Abstract
Lonicera caerulea fruits are a rich source of vitamins, organic acids, and phenolic compounds, which are characterised by their health-promoting properties. The content of bioactive compounds in this fruit may vary depending on the cultivar and the harvest date. The fruits of the L. caerulea var. kamtschatica cultivars ‘Duet’ and ‘Aurora’ and the L. caerulea var. emphyllocalyx cultivars ‘Lori’, ‘Colin’ and ‘Willa’ were used in this study. L. emphyllocalyx fruit, especially the cultivar ‘Willa’, was characterised as having a higher acidity by an average of 29.96% compared to L. kamtschatica. The average ascorbic acid content of the L. kamtschatica fruit was 53.5 mg·100 g−1 f.w., while L. emphyllocalyx fruit had an average content that was 14.14% lower. The antioxidant activity (determined by DPPH, FRAP, and ABTS) varied according to the cultivar and the species of fruit analysed. The total polyphenol content differed significantly depending on the cultivar analysed; fruits of the L. emphyllocalyx cultivar ‘Willa’ were characterised by the lowest content of total polyphenols—416.94 mg GAE·100 g−1 f.w.—while the highest content of total polyphenols—747.85 GAE·100 g−1 f.w.—was found in the fruits of the L. emphyllocalyx cultivar ‘Lori’. Lonicera caerulea fruits contained 26 different phenolic compounds in their compositions, of which the highest content was characterised by cyanidin 3-O-glucoside (average: 347.37 mg·100 g−1). On the basis of this study, it appears that both L. kamtschatica fruits and L. emphyllocalyx fruits, especially of the cultivars ‘Lori’ and ‘Willa’, can be used in food processing.
1. Introduction
The blue honeysuckle (Lonicera caerulea L.) belongs to the genus Lonicera (Caprifoliaceae), which contains more than 200 species, native to the cold lands of the Far East and central Asia. Most of them are ornamental plants; only about 17 are edible fruit-producing species [1]. Lonicera caerulea has numerous varieties, several of which are widely cultivated, originating from Russia (L. caerulea var. edulis, L. caerulea var. kamtschatica, L. caerulea var. altaica, L. caerulea var. boczkarnikovae) and the island of Hokkaido in Japan (L. caerulea var. emphyllocalyx). They are long-living (25–30 years) shrubs that can reach 0.8–3.0 m height. They need an outside pollinator to bear fruits one year after planting. After three years, approx. 500 g of fruit can be obtained from one plant. Berries are dark blue or dark purple in colour, with a size of 1.5–3.0 cm, and a mostly cylindrical shape with a wax coating. They are plants that are very resistant to frost, able to withstand temperatures up to −46 °C [2]. Lonicera caerulea var. kamtschatica is a variety of honeysuckle, commonly known as Kamchatka berry, which is one of the most popular fruits in Poland, the Czech Republic, Canada, and Russia. Several varieties native to the species have been selected, which differ, among other things, in terms of flowering time, prolificacy, and content of bioactive substances [3]. A lesser-known variety is Lonicera caerulea var. emphyllocalyx (Maxim.) Nakai, commonly referred to as Japanese haskap. This is native to the island of Hokkaido in northern Japan and is also cultivated in China, Korea, or Russia [4]. The word ‘haskap’, from the Japanese hasukappu, hascup, haskappu, or hasakapu, in the language of the natives literally means a lot of small objects on the tops of the branches. In the regions where the plant originates, the fruit is recognised as a medicine and immune booster, used to treat stomach ailments and protect against many diseases. The native Ainu people of the island of Hokkaido called the fruit the ‘elixir of life’, making it an important part of their diet. They were eaten fresh, preserved with sugar and salt and were also used to prepare spirits and stockpile valuable raw material for winter [5,6].
The L. caerulea fruit is rich in sugars, organic acids, and polyphenols; this has a significant impact not only on sensory perception, but also on the health-promoting properties of the fruit. The content of polyphenols, the main group showing biological activity, can vary depending on the cultivar and the harvest date. The cultivation conditions (soil type and fertilisation method) do not significantly affect the content of active compounds; fruit from different sites will be characterised by similar contents of bioactive compounds [6]. Lonicera caerulea berries are characterised by a high content of phenolic compounds, ranging from 428.14 to 622.52 mg GAE·100 g−1 f.w. [7,8]. L. caerulea is an important source of phenolic compounds such as anthocyanins, flavonoids, proanthocyanidins and phenolic acids [6], the predominant group of phenolic compunds being anthocyanins, mainly cyanidin 3-O-glucoside. This is a widely used compound in the plant world, accounting for about 79–92% of the the anthocyanin content of L. caerulea fruit [9]. Fruits are a valuable source of vitamin C (ascorbic acid), at around 30.5–186.6 mg·100 g−1 [10]. The fruit is a source of minerals, including magnesium (79–163 mg·kg), potassium (3000–5000 mg·kg), phosphorus (486–2252 mg·kg), and calcium (1077 mg·kg) [11]. The berries are characterised by anti-inflammatory, antimicrobial, and antioxidant properties [6]. The fruits of L. caerulea has many uses in food processing. Pulp made from L. caerulea berries has a high juice yield and tiny seeds, making it excellent source of fruit juice [12]. Due to the very dense colour of the fruit juice, it is suitable for the production of food products that require the addition of juice. It also has high antioxidant properties [13,14]. Canned food and spreads are a relatively good way to improve the utility value of L. caerulea; however, due to the high content of sugars, the amount of added sugar should be controlled during the production stage [15]. The processing of fruits might change the levels of bioactive compounds. Fresh-processed foods have more health-promoting properties than the thermally treated foods. The concentration of some compounds might increase after water loss caused by fruit processing methods, e.g., heating. After freeze drying or pressing the contents of some compounds might change [15].
