A Comparative Study on CO2-Switchable Foams Stabilized by C22- or C18-Tailed Tertiary Amines
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Optimum Concentration
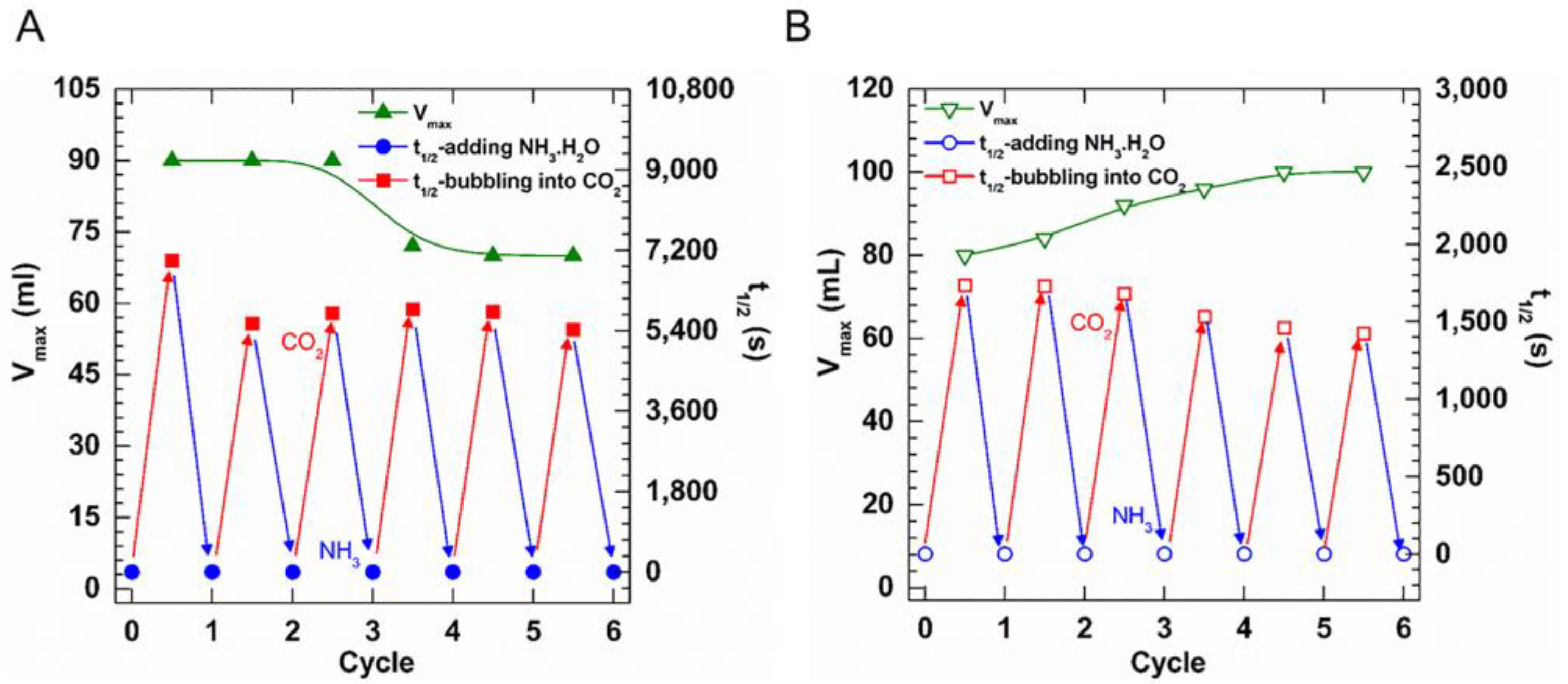
2.2. A Comparison of the Foams Switchability
2.3. Comparison of the Effect of External Factors on Foam Properties
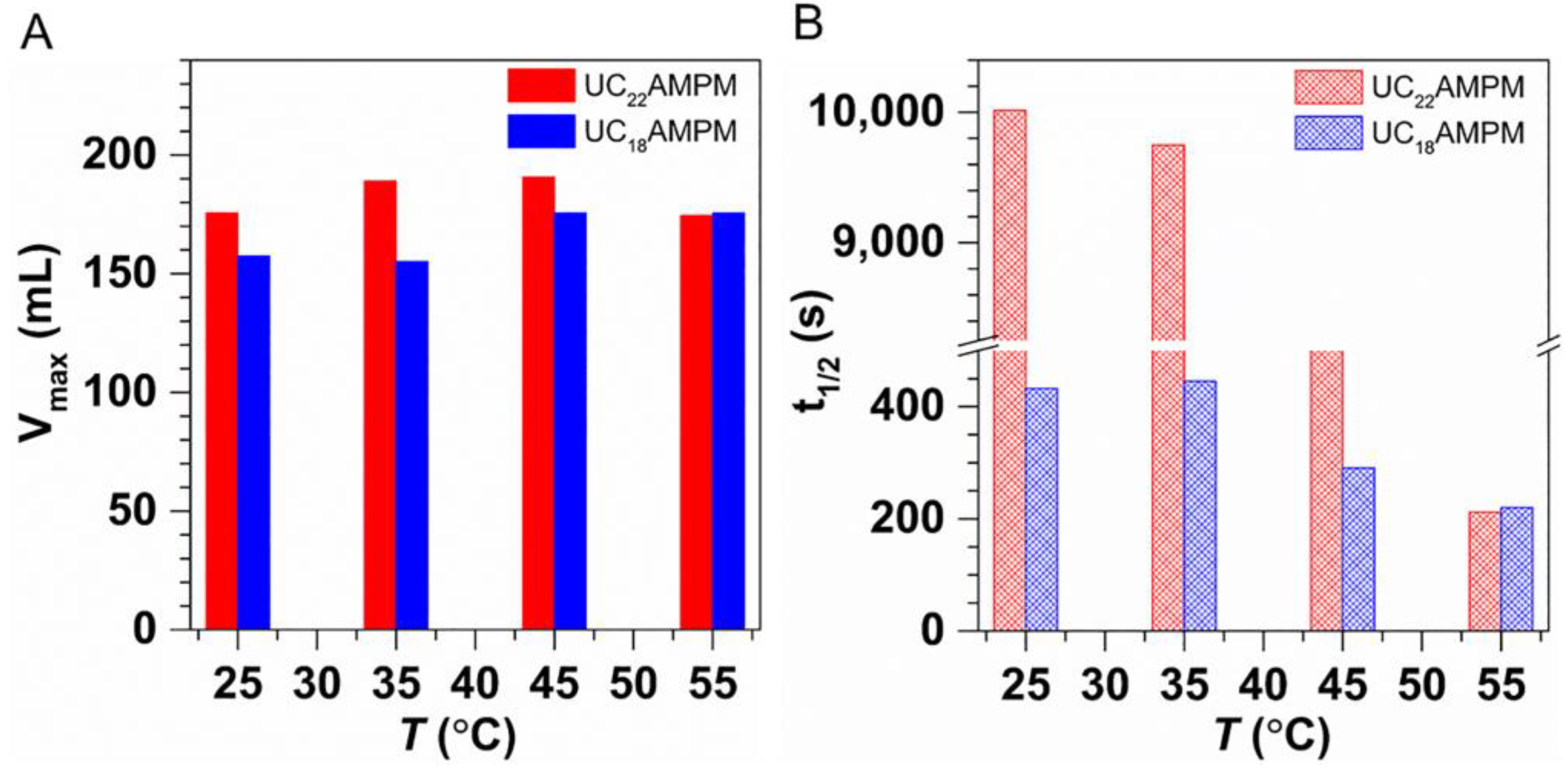
2.3.1. Effect of Temperature
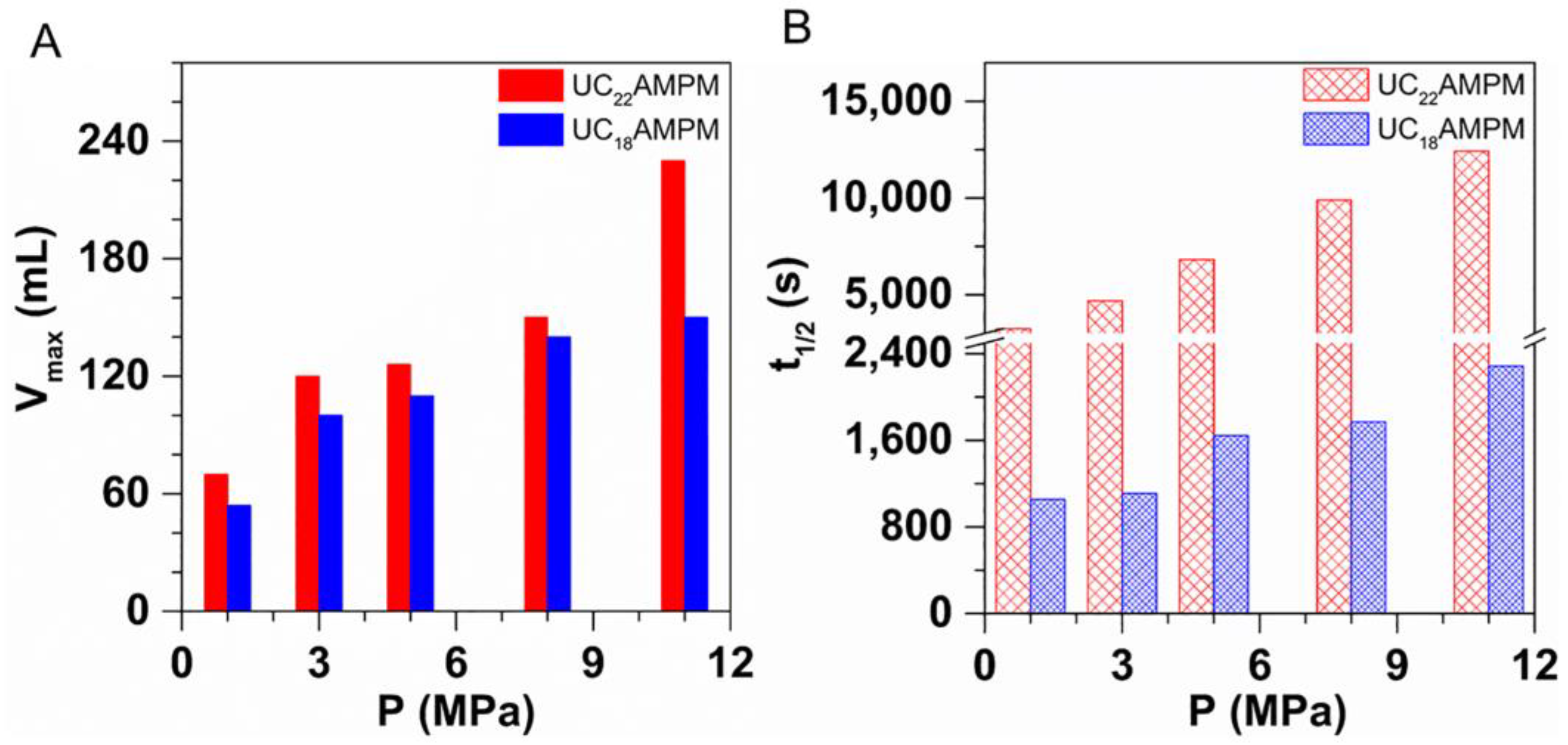
2.3.2. Effect of Pressure
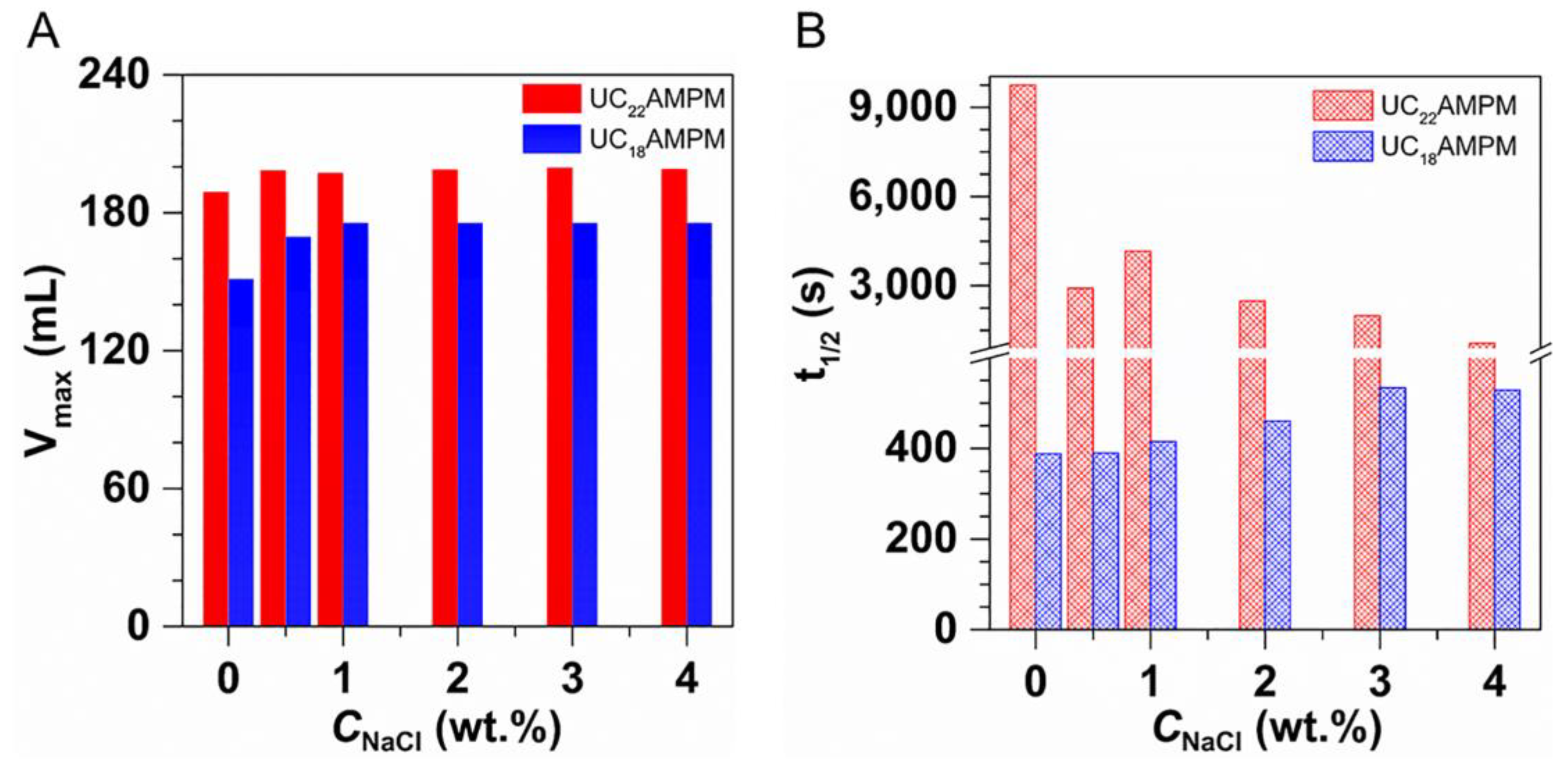
2.3.3. Effect of Salinity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Foaming Solution
3.3. Evaluation of Aqueous Foams at Atmospheric Pressure
3.4. Evaluation of Aqueous Foams at High Pressure
3.5. Characterization of Switchability of Aqueous Foams
3.6. Rheological Test of Foaming Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Langevin, D. Aqueous foams: A field of investigation at the frontier between chemistry and physics. Chemphyschem 2008, 9, 510–522. [Google Scholar] [CrossRef]
- Wei, P.; Guo, K.; Pu, W.; Xie, Y.; Huang, X.; Zhang, J. Aqueous Foam Stabilized by an in Situ Hydrophobic Polymer via Interaction with Alkyl Polyglycoside for Enhancing Oil Recovery. Energy Fuels 2020, 34, 1639–1652. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Hu, Y.; Yu, X.; He, G.; Fu, W. Froth flotation separation of lepidolite ore using a new Gemini surfactant as the flotation collector. Sep. Purif. Technol. 2022, 282, 119122–119136. