydroxylated Biphenyls
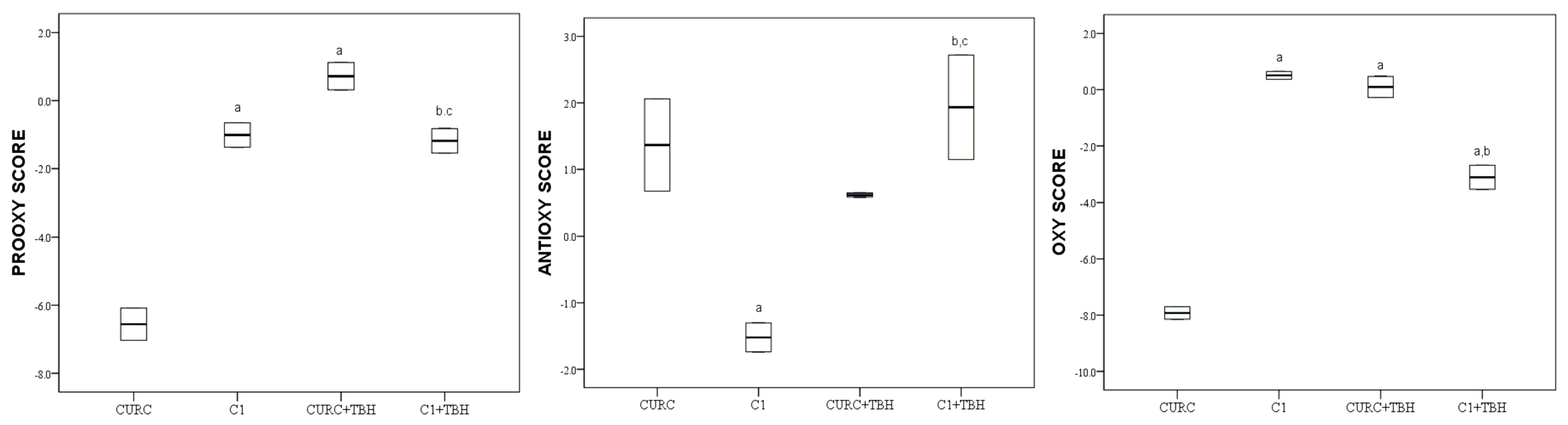
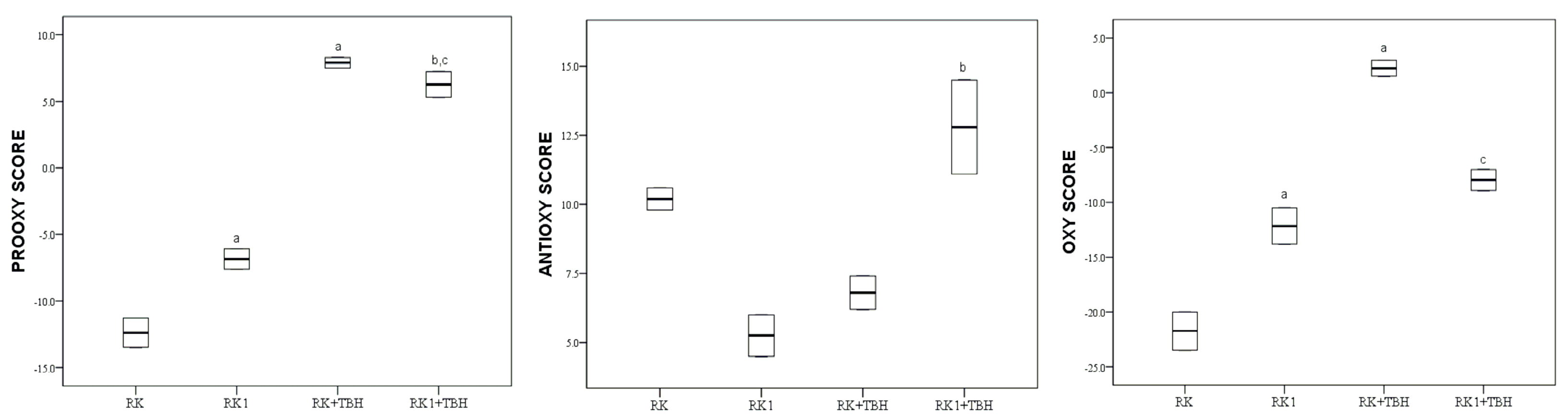
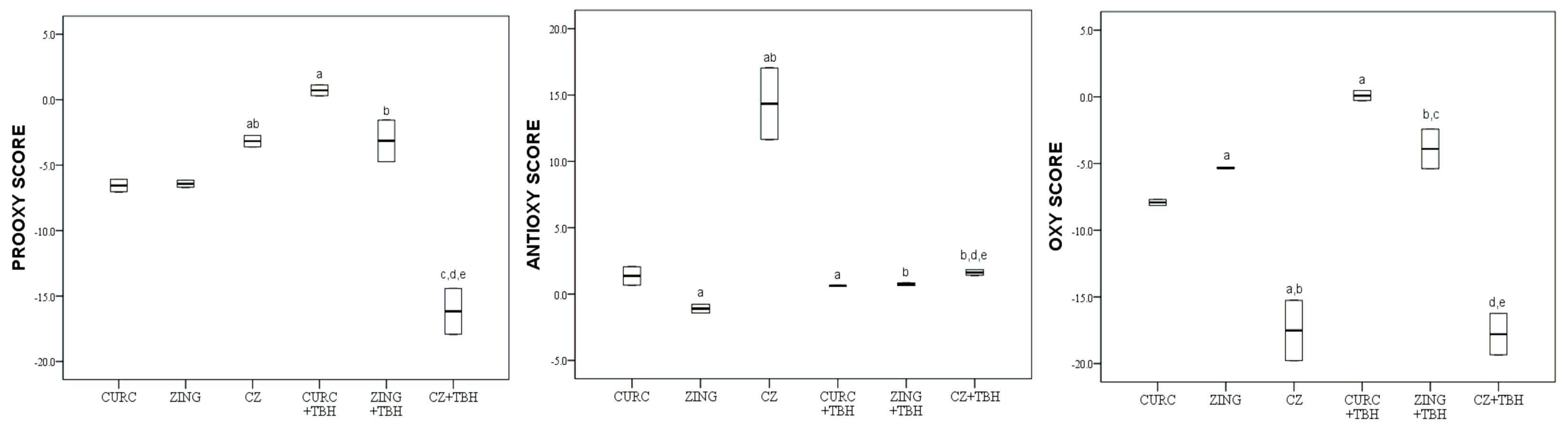
2. Results
3. Discussion
3.1. Phenols as Antioxidants
3.2. Antioxidative Properties of Tested Compounds
3.3. Antioxidative Properties of Tested Compound under Condition of Oxidative Stress
3.4. Antioxidative Properties of Mixture of Natural Phenolic Compounds
4. Material and Methods
4.1. General
4.2. Chemistry
Synthesized Compounds
4.3. Evaluation of In Vitro Antioxidant Potential (Pro-oxidant/Antioxidant Activity) of the Compounds in Biological Matrix (Human Serum Pool)
4.3.1. Sample Collection
4.3.2. Total Oxidative Potency (TOP)
4.3.3. Pro-oxidative–Antioxidative Balance (PAB)
4.3.4. Total Sulphydryl Groups (SHG)
4.3.5. Total Antioxidant Capacity (TAC)
4.3.6. Pro-oxidative Score, Antioxidative Score and Oxy Score
4.3.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Ann. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.S.; Rani, V.; Deep, G.; Singh, R.K.; Palle, K. Oxidative Stress in Metabolic Disorders: Pathogenesis, Prevention, and Therapeutics. Oxidative Med. Cell. Longev. 2016, 2016, e9137629. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Mitra, A.K. Antioxidants: A Masterpiece of Mother Nature to Prevent Illness. J. Chem. Rev. 2020, 2, 243–256. [Google Scholar] [CrossRef]
