1. Introduction
Thrombotic diseases seriously endanger the health and life of patients. It is believed that the best way to improve the survival rate and reduce the mortality rate of patients is immediate, early detection, and effective thrombolytic therapy [
1]. In recent years a variety of technical methods have emerged, including drug thrombolytic therapy, interventional thrombolytic therapy, and interventional thrombolysis. However, thrombolytic therapy is still an important, irreplaceable, and fundamental measure for the treatment of thromboembolism [
2]. Thrombolytic agents in clinical use are Plasminogen activators (PAs) to treat thromboembolism, including streptokinase (SK), urokinase (UK), alteplase (RT-PA), tissue plasminogen activator (tPA) and tenepase (TNK-TPA), etc. UK is an effective thrombolytic drug that has a low price and is widely used in primary hospitals [
3]. However, due to the short half-life (2–20 min) [
4], PAs need more doses to be administered. More doses of PAs could cause more serious system hemorrhage because the low thrombus-specificity of Pas activates both fibrin-bound and circulating plasminogen creating a serious risk of hemorrhage. These factors could cause a side effect risk of harmful bleeding complications and lead to the aggravation of the disease. Bleeding complications of Pas bring difficulties and risks to the clinical application of Pas including r-tPA with fibrin specificity [
5,
6]. On the whole, decreasing the bleeding complications of PAs could be very significant and is urgently needed for the clinical application of PAs.
Targeted delivery systems of PAs could target PAs to the site of the thrombus and at the same time reduce the dose of PAs. So, the systematic generation of broad matrix-specific fibrinolytic enzymes associated with bleeding complications could decrease and targeted delivery systems of PAs could be a better way to avoid or resolve dose-induced side effects [
4]. Many targeted delivery systems have been performed to target PAs to the site of the thrombus, release PAs and perform effective thrombolytic treatment. Targeted delivery systems were generally prepared by directly bonding the thrombus-targeted ligands to PAs or the surface of drug carriers, such as liposomes [
7,
8], magnetic targeting delivery systems [
9,
10], microbubbles [
11,
12,
13], polymer nanoparticles [
2,
9,
14,
15,
16] and new inorganic-organic hybrid nanoparticles for deeper or continuous release in recent years [
9,
10]. The polymers in local delivery systems of PAs include PLGA Polymers, polyglutamic acid peptide dendrimer, chitosan derivatives, PEG, etc. [
9,
16,
17,
18]. These thrombus-targeted ligands include fibrin-specific anti-fibrin antibodies [
19,
20], vMF factor-specific alkaline gelatin [
21], p-selectin-specific fucoidan [
2,
4], activated platelets-specific RGD sequence peptide, etc. [
8,
9,
12,
13,
16]. Then, by wrapping or bonding PAs, these targeted delivery systems were constructed into thrombolytic targeting delivery systems. In addition, thrombin-sensitive peptides and pH-sensitive phenyl imine bonds also were used to develop PAs delivery systems and could be ruptured at the thrombus of the stroke to perform targeted thrombolytic therapy [
3,
4,
18,
21].
RGD sequence peptides exist in the α-chain of the fibrinogen and could specifically recognize Glycoprotein (GP) IIb/IIIa receptor on the activated platelet membrane. RGD sequence peptides have drawn much attention from researchers in the diagnosis of thrombosis and targeted thrombolytic therapy [
22]. The cyclic RGD (cRGD) functionalized liposome has been used to carry urokinase to target thrombus in vivo thrombolysis study [
8]. However, intravascular liposomes are limited due to stability problems. The major challenges in the development of liposomal PAs-targeting delivery systems are cost and still ineffective treatment [
7,
8]. In other research, it was shown that thrombolytic therapy by targeted microbubbles containing RGD sequence peptides under ultrasound could destroy the fibrillary network structure of the thrombus [
13] and enhance the dissolution of the thrombus, while targeted nano-bubbles have a higher thrombolytic rate and penetrate deeper into thrombus than targeted micron bubbles [
12]. Additionally, polymer nanoparticles are relatively stable carriers. Targeted nanoparticles, such as mesoporous carbon nanomaterials [
10], poly(lactic-co-glycolic acid) magnetic nanoparticles [
6], and chitosan nanoparticles [
16]. Furthermore, non-bonding complex PAs-targeting delivery systems, such as the PAs complex [
23,
24], were used to target thrombus.