The purpose of this study was to compare the chemical compositions of the fruits of L. caerulea var. kamtschatica ( ‘Duet’ and ‘Aurora’ cultivars) and L. caerulea var. emphyllocalyx ( ‘Lori’, ‘Colin’, and ‘Willa’ cultivars) and the potential use of L. emphyllocalyx fruit in food processing.
2. Results and Discussion
2.1. pH and Acidity of Fruits
The organic acid content of the fruit decreases with successive stages of ripening. The organic acids contained in the fruit are perishable and, under the influence of various factors, e.g., temperature, can change in terms of concentration in the plant material [16,17]. L. caerulea fruit are rich in organic acids (e.g., malic acid, citric acid, quinic acid and fumaric acid; [17]). The contents of individual organic acids significantly influence the taste qualities of ripe fruit and the acceptability of the consumer. The average pH values of the L. kamtschatica and the L. emphyllocalyx fruits were similar: 3.07–3.32 and 3.13–3.52, respectively (Figure 1).
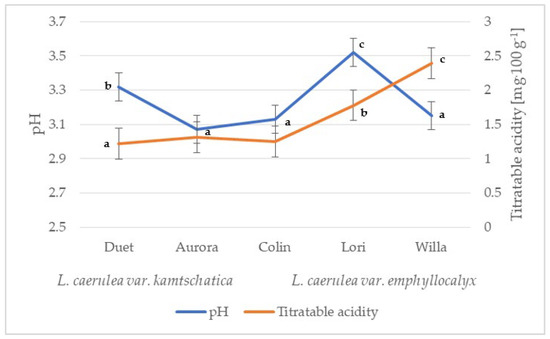
Figure 1.
pH and total acidity of L. caerulea var. kamtschatica and L. caerulea var. emphyllocalyx fruits (data are expressed as mean values (n = 3) ± SD; SD—standard deviation). Mean values with different letters are significantly different (p < 0.05).
These results are comparable to those obtained by other authors. In a study by Gerbrand et al. [18], the fruit pH of Lonicera caerulea ranged from 2.42 to 3.10; in an experiment of Auzanneau et al. [19], the fruit pH of the same species ranged from 2.70 to 3.30, depending on the growing year and harvest date. Compared to Miyashita et al. [20] and Oszmiański et al. [21], we found a lower average pH value in L. kamtschatica and L. emphyllocalyx berries: 2.60 and 2.65, respectively. The pH value is diversified compared to other species grown in Poland, e.g., saskatoon berry (4.12–5.03) [22], red currant (3.20 and 3.27) [23], sea buckthorn (3.02–3.19) [24], highbush blueberry fruit (2.76–3.33) [25], raspberry (3.72) and mulberry (5.17) [26]. In general, both L. kamtschatica and L. emphyllocalyx are rich in organic acids that give a taste resembling blueberries, with a distinct hint of sourness.
The difference in acidity between varieties may have been caused by different climatic conditions. The acidity of the L. kamtschatica fruit was at the level of 1.22–1.31 g·100 g−1, while the L. emphyllocalyx fruit, especially the ‘Willa’ cultivar, had a higher acidity by 29.96% on average compared to the L. kamtschatica fruit (Figure 1). The results in this study are similar to a study by MacKenzie et al. [27], where the total acidity in L. caerulea fruit ranged from 1.6 to 3.0 g·100 g−1, depending on the year of cultivation. The obtained results are comparable with berries grown in Poland, e.g., cranberry (1.56–1.60 g·100 g−1) [28], saskatoon berry (0.3–1.5 g·100 g−1) [22], red currant (0.7–1.6 g·100 g−1) [29], mulberry fruit (0.26 g·100 g−1) and raspberry (0.63 g·100 g−1) [26].
2.2. Contents of Ascorbic Acid and Antioxidant Activity in L. kamtschatica and L. emphyllocalyx Fruit
Ascorbic acid is a powerful antioxidant that is required for the activation of many enzymes. This compound is essential for the functioning of the human body, influencing the immune and circulatory systems, accelerating wound healing, slowing skin ageing, and regulating collagen production [30]. The average ascorbic acid content of the L. kamtschatica fruit was 53.5 mg·100 g−1 f.w., while the L. emphyllocalyx fruit had an average ascorbic acid content of 45.93 mg·100 g−1 f.w. (Table 1). The content ascorbic acid of L. caerulea fruit differs from that reported by Celli et al. [10]; we obtained results ranging from 30.5 to 186.6 mg·100 g−1 f.w. According to Jurnikova et al. [31], the content of ascorbic acid in Lonicera caerulea, higher than 70 mg·100 g−1 f.w., is associated with a lower accumulation of phenolics. The ascorbic acid content of L. kamtschatica and L. emphyllocalyx fruit was at a similar level to popular berries grown in Poland, e.g., strawberries (average: 50.1 mg·100 g−1), raspberries (average: 30.6 mg·100 g−1 f.w.), blueberries (average: 60.1 mg·100 g−1 f.w.) [32,33], plum blackthorn (21.94 mg·100 g−1 f.w.), blackberry (33.85 mg·100 g−1 f.w.) [33]; red currant (31.2 mg·100 g−1–44.1 mg·100 g−1 f.w.) [23], sea buckthorn (4.0–9.1 mg·100 g−1 f.w.) [24]. According to Senica et al. [14], spreads and smoothies made out of L. caerulea berries increase the concentration of ascorbic acid by 100%. Heating plant material before crushing may influence the stability of ascorbic acid, preserving its contents. This can be used in the food industry in the production of L. caerulea products [14].

