Comprehensive Analysis of Secondary Metabolites of Four Medicinal Thyme Species Used in Folk Medicine and Their Antioxidant Activities In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Polyphenol and Flavonoid Content
2.2. Antioxidant Activity
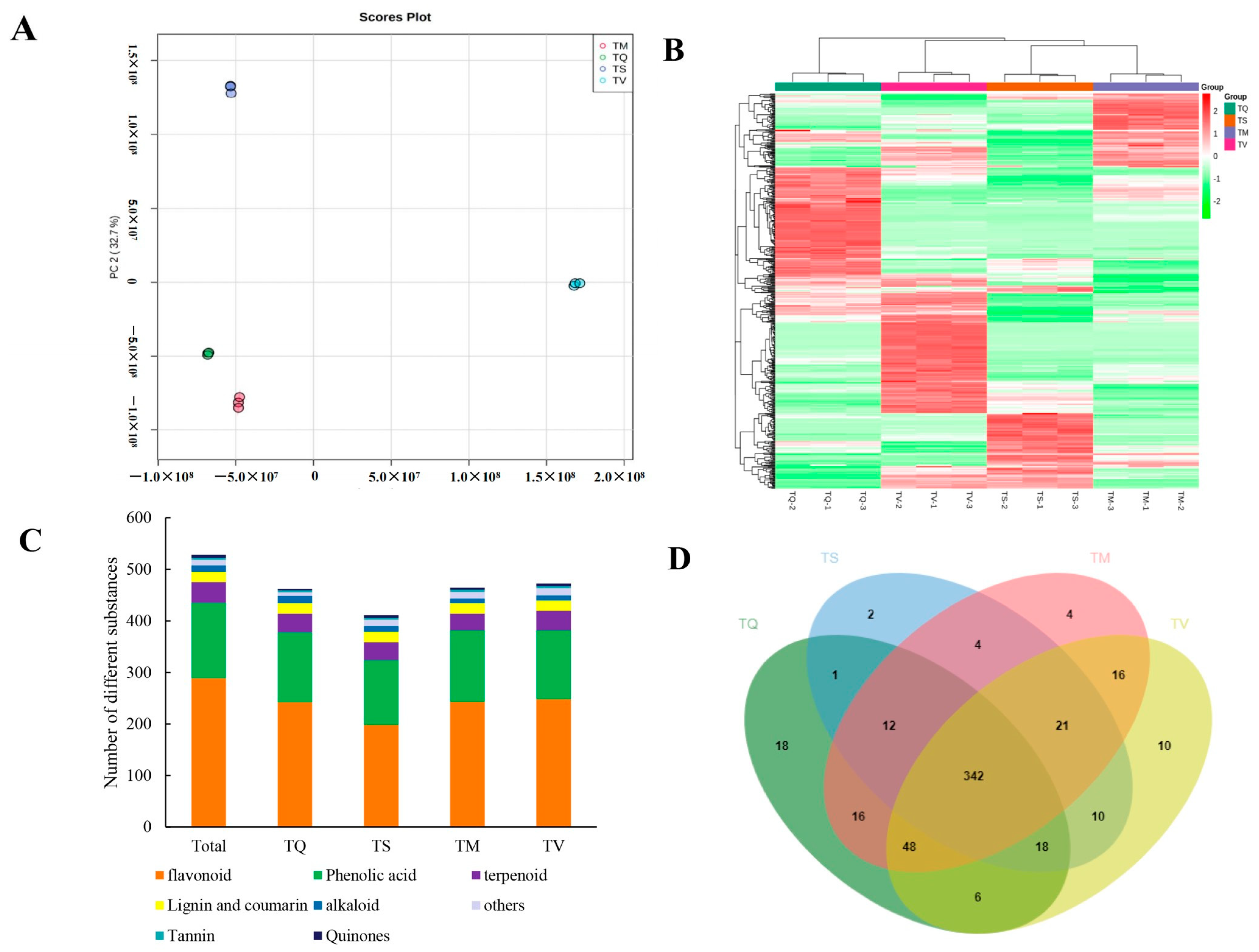
2.3. Profiling of Secondary Metabolites
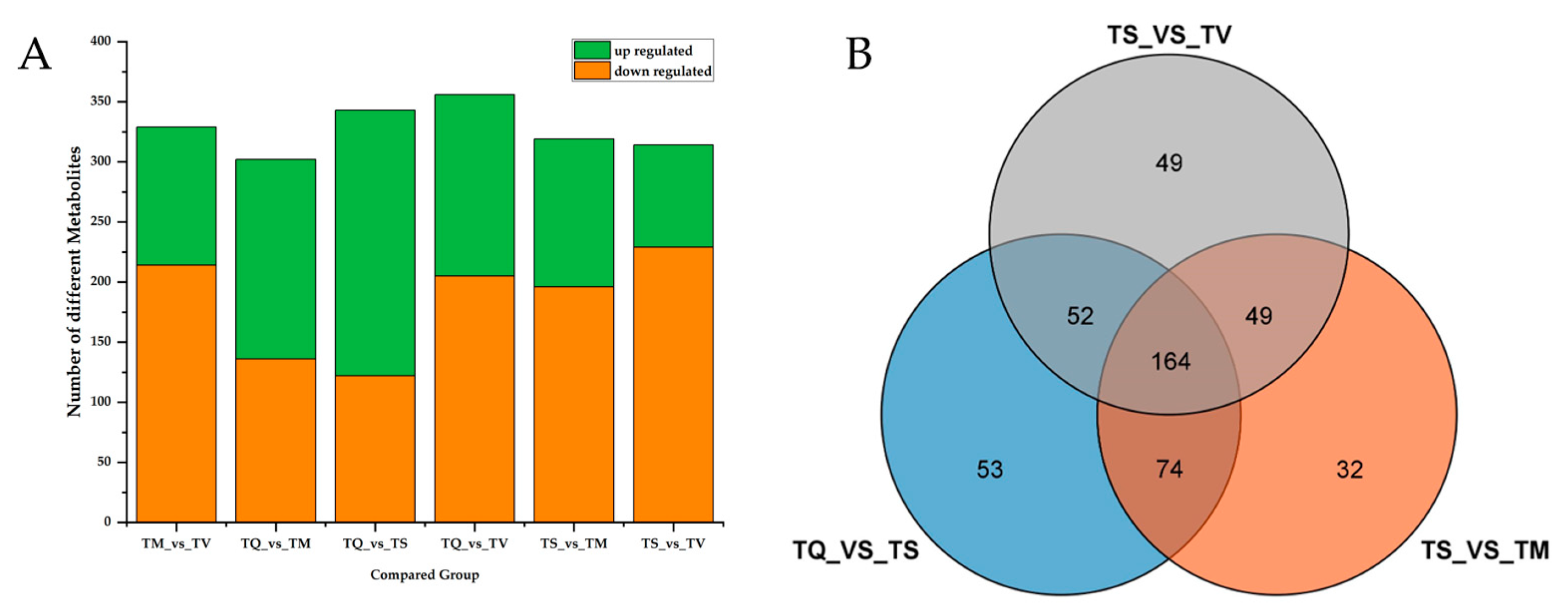
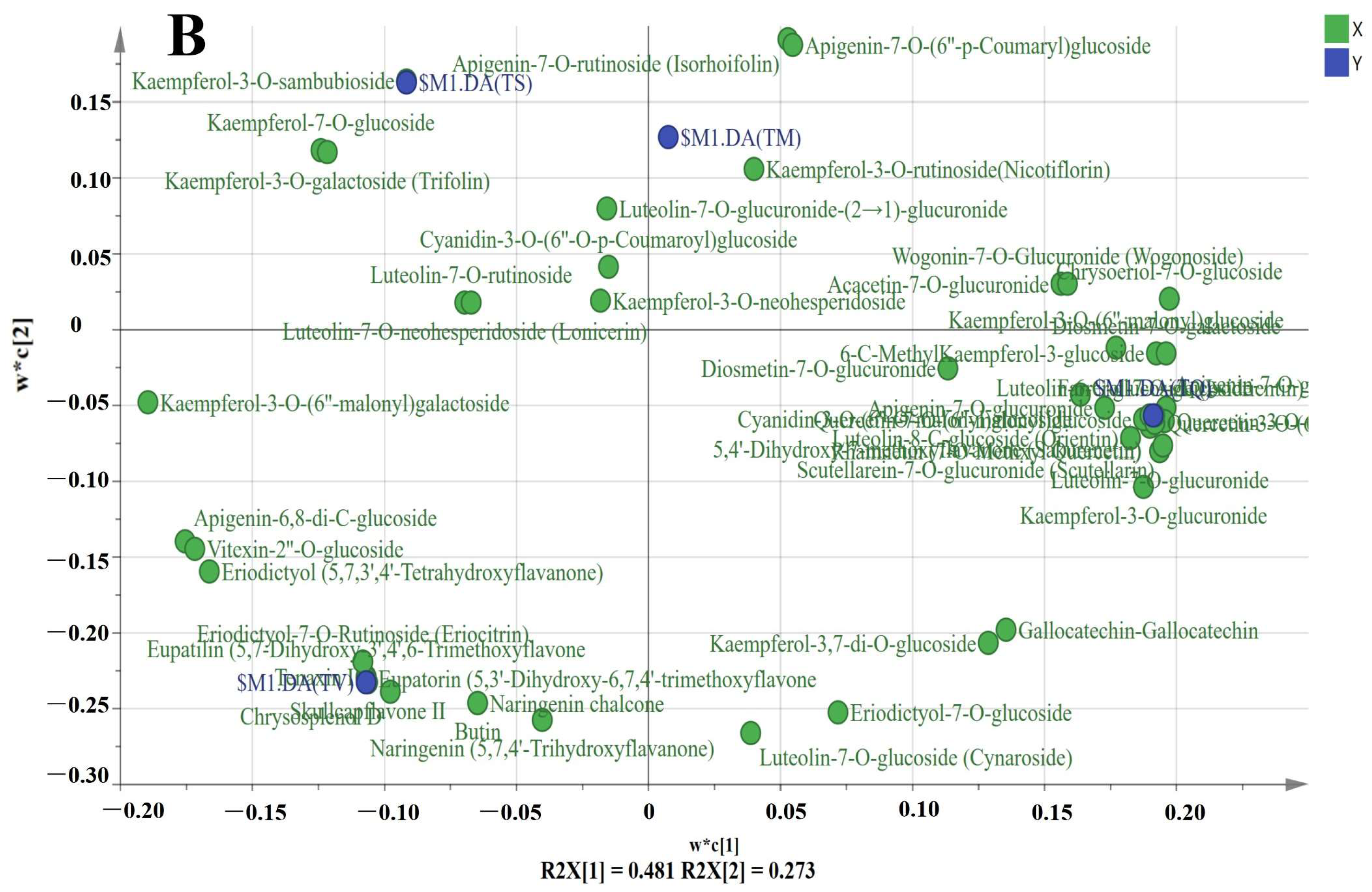
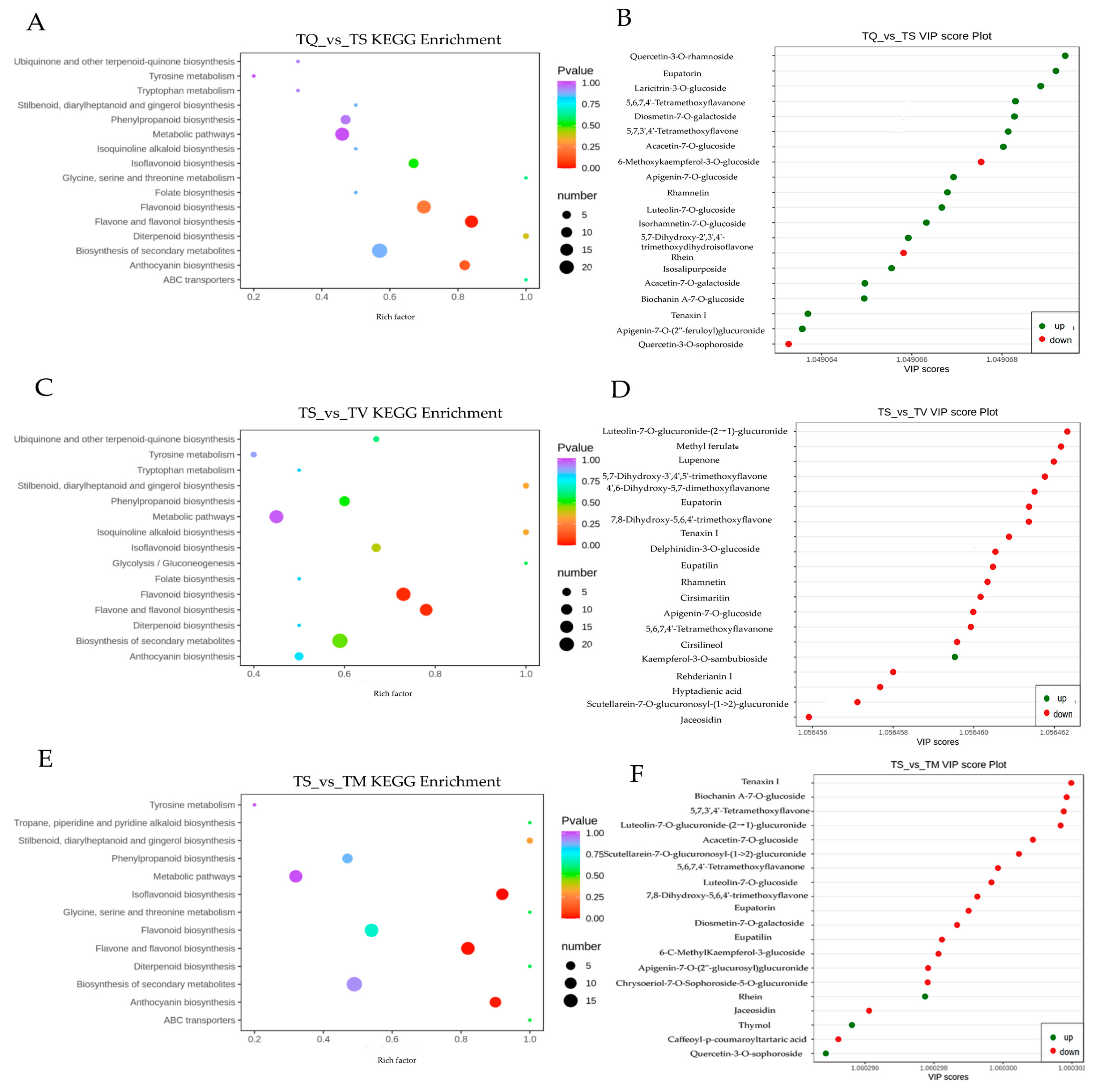
2.4. Difference Analysis of Secondary Metabolites
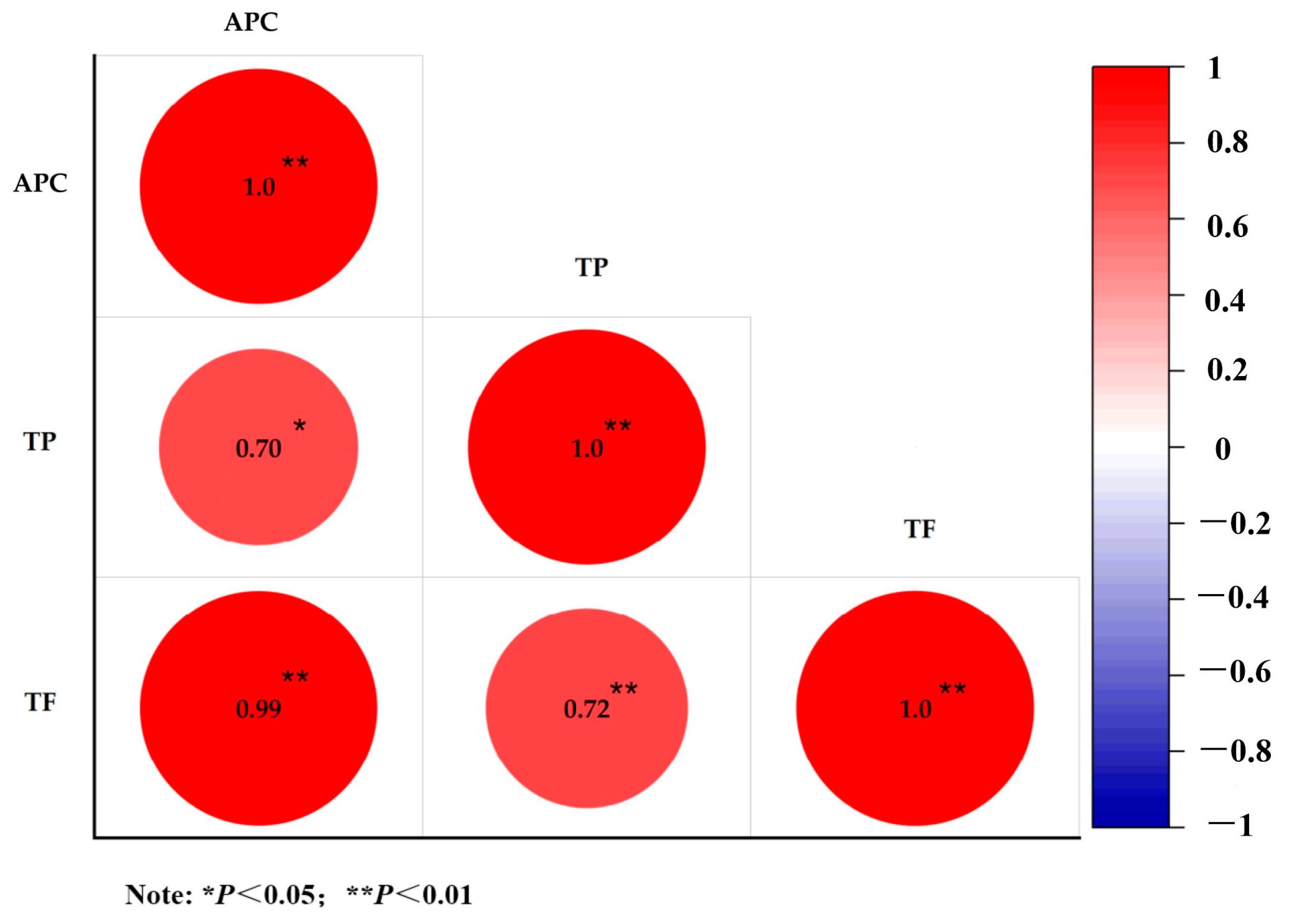
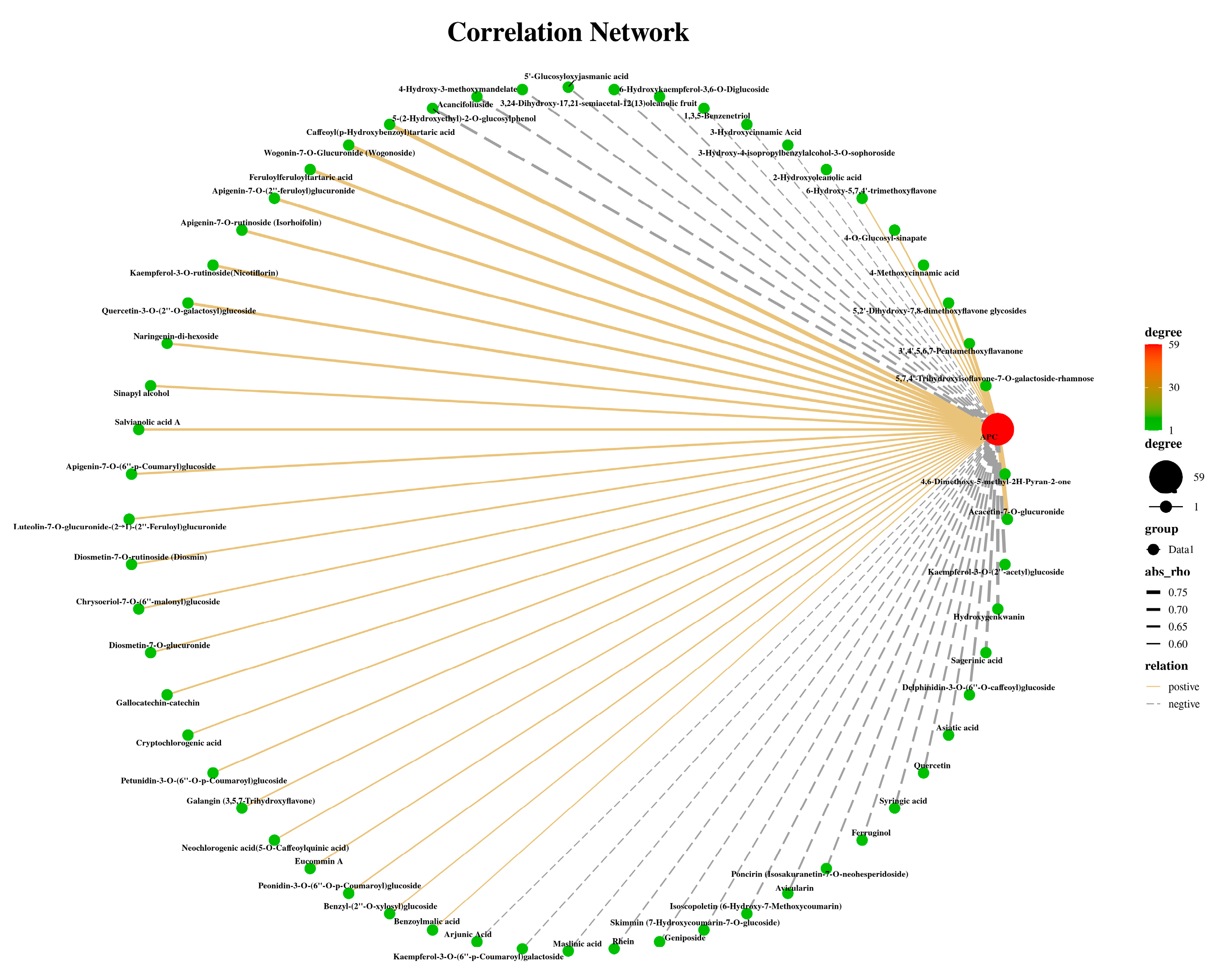
2.5. Correlation Analysis of Secondary Metabolites and Antioxidant Capacity
3. Materials and Methods
3.1. Plant Materials
3.2. Sample Preparation
3.3. Determination of Total Polyphenols and Total Flavonoids
3.4. Antioxidant Activities
3.5. UPLC-ESI-Q TRAP-MS/MS
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. Plants—Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Yaagoubi, M.E.; Mechqoq, H.; Hamdaoui, A.E.; Mukku, V.J.; Mousadik, A.E.; Msanda, F.; Aouad, N.E. A review on Moroccan Thymus species: Traditional uses, essential oils chemical composition and biological effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef] [PubMed]
- Nikoli, M.; Glamoclija, J.; Ferreira, I.; Calhelha, R.C.; Fernandes, A.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Massimo, N.; Oliviero, M.; Beatrice, M.M.; Giovanna, N.; Romilde, I.; Consuelo, A.; Giorgio, S.; Filippo, M. Thyme extract increases mucociliary-beating frequency in primary cell lines from chronic obstructive pulmonary disease patients. Biomed. Pharmacother. 2018, 105, 1248. [Google Scholar]