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Wang, Q.; Zhao, Y.; Liu, X. Study of environmental-friendly firefighting foam based on the mixture of hydrocarbon and silicone surfactants. Fire Technol. 2020, 56, 1059–1075. [Google Scholar] [CrossRef]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. Foamability of sodium dodecyl sulfate solutions: Anomalous effect of dodecanol unexplained by conventional theories. Colloids Surf. A 2016, 495, 110–117. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Krastev, R.; Zitha, P.L.J. Foam films stabilized with alpha olefin sulfonate (AOS). Colloids Surf. A 2008, 324, 35–40. [Google Scholar] [CrossRef]
- Saint-Jalmes, A. Physical chemistry in foam drainage and coarsening. Soft Matter 2006, 2, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef]
- Langevin, D. Aqueous foams and foam films stabilised by surfactants. Gravity-free studies. C. R. Mécanique 2017, 345, 47–55. [Google Scholar] [CrossRef]
- Heerschap, S.; Marafino, J.N.; McKenna, K.; Caran, K.L.; Feitosa, K. Foams stabilized by tricationic amphiphilic surfactants. Colloids Surf. A 2015, 487, 190–197. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Wang, P. Experimental Study of the Stabilization of CO2 foam by sodium dodecyl sulfate and hydrophobic nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 1243–1253. [Google Scholar] [CrossRef]
- Fukuoka, K.; Tomikawa, A.; Nakamura, Y.; Fujii, S. Aqueous foams stabilized with several tens of micrometer-sized polymer particles: Effects of surface hydrophilic–hydrophobic balance on foamability and foam stability. Chem. Lett. 2016, 45, 667–669. [Google Scholar] [CrossRef]