Table 1.
Ascorbic acid content and antioxidant activity of L. kamtschatica and L. emphyllocalyx fruit.
Lonicera caerulea fruit extract containing anthocyanins is a highly effective antioxidant agent, effectively reducing reactive oxygen species (ROS), which are produced by immune cells as a result of inflammation. Persistent inflammation can lead to cell damage and chronic disease. The fruit extract further reduces lipid peroxidation, which affects cellular damage under oxidative stress conditions, which can contribute to the reduction in diseases related to oxidative stress [34]. The antioxidant activity of L. kamtschatica and L. emphyllocalyx fruit, determined by the DPPH method, was at the level of 68.68–89.62% inhibition, of which the L. kamtschatica cultivar ‘Duet’ had the highest free radical scavenging activity (on average 13.4–30.4% more than the other cultivars analysed). These results are comparable to those obtained by Kula et al. [2] and Khattab et al. [35], in which the antiradical values of Lonicera caerluea were, respectively, 85% and 78.70%. The antioxidant activity of L. caerulea fruits is significantly higher compared to other species, e.g., sea buckthorn (74%), bilberry (37–91%), and garden rhubarb (48–98%) [36]. The iron reduction capacity (FRAP method) of L. kamtschatica range from 30.52 to 37.67 μM Fe2+·g−1 f.w. and L. emphyllocalyx range from 30.52 to 37.67 μM Fe2+·g−1 f.w. Results comparable to this study were obtained by Rupasinghe et al. [7], with an antioxidant value (FRAP) ranging from 27.96 to 46.90 μM Fe2+·g−1. According to studies, L. caerulea had more antioxidative properties (FRAP) compared to other species grown in Poland, e.g., strawberries (8.00 µM TE·g−1 f.w), blackberries (15.03 µM TE·g−1 f.w.), highbush blueberry (16.24 µM TE·g−1 f.w.), elderberry (29.56 mM Fe2+·g−1 f.w.), blackthorn (14.74 mM Fe2+·g−1 f.w.) and wild strawberry (10.99 Fe2+·g−1 f.w.) [7,33]. Lonicera caerulea fruits are characterised by a higher antioxidant capacity. The antioxidant activity of the fruits of L. kamtschatica and L. emphyllocalyx, determined by the ABTS method, ranged from 1.91 to 2.21 mM TE·100 g−1 f.w. As reported by Rop et al. [37], the ABTS antiradical activity of ABTS of Lonicera caerulea was 0.30 µM·g−1. The values obtained in this study are different to other species cultivated in Poland, e.g., highbush blueberry (16.87 mM TE·g−1 f.w.), elderberry (15.88 mM TE·g−1 f.w.), blackberry (9.55 mM TE·g−1 f.w.), strawberries (5.61 TE·g−1 f.w.) [7,33], red currant (11.83–12.59 µM TE·g−1 d.m.) [13], mulberry (0.14 mM TE·100 g−1 f.w.) and raspberry (0.08 mM TE·100 g−1 f.w.) [26]. The high level of antioxidant capacity in L. kamtschatica and L. emphyllocalyx makes them very valuable in terms of bioactivity. Research on the antioxidant activity of extracts with L. caerulea revealed that the fruits of this plant are characterised by stronger antioxidant properties from other berries commonly regarded as effective antioxidants. Considering the relationship between modern-day diseases and long-term oxygen stress, strong antioxidant properties may indicate the potential importance of this fruit, not only in prophylaxis, but also in the treatment of many diseases [2], thus making L. caerulea products more valuable for food industry.
2.3. Polyphenolic Compound Content in L. kamtschatica and L. emphyllocalyx Fruit
The phenolic compounds, mainly anthocyanins, contained in L. caerulea fruit extract exhibit anti-inflammatory effects. They reduce cellular damage under conditions of oxidative stress in in vitro cultures of rat microsomes and reduce ROS production in cultures of pro-inflammatory gingival fibroblasts [6,38]. The contents of total polyphenols differed significantly depending on the cultivar analysed; fruits of the L. emphyllocalyx cultivar ‘Willa’ were characterised by the lowest total polyphenol content—416.94 mg GAE·100 g−1 f.w.—while the highest total polyphenol content of total polyphenols—747.85 GAE·100 g−1 f.w.—was also found in fruits of L. emphyllocalyx but of the cultivar ‘Lori’ (Table 2). The results obtained in this study are comparable to those reported by Rop et al. [37]; the content of total polyphenols in the L. kamtschatica fruit ranged from 575 to 903 mg GAE·100 g−1 f.w, while in the study by Oszmiański et al. [21], the polyphenol content of the L. kamtschatica fruit was 12.29 g·100 g−1 f.w. The analysed fruits of L. emphyllocalyx and L. kamtschatica were characterised by significantly higher total polyphenol contents compared to fruits grown in Poland: raspberries—445.5 mg GAE·100 g−1 f.w.; strawberries—238.0 mg GAE·100 g−1 f.w.; sea buckthorn—302.72 mg GAE·100 g−1 f.w. [6,39,40]; blackberry—247.25 mg GAE·100 g−1 f.w.; blackthorn—402.67 mg GAE·100 g−1 f.w.; highbush blueberry—424.72 mg GAE·100 g−1 f.w.; and elderberry—535.98 mg GAE·100 g−1 f.w. [33].

