Volatile Metabolites of Piper eriopodon (Miq.) C.DC. from Northern Region of Colombia and Assessment of In Vitro Bioactivities of the Leaf Essential Oil
Abstract
1. Introduction
2. Results
2.1. Identity of Plant
2.2. Chemical Composition of the Volatile Metabolites
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno, L.A.; Andrade, G.I.; Ruíz-Contreras, L.F. Biodiversity 2016: Status and Trends of Colombian Continental Biodiversity; Research Institute of Biological Resources Alexander von Humboldt: Bogotá, Colombia, 2016; 106p. [Google Scholar]
- Arbeláez-Cortés, E. Knowledge of Colombian biodiversity: Published and indexed. Biodivers. Conserv. 2013, 22, 2875–2906. [Google Scholar] [CrossRef]
- Rangel-Churio, J.O. La riqueza de las plantas con flores de Colombia. Caldasia 2015, 37, 279–307. [Google Scholar] [CrossRef]
- Quijano-Abril, M.A.; Callejas-Posada, R.; Miranda-Esquivel, D.R. Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae). J. Biogeogr. 2006, 33, 1266–1278. [Google Scholar] [CrossRef]
- The Plant List. Available online: http://www.theplantlist.org/tpl/record/kew-2558272 (accessed on 1 November 2020).
- POWO—Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:681318-1 (accessed on 2 November 2020).
- Saavedra Barrera, R.A.S. Análisis y Estudio Comparativo de los Extractos Obtenidos con Fluido Supercrítico y de los Aceites Esenciales de Diferentes Plantas del Género Piper. Bachelor’s Thesis, Universidad Industrial de Santander, Bucaramanga, Colombia, 2015. [Google Scholar]
- Xiang, C.-P.; Shi, Y.-N.; Liu, F.-F.; Li, H.-Z.; Zhang, Y.-J.; Yang, C.-R.; Xu, M. A survey of the chemical compounds of Piper spp. (Piperaceae) and their biological activities. Nat. Prod. Commun. 2016, 11, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Chahal, J.; Ohlyan, R.; Kandale, A.; Walia, A.; Puri, S. Introduction, phytochemistry, traditional uses and biological activity of genus Piper: A review. Int. J. Curr. Pharmac. Rev. Res. 2011, 2, 130–144. [Google Scholar]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Castañeda Muñoz, M.L. Estudio de la Composición Química y la Actividad Biológica de los Aceites Esenciales de Diez Plantas Aromáticas Colombianas. Bachelor’s Thesis, Universidad Industrial de Santander, Bucaramanga, Colombia, 2007. [Google Scholar]
- Tangarife-Castaño, V.; Correa-Royero, J.B.; Roa-Linares, V.C.; Pino-Benitez, N.; Betancur-Galvis, L.A.; Durán, D.C.; Stashenko, E.E.; Mesa-Arango, A.C. Anti-dermatophyte, anti-Fusarium and cytotoxic activity of essential oils and plant extracts of Piper genus. J. Essent. Oil Res. 2014, 26, 221–227. [Google Scholar] [CrossRef]
- Ustáriz Fajardo, F.J.; Lucena de Ustári, M.E.; Urbina Carmona, F.G.; Villamizar Sánchez, D.M.; Rojas Fermín, L.B.; Cordero de Rojas, Y.E.; Ustáriz Lucena, J.E.; González Ramírez, L.C.; Araujo Baptista, L.M. Composition and antibacterial activity of the Piper eriopodon (Miq.) C.DC. essential oil from the Venezuelan Andes. PharmacologyOnLine 2020, 2, 13–22. [Google Scholar]
- Guzman, J.D.; Gupta, A.; Evangelopoulos, D.; Basavannacharya, C.; Pabon, L.C.; Plazas, E.A.; Muñoz, D.R.; Delgado, W.A.; Cuca, L.E.; Ribon, W.; et al. Anti-tubercular screening of natural products from Colombian plants: 3-methoxynordomesticine, an inhibitor of MurE ligase of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 2101–2107. [Google Scholar] [CrossRef]
- Muñoz, D.R.; Sandoval-Hernández, A.G.; Delgado, W.A.; Arboleda, G.H.; Cuca, L.E. In vitro anticancer screening of Colombian plants from Piper genus (Piperaceae). J. Pharmacogn. Phytother. 2018, 10, 174–181. [Google Scholar] [CrossRef]
- Muñoz, D.R. Estudio Fitoquímico y Evaluación de la Actividad Fungicida e Insecticida de la Especie Piper eriopodon (Piperaceae). Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2008. [Google Scholar]