- Kim, Y.-S.; Lee, S.-J.; Hwang, J.-W.; Kim, E.-K.; Kim, S.-E.; Kim, E.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. In vitro protective effects of Thymus quinquecostatus Celak extracts on t-BHP-induced cell damage through antioxidant activity. Food Chem. Toxicol 2021, 91, 153673. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2016, 387, 95–105. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: Ros as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radic. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Fota, M.; Tatar, B.; Kroniak, M. The Comprehensive toxicological assessment of total chromium impurities in traditional herbal medicinal product with Thymi herba (Thymus vulgaris L. and Thymus zygis L. Available in pharmacies in poland: Short communication. Insect Biochem. Mol. 2021, 200, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Jaric, S.; Mitrovic, M.; Pavlovic, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Integr. Complement. Med. 2015, 2015, 101978. [Google Scholar]
- Fan, S.S.; Liu, X.Y.; Wang, Y.; Ren, X.Y.; Liu, Y.; Dong, Y.; Fan, Q.Q.; Wei, J.M.; Yu, A.; Song, R.; et al. Thymus quinquecostatus Celak. ameliorates cerebral ischemia-reperfusion injury via dual antioxidant actions: Activating Keap1/Nrf2/HO-1 signaling pathway and directly scavenging ROS. Phytomedicine 2021, 91, 153673. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.L.; Ji, Q.L.; Xing, S.L.; Zhang, P.H.; Zhu, G.L.; Wang, X.H. Chemical composition and antioxidant, antimicrobial activities of the essential oils of Thymus Marschallianus Will. and Thymus Proximus Serg. J. Food Sci 2010, 75, E59–E65. [Google Scholar] [CrossRef]