- Pu, W.-F.; Wei, P.; Sun, L.; Jin, F.-Y.; Wang, S. Experimental investigation of viscoelastic polymers for stabilizing foam. Ind. Eng. Chem. Ind. Ed. 2017, 47, 360–367. [Google Scholar] [CrossRef]
- Rouimi, S.; Schorsch, C.; Valentini, C.; Vaslin, S. Foam stability and interfacial properties of milk protein-surfactant systems. Food Hydrocoll. 2005, 19, 467–478. [Google Scholar] [CrossRef]
- Murray, B.S.; Ettelaie, R. Foam stability: Proteins and nanoparticles. Curr. Opin. Colloid Interface Sci. 2004, 9, 314–320. [Google Scholar] [CrossRef]
- Davis, J.P.; Foegeding, E.A. Comparisons of the foaming and interfacial properties of whey protein isolate and egg white proteins. Colloids Surf. B 2007, 54, 200–210. [Google Scholar] [CrossRef]
- Stocco, A.; Rio, E.; Binks, B.P.; Langevin, D. Aqueous foams stabilized solely by particles. Soft Matter 2011, 7, 1260–1267. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Schad, T.; Preisig, N.; Blunk, D.; Piening, H.; Drenckhan, W.; Stubenrauch, C. Less is more: Unstable foams clean better than stable foams. J. Colloid Interface Sci. 2021, 590, 311–320. [Google Scholar] [CrossRef]
- Alzobaidi, S.; Da, C.; Tran, V.; Prodanovic, M.; Johnston, K.P. High temperature ultralow water content carbon dioxide-in-water foam stabilized with viscoelastic zwitterionic surfactants. J. Colloid Interface Sci. 2017, 488, 79–91. [Google Scholar] [CrossRef]