- Moreno López, J.P. Actividad Antifúngica de los Extractos Vegetales de Piper eriopodon y Zanthoxylum monophyllum y sus Metabolitos Secundarios Mayoritarios Sobre dos Hongos Fitopatógenos de Clavel (Dianthus caryophyllus). Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2011. [Google Scholar]
- Cuervo Cuervo, A.; García Vásquez, D.; Orozco Gómez, L.; Ramírez Ospino, D. Efectividad del Aceite Esencial de Piper eriopodon en Inhibición del Crecimiento de Trichophyton rubrum y Trichophyton mentagrophytes; Anuario de Investigación; Fundación Universitaria Juan. M. Corpas: Bogotá, Colombia, 2017. [Google Scholar]
- Velandia, S.A.; Quintero, E.; Stashenko, E.E.; Ocazionez, R.E. Actividad antiproliferativa de aceites esenciales de plantas cultivadas en Colombia. Acta Biol. Colomb. 2018, 23, 189–198. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Güete-Fernández, J.; Stashenko, E.E. Acute toxicity against Artemia franciscana of essential oils isolated from plants of the genus Lippia and Piper collected in Colombia. Bol. Latinoam. Caribe Plantas Med. Aromát. 2009, 8, 419–427. [Google Scholar]
- Amaya, C.; Acevedo, A.C. Effect of the extracts of Piper cumanense and Piper eriopodon in the behavior of genes involved in oxidation process of the skin. Planta Med. 2011, 77, PI6. [Google Scholar] [CrossRef]
- Mendoza Forero, C.; Celis, A.; Pachón, M.E. Herbicide effects of Piper extracts on a seed bank in Fusagasuga (Colombia). Acta Hort. 2014, 1030, 77–82. [Google Scholar] [CrossRef]
- Correa Navarro, Y.M.; Palomino García, L.R.; Marino Mosquera, O. Actividad antioxidante y antifúngica de Piperaceaes de la flora colombiana. Rev. Cubana Plant. Med. 2001, 20, 167–181. [Google Scholar]
- Blandón, A.M.; Mosquera, O.M.; Sant’ana, A.E.G.; dos Santos, A.F.; Pires, L.L.S. Antioxidant activity of plant extracts from Colombian coffee-growing eco-region. Rev. Fac. Cienc. Bás. 2017, 13, 56–59. [Google Scholar] [CrossRef]
- Rincón Santana, E.J. Estudio Fitoquímico de la Madera de Piper eriopodon y Evaluación de su Actividad Citotóxica en Células de Cáncer de Mama. Bachelor’s Thesis, Universidad de Ciencias Ambientales y Aplicadas, Bogotá, Colombia, 2021. [Google Scholar]
- Muñoz, D.; Brucoli, M.; Zecchini, S.; Sandoval-Hernandez, A.; Arboleda, G.; Lopez-Vallejo, F.; Delgado, W.; Giovarelli, M.; Coazzoli, M.; Catalani, E.; et al. XIAP as a target of new small organic natural molecules inducing human cancer cell death. Cancers 2019, 11, 1336. [Google Scholar] [CrossRef]
- Mesa Vanegas, A.M.; Wagner Arenas, J.; Ocampo Jiménez, O.; Monsalve Fonnegra, Z. Nematicidal activity and in vitro radical scavenging from Piper cumbricola and Piper eriopodon. Biocatal. Agric. Biotech. 2023, 47, 102595. [Google Scholar] [CrossRef]
- Castañeda, M.L.; Muñoz, A.; Martínez, J.R.; Stashenko, E.E. Estudio de la composición química y la actividad biológica de los aceites esenciales de diez plantas aromáticas colombianas. Sci. Technol. 2007, 33, 165–166. [Google Scholar]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T. Geographical impact on essential oil composition of endemic Kundmannia anatolica Hub.-Mor. (Apiaceae). Afr. J. Tradit. Complement. Altern. Med. 2016, 23, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.-Y.; Fan, R.; Qin, X.-W.; Hu, L.-S.; Tan, L.-H.; Xu, F.; Wu, B.-D. Characterization of volatile compounds in ten Piper species cultivated in Hainan Island, South China. Int. J. Food Prop. 2018, 21, 633–644. [Google Scholar] [CrossRef]
- Liu, L.; Song, G.; Hu, Y. GC-MS Analysis of the essential oils of Piper nigrum L. and Piper longum L. Chromatographia 2007, 66, 785–790. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J. Chromatogr. A 2002, 976, 265–275. [Google Scholar] [CrossRef]