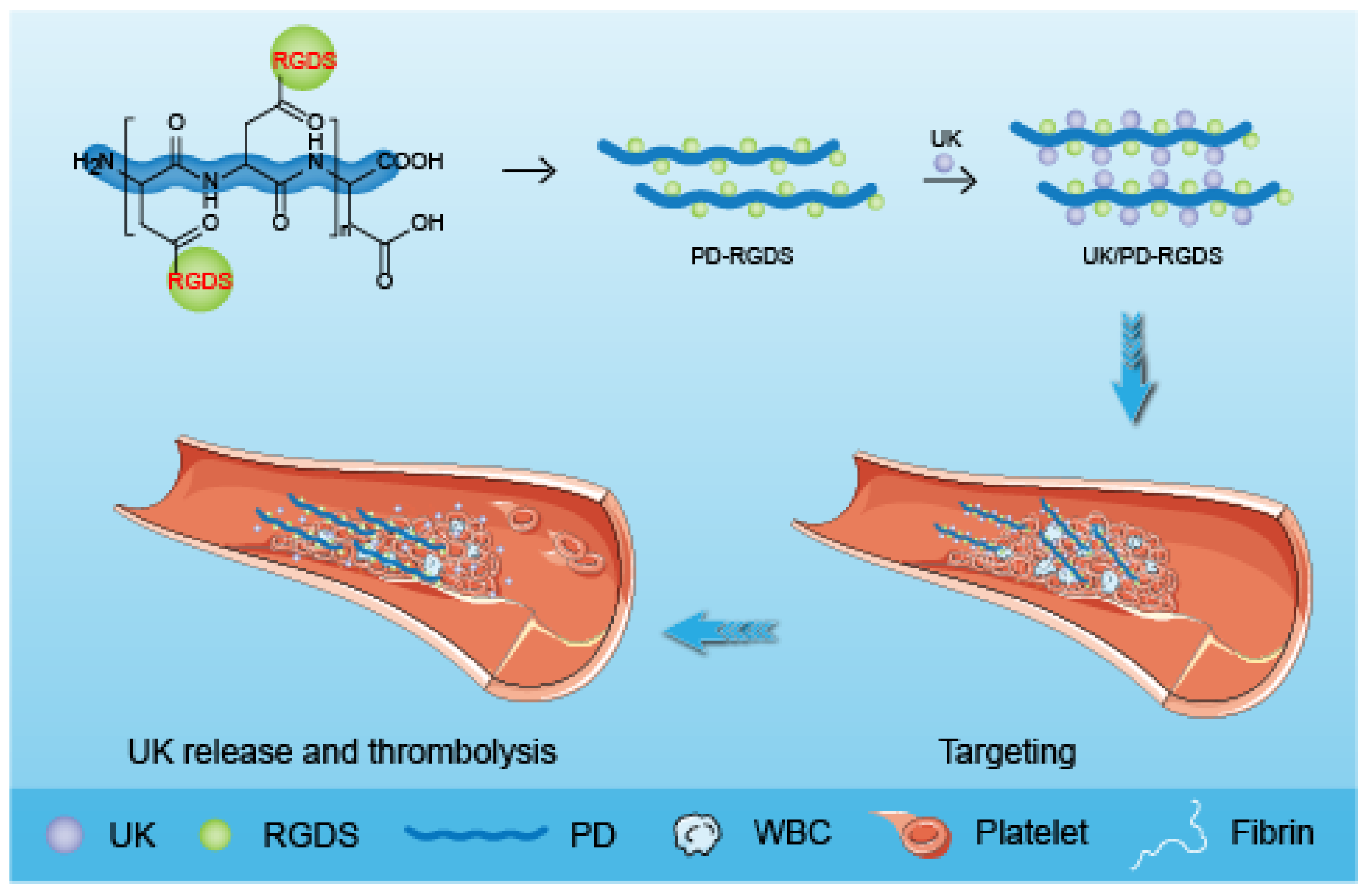
In our previous work, based on the specificity of RGD sequence peptides on activated platelets, multiple RGDS molecules have been bonded to the highly biodegradable poly-
α,
β-
d,
l-aspartic acid (PD) [
25,
26,
27]. PD-RGDS with a high grafting rate of 46% was prepared to have a specific affinity for activating platelets [
26]. In this study, we based on the interaction between proteins and constructed a UK/PD-RGDS complex delivery system (
Scheme 1). We characterized the UK/PD-RGDS complex delivery system by Zeta Sizer and TEM. The thrombolytic potency of the UK/PD-RGDS complex was measured by the bubble-rising method. The in vivo thrombolytic activity, the bleeding complications, and organ distribution of UK/PD-RGDS were evaluated to investigate the thrombolysis efficacy and the side effects via male Wistar rats. Our study provided a foundation for the development of novel delivery systems for thrombolytic therapy.
3. Discussion
Thrombolytic therapy is still the most basic treatment method and the fundamental measure to treat thrombosis. Plasminogen activator is currently an effective thrombolytic drug in clinical practice. Plasminogen activators (PAs) systematically activate plasminogen to become plasmin, then the produced plasmins degrade fibrinogen and fibrin in the clots, and decompose the thrombus. When excessive plasmins are produced, bleeding of different degrees occurs. Because PAs was a group of proteases and would be degraded quickly in the blood, PAs have short half-life periods (2–20 min). To achieve the effect of thrombolytic therapy, large doses of drugs were used to reach the high blood concentration for treatment, which increases the risks of bleeding side effects and non-specific toxicity. Reducing bleeding side effects could improve the safety of the medication and reduce pressure for doctors and the risk of bleeding for patients, which was urgent and meaningful.
A targeted delivery system could target thrombolytic drugs to the thrombus site and release thrombolytic drugs for effective thrombolysis. So, targeted delivery systems could reduce the dose to achieve the treatment effect of thrombolytic therapy. Meanwhile, a targeted delivery system will also reduce the bleeding side effects caused by the increasing dose of PAs, and is the best way to avoid or solve the side effects caused by the dose [
1].