- Wang, J.; Luo, X.; Rogers, S.; Li, P.; Feng, Y. Stabilization of CO2 aqueous foams at high temperature and high pressure: Small-angle neutron scattering and rheological studies. Colloids Surf. A 2022, 647, 129015–129026. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Worthen, A.J.; Reddy, P.P.; Ou, A.M.; Hirasaki, G.J.; Nguyen, Q.P.; Biswal, S.L.; Johnston, K.P. High Temperature CO2-in-water foams stabilized with cationic quaternary ammonium Surfactants. J. Chem. Eng. Data 2016, 61, 2761–2770. [Google Scholar] [CrossRef]
- Elhag, A.S.; Da, C.; Chen, Y.; Mukherjee, N.; Noguera, J.A.; Alzobaidi, S.; Reddy, P.P.; AlSumaiti, A.M.; Hirasaki, G.J.; Biswal, S.L.; et al. Viscoelastic diamine surfactant for stable carbon dioxide/water foams over a wide range in salinity and temperature. J. Colloid Interface Sci. 2018, 522, 151–162. [Google Scholar] [CrossRef]
- Xue, Z.; Worthen, A.; Qajar, A.; Robert, I.; Bryant, S.L.; Huh, C.; Prodanovic, M.; Johnston, K.P. Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J. Colloid Interface Sci. 2016, 461, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y. Vegetable-derived long-chain surfactants synthesized via a “Green” route. ACS Sustain. Chem. Eng. 2012, 1, 75–79. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, Z. A Facile route towards the preparation of ultra-long-chain amidosulfobetaine surfactants. Synlett 2009, 2009, 2655–2658. [Google Scholar] [CrossRef]
- Zhang, P.; Bai, G.; Cui, G.; Zhang, L.; Peng, X.; Pei, S.; Ren, S. Enhanced CO2 foam based on amide and amine surfactants and synergistically coupled with sodium dodecyl sulfate at high temperature and high pressure. J. Pet. Sci. Eng. 2019, 179, 266–275. [Google Scholar] [CrossRef]