- Chung, M.Y.; López-Pujol, J.; Chung, J.M.; Kim, K.J.; Park, S.J.; Chung, M.G. Polyploidy in Lilium lancifolium: Evidence of autotriploidy and no niche divergence between diploid and triploid cytotypes in their native ranges. Flora 2015, 213, 57–68. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Sun, M.Y.; Zhang, Y.N.; Zhu, L.; Liu, N.; Bai, H.T.; Sun, G.F.; Zhang, J.Z.; Shi, L. Chromosome-level assembly and analysis of the Thymus genome provide insights into glandular secretory trichome formation and monoterpenoid biosynthesis in thyme. Plant Commun. 2022, 3, 100431. [Google Scholar] [CrossRef]
- Sun, M.Y.; Zhang, Y.N.; Bai, H.T.; Sun, G.F.; Zhang, J.Z.; Shi, L. Population diversity analyses provide insights into key horticultural traits of Chinese native thymes. Hortic. Res. 2022, 10, uhac262. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, B.; He, B.; Zhang, C.; Chen, L.; Wang, Z.; Chen, Y.; Abliz, Z. Multidimensional molecular differences between artificial cultivated and wild Artemisia rupestris L. based on metabolomics-transcriptomics integration strategy. Ind. Crops Prod. 2021, 170, 113732. [Google Scholar] [CrossRef]
- Shyur, L.; Yang, N. Metabolorn cs for phytomedicine research and drug development. Curr Opin Chem Biol. 2008, 12, 66–71. [Google Scholar] [CrossRef]
- Al-Shahrani, M.H.; Mahfoud, M.; Anvarbatcha, R.; Athar, M.T.; Al-Asmari, A. Evaluation of antifungal activity and cytotoxicity of Thymus vulgaris essential oil. Pharmacogn. Commun. 2017, 7, 34–40. [Google Scholar] [CrossRef]