- Terry, I.; Walter, G.H.; Moore, C.; Roemer, R.; Hull, C. Odor-mediated push-pull pollination in cycads. Science 2007, 318, 70. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Himanen, S.J.; Yuan, J.S.; Chen, F.; Stewart, C.N. Ecological functions of terpenoids in changing climates. In Natural Products. Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2913–2940. [Google Scholar] [CrossRef]
- Cheng, A.-X.; Lou, Y.-G.; Mao, Y.-B.; Lu, S.; Wang, L.-J.; Chen, X.-Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant. Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kung, T.L.; Chen, Y.J.; Chao, L.K.; Wu, C.S.; Lin, L.Y.; Chen, H.C. Analysis of volatile constituents in Platostoma palustre (Blume) using headspace solid-phase microextraction and simultaneous distillation extraction. Foods 2019, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Orjala, J.; Mian, P.; Rali, T.; Sticher, O. Gibbilimbols A−D, cytotoxic and antibacterial alkenylphenols from Piper gibbilimbum. J. Nat. Prod. 1998, 61, 939–941. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Mesquita, J.T.; Tempone, A.G.; Lago, J.H.G.; Guimarães, E.F.; Kato, M.J. Leishmanicidal activity of an alkenylphenol from Piper malacophyllum is related to plasma membrane disruption. Exp. Parasit. 2012, 132, 383–387. [Google Scholar] [CrossRef]
- Teixeira, S.D. Estudo Fitoquímico De Piper gaudichaudianum e Sua Interação Com Morcegos Frugívoros. Ph.D. Thesis, Universidade Federal Do Paraná, Curitiba, Brazil, 2003. [Google Scholar]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Celotti, L.; Bianchi, V. Applications of human peripheral blood lymphocytes in genotoxicity and cytotoxicity. Altern. Lab. Anim. 1990, 18, 231–234. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Duarte-Restrepo, E.; Pino-Benítez, N. Evaluación de la actividad repelente de aceites esenciales de plantas Piperáceas del departamento de Chocó, Colombia. Rev. Toxicol. 2015, 32, 112–116. [Google Scholar]
- Xiang, C.-P.; Han, J.-X.; Li, X.-C.; Li, Y.-H.; Zhang, Y.; Chen, L.; Qu, Y.; Hao, C.-Y.; Li, H.-Z.; Yang, C.-R.; et al. Chemical composition and acetylcholinesterase inhibitory activity of essential oils from Piper species. J. Agric. Food Chem. 2017, 65, 3702–3710. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C. Review: Methods use to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Godefroot, M.; Sandra, P.; Verzele, M. New method for quantitative essential oil analysis. J. Chromatogr. A 1981, 203, 325–335. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; González, M.C.; Stashenko, E.E. Volatile fractions and essential oils of the leaves and branches of Dalea carthagenensis (Jacq.) J.F. Macbr. from northern region of Colombia. J. Essent. Oil Bear. Plants 2019, 22, 774–788. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; Aristizabal-Córdoba, S.; Rodríguez, J.D.; Torres, E.A.; Molina, A.M.; Gutiérrez, R.G.; Kouznetsov, V.V. Citotoxicidad/capacidad antiradicalaria in-vitro y caracterización estructural por GC-MS/1H-13C-RMN de los aceites esenciales de hojas de árboles joven/adulto de Annona purpurea Moc. & Sessé ex Dunal de Repelón (Atlántico, Colombia). Bol. Latinoam. Caribe Plantas Med. Aromát. 2016, 15, 99–111. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; 804p. [Google Scholar]
- Joulian, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpenes Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998; 658p. [Google Scholar]
- NIST Chemistry WebBook. National Institute of Standards and Technology. Available online: http://webbook.nist.gov/chemistry/ (accessed on 1 November 2022).