- Salonen, A.; In, M.; Emile, J.; Saint-Jalmes, A. Solutions of surfactant oligomers: A model system for tuning foam stability by the surfactant structure. Soft Matter 2010, 6, 2271–2281. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; He, X.; Li, C.; Li, Z.; Cao, X.; Xin, X.; Somasundaran, P. Structure-behavior-property relationship study of surfactants as foam stabilizers explored by experimental and molecular simulation approaches. J. Phys. Chem. B 2012, 116, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Fameau, A.-L.; Ventureira, J.; Novales, B.; Douliez, J.-P. Foaming and emulsifying properties of fatty acids neutralized by tetrabutylammonium hydroxide. Colloids Surf. A 2012, 403, 87–95. [Google Scholar] [CrossRef]
- Oh, S.G.; Shah, D.O. Relationship between micellar lifetime and foamability of sodium dodecyl sulfate and sodium dodecyl sulfate/1-hexanol mixtures. Langmuir 1991, 7, 1316–1318. [Google Scholar] [CrossRef]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of aqueous solutions: Role of surfactant type and concentration. Adv. Colloid Interface Sci. 2020, 276, 102084–102103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qi, X.; Sun, H.; Zhao, H.; Li, Y. Understanding about How Different Foaming Gases Effect the Interfacial Array Behaviors of Surfactants and the Foam Properties. Langmuir 2016, 32, 7503–7511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qu, C.; Lu, G.; Li, Z.; Wang, X.; Yin, H.; Feng, Y. Deliquification of low-productivity natural gas wells with in situ generated foams and heat. Energy Fuels 2021, 35, 9873–9882. [Google Scholar] [CrossRef]