- Berdowska, I.; Zieliński, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, O.; Fu, H.Y.; Ouyang, L.Q.; Gong, X.D.; Meng, P.; Wang, Z.; Dai, M.; Guo, X.M.; Wang, Y. UPLC–Q-TOF/MS-based untargeted metabolomics coupled with chemometrics approach for Tieguanyin tea with seasonal and year variations. Food Sci. Technol. 2019, 283, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Liu, Y.J.; He, G.R.; Cao, Y.W.; Bi, M.M.; Song, M.; Yang, P.P.; Xu, L.F.; Ming, J. Comprehensive analysis of secondary metabolites in the extracts from different lily bulbs and their antioxidant ability. Antioxidants 2021, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Vanlalneihi, B.; Saha, P.; Kalia, P.; Singh, S.; Saha, N.D.; Kundu, A.; Singh, N.; Bhowmik, A. Genetic and principal component analysis for agro-morphological traits, bioactive compounds, antioxidant activity variation in breeding lines of early indian cauliflower and their suitability for breeding. J. Hortic. Sci. Biotechnol. 2020, 95, 93–105. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. and Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, L. Evaluation of the antioxidant potential of mixed fruit-based beverages: A new insight on the Folin-Ciocalteu method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2021, 19, 671. [Google Scholar] [CrossRef]
- Kaplan, L. What is the Origin of the Common Bean? Econ. Bot. 1981, 35, 240–254. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, L.; Liu, B.; Wang, Z.; Jin, Z.; Sun, H. The Genetic Diversity of Cultivated Soybean Grown in China. Appl. Genet. 2004, 108, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Pongsuwan, W.; Fukusaki, E.; Bamba, T.; Yonetani, T.; Yamahara, T.; Kobayashi, A. Prediction of Japanese Green Tea Ranking by Gas chromatography/mass Spectrometry-Based Hydrophilic Metabolite Fingerprinting. J. Agric. Food Chem. 2007, 55, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ertas, A.; Boga, M.; Yilmaz, M.A.; Yesil, Y.; Tel, G.; Temel, H.; Hasimi, N.; Gazioglu, I.; Ozturk, M.; Ugurlu, P. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): Rosmarinic acid. Ind. Crops Prod. 2015, 67, 336–345. [Google Scholar] [CrossRef]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Ciocarlan, N.; Spinu, M. Phytochemical profile and antimicrobial potential of extracts obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Maggi, F.; Bendif, H.; Miara, M.D.; Cinque, B.; Lizzi, A.R.; Brisdelli, F.; Celenza, G. Thymus lanceolatus ethanolic extract protects human cells from t-BHP induced oxidative damage. Food Funct. 2018, 9, 3665–3672. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.P.; Kang, J.M.; Guo, X.Q.; Yang, R.; Chen, Y.L.; Li, J.Y.; Shi, L. Comparison of nutritional compositions and essential oil profiles of different parts of a dill and two fennel cultivars. Foods 2021, 10, 1784. [Google Scholar] [CrossRef]
- Liu, M.; Lv, Q.; Xu, J.; Liu, B.; Zhou, Y.; Zhang, S.; Shen, X.; Wang, L. Isoflavone glucoside genistin, an inhibitor targeting Sortase A and Listeriolysin O, attenuates the virulence of Listeria monocytogenes in vivo and in vitro. Biochem. Pharmacol. 2023, 209, 115447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Wang, J.H.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Wang, J.H.; Zhang, Z. Salvianolic acid A protects the kidney against oxidative stress by activating the Akt/GSK-3β/Nrf2 signaling pathway and inhibiting the NF-κB signaling pathway in 5/6 nephrectomized rats. Oxid. Med. Cell Longev. 2019, 2853534. [Google Scholar] [CrossRef]