The RGDS sequence peptide exists at 572–575 on the alpha chain of fibrinogen. It can specifically recognize the glycoprotein (GP) IIb/IIIa receptor on activated platelet membranes, which has been widely studied for the diagnosis of thrombosis and targeted thrombolytic therapy in recent years. RGDS can competitively bind activated platelets, thus preventing the fibrin bridging, and has the effect of inhibiting platelet aggregation. Here, PD-RGDS was a safe and biodegradable polyamino acid carrier; the main chain is biodegradable poly-
α,
β-
d,
l-aspartic acid, and the side chain consists of amino-group of poly-
α,
β-
d,
l-aspartic acid bonded with the carboxyl group of RGDS. So PD-RGDS also could specifically recognize the glycoprotein (GP) IIb/IIIa receptor on the activated platelet membrane. Moreover, in this study, the PD-RGDS carried plenty of RGDS motifs (grafting ratio 46%). The Effect of PD-RGDS on GPIIb/IIIa expression results also confirmed that PD-RGDS (10
−5 M) could reduce to one-thousandth of the concentration of RGDS while achieving the equivalent binding to the GPIIb/IIIa receptors on the platelet surface as RGDS (2.5 × 10
−2 M) [
26]. Because plenty of RGDS motifs are endowed with high specific binding to activated platelets, PD-RGDS could target thrombus better. In addition, the transmission electron microscopy results showed that PD-RGDS existed as nanoparticles of 60–108 nm which was more beneficial to construct a nano-sized drug delivery system than a micron-sized carrier.
The preparation method of the UK/PD-RGDS complex was simple, green, and has a short mixing time, which better protected the activity of UK than the UK conjugation delivery system. The z-coverage sizes and zeta potentials results implied that the UK/PD-RGDS complex could exist stably for 3 days. Moreover, in UK/PD-RGDS complexes UK and PD-RGDS had a close interaction because the UK/PD-RGDS complex has smaller sizes and narrow size distribution than UK and PD-RGDS. The zeta potential results showed both UK and PD-RGDS are exposed on the surface of the UK/PD-RGDS complex. We think the complexation and closer binding of UK and PD-RGDS could cause the changing of UK and PD-RGDS conformation and be in favor of the increase in UK potency. This may be the reason for the increasing thrombolytic potency of the UK/PD-RGDS complex. In addition, the transmission electron microscopy results showed that UK/PD-RGDS existed as nanoparticles of 18–131 nm. Fibrin clots highly inhibit the penetration of particles of 1 μm or larger into fibrin clots [