- Tan, F. Trease and Evans Pharmacognosy, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.u.R. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-Methoxyphenyl)-2-Butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef]
- Kim, M.K.; Chung, S.W.; Kim, D.H.; Kim, J.M.; Lee, E.K.; Kim, J.Y.; Ha, Y.M.; Kim, Y.H.; No, J.-K.; Chung, H.S.; et al. Modulation of Age-Related NF-KappaB Activation by Dietary Zingerone via MAPK Pathway. Exp. Gerontol. 2010, 45, 419–426. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Harjai, K. Zingerone Inhibit Biofilm Formation and Improve Antibiofilm Efficacy of Ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia 2013, 90, 73–78. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in Various Cancers. Biofactors 2013, 39, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in Inflammatory Diseases. Biofactors 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and Neurodegenerative Diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and Curcumin Inhibit the Growth of Helicobacter Pylori, a Group 1 Carcinogen. Anticancer. Res. 2002, 22, 4179–4181. [Google Scholar] [PubMed]
- Pyun, C.-W.; Kim, J.-H.; Han, K.-H.; Hong, G.-E.; Lee, C.-H. In Vivo Protective Effects of Dietary Curcumin and Capsaicin against Alcohol-Induced Oxidative Stress. Biofactors 2014, 40, 494–500. [Google Scholar] [CrossRef]
- A Study on the Genus Rubus of China. Available online: https://www.jse.ac.cn/EN/Y1983/V21/I1/13 (accessed on 26 January 2023).