Table 2.
Individual phenolic compounds identified by UPLC-PDA-MS/MS in L. kamtschatica i L. emphyllocalyx.
The analysis of phenolic compounds using the UPLC-PDA-MS/MS method allowed for the determination of the differences between the contents of individual groups of polyphenolic compounds contained in the fruit of the analysed cultivars of L. kamtschatica and L. emphyllocalyx (Table 2). Berries of the L. kamtschatica cultivars ‘Duet’ and ‘Aurora’, and L. emphyllocalyx cultivars ‘Lori’, ‘Willa’ and ‘Colin’ were characterised by different contents of individual polyphenolic compounds. The total content of phenolic compounds depends, among other things, on the cultivar, the degree of fruit maturity, and also on the harvest date. The identification of compounds was carried out based on retention time (Rt), MS and MS/MS with available publications [41,42,43,44,45]. The extract prepared from the fruit contained 26 different compounds in its composition, including 14 anthocyanins, 8 flavonoids, 2 phenolic acids and 1 flavan-3-ol (Table 2).
The highest anthocyanin content in the analysed fruits was found in the berries of L. kamtschatica ‘Duet’, at 456.3 mg·100 g−1 (Table 2). Among the phenolic compounds found in the fruits of L. kamtschatica (509.29–597.29 mg GAE·100 g−1) and L. emphyllocalyx (416.94–747.85 mg GAE·100 g−1), anthocyanins represented, on average, 94% of all polyphenols, and the main representative was cyanidin 3-O-glucoside—C3G (the compound represented, on average, 82.2% of the total anthocyanin content detected in the fruits). Among the varieties analysed, the L. kamtschatica fruits of the ‘Duet’ variety contained the highest concentration of C3G in their composition—382.18 mg·100 g−1 (Table 2). The results obtained in this study are comparable to those obtained by Rupasinghe et al. [9]; the C3G content of Lonicera caerulea fruit ranged from 68 to 649 mg·100 g−1. C3G content was comparable to that reported by Khattab et al. [35], and the C3G content in the fruit of the cultivars ‘Tundra’, ‘Berry Blue’ and ‘Indigo gem’ reached 79–88% of the total anthocyanin content. The C3G content of the Lonicera caerulea fruit was significantly higher compared to the strawberry fruit (3.7 mg·100 g−1), blueberry (3.0 mg·100 g−1), and the cranberry (0.7 mg·100 g−1) [9]. C3G is the most prevalent anthocyanin in edible fruits and has been shown to have anti-inflammatory, antioxidant, chemotherapeutic, and epigenetic effects [9]. Fruits with a larger diameter and harvested at the optimal harvest time will have a higher anthocyanin content due to the larger skin area [15]. In the study of Senica et al. [15], spreads and smoothies made out of L. caerulea had higher concentrations of C3G (6.48, 5.00 mg·100 g−1) in comparison to fresh fruit. The fruits of this species play important roles in a wide range of physiological processes, e.g., protective effect against UV radiation for skin, protection against pathogenic strains, etc..
The total flavonoid contents ranged from 11.11 mg·100 g−1 in the L. emphyllocalyx cv. cultivar ‘Colin’ to 24.4 mg·100 g−1 in the case of the L. kamtschatica cv. cultivar ‘Duet’ (Table 2). In the L. kamtschatica and L. emphyllocalyx fruits analysed, the average content of flavonoids accounted for 3% of all phenolic compounds and the main representative was quercetin 3-O-rutinoside, which represented an average of 41.6% of all flavonoids (Table 2). Quercetin 3-O-rutinoside content in our study was significantly higher compared to the results of Oszmiański et al. [21]; the L. kamtschatica fruits of the cultivar ‘Wojtek’ were characterised by a quercetin 3-O-rutinoside content of 0.21 mg·100 g−1 f.w. Quercetin 3-O-rutinoside shows a protective effect on the liver or blood vessels and has anti-inflammatory and antidiabetic properties [46].
Two phenolic acids, chlorogenic acid and neochlorogenic acid, were determined in the composition of the L. kamtschatica and L. emphyllocalyx fruits. The fruits of L. kamtschatica cultivars were characterised by a higher content of phenolic acids than those of L. emphyllocalyx cultivars. Of the L. kamtschatica and L. emphyllocalyx cultivars analysed, the L. kamtschatica cultivar ‘Duet’ was characterised by the highest contents of phenolic acids, on average 14.62 mg·100 g−1, which was 51% higher than in the fruit of the L. emphyllocalyx cultivar ‘Colin’ (Table 2). Chlorogenic acid, with an average of 9.77 mg·100 g−1, was present in the analysed fruits at a significantly higher concentration than neochlorogenic acid (Table 2). The chlorogenic acid contained in the fruits analysed constituted 91.8% of all phenolic acids, which is comparable to the results of Kithama et al. [47], who determined that, in the cultivars ‘Aurora’, ‘Evie’, ‘Larissa’ and ‘Rebecca’, the content of chlorogenic acid made up 95% of all phenolic acids contained in L. kamtschatica fruits.