- Muñoz-Acevedo, A.; González, M.C.; Rodríguez, J.D.; De Moya, Y.S. New chemovariety of Lippia alba from Colombia: Compositional analysis of the volatile secondary metabolites and some in vitro biological activities of the essential oil from plant leaves. Nat. Prod. Commun. 2019, 3, 563–566. [Google Scholar] [CrossRef]
- Tapondjou, A.L.; Adler, C.; Fontem, D.A.; Bouda, H.; Reichmuth, C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 2005, 41, 91–102. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Muñoz-Acevedo, A.; Vargas Méndez, L.Y.; Stashenko, E.E.; Kouznetsov, V.V. Improved Trolox® equivalent antioxidant capacity assay for efficient and fast search of new antioxidant agents. Anal. Chem. Lett. 2011, 1, 86–102. [Google Scholar] [CrossRef]
- Felter, S.P.; Zhang, X.; Thompson, C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharm. 2021, 121, 104887. [Google Scholar] [CrossRef]
| No. Peak | Compounds | Retention Index | Relative Amounts, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HS-SPME | SDE | EO | EA-E | ||||||||
| Calc. | Lit. | INF | L | INF | FR | L | L | INF | L | ||
| 1 | α-Pinene | 933 | 930 | 10.3 | 1.9 | 13.6 | 4.6 | 6.4 | ---- | 1.6 | ---- |
| 2 | β-Pinene | 969 | 970 | 19.2 | 2.4 | 23.3 | 10.0 | 8.9 | tr | 3.6 | tr |
| 3 | Myrcene | 984 | 981 | 19.6 | tr | 31.0 | 9.3 | 6.8 | tr | 1.9 | ---- |
| 4 | δ-3-Carene | 1004 | 1005 | 3.5 | 0.6 | ---- | 1.2 | 2.0 | tr | tr | ---- |
| 5 | α-Tolualdehyde | 1006 | 1011 | ---- | ---- | 1.2 | tr | tr | ---- | ---- | ---- |
| 6 | p-Cymene | 1010 | 1011 | tr | ---- | 0.5 | tr | tr | ---- | tr | ---- |
| 7 | β-Phellandrene | 1017 | 1023 | 0.5 | ---- | 1.5 | tr | 0.4 | ---- | ---- | |
| 8 | Limonene | 1019 | 1020 | 1.5 | tr | 3.5 | 0.9 | 1.1 | 0.1 | 0.6 | ---- |