29], which has allowed the nanosized system to accelerate thrombolytic therapy without causing microbubbles and holes. The nanosized UK/PD-RGDS was more beneficial to penetrate the thrombus for thrombolysis than the micron-sized carrier.
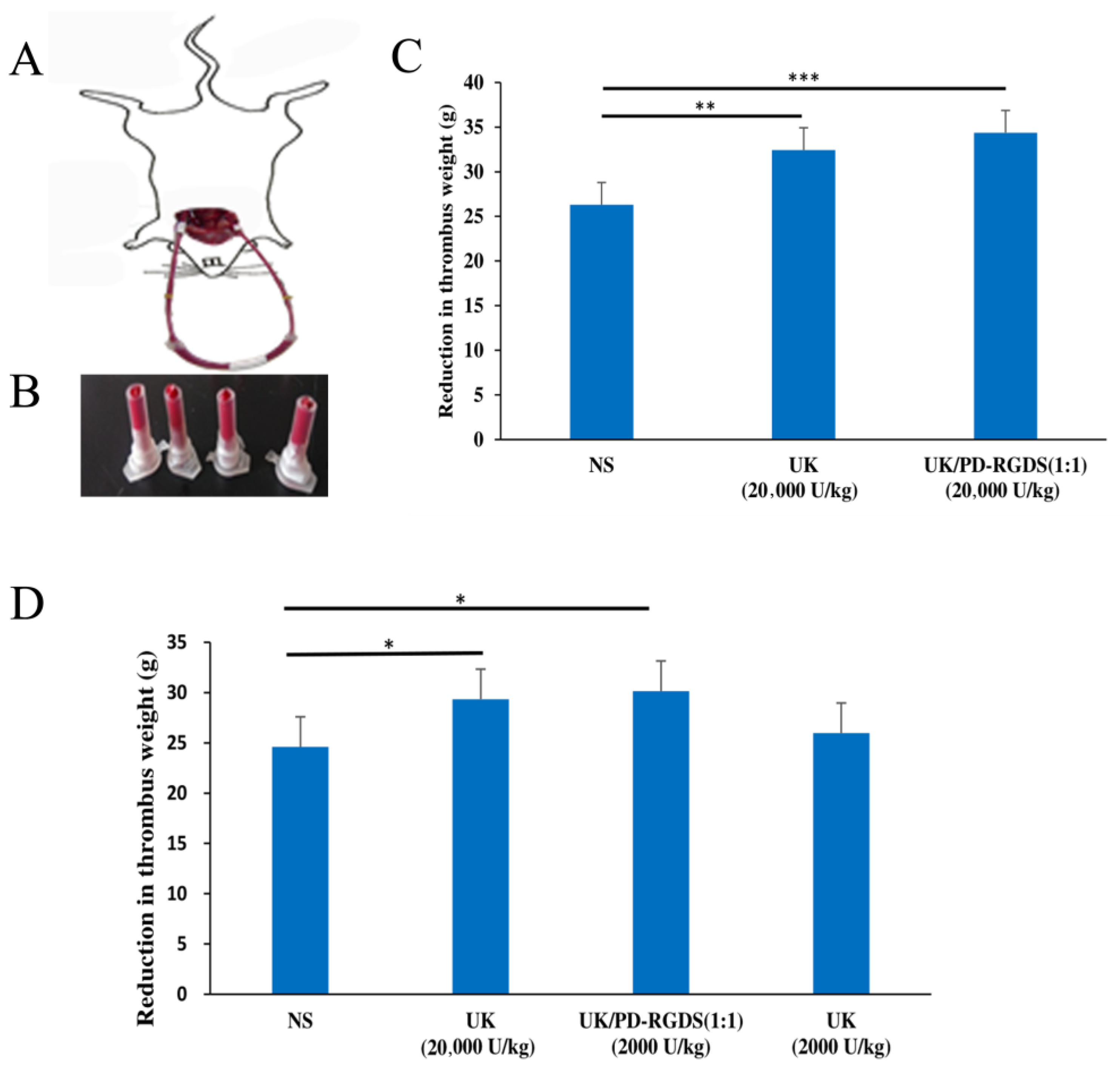
Platelets are the main targets of thrombus [
7]. RGD sequence peptides could specifically bind to activated platelets by targeting GPIIb/IIIa on the surface of platelets [
30,
31]. PD-RGDS containing 46% RGDS has specifically adhered to activated platelets [
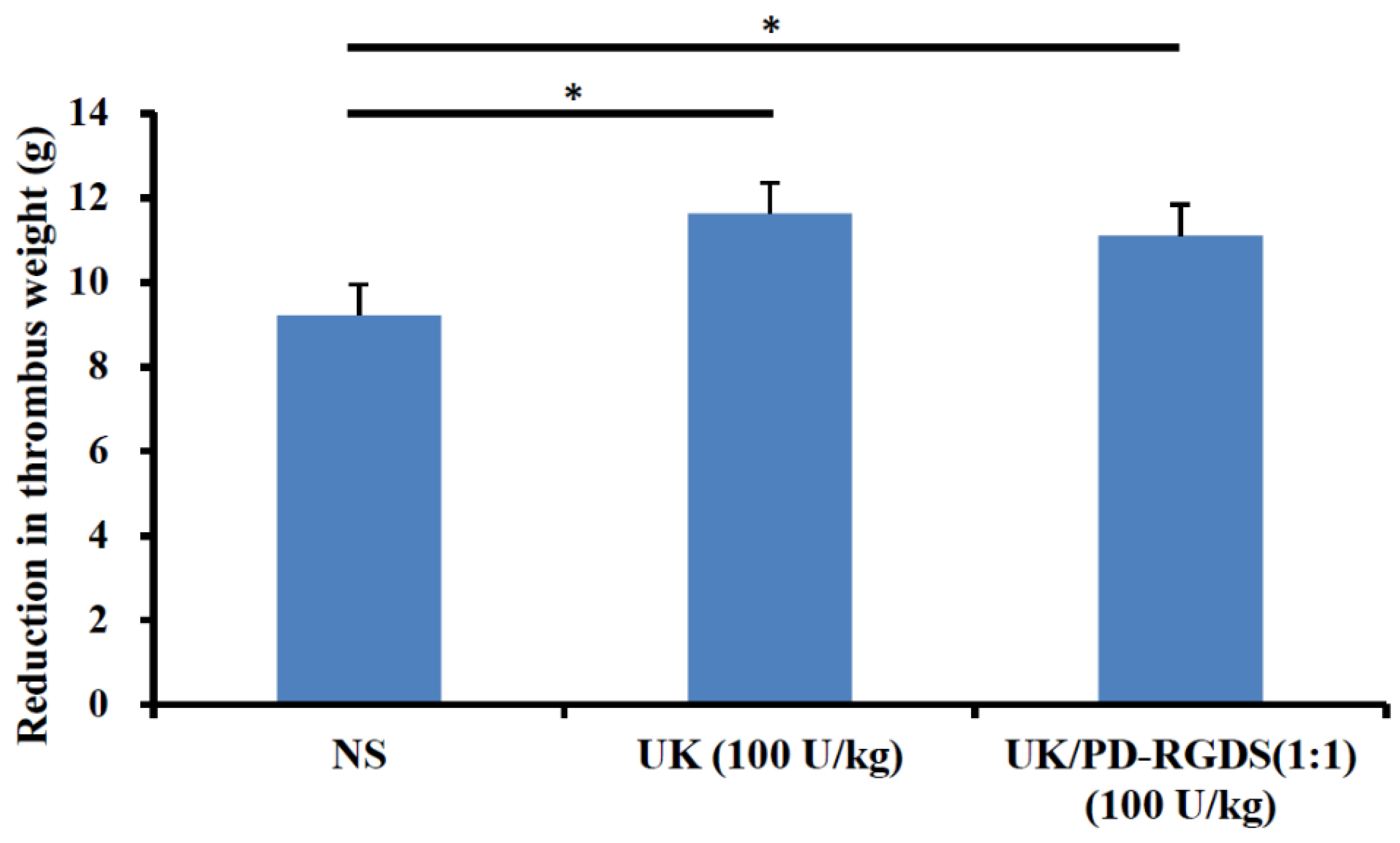
26]. Then PD-RGDS containing these RGDS motifs loaded UK specifically to the thrombus site and then UK was concentrated to dissolve the local thrombus. Therefore, less dose of the UK/PD-RGDS complex (2000 IU/kg) could show significant thrombolytic activity as the free urokinase at the dose of 20,000 IU/kg in a rat model of carotid arteriovenous bypass thrombolysis. It could be explained by the increasing thrombolytic potency, high ratio targeting factors, and nano-sized particles. Firstly, the thrombolytic potency of the UK/PD-RGDS complex increased compared with the UK by the Bubble-rising method. That is to say, the structure of the UK/PD-RGDS complex could help to increase the thrombolytic potency of the UK and cause the stronger thrombolytic activity of the UK/PD-RGDS complex group in vivo to a certain extent. Moreover, the grafting rate of RGDS in PD-RGDS is very high and reaches up to 46%. The high grafting rate of RGDS in PD-RGDS could help UK better to concentrate on the thrombus and increase the thrombolytic activity. After complexation with PD-RGDS, UK at the dose of 2000 IU/kg had significant thrombolytic activity. However, the in vivo UK group (2000 IU/kg) has no significant thrombolytic activity compared with the NS group. It suggested that the thrombolytic activity of the UK/PD-RGDS complex group (2000 IU/kg) was 10 times of the UK group. In addition, the nanoscale UK/PD-RGDS complex system could be beneficial to dissolve thrombus. It showed that polymer conjugation by grafting with targeted motifs is a good method as the drug carrier when we construct a targeted nano-delivery system.