- Wu, Y.; Pegan, S.D.; Crich, D.; Desrochers, E.; Starling, E.B.; Hansen, M.C.; Booth, C.; Nicole Mullininx, L.; Lou, L.; Chang, K.Y.; et al. Polyphenols as alternative treatments of COVID-19. Comput. Struct. Biotechnol. 2021, 19, 5371–5380. [Google Scholar] [CrossRef]
- Martinez, S.; Fuentes, C.; Carballo, J. Antioxidant activity, total phenolic content and total flavonoid content in sweet chestnut (Castanea sativa Mill.) cultivars grown in northwest spain under different environmental conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Al-Bataineh, N.; Al-Balawi, S.S.; Lahham, J.N.; Al-Momani, I.; Al-Sheraideh, M.S.; Mayyas, A.S.; Orabi, S.T.; Al-Qudah, M.A. LC-MS/MS screening, total phenolic, flavonoid and antioxidant contents of crude extracts from three asclepiadaceae species growing in Jordan. Molecules 2022, 27, 859. [Google Scholar] [CrossRef] [PubMed]
- Ickovski, J.D.; Arsic, B.B.; Mitic, M.N.; Stojkovic, M.B.; Dordevic, M.M.; Stojanovic, G.S. Chemometric approach to the composition of flavonoid compounds and phenolic ccids and antioxidant potential of artemisia species from different habitats. Insect. Biochem. Mol. 2022, 19, e202200365. [Google Scholar]
- Li, G.F.; Yan, N.; Li, G.Q. The effect of in vitro gastrointestinal digestion on the antioxidants, antioxidant activity, and hypolipidemic activity of green jujube vinegar. Foods 2022, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Fortuna, T. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009, 113, 568–574. [Google Scholar] [CrossRef]
- Huang, S.A.; Fu, Y.A.; Xu, B.A.; Liu, C.A.; Wang, Q.A.; Luo, S.A. Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the mlck/pmlc2 pathway. Phytomedicine 2020, 68, 153179. [Google Scholar] [CrossRef]