| 9 | (Z)-β-Ocimene | 1028 | 1032 | 7.8 | ---- | 0.7 | 2.6 | 3.9 | 2.0 | 0.9 | tr |
| 10 | (E)-β-Ocimene | 1038 | 1036 | 1.4 | ---- | ---- | 0.4 | 0.5 | 0.5 | tr | tr |
| 11 | (E)-Hex-2-enoic acid | 1040 | 1042 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 4.8 |
| 12 | α-Copaene | 1368 | 1376 | 3.3 | 4.2 | 2.6 | 1.0 | 0.4 | 0.4 | 1.3 | 3.5 |
| 13 | β-Caryophyllene | 1407 | 1421 | 22.8 | 42.6 | 6.5 | 5.7 | 10.6 | 8.6 | 7.0 | 19.4 |
| 14 | β-Copaene | 1415 | 1437 | 0.8 | 1.4 | ---- | tr | tr | tr | tr | tr |
| 15 | Aromadendrene | 1429 | 1439 | ---- | 1.1 | ---- | tr | tr | tr | tr | |
| 16 | α-Humulene | 1439 | 1454 | 1.5 | 5.0 | 0.5 | 0.4 | 1.2 | 1.1 | 0.5 | 1.5 |
| 17 | γ-Muurolene | 1462 | 1471 | 0.4 | 1.2 | ---- | tr | tr | tr | tr | tr |
| 18 | Selina-4,11-diene | 1468 | 1475 | tr | 1.2 | ---- | tr | tr | tr | ---- | ---- |
| 19 | β-Selinene | 1470 | 1483 | 1.2 | 20.2 | 1.0 | 0.4 | 3.5 | 3.4 | 0.6 | 9.8 |
| 20 | α-Selinene | 1481 | 1491 | 0.8 | 5.2 | ---- | tr | 0.8 | 0.9 | tr | 1.5 |
| 21 | α-Muurolene | 1485 | 1494 | 0.7 | 2.2 | ---- | tr | tr | tr | 0.4 | 2.3 |
| 22 | (E),(E)-α-Farnesene | 1492 | 1498 | 0.6 | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- |
| 23 | γ-Cadinene | 1495 | 1507 | ---- | 0.6 | ---- | ---- | ---- | ---- | ---- | ---- |
| 24 | trans-Calamenene | 1498 | 1502 | tr | 1.2 | ---- | ---- | tr | tr | ---- | ---- |
| 25 | 7-epi-α-Selinene | 1501 | 1511 | tr | 4.3 | ---- | tr | 0.8 | 0.9 | tr | 2.5 |
| 26 | (E)-Nerolidol | 1539 | 1549 | ---- | ---- | ---- | tr | 0.5 | 1.0 | ---- | tr |
| 27 | Caryophyllene oxide | 1551 | 1558 | ---- | 1.8 | ---- | tr | 0.6 | 0.9 | ---- | 2.9 |
| 28 | Dillapiole | 1580 | 1589 | ---- | ---- | ---- | ---- | ---- | 2.2 | ---- | tr |
| 29 | τ-Cadinol | 1622 | 1628 | ---- | ---- | ---- | ---- | tr | 0.5 | ---- | ---- |