- Wang, J.; Liang, M.; Tian, Q.; Feng, Y.; Yin, H.; Lu, G. CO2-switchable foams stabilized by a long-chain viscoelastic surfactant. J. Colloid Interface Sci. 2018, 523, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dehdari, B.; Parsaei, R.; Riazi, M.; Rezaei, N.; Zendehboudi, S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755–112768. [Google Scholar] [CrossRef]
- Pang, Z.; Wu, Y.; Zhao, M. Novel evaluation method of foam agents for thermal recovery in heavy oil Reservoirs. Energy Fuels 2016, 30, 2948–2957. [Google Scholar] [CrossRef]
- Hill, C.; Eastoe, J. Foams: From nature to industry. Adv. Colloid Interface Sci. 2017, 247, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, Y.; Chen, Q.; Wang, J.; Li, B.; Feng, Y. Synthesis and surface activities of amidobetaine surfactants with ultra-long unsaturated hydrophobic chains. J. Surfactants Deterg. 2012, 15, 657–661. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Agrawal, N.R.; Raghavan, S.R. Wormlike micelles versus water-soluble polymers as rheology-modifiers: Similarities and differences. Phys. Chem. Chem. Phys. 2017, 19, 24458–24466. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, Z.; Dreiss, C.A.; Wang, Y.; Fei, C.; Feng, Y. Smart wormlike micelles switched by CO2 and air. Soft Matter 2013, 9, 6217–6221. [Google Scholar] [CrossRef]
- Langevin, D. On the rupture of thin films made from aqueous surfactant solutions. Adv. Colloid Interface Sci. 2020, 275, 102075–102084. [Google Scholar] [CrossRef]