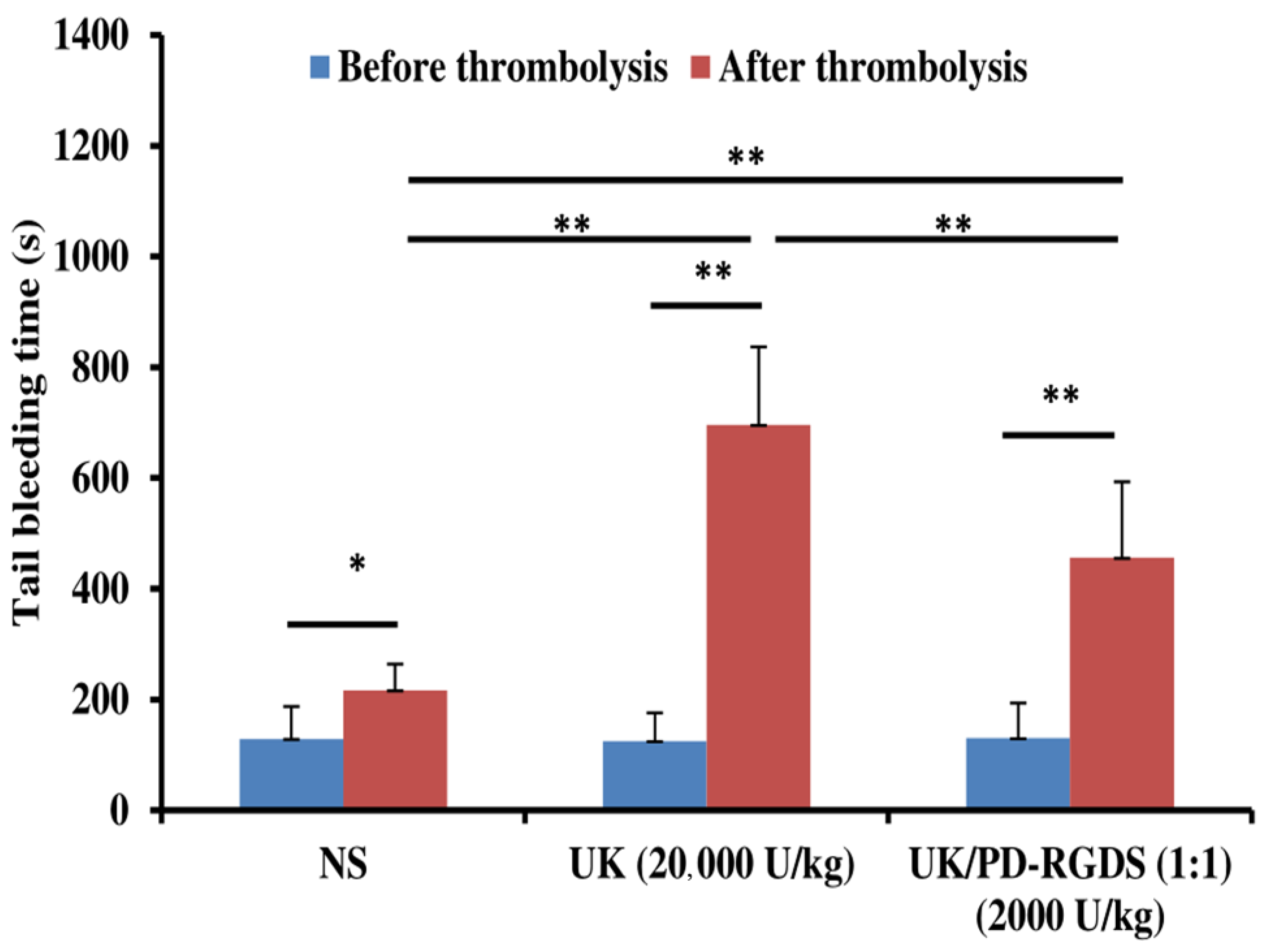
The tail bleeding time results indicate that the UK/PD-RGDS complex could reduce the side effect of bleeding. Meanwhile, the UK/PD-RGDS complex could improve thrombolytic activity. Therefore, this study achieved the purpose of our expected research design. The tail bleeding times after NS administration was significantly higher than that before NS administration (
p < 0.05, n = 5) (
Figure 4), indicating that the tail bleeding time of the blank control group was increased. It was perhaps caused by heparin (140 U/kg) added to the carotid arteriovenous bypass of rats.
The interaction between UK and PDRGDS could not be analyzed because the determination of protein interactions by isothermal calorimetric titration requires the unavailable UK sample with a single molecular weight; our UK is a mixture of high molecular weight UK (Mw 54,000) and low molecular weight UK (Mw 33,000).