| 30 | Ylangenol * | 1625 | 1666 | ---- | ---- | ---- | tr | 0.4 | 1.1 | ---- | ---- |
| 31 | Unidentified compound (M+• 168.07, BP 124.06) | 1679 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 8.7 |
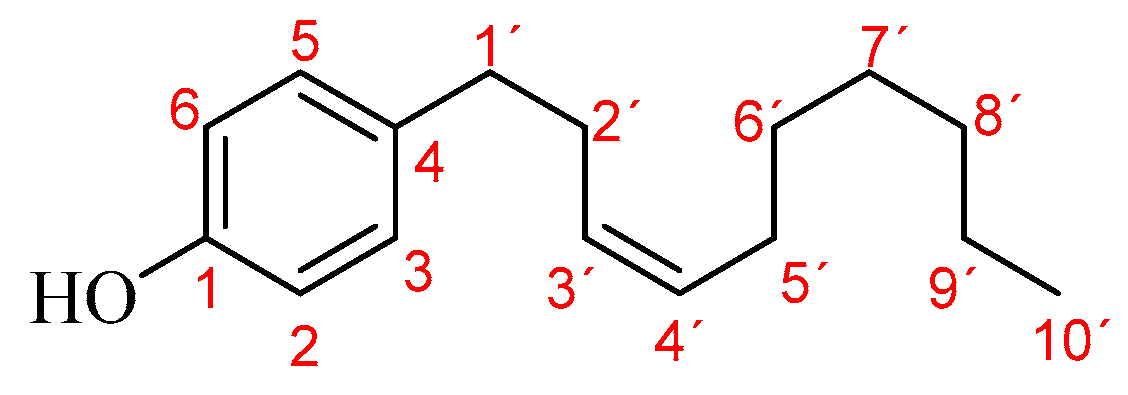
| 32 | Gibbilimbol B | 1915 | 1997 | 0.9 | ---- | 14.2 | 60.1 | 45.5 | 71.7 | 70.0 | 10.3 |
| 33 | Palmitic acid | 1938 | 1970 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 2.9 |
| 34 | Ethyl palmitate | 1945 | 1978 | ---- | 1.1 | ---- | ---- | ---- | ---- | 0.4 | 7.7 |
| 35 | Stearyl alcohol | 1982 | 2066 | ---- | ---- | ---- | ---- | ---- | 0.5 | ---- | ---- |
| 36 | Phytol | 1991 | 2102 | ---- | ---- | ---- | ---- | ---- | tr | tr | 11.3 |
| 37 | Alkenylphenol (M+• 260.21, BP 107.07) | 1996 | ---- | ---- | ---- | ---- | tr | ---- | tr | 2.4 | tr |
| 38 | Eriopodol A * (M+• 248.18, BP 123.05) | 2015 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 1.2 | ---- |
| 39 | Ethyl linoleate | 2035 | 2139 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 2.8 |
| 40 | Ethyl linolenate | 2040 | 2145 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 3.9 |
| 41 | Ethyl oleate | 2045 | 2150 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 1.7 |
| 42 | Ethyl stearate | 2070 | 2175 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 2.5 |
| Total relative amount, % | 96.8 | 98.2 | 100 | 96.6 | 94.3 | 96.0 | 92.8 | 99.9 | |||
| † Cytotoxicity, µg/mL * | ||||
| HC50 | LC50 | |||
| Erythrocytes | Lymphocytes | Hep-2 line | ||
| Positive controls | 100 ± 0% (1,000 µg/mL) | 99 ± 1% (7.5 µg/mL) | 96.0 ± 0.7% (0.1/1 µg/mL) | |
| EO | 115 ± 3 | 71 ± 4 | 33 ± 2 | |
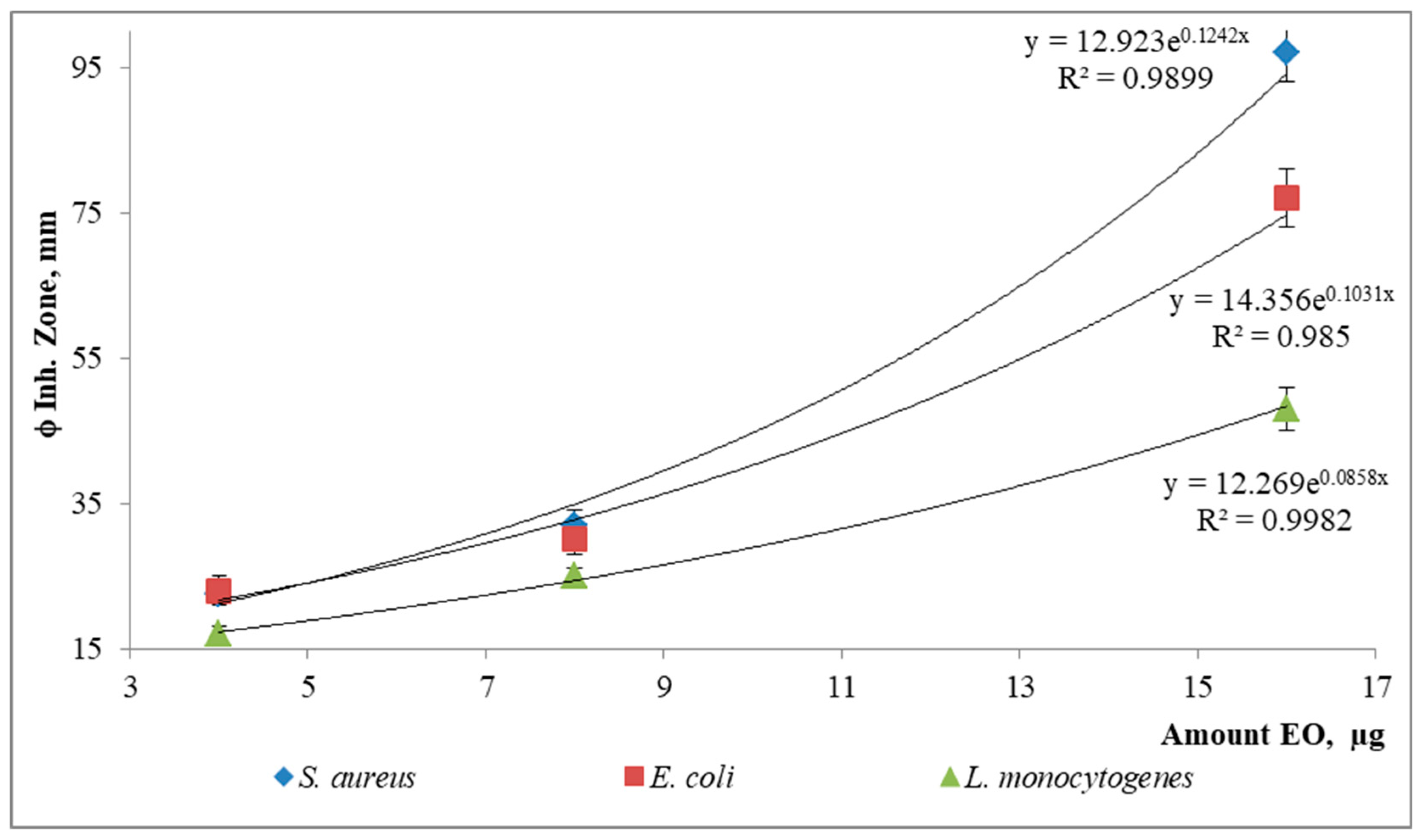
| ‡Antibacterial susceptibility-ϕ inhibition zone, mm * | ||||
| S. aureus | E. coli | L. monocytogenes | ||