- Qu, C.; Wang, J.; Yin, H.; Lu, G.; Li, Z.; Feng, Y. Condensate oil-tolerant foams stabilized by an anionic-sulfobetaine surfactant mixture. ACS Omega 2019, 4, 1738–1747. [Google Scholar] [CrossRef]
- Fan, C.; Jia, J.; Peng, B.; Liang, Y.; Li, J.; Liu, S. Molecular dynamics study on CO2 foam films with sodium dodecyl sulfate: Effects of surfactant concentration, temperature, and pressure on the interfacial tension. Energy Fuels 2020, 34, 8562–8574. [Google Scholar] [CrossRef]
- Maiti, K.; Mitra, D.; Guha, S.; Moulik, S.P. Salt effect on self-aggregation of sodium dodecylsulfate (SDS) and tetradecyltrimethylammonium bromide (TTAB): Physicochemical correlation and assessment in the light of Hofmeister (lyotropic) effect. J. Mol. Liq. 2009, 146, 44–51. [Google Scholar] [CrossRef]
- Kumar, B.; Tikariha, D.; Ghosh, K.K. Effects of Electrolytes on Micellar and Surface Properties of Some Monomeric Surfactants. J. Dispers. Sci. Technol. 2012, 33, 265–271. [Google Scholar] [CrossRef]
- McCoy, T.M.; Valiakhmetova, A.; Pottage, M.J.; Garvey, C.J.; Campo, L.; Rehm, C.; Kuryashov, D.A.; Tabor, R.F. Structural Evolution of Wormlike Micellar Fluids Formed by Erucyl Amidopropyl Betaine with Oil, Salts, and Surfactants. Langmuir 2016, 32, 12423–12433. [Google Scholar] [CrossRef]
- Parker, A.; Fieber, W. Viscoelasticity of anionic wormlike micelles: Effects of ionic strength and small hydrophobic molecules. Soft Matter 2013, 9, 1203–1213. [Google Scholar] [CrossRef]