4. Materials and Methods
4.1. Materials
All amino acids were purchased from Sichuan Sangao Biochemical Co., Ltd. (Chengdu, China). Urokinase for injection (100,000 units) was purchased from Peking University Gaoke Huatai Pharmaceutical. Bovine fibrinogen Standard (Lot 140607-201841), Bovine thrombin Standard (Lot 140605-201526), Bovine fibrinogen Standard (Lot 140606-201826), bovine fibrinogen Standard (Lot 140606-201826), Bovine fibrinogen standard (Lot 140606-201826), Bovine fibrinogen standard (Lot 140606-201826), Bovine fibrinogen standard (Lot 140606-201826) and Urokinase standard (Batch No. 140604-201224) were purchased from China National Institute for Food and Drug. Bovine thrombin(SLBV3604) and Fluorescent isothiocyanate yellow (FITC)were purchased from Sigma Company (Shanghai, China). Agarose (Batch No. 424G056) was acquired from Beijing Solebo Technology Co., Ltd. (Beijing, China). Barbiturate-sodium chloride buffer (pH 7.8, DZ331) was purchased from Xi’an Hutt Biological Company (Xian, China). Trimethylol aminomethane buffer (pH 9.0) was purchased from Beijing Regen Biotechnology Co., Ltd. (Beijing, China). Other reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
4.2. Preparation of Poly-α, β-d, l-Aspartyl-Arg-Gly-Asp-Ser (PD-RGDS)
The preparation of poly-
α,
β-
d,
l-aspartyl-Arg-Gly-Asp-Ser was carried out according to the method in the literature [
26]. In short, 82.8 mg of PD was dissolved in 1 mL of anhydrous DMF, then 97 mg of HoBt and 360 mg of HCl·EDC were added to an ice bath. After 0.5 h, HCl·Arg(Tos)-Gly-Asp(OBzl)-Ser(Bzl)-OBzl was added and the pH value was adjusted to 9. After 24 h, the reaction solution was dried, extracted by ether, and washed separately with 5% KHSO
4, and water three times. The solid was dried at 37 °C under reduced pressure for 48 h and provided 197 mg of the yellowish powder. The yellowish powder was dissolved and mixed in 8 mL of CF
3CO
2H:CF
3SO
3H (3:1) at 0 °C for 75–90 min. Then it was triturated with 150 mL of ether and the residue was mixed with water and dissolved until the pH value was adjusted to 7. After centrifugation for 30 min, the supernatant was dialyzed for 3 days with ultrapure water and lyophilized to provide 197 mg (72%) of the title compound as a white powder.
4.3. Preparation of UK/PD-RGDS Complex
An amount of 8 mg of PD-RGDS and 8 mg of UK were mixed in 3 mL of pH 7.4 PBS buffer (10 mM) and stirred at 4 °C for 1 h to prepare the solution of 6.667 IU/mL UK/PD-RGDS complex.
4.4. Morphology of UK/PD-RGDS Complex
The sizes and Zeta potentials of samples (2.67 mg/mL)in pH 7.4 PBS buffer (10 mM) were determined in the automatic measurement mode on Malvern’s Zeta Sizer (Nano-ZS90). The sizes and morphology of UK/PD-RGDS complex particles were observed by transmission electron microscopy (JEM-2100, Japan). The solutions of the UK/PD-RGDS complex (103, 102, 10−2, 10−5, 10−7, 10−9 nM) were prepared and dropped onto a formvar-coated copper grid as TEM samples. Then a drop of ethanol was added. The copper grid is first placed in the air to dry completely. The samples were observed by transmission electron microscopy (JSM-6360 LV, JEOL, Tokyo, Japan) with an electron beam acceleration voltage of 120 kV. All samples were prepared in three copies.
4.5. Bioassays of UK/PD-RGDS Nanosystem
4.5.1. Bubble-Rising Method
The test of the Bubble-rising method was performed according to Pharmacopoeia of the People’s Republic of China, Part II (2015 Edition); 6.67 mg/mL bovine fibrinogen standard in barbiturate-sodium chloride buffer (pH 7.8), 6.0 bp/mL bovine thrombin standard in barbiturate-sodium chloride buffer (pH 7.8), and 1 casein unit/mL bovine plasminogen in Tris (Hydroxymethyl) aminomethane buffer solution (pH 9.0) was prepared first. Bovine thrombin and bovine plasminogen were mixed with equal volume and the mixed solution was obtained. A 60 units/mL standard solution of urokinase in a barbiturate-sodium chloride buffer (pH 7.8) was also prepared.
The UK/PD-RGDS complex prepared by method 2.2 was quantitatively diluted with barbiturate-sodium chloride buffer (pH 7.8) to the concentration of 60 units/mL UK. The sample of UK control was also prepared according to method 2.2; 0.3 mL of bovine fibrinogen was added to each tube and put at 37 ± 0.5 °C in a water bath. Then 0.9 mL, 0.8 mL, 0.7 mL, and 0.6 mL barbiturate-sodium chloride buffer (pH 7.8) were added, respectively; 0.1 mL, 0.2 mL, 0.3 mL, and 0.4 mL of UK standard solution were added successively. After that 0.4 mL of the mixed solution was added, and each tube fully oscillated until the reaction system was full of bubbles and the bubbles stay in the system. The reaction system usually condenses in 30~40 s. The end of timing was recorded when the small bubbles rose to half the volume of the reaction system in the clot. All the samples were carried out in triplicate. The potency of the UK samples and UK/PD-RGDS complex samples were measured and determined by converting measurements to the thrombolytic potency through a standard curve between the logarithm of time versus the logarithm of the concentration of urokinase.