- Benjamin, E.U.; Chikako, H.; Hideo, B.; Satoru, K.; Masato, W.; Shozo, K.; Takaya, M. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of uv-b and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Seeram, N.P.; Nair, M.G. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J. Agric. Food Chem. 2002, 50, 5308–5312. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; van Buren, L.; Wagner, E.; Wiseman, S.; van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Ielciu, I.; Frederich, M.; Hanganu, D.; Angenot, L.; Olah, N.K.; Ledoux, A.; Crisan, G.; Paltinean, R. Flavonoid analysis and antioxidant activities of the Bryonia Alba L. aerial parts. Antioxidants 2019, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Du, Y.M.; Yu, X.T.; Shi, J.; Yuan, X.L.; Liu, X.M.; Liu, Y.H.; Zhang, H.B.; Zhang, Z.F.; Yan, N. Dynamics of antioxidant activities, metabolites, phenolic acids, flavonoids, and phenolic biosynthetic genes in germinating chinese wild rice (Zizania Latifolia). Food Chem. 2020, 318, 126483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.L.; Liu, E.H. Chemical and biological comparison of the fruit extracts of Citrus Wilsonii Tanaka and Citrus Medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]


| Species | TP (mg GAE/g DW) | TF (mg RU/g DW) |
|---|---|---|
| T. serpyllum | 49.80 ± 0.10 b | 182.0 ± 0.24 d |
| T. vulgaris | 64.4 ± 2.10 a | 217.4 ± 1.18 c |
| T. quinquecostatus | 64.78 ± 0.20 a | 225.3 ± 1.20 b |
| T. marschallianus | 64.50 ± 0.31 a | 279.0 ± 0.56 a |
| p-value | p < 0.01 | p < 0.01 |