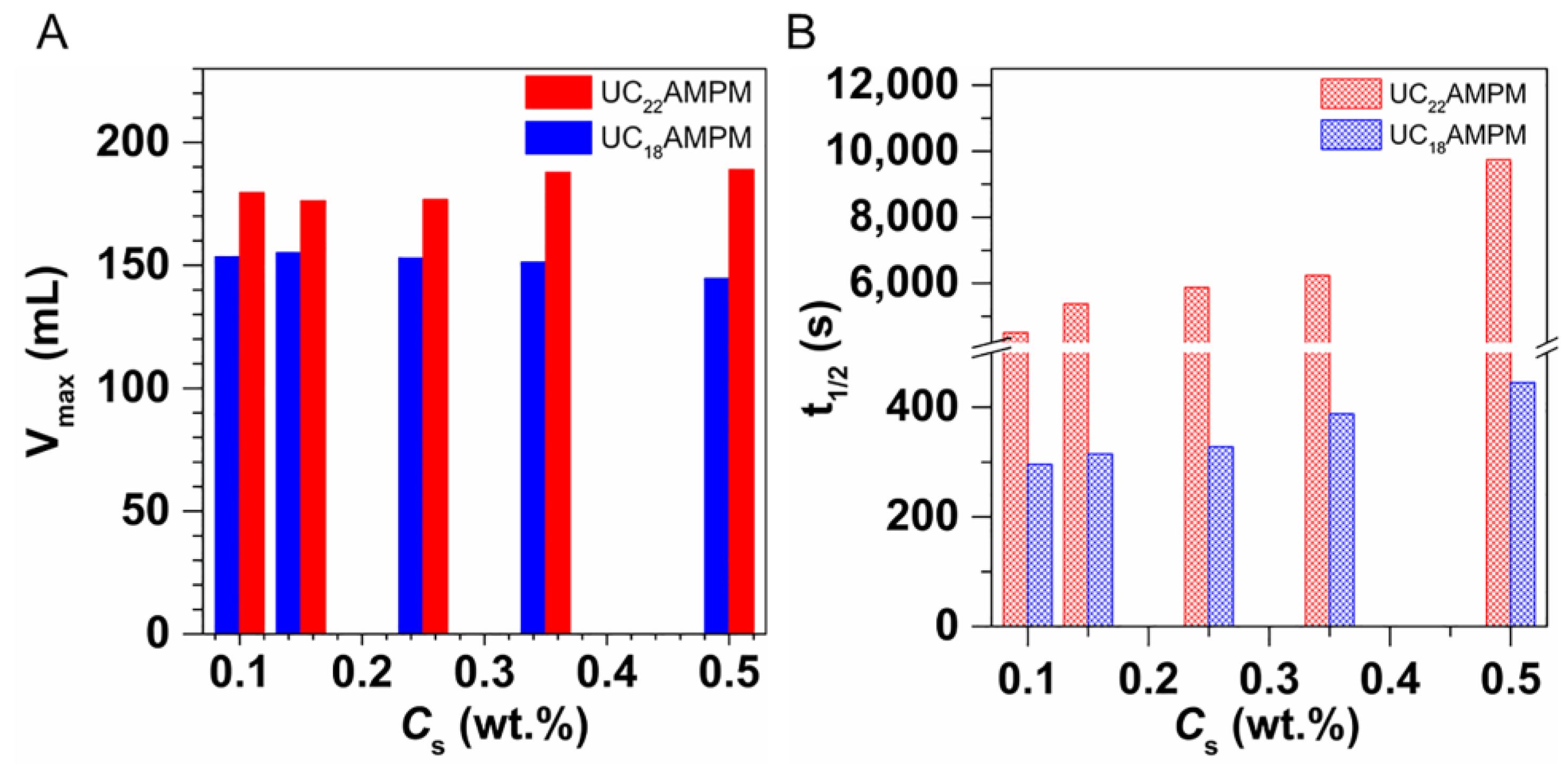
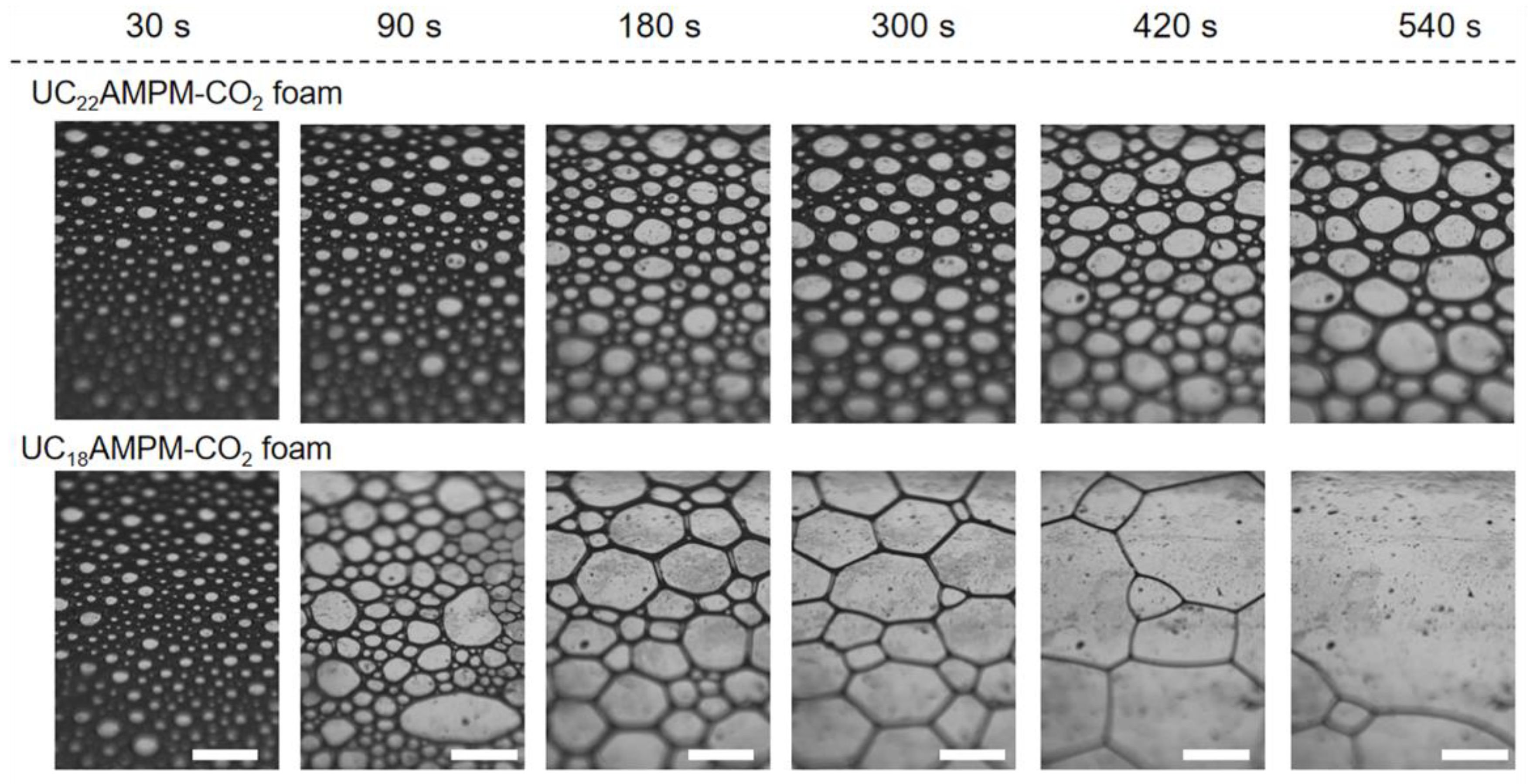
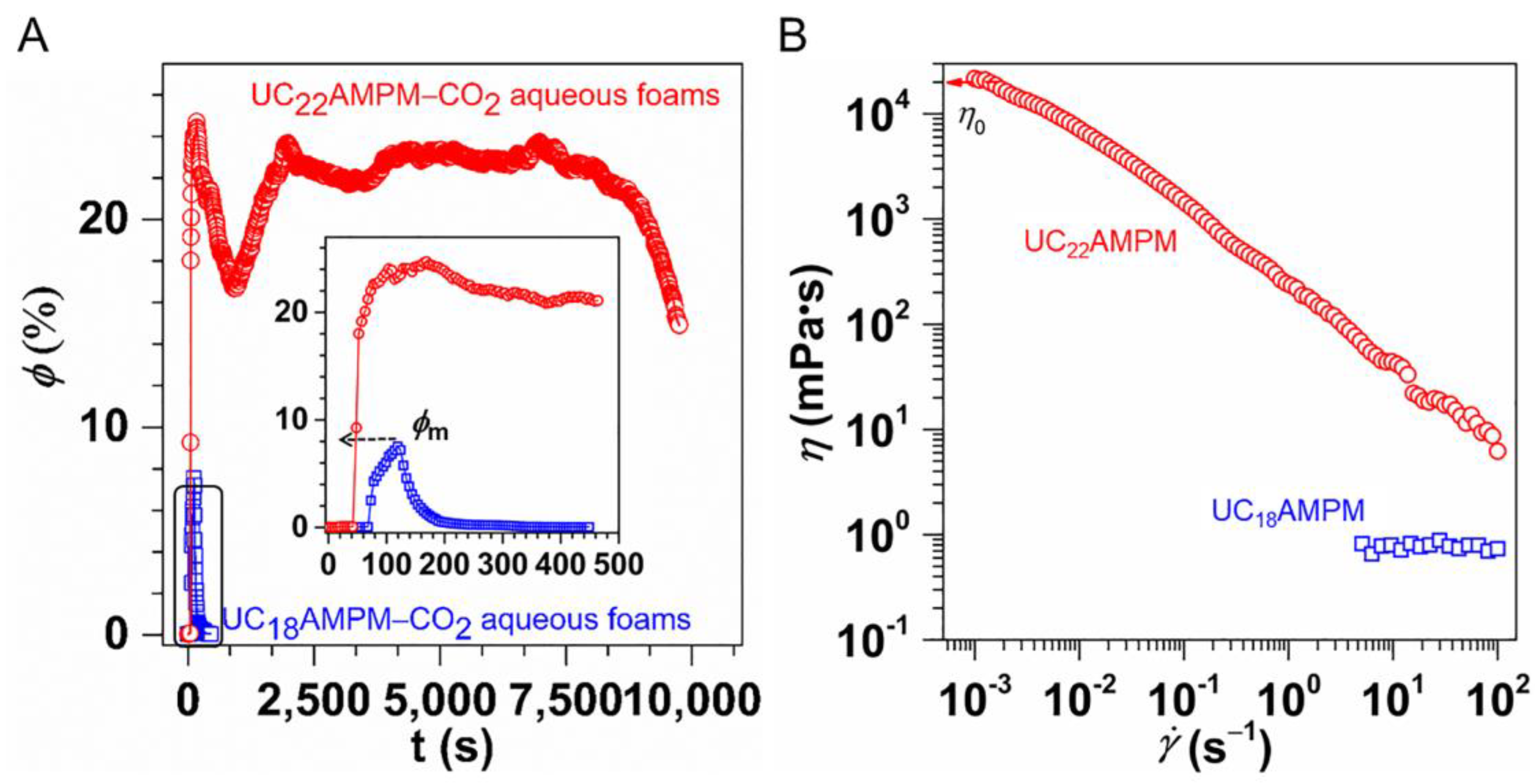

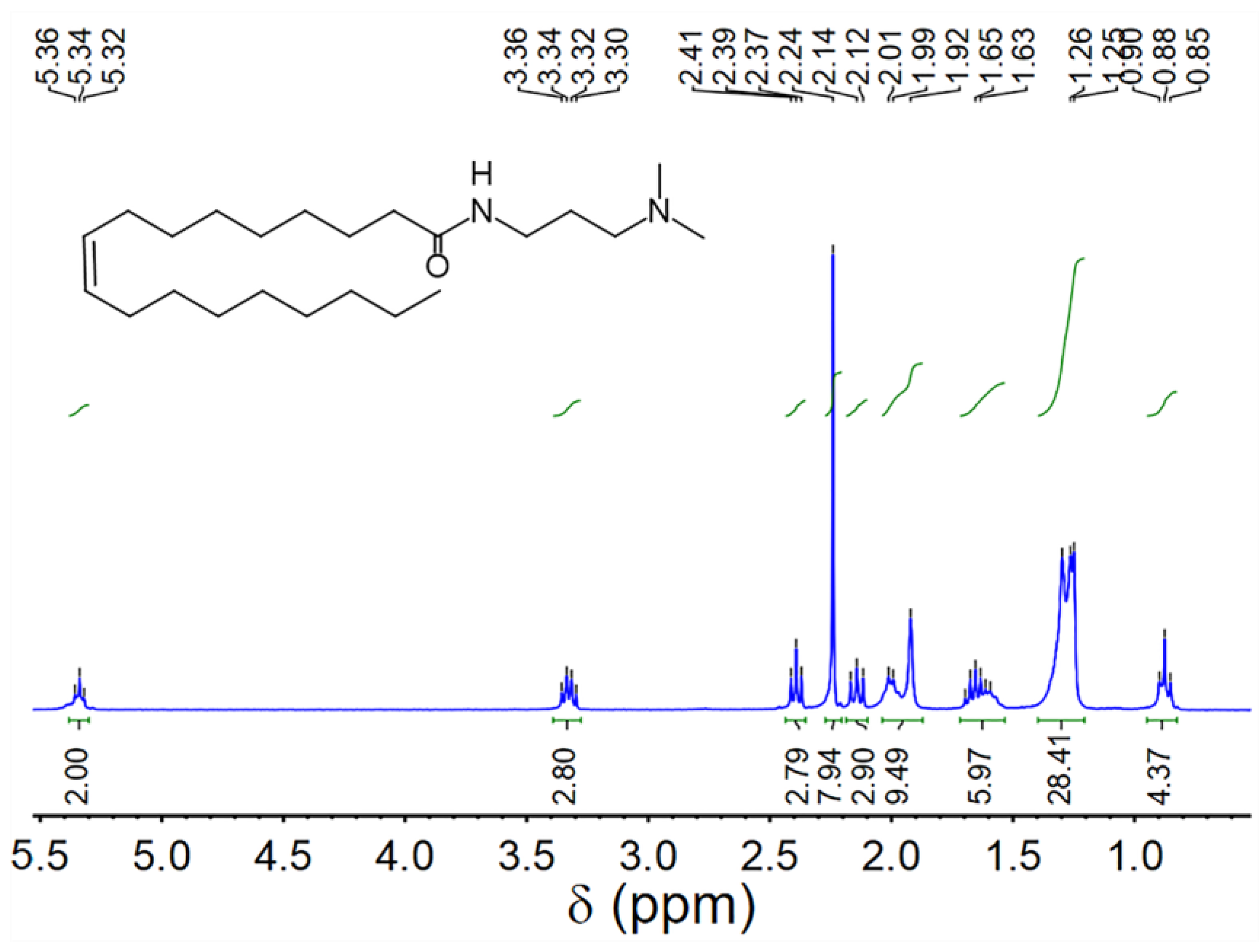
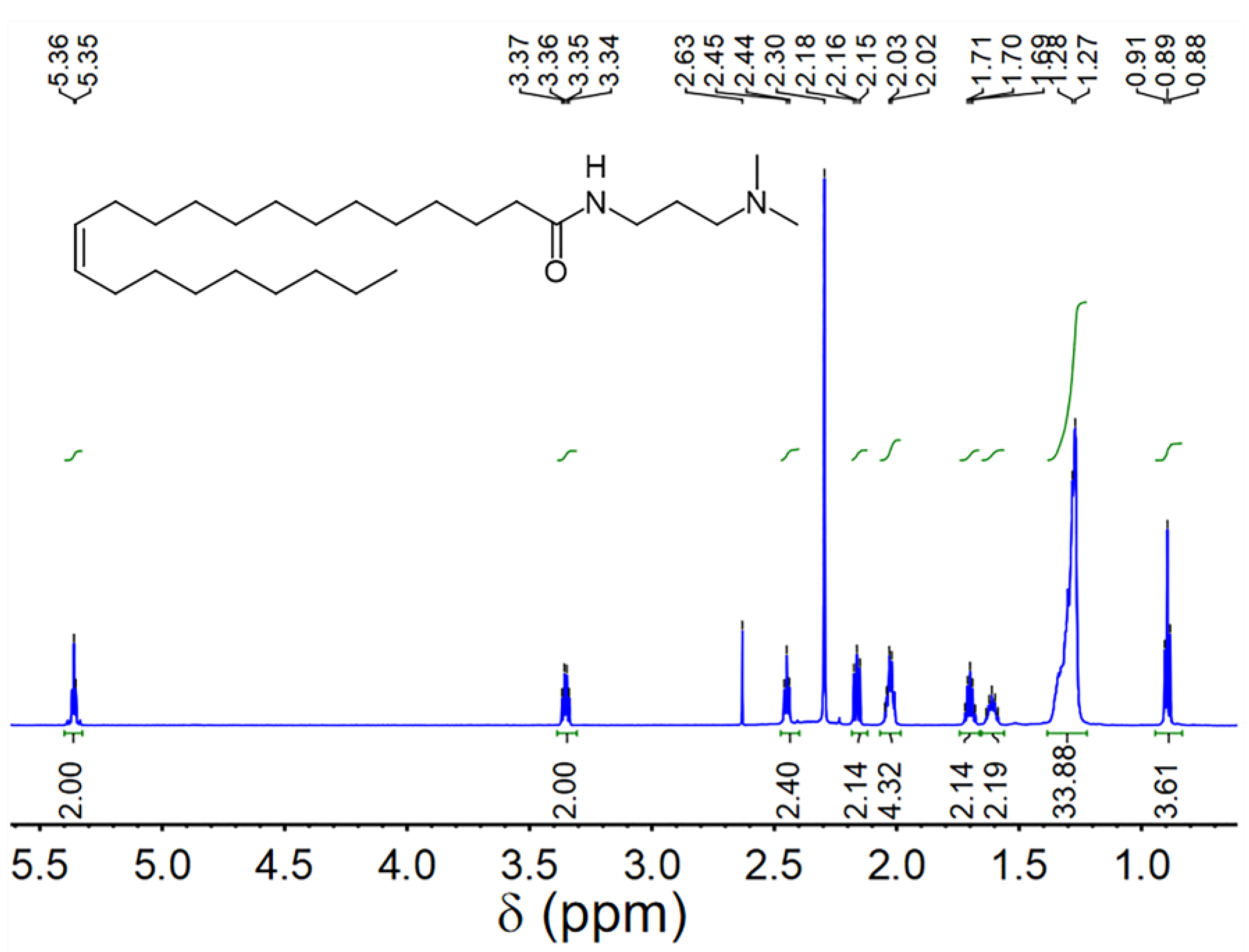
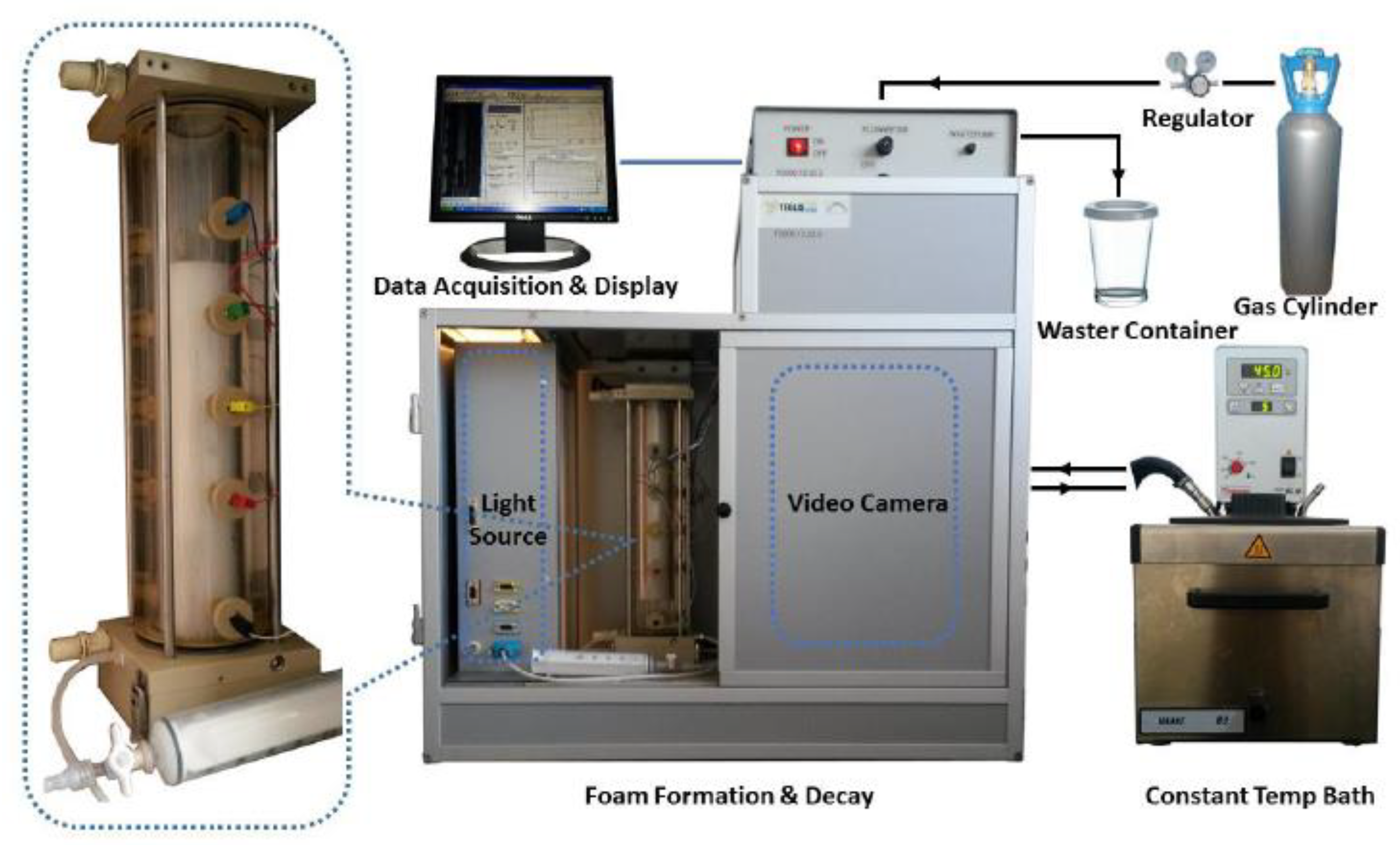
| Cs (wt.%) | t1/2 (s) | Vmax (mL) | FCI (s·mL) | |
|---|---|---|---|---|
| UC18AMPM | 0.1 | 296 | 153 | 33,621 |
| 0.15 | 315 | 155 | 36,618 | |
| 0.25 | 328 | 153 | 37,638 | |
| 035 | 388 | 151 | 43,941 | |
| 0.5 | 445 | 144 | 49,140 | |
| UC22AMPM | 0.1 | 4509 | 179 | 605,333 |
| 0.15 | 5379 | 176 | 710,028 | |
| 0.25 | 5872 | 176 | 775,104 | |
| 035 | 6240 | 187 | 879,372 | |
| 0.5 | 9750 | 189 | 1,382,062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Zhao, X.; Wang, J.; Feng, Y. A Comparative Study on CO2-Switchable Foams Stabilized by C22- or C18-Tailed Tertiary Amines. Molecules 2023, 28, 2567. https://doi.org/10.3390/molecules28062567
Liang M, Zhao X, Wang J, Feng Y. A Comparative Study on CO2-Switchable Foams Stabilized by C22- or C18-Tailed Tertiary Amines. Molecules. 2023; 28(6):2567. https://doi.org/10.3390/molecules28062567
Chicago/Turabian StyleLiang, Meiqing, Xuezhi Zhao, Ji Wang, and Yujun Feng. 2023. "A Comparative Study on CO2-Switchable Foams Stabilized by C22- or C18-Tailed Tertiary Amines" Molecules 28, no. 6: 2567. https://doi.org/10.3390/molecules28062567
APA StyleLiang, M., Zhao, X., Wang, J., & Feng, Y. (2023). A Comparative Study on CO2-Switchable Foams Stabilized by C22- or C18-Tailed Tertiary Amines. Molecules, 28(6), 2567. https://doi.org/10.3390/molecules28062567