4.5.2. Agarose-Fibrin Plate Method
A concentration of 8 mg/mL bovine fibrinogen standard, 1 mg/mL bovine thrombin standard, and 8 mg/mL agarose standard in 10 mM PBS buffer (pH 7.4) were prepared separately. Then 8 mg/mL agarose was heated in a microwave oven until boiled. After the agarose was completely dissolved, the agarose was placed in a hot water bath at 52 °C for later use; 800 U/mL, 600 U/mL, 400 U/mL, and 200 U/mL UK standard in 10 mM PBS buffer solution (pH 7.4) were prepared separately. UK samples and UK/PD-RGDS samples were diluted to an appropriate concentration with 10 mM PBS buffer (pH 7.4). Three Petri dishes (9 cm in diameter) were taken and numbered; 18 mL of agarose solution was added to a small beaker. Then 1 mL of bovine thrombin and 1 mL of bovine fibrinogen were added to the beaker. They were shaken thoroughly and quickly poured into a disposable Petri dish. A homemade punch was used and the mixtures stayed at room temperature for 1 h. After the agarose was completely solidified, the liquid in the well was drained; 5 μL of 800 U/mL, 600 U/mL, 400 U/mL, and 200 U/mL UK standard was added separately into the holes. After the mixture has been placed at room temperature for 24 h, transparent rings on the agarose-fibrin plate were observed. The potency of UK samples and UK/PD-RGDS complex samples were measured and determined by converting measurements to the thrombolytic potency through a standard curve between concentrations of UK versus the area of the transparent rings.
4.6. In Vitro Thrombus Clot Lysis Assay
The in vitro thrombus, clot lysis assay was carried out according to the procedure reported earlier [
28]. Male Wistar rats (220 g ± 10 g) were anesthetized with pentobarbital sodium (20%, 7 mL·kg
−1, i.p.). The right carotid artery was isolated and the whole blood was collected in centrifuge tubes. The whole blood was injected into a flexible rubber hose (D 1.7 cm) containing a helix (L 15 mm; D 1.0 mm). After 40 min the thrombus with helix was carefully removed and suspended in the tri-distilled water for 1 h at room temperature. The surface water was removed using filter paper and the thrombus was weighed precisely. Then they were immersed into 8 mL NS, UK (100 IU/mL), or UK/PD-RGDS complex (100 IU/mL), respectively, at 37 °C at 70 rpm in a shaker for 3 h. The thrombi were removed and the surface water was gently removed by filter paper. The reduced weight of the thrombus was used to compare the degree of thrombus clot lysis.
4.7. In Vivo Thrombolytic Activity
Male Wistar rats (210–250 g) were anesthetized with pentobarbital sodium (20%, 7 mL·kg−1, i.p.). The right common carotid artery and the left vein were operated on and isolated. The whole blood was collected from the right common carotid artery and used to prepare the thrombus clots for 40 min. The surface blood of the thrombus with helix was removed using filter paper and the thrombus was weighed precisely. The thrombus was put into a polyethylene tube as an external circulation pipeline between the right common carotid artery and the left vein. These pipelines were filled with heparin sodium (50 IU/mL NS solution). One end was inserted into the left internal jugular vein and after the heparin sodium (200 U/kg) was injected the other end was inserted into the right carotid artery. NS (3 mL/kg), UK (20,000 IU/kg), or UK/PD-RGDS complex (2000 IU/kg) were injected near the venous end. After the blood was circulated for 60 min, the thrombus was taken out and weighed after the surface blood was absorbed. The reduced weight of the thrombus was used to represent their thrombolytic activity in vivo.
4.8. Determination of the Tail Bleeding Time
The tail bleeding time was assayed as described previously with a few small modifications [
32]. The operating method was referred to in 4.7. The difference is that the dose of heparin sodium (200 U/kg) was adjusted to 140 U/kg and only used to fill the tube instead of intravenous injection. Bleeding times were measured at 40 min before and after the thrombolytic treatment. The rat tail was cut off at 1 mm near the tail tip and placed in 25 mL of normal saline at 37 °C. The occurrence of uniform and continuous bloodlines was taken as the beginning of timing. The complete cessation of bleeding was recorded as the bleeding time. If the bleeding does not stop at 1800 s, it is classified as 1800 s.
4.9. Organ Distribution Study
Firstly, UK was labeled by FITC as in previous articles [
33]; 10 mg of urokinase was dissolved in 1 mL of 10 mmol/L PBS (pH 7.1); 2.6 mg of fluorescence isothiocyanate yellow (FITC) was dissolved in 100 μL of 0.5 mol/L carbonate buffer (pH 9.5). FITC was dropped into urokinase and stirred in a shading environment at room temperature for 4 h. Then the mixture was centrifuged at 2500 r/min for 25 min. The supernatant was dialyzed in 10 mmol/L PBS buffer (pH 8.0) for 2–4 h. After dialyzation, FITC-UK was purified by Sephadex G50 column and eluted with 10 mmol/L PBS buffer (pH 7.1). The fluorescence of FITC-UK was measured by F-2500 Fluorescence Spectrophotometer. FITC-UK was diluted appropriately until its OD280 was close to 1.0. Its OD value was read at 495 nm and 280 nm. F/P value was calculated according to the following formula: F/P = 2.87 × OD
495/(OD
280 − 0.35 × OD
495).