- Kshatriya, D.; Li, X.; Giunta, G.M.; Yuan, B.; Zhao, D.; Simon, J.E.; Wu, Q.; Bello, N.T. Phenolic-Enriched Raspberry Fruit Extract (Rubus Idaeus) Resulted in Lower Weight Gain, Increased Ambulatory Activity, and Elevated Hepatic Lipoprotein Lipase and Heme Oxygenase-1 Expression in Male Mice Fed a High-Fat Diet. Nutr. Res. 2019, 68, 19–33. [Google Scholar] [CrossRef]
- Lin, V.C.-H.; Ding, H.-Y.; Kuo, S.-Y.; Chin, L.-W.; Wu, J.-Y.; Chang, T.-S. Evaluation of in Vitro and in Vivo Depigmenting Activity of Raspberry Ketone from Rheum officinale. Int. J. Mol. Sci. 2011, 12, 4819–4835. [Google Scholar] [CrossRef]
- Wang, L.; Meng, X.; Zhang, F. Raspberry Ketone Protects Rats Fed High-Fat Diets against Nonalcoholic Steatohepatitis. J. Med. Food 2012, 15, 495–503. [Google Scholar] [CrossRef]
- Zhu, L.; Ge, G.; Zhang, H.; Liu, H.; He, G.; Liang, S.; Zhang, Y.; Fang, Z.; Dong, P.; Finel, M.; et al. Characterization of Hepatic and Intestinal Glucuronidation of Magnolol: Application of the Relative Activity Factor Approach to Decipher the Contributions of Multiple UDP-Glucuronosyltransferase Isoforms. Drug Metab. Dispos. 2012, 40, 529–538. [Google Scholar] [CrossRef]
- Rajan, I.; Narayanan, N.; Rabindran, R.; Jayasree, P.R.; Manish Kumar, P.R. Zingerone Protects Against Stannous Chloride-Induced and Hydrogen Peroxide-Induced Oxidative DNA Damage In Vitro. Biol. Trace Elem. Res. 2013, 155, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Parray, H.A.; Lone, J.; Park, J.P.; Choi, J.W.; Yun, J.W. Magnolol Promotes Thermogenesis and Attenuates Oxidative Stress in 3T3-L1 Adipocytes. Nutrition 2018, 50, 82–90. [Google Scholar] [CrossRef]
- Fujisawa, S.; Atsumi, T.; Murakami, Y.; Kadoma, Y. Dimerization, ROS Formation, and Biological Activity of o-Methoxyphenols. Arch. Immunol. Ther. Exp. 2005, 53, 28–38. [Google Scholar]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Janiak, M.; Amarowicz, R. Protective Effects of Equimolar Mixtures of Monomer and Dimer of Dehydrozingerone with α-Tocopherol and/or Ascorbyl Palmitate during Bulk Lipid Autoxidation. Food Chem. 2014, 157, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, P.J.; Bures, M.; Praestgaard, J.; Fesik, S.W. Privileged Molecules for Protein Binding Identified from NMR-Based Screening. J. Med. Chem. 2000, 43, 3443–3447. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F. Antioxidant Activity of O-Bisphenols: The Role of Intramolecular Hydrogen Bonding. J. Org. Chem. 2003, 68, 5198–5204. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Angelova, S.; Dettori, M.; Nechev, J.; Delogu, G. Antiradical and Antioxidant Activities of New Natural-like Hydroxylated Biphenyls of Dehydrozingerone, Zingerone and Ferulic Acid. Comptes Rendus De L’académie Bulg. Des Sci. Sci. Mathématiques Et Nat. 2013, 66, 361–368. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of Curcuminoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-like Molecules: From Spice to Drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-Breaking Antioxidant Activity of Hydroxylated and Methoxylated Magnolol Derivatives: The Role of H-Bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free. Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Davies, M.J. Detection of Peroxyl and Alkoxyl Radicals Produced by Reaction of Hydroperoxides with Rat Liver Microsomal Fractions. Biochem. J. 1989, 257, 603–606. [Google Scholar] [CrossRef]
- Crane, D.; Häussinger, D.; Graf, P.; Sies, H. Decreased Flux through Pyruvate Dehydrogenase by Thiol Oxidation during T-Butyl Hydroperoxide Metabolism in Perfused Rat Liver. Hoppe Seylers Z Physiol. Chem. 1983, 364, 977–987. [Google Scholar] [CrossRef]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-Catalyzed Oxidation of the Leukoderma-Inducing Agent Raspberry Ketone Produces (E)-4-(3-Oxo-1-Butenyl)-1,2-Benzoquinone: Implications for Melanocyte Toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef]