A chemical compound from the flavan-3-ol group—B-type procyanidins—was present among the fruits tested of L. kamtschatica and L. emphyllocalyx cultivars. The highest concentration of B-type procyanidins was determined in the berries of the L. emphyllocalyx cultivar ‘Willa’—an average of 2.35 mg·100 g−1, which was on average 62.1% higher than in the fruit of the cultivar ‘Colin’ (Table 2). In a study by Raudonė [48], L. kamtschatica fruits of the cultivars ‘Wojtek’, ‘Indigo Gem’, ‘Iga’, ‘Leningradskij Velikan’, ‘Nimfa’, ‘Amphora’, ‘Tola’ and ‘Tundra’ contained from 9.76 mg·100 g−1 f.w. to 27.1 mg·100 g−1 f.w. of procyanidin type B procyanidin in their compositions. L. kamtschatica fruits of the cultivar ‘Wojtek’ in the study by Oszmiański et al. [21] were characterised by a procyanidin dimer content of 7.05 mg·100 g−1 f.w.
2.4. Sugar Content of L. kamtschatica and L. emphyllocalyx Fruit
Of the L. kamtschatica berry cultivars analysed, the ‘Duet’ cultivar had the highest total sugar content, with an average of 4968 mg·100 g−1, and glucose was the dominant sugar in the fruit of all the cultivars (Table 3). A significant difference in the contents of total sugars was observed between the fruits of the L. kamtschatica and the L. emphyllocalyx cultivars. The L. kamtschatica fruits of the cultivar ‘Aurora’ were characterised by the highest glucose content, on average 2963 mg·100 g−1, while the significantly lower glucose content between the analysed cultivars was characteristic of L. emphyllocalyx fruits of the cultivar ‘Willa’ (Table 3). However, this cultivar was characterised by a high fructose content (comparable to that of the L. kamtschatica cultivar ‘Duet’) and significantly higher compared to the other cultivars (Table 3). The sucrose content of the fruits analysed ranged from 1325 mg·100 g−1 (L. emphyllocalyx cultivar ‘Willa’) to 2750 mg·100 g−1 (L. kamtschatica cultivar ‘Aurora’; Table 3). The sugar content results are comparable to those obtained by Senica et al. [49], who obtained a total sugar content ranging from 1557.37 to 2585.45 mg· 100 g−1 f.w. The results obtained by Gołba et al. [11] were significantly lower compared to those of this study, the total sugar content of the Lonicera caerulea fruit ranged from 1500 mg·100 g−1 to 2585 mg·100 g−1. The average fructose content of Lonicera caerulea reported by Sharma et al. [17] was comparable with this study and ranged from 1047.53 to 1363.67 mg·100 g−1 f.w., but the content of glucose was much smaller and ranged from 750.89 to 1129.35 mg·100 g−1 f.w. A range of 3.72–126.12 mg·100 g−1 f.w. of sucrose has also been reported by Cheng et al. [50]; these values are comparable to those obtained in the experiment. As reported by Wojdyło et al. [51], Sorbitol was previously found in Polish L. kamtschatica cultivars, with a concentration ranging from 0.1 to 0.4 mg·100 g−1 f.w. The sugar content of L. kamtschatica and L. emphyllocalyx fruit depends on environmental conditions, light intensity, fruit maturity and species, among other factors [16].

Table 3.
Total sugar content of the fruits of L. kamtschatica and L. emphyllocalyx.
3. Materials and Methods
3.1. Material
Fruits of the L. caerulea var. kamtschatica cultivars ‘Duet’ and ‘Aurora’ were obtained from a nursery crop located in Tyczyn (49°57′52″ N 22°2′47″ E, Subcarpathian Voivodship, Poland) in the year 2022. Fruits of the L. caerulea var. emphyllocalyx cultivars ‘Lori’, ‘Willa’ and ‘Colin’ were obtained from ‘Korfanty’ (49°41′41″ N 22°5′3″ E, Grabownica Starzeńska, Subcarpathian Voivodeship, Poland) in 2022. Both species were grown in containers filled with a peat substrate containing sand and perlite in a ratio of 20:1:1, with the addition of fertilizer Osmocote Exact 3–4 m (ICL, Sydney, Autralia) at concentration of 2.0 kg for 1 m3 of substrate.
The average monthly temperatures in the period from March to June in Tyczyn were, respectively, 3.3, 7.0, 14.8, and 19.8 °C, and in Grabownica Starzeńska were, respectively, 2.2, 6.0, 13.7, and 18.5 °C. The average monthly rainfall values in the period from March to June in Tyczyn were, respectively, 20 mm, 60 mm, 50 mm, and 20 mm, and in Grabownica Starzeńska the average monthly rainfall in the period from March to June was 50 mm.
The fruits of the analysed cultivars were harvested by hand at the stage of their harvest maturity (first decade of June), 1000 g each. Immediately after harvest, the fruits were subjected to chemical analysis.
3.2. Determination of pH and Acidity
The total acidity (as citric acid) and the pH of the L. kamtschatica and L. emphyllocalyx fruit were determined through the potentiometric titration of the sample for analysis with a standard 0.1 M NaOH solution at pH = 8.1 using a TitroLine 5000 (SI Analytcs, Weilheim, Germany) according to the method given in PN-EN 12147:2000 [52]. The results are expressed as g of citric acid per 100 g of fruit. The analyses were performed in triplicate.