| Positive control | 18.2 ± 0.2 (30 µg) | 18.16 ± 0.01 (4 µg) | 16.55 ± 0.07 (8 µg) | |
| EO | 16 µg | 97 ± 4 | 77 ± 4 | 48 ± 3 |
| 8 µg | 32 ± 2 | 30 ± 2 | 25 ± 1 | |
| 4 µg | 22.5 ± 0.4 | 23 ± 2 | 17 ± 1 | |
| Insecticidal/Repellency capacity | ||||
| † AChE, µg/mL * | ‡ Repellency, % *-1 µg/cm2 | |||
| IC50 | 2 h | 4 h | ||
| Positive controls | 0.59 ± 0.02 | 58 ± 5 | 58 ± 5 | |
| EO | 13 ± 1 | 20 ± 0 | 37 ± 6 | |
| ¥ABTS+• radical-cation reactivity—TAA, mmol Trolox®/kg | ||||
| BHA | 2157 ± 63 | EO | 2249 ± 130 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Acevedo, A.; González, M.C.; De Moya, Y.S.; Rodríguez, J.D. Volatile Metabolites of Piper eriopodon (Miq.) C.DC. from Northern Region of Colombia and Assessment of In Vitro Bioactivities of the Leaf Essential Oil. Molecules 2023, 28, 2594. https://doi.org/10.3390/molecules28062594
Muñoz-Acevedo A, González MC, De Moya YS, Rodríguez JD. Volatile Metabolites of Piper eriopodon (Miq.) C.DC. from Northern Region of Colombia and Assessment of In Vitro Bioactivities of the Leaf Essential Oil. Molecules. 2023; 28(6):2594. https://doi.org/10.3390/molecules28062594
Chicago/Turabian StyleMuñoz-Acevedo, Amner, María C. González, Yurina Sh. De Moya, and Juan D. Rodríguez. 2023. "Volatile Metabolites of Piper eriopodon (Miq.) C.DC. from Northern Region of Colombia and Assessment of In Vitro Bioactivities of the Leaf Essential Oil" Molecules 28, no. 6: 2594. https://doi.org/10.3390/molecules28062594
APA StyleMuñoz-Acevedo, A., González, M. C., De Moya, Y. S., & Rodríguez, J. D. (2023). Volatile Metabolites of Piper eriopodon (Miq.) C.DC. from Northern Region of Colombia and Assessment of In Vitro Bioactivities of the Leaf Essential Oil. Molecules, 28(6), 2594. https://doi.org/10.3390/molecules28062594