- Hussain, N.; Hashmi, A.-S.; Wasim, M.; Akhtar, T.; Saeed, S.; Ahmad, T. Synergistic Potential of Zingiber officinale and Curcuma longa to Ameliorate Diabetic-Dyslipidemia. Pak. J. Pharm. Sci. 2018, 31, 491–498. [Google Scholar] [PubMed]
- Yin, F.; Sun, X.; Zheng, W.; Luo, X.; Zhang, Y.; Yin, L.; Jia, Q.; Fu, Y. Screening of Highly Effective Mixed Natural Antioxidants to Improve the Oxidative Stability of Microalgal DHA-Rich Oil. RSC Adv. 2021, 11, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Noguchi, N.; Tsuchihashi, H.; Gotoh, N. Interaction among Vitamin C, Vitamin E, and Beta-Carotene. Am. J. Clin. Nutr. 1995, 62, 1322S–1326S. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Mitchell, M.S.; Mohan, R.S. Isolation of Curcumin from Turmeric. J. Chem. Educ. 2000, 77, 359. [Google Scholar] [CrossRef]
- Marchiani, A.; Mammi, S.; Siligardi, G.; Hussain, R.; Tessari, I.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sanna, D.; et al. Small Molecules Interacting with α-Synuclein: Antiaggregating and Cytoprotective Properties. Amino Acids 2013, 45, 327–338. [Google Scholar] [CrossRef]
- Sun, X.-L.; Zhu, M.-L.; Dai, Y.-Q.; Li, H.-M.; Li, B.-H.; Ma, H.; Zhang, C.-H.; Wu, C.-Z. Semi-Synthesis and In Vitro Anti-Cancer Evaluation of Magnolol Derivatives. Molecules 2021, 26, 4302. [Google Scholar] [CrossRef]
- Brboric, J.; Klisic, A.; Kotur-Stevuljevic, J.; Delogu, G.; Ackova, D.G.; Kostic, K.; Dettori, M.A.; Fabbri, D.; Carta, P.; Saso, L. Natural and Natural-like Polyphenol Compounds: In Vitro Antioxidant Activity and Potential for Therapeutic Application. Arch. Med. Sci. 2021, 19, 135379. [Google Scholar] [CrossRef]
- Long, C.-Y.; Chen, H.; Ma, C.; Zhao, B.-W.; Li, S.-H.; Cui, Y.; Yang, X.; Ni, S.-F.; Wang, X.-Q. Highly Chemoselective Ni-Catalyzed Protecting-Group-Free 2,2′-Biphenol Synthesis and Mechanistic Insights. Org. Lett. 2022, 24, 4155–4159. [Google Scholar] [CrossRef]
- Kotur-Stevuljevic, J.; Bogavac-Stanojevic, N.; Jelic-Ivanovic, Z.; Stefanovic, A.; Gojkovic, T.; Joksic, J.; Sopic, M.; Gulan, B.; Janac, J.; Milosevic, S. Oxidative Stress and Paraoxonase 1 Status in Acute Ischemic Stroke Patients. Atherosclerosis 2015, 241, 192–198. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Paletas, K.; Pegiou, T.; Sarigianni, M.; Befani, C.; Koliakos, G. A Novel Assay for the Evaluation of the Prooxidant–Antioxidant Balance, before and after Antioxidant Vitamin Administration in Type II Diabetes Patients. Clin. Biochem. 2007, 40, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, K.; Brborić, J.; Delogu, G.; Simić, M.R.; Samardžić, S.; Maksimović, Z.; Dettori, M.A.; Fabbri, D.; Kotur-Stevuljević, J.; Saso, L. Antioxidant Activity of Natural Phenols and Derived Hydroxylated Biphenyls. Molecules 2023, 28, 2646. https://doi.org/10.3390/molecules28062646
Kostić K, Brborić J, Delogu G, Simić MR, Samardžić S, Maksimović Z, Dettori MA, Fabbri D, Kotur-Stevuljević J, Saso L. Antioxidant Activity of Natural Phenols and Derived Hydroxylated Biphenyls. Molecules. 2023; 28(6):2646. https://doi.org/10.3390/molecules28062646
Chicago/Turabian StyleKostić, Kristina, Jasmina Brborić, Giovanna Delogu, Milena R. Simić, Stevan Samardžić, Zoran Maksimović, Maria Antonietta Dettori, Davide Fabbri, Jelena Kotur-Stevuljević, and Luciano Saso. 2023. "Antioxidant Activity of Natural Phenols and Derived Hydroxylated Biphenyls" Molecules 28, no. 6: 2646. https://doi.org/10.3390/molecules28062646
APA StyleKostić, K., Brborić, J., Delogu, G., Simić, M. R., Samardžić, S., Maksimović, Z., Dettori, M. A., Fabbri, D., Kotur-Stevuljević, J., & Saso, L. (2023). Antioxidant Activity of Natural Phenols and Derived Hydroxylated Biphenyls. Molecules, 28(6), 2646. https://doi.org/10.3390/molecules28062646