3.3. Determination of the Contents of Bioactive Compounds in Fruit and Determination of Their Antioxidant Activity
Vitamin C (ascorbic acid) was determined according to PN-A-04019:1998 [53]. Total polyphenol content (mg GAE·100 g−1 f.w.) was determined using the Folin–Ciocalteu method according to the methodology described by Bakowska-Barczak et al. [8]. The identification of the polyphenolic profile in L. kamtschatica and L. emphyllocalyx fruit was determined according to the methodology reported by Gorzelany et al. [24].
The ability of the fruit to reduce iron ions (FRAP method) was determined according to the methodology given by Rupasinghe et al. [26], and the results are given in μM Fe2+·g−1 f.w. The antioxidant activity of the fruit was determined using DPPH methods according to the methodology given by Jurčaga et al. [54], and the result is expressed as % inhibition of DPPH radicals, and through the ABTS method according to the methodology given by Gawroński et al. [1], and the results are expressed in mM TE·100 g−1 f.w. All analyses were performed in triplicate.
3.4. Determination of Sugars in L. kamtschatica and L. emphyllocalyx Fruit
The sugar content was measured using the HPLC method with refractive index detection. The chromatographic equipment SYKAM (Sykam GmbH, Eresing, Germany), consisting of sample injector S5250, pump system S1125, column oven S4120 and RI detector S3590, was used. Separation was carried out using a Cosmosil Sugar-D column 250 × 4.6 mm ( Nacalai, Kyoto, Japan). The separation was achieved with a mobile phase of 70% of acetonitrile in water in isocratic mode. The flow rate was 0.5 mL/min at column temperature set at 30 °C. The volume of injected sample was 20 µL and 15 min was needed to complete the analysis. Samples before injection were centrifuged at 5000 rpm for 10 min using Centrifuge 5430 (Eppendorf, Hamburg, Germany) and diluted with mobile phase 1:4 (v/v). All determinations were performed in triplicate.
3.5. Statistical Analysis
Using Statistica 13.3. software (TIBCO Software Inc., Tulsa, OK, USA), a statistical analysis of the results obtained was performed that included the analysis of variance (ANOVA) and NIR significance test at a significance level of α = 0.05.
4. Conclusions
Based on this study, differences in fruit composition were found in both individual species and the L. caerulea cultivars. The fruits of the L. kamtschatica cultivar ‘Aurora’ contained the highest amount of ascorbic acid in their composition, approximately 22% more compared to ‘Lori’, which had the highest content of this compound among L. emphyllocalyx cultivars. The highest total polyphenol content was found in the L. emphyllocalyx cultivar ‘Lori’, while the predominant polyphenolic compound was cyanidin 3-O-glucoside. On the basis of the results obtained, it can be concluded that the L. emphyllocalyx cultivars ‘Lori’ and ‘Willa’ can find applications in various food industries. Despite the diversity of chemical composition, the fruits have high antioxidant properties compared to the well-known varieties ‘Duet’ and ‘Aurora’. The variety ‘Lori’ has the highest content of phenolic compounds among the tested varieties. ‘Willa’ has the highest concentration of C3G comparable to the widely known varieties of L. kamtschatica and the highest sugar content among L. emphyllocalyx. The use of Lonicera as an ingredient of functional foods, natural colourant or source of natural antioxidant seems to be promising. Compared to other types of berries grown in Poland, e.g., raspberries and red currant, L. kamtschatica and L. emphyllocalyx can be a good source of bioactive substances and sugar, making them good in food industry and processing, including juices, wines, spreads and dried fruits.
Author Contributions
Conceptualisation, O.B. and J.G.; methodology, J.B., I.K. and O.B.; validation, J.B., O.B. and J.G.; formal analysis, J.B. and K.P.; investigation, O.B. and K.P.; writing—original draft preparation, O.B. and J.B.; writing—review and editing, J.G., O.B. and I.K.; visualisation, J.B.; supervision, O.B. and J.B.; project administration, O.B. and J.B.; funding acquisition, J.G. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds used in research are available from the authors.
References
- Gawronski, J.; Hortynski, J.; Kaczmarska, E.; Dyduch-Sieminska, M.; Marecki, W.; Witorozec, A. Evaluation of phenotypic and genotypic diversity of some Polish and Russian blue honeysuckle (Lonicera caerulea L.) cultivars and clones. Acta Sci. Polonorum. Hortorum Cultus 2014, 13, 157–169. [Google Scholar]
- Kula, M.; Krauze-Baranowska, M. Blue Honeysuckle (Lonicera caerulea L.)—The current state of phytochemical research and biological activity. Post Fitoter. 2016, 17, 111–118. [Google Scholar]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Maxine, T.M. Introducing haskap, Japanese Blue honeysuckle. J. Am. Pomol. Soc. 2006, 60, 164–168. [Google Scholar]
- Minami, M.; Takase, H.; Nakamura, M.; Makino, T. Methanol extract of Lonicera caerulea var. emphyllocalyx fruit has anti-motility and anti-biofilm activity against enteropathogenic Escherichia coli. Drug Discov. Ther. 2019, 19, 335–342. [Google Scholar] [CrossRef]
- Ochmian, D.; Skupień, K.; Grajkowski, J.; Smolik, M.; Ostrowska, K. Chemical Composition and Physical Characteristics of Fruits of Two Cultivars of Blue Honeysuckle (Lonicera caerulea L.) in Relation to their Degree of Maturity and Harvest Date. Not. Bot. Horti. Agrobot. 2012, 40, 155–162. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Yu, L.J.; Bhullar, K.S.; Bors, B. Short Communication: Haskap (Lonicera caeruela): A new berry crop with high antioxidant capacity. Can. J. Plant Sci. 2012, 21, 4334. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Marianchuk, M.; Kolodziejczak, P. Survey of bioactive components in Western Canadian berries. Can. J. Physiol. Pharmacol. 2007, 85, 1139–1152. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap Berries (Lonicera caerulea L.)—A Critical Review of Antioxidant Capacity and Health-Related Studies for Potential Value-Added Products. Food Bioprocess Technol. 2014, 7, 1541–1554. [Google Scholar] [CrossRef]
- Khattab, R.; Ghanem, A.; Brooks, M.S.L. Quality of dried haskap berries (Lonicera caerulea L.) as affected by prior juice extraction, osmotic treatment, and drying conditions. Dry. Technol. 2017, 35, 375–391. [Google Scholar] [CrossRef]
- Gołba, M.; Sokół-Łętowska, A.; Kucharska, A. Health Properties and Composition of Honeysuckle Berry Lonicera caerulea L. An U date on Recent Studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef]
- Belyaeva, O.V.; Sergeeva, I.Y.; Belyaeva, E.E.; Chernobrovkina, E.V. Study of antioxidant activity of juices and beverages from blue honeysuckle and black chokeberry. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 052008. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. The effect of the addition of blue honeysuckle berry juice to apple juice on the selected quality characteristics, anthocyanin stability, and antioxidant properties. Biomolecules 2019, 9, 744. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk. J. Agric. For. 2019, 43, 576–585. [Google Scholar] [CrossRef]
- Jurikova, T.; Matuškovič, J.; Gazdik, Z. Effect of irrigation on intensity of respiration and study of sugar and organic acids content in different development stages of Lonicera kamtschatica and Lonicera edulis berries. HortScience 2009, 36, 14–20. [Google Scholar]
- Sharma, A.; Hae-Jeung, L. Lonicera caerulea: An updated account of its phytoconstituents and health-promoting activities. Trends Food Sci. Technol. 2021, 107, 130–149. [Google Scholar] [CrossRef]
- Gerbrand, E.M.; Bors, R.H.; Meyer, D.; Wilen, R.; Chibbar, R. Fruit quality of Japanese, Kuril and Russian blue honeysuckle (Lonicera caerulea L.) germplasm compared to blueberry, raspberry and strawberry. Euphytica 2020, 216, 59. [Google Scholar] [CrossRef]
- Auzanneau, N.; Weber, P.; Kosińska-Cagnazzo, A.; Audlauer, W. Bioactive compounds and antioxidant capacity of Lonicera caerulea berries: Comparison of seven cultivars over three harvesting years. J. Food Compos. Anal. 2018, 66, 81–89. [Google Scholar] [CrossRef]
- Miyashita, T.; Hoshino, Y. Intersecific hybridization in Lonicera caerulea and Lonicera gracilipes: The occurrence of green/albino plants by reciprocal crossing. Sci. Hortic. 2010, 125, 692–699. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S. Effect of dried powder preparation process on polyphenolic content and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L. var. kamtschatica). LWT Food Sci. Technol. 2015, 64, 214–222. [Google Scholar] [CrossRef]
- Gorzelany, J.; Kapusta, I.; Zardzewiały, M.; Belcar, J. Effects of ozone application on microbiological stability and content of sugars and bioactive compounds in the fruit of the saskatoon berry (Amelanchier alnifolia Nutt.). Molecules 2022, 27, 6446. [Google Scholar] [CrossRef]
- Kużniar, P.; Belcar, J.; Zardzewiały, M.; Basara, O.; Gorzelany, J. Effect of Ozonation on the Mechanical, Chemical, and Microbiological Properties of Organically Grown Red Currant (Ribes rubrum L.) Fruit. Molecules 2022, 27, 8231. [Google Scholar] [CrossRef]
- Gorzelany, J.; Basara, O.; Kuźniar, P.; Pawłowska, M.; Belcar, J. Effect of ozone treatment on mechanical and chemical properties of sea-buckthorn (Hippophae rhamnoides L.) fruit. Acta Univ. Cibinensis Ser. E Food Technol. 2022, 2, 183–194. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, B.; Yuan, F. Volatile composition of eight blueberry cultivars and their relationship with sensory attributes. Flavour Fragr. J. 2020, 35, 443–453. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.-H.; Kim, S.-D.; Chang, M.-S.; Jo, I.-S.; Kim, S.-J.; Hwang, K.T.; Jo, H.-B.; Kim, J.-H. Chemical composition, functional constituents, and antioxidant activities of berry fruits produced in Korea. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1295–1303. [Google Scholar] [CrossRef]
- MacKenzie, J.O.; Eiford, E.M.A.; Subramanian, J.; Brandt, R.W.; Stone, K.E.; Sulivan, J.A. Performance of five haskap (Lonicera caeurlea L.) cultivars and effect of hexanal on postharvest quality. Can. J. Plant Sci. 2018, 98, 432–443. [Google Scholar] [CrossRef]
- Gorzelany, J.; Belcar, J.; Kuźniar, P.; Niedbała, G.; Pentoś, K. Modelling of Mechanical Properties of Fresh and Stored Fruit of Large Cranberry Using Multiple Linear Regression and Machine Learning. Agriculture 2022, 12, 200. [Google Scholar] [CrossRef]
- Djordjević, B.S.; Djurovic, D.B.; Zec, G.D.; Meland, M.O.; Fotiric Aksic, M.M. Effect of shoot age on biological and chemical properties of red currant (Ribes rubrum L.) cultivars. Folia Hort. 2020, 32, 291–305. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Jurnikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profiles of edible honeysuckle berries (Genus Lonicera) and Their Biological Effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J. Fruit Ornam. Plant Res. 2009, 17, 115–120. [Google Scholar]
- Paliková, I.; Valentová, K.; Oborná, I.; Ulrichová, J. Protectivity of blue honeysuckle extract against oxidative human endothelilal cells and rat hepatocyte damage. J. Agric. Food Chem. 2009, 57, 6584–6589. [Google Scholar] [CrossRef]
- Khattab, R.; Brooks, M.S.-L.; Ghanem, A. Phenolic analyses of haskap berries (Lonicera caerulea L.): Spectrophotometry Versus High Performance Liquid Chromatography. Int. J. Food Prop. 2016, 19, 1708–1725. [Google Scholar] [CrossRef]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremäe, K.; Pedastsarr, P.; Mäesaar, M.; Raal, A.; Laikoja, K.; Pȕssa, T. The antioxidative and antimicrobial properties of the blue honeysuckle (Lonicera caerulea L.), Siberian rhubarb (Rheum rhaponticum L.) and some other plants, compared to ascorbic acid and sodium nitrate. Food Control 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Rop, O.; Řezníček, V.; Mlček, J.; Juríková, T.; Balík, J.; Sochor, J.; Kramářová, D. Antioxidant and radical oxygen species scavenging activities of 12 cultivars of blue honeysuckle fruit. Hort. Sci. 2011, 38, 63–70. [Google Scholar] [CrossRef]
- Zdarilová, A.; Svobodvaá, A.R.; Chytilová, K.; Simánek, V.; Ulrichová, J. Polyphenolic fraction of Lonicera caerulea L. fruits reduces oxidative stress and inflammatory markers induced by lipopolysaccharide in gingival fibroblasts. Food Chem. Toxicol. 2010, 48, 1555–1561. [Google Scholar] [CrossRef]
- Kant, V.; Mehta, M.; Varshneya, C. Antioxidant potential and total phenolic contents of seabuckthorn (Hippophae rhamnoides) pomace. Free. Radic. Antioxid. 2012, 2, 79–86. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compound and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 4, 816–823. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2017, 244, 705–719. [Google Scholar] [CrossRef]
- Truong, V.D.; McFeeters, R.F.; Thompson, R.T.; Dean, L.L.; Shofran, B. Phenolic Acid Content and Composition in Leaves and Roots of Common Commercial Sweet Potato (Ipomea batatas L.) Cultivars in the United States. J. Food Sci. 2007, 72, 343–349. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.H.; Koo, B.; Kim, H.-B.; Jo, Y.-Y.; Kweon, H.; Ju, W.-T. Flavonoids analysis in leaves and fruits of Korean mulberry cultivar, Baekokwang having white fruits. Int. J. Ind. Entomol. 2020, 41, 45–50. [Google Scholar] [CrossRef]
- Ju, W.-T.; Kwon, O.-C.; Kim, H.-B.; Sung, G.-B.; Kim, H.-W.; Kim, Y.-S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limiatation of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Kithama, A.; De Silva, H.; Rupasinghe, H.P.V. Polyphenols composition and anti-diabetic properties in vitro oh haskap (Lonicera caerulea L.) beries in relation to cultivar harvesting date. J. Food Compos. Anal. 2019, 88, 103402. [Google Scholar] [CrossRef]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žwikas, V.; Viškelis, P. Phenolic profiles, antioxidant activity and phenotypic characterization of Lonicera caerulea L. berries, cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera cearulea L. subs. Edulis) berry; A rich source of some nutrients and their differences among four different cultivars. Sci. Hortic. 2018, 19, 215–221. [Google Scholar] [CrossRef]
- Cheng, Z.; Bao, Y.; Li, Z.; Wang, J.; Wang, M.; Wang, S.; Wang, Y.; Wang, Y.; Li, B. Lonicera caerulea (Haskap berries): A review of development traceability, functional value, product development status, future opportunities and challenges. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, A.A.; Oszmiański, J.; Golis, T. Varability of Phytochemical Properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica Berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- PN-EN 12147:2000; Fruit and Vegetable Juices—Determination of Titrable Acidity. Polish Committee for Standardization: Warsaw, Poland, 2000.
- PN-A-04019:1998; Food Products—Determination of Vitamin C Content. Polish Committee for Standardization: Warsaw, Poland, 1998.
- Jurčaga, L.; Bobko, M.; Kolesárová, A.; Bobková, A.; Demianová, A.; Hašćík, P.; Belej, L.; Mendelová, A.; Bučko, O.; Kročko, M.; et al. Blackcurrant (Ribes nigrum L.) and Kamchatka Honeysuckle (Lonicera caerulea var. Kamtschatica) Extract Effects on Technological Properties, Sensory Quality, and Lipid