| Species | DPPH (% Inhibition) | FRAP (μmol TE/g DW) | ABTS (mmol TE/g DW) | APC (% Inhibition) |
|---|---|---|---|---|
| T. serpyllum | 76.3 ± 0.87 b | 152.33 ± 1.63 d | 1.41 ± 0.21 d | 87.6.0 ± 6.67 b |
| T. vulgaris | 74.2 ± 0.22 dc | 165.00 ± 1.74 b | 1.50 ± 0.03 c | 90.8 ± 2.54 b |
| T. quinquecostatus | 74.8 ± 0.32 c | 160.67 ± 1.32 c | 1.62 ± 0.16 a | 92.8 ± 7.20 b |
| T. marschallianus | 80.7 ± 0.65 a | 187.67 ± 1.65 a | 1.54 ± 0.02 b | 98.4 ± 2.83 a |
| p value | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Dong, Y.; Gao, F.; Li, J.; Stevanovic, Z.D.; Li, H.; Shi, L. Comprehensive Analysis of Secondary Metabolites of Four Medicinal Thyme Species Used in Folk Medicine and Their Antioxidant Activities In Vitro. Molecules 2023, 28, 2582. https://doi.org/10.3390/molecules28062582
Yang R, Dong Y, Gao F, Li J, Stevanovic ZD, Li H, Shi L. Comprehensive Analysis of Secondary Metabolites of Four Medicinal Thyme Species Used in Folk Medicine and Their Antioxidant Activities In Vitro. Molecules. 2023; 28(6):2582. https://doi.org/10.3390/molecules28062582
Chicago/Turabian StyleYang, Rui, Yanmei Dong, Fei Gao, Jingyi Li, Zora Dajic Stevanovic, Hui Li, and Lei Shi. 2023. "Comprehensive Analysis of Secondary Metabolites of Four Medicinal Thyme Species Used in Folk Medicine and Their Antioxidant Activities In Vitro" Molecules 28, no. 6: 2582. https://doi.org/10.3390/molecules28062582
APA StyleYang, R., Dong, Y., Gao, F., Li, J., Stevanovic, Z. D., Li, H., & Shi, L. (2023). Comprehensive Analysis of Secondary Metabolites of Four Medicinal Thyme Species Used in Folk Medicine and Their Antioxidant Activities In Vitro. Molecules, 28(6), 2582. https://doi.org/10.3390/molecules28062582