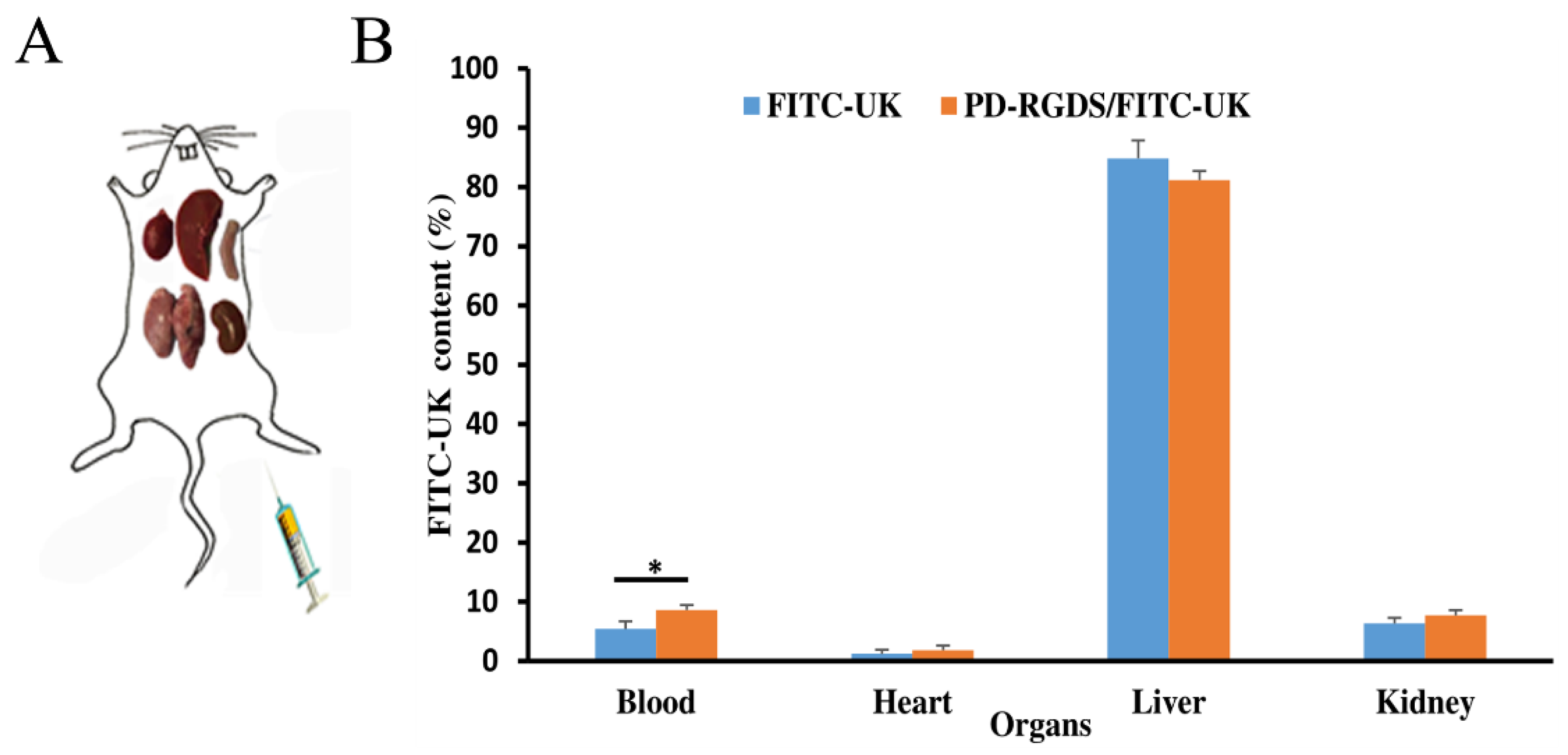
In vivo organ distribution was evaluated as in previous articles [
34]. Male Wistar rats (250–300 g) were anesthetized with pentobarbital sodium (20%, 7 mL·kg
−1, i.p.). After the right common carotid artery was separated, filter paper (1.3cm wide) soaked in 25% FeCl
3 saturated solution and a small piece of Para membrane (1.7 cm wide) was put under the artery for 15 min to induce the formation of carotid artery thrombosis. After blood reperfusion for one hour, NS solution (0.3 mL/kg), FITC-UK/PD-RGDS complex solution (8000 U/kg), or FITC-UK solution (8000 U/kg) were injected via the femoral vein. The rats were sacrificed 1-h post-administration. The liver, spleen, kidneys, lungs, heart, and thrombus clots are separated and taken out. About 1 g of each tissue was added into 3 mL homogenizing buffer (0.32 M sucrose, 100 mM HEPES, pH 7.4), and homogenized in a glass homogenizer. After homogenization, the liquid was centrifuged (4000 rpm for 15 min) to obtain the supernatant samples of each tissue. The supernatant powder samples of each tissue were obtained by freeze-drying. The appropriate amount of supernatant powder samples of each tissue was dissolved in an appropriate solution (1% Triton X-100, 100 mM NaCl, 0.1% SDS, 0.5% Na-Deoxycholate) and cultured at 4 degrees for 30 min to obtain the final test samples of organs.
Standard solutions of 20, 10, 5, 2.5, 1, 0.5, 0.25, and 0.1 mg/L FITC-UK were prepared with 10 mM PBS buffer solution (pH 7.4). The fluorescence intensity was measured at the excitation wavelength (493.0 nm) and emission wavelength (524.0 nm). The concentration of FITC-UK in each tissue was determined by converting measurements to concentrations through a standard curve between the fluorescence intensity versus the concentration of the UK.
The described assessments were approved by the Ethics Committee of Capital Medical University. The committee assures the welfare of the animals was maintained under the requirements of the Animal Welfare Act and according to the guideline for the care and use of laboratory animals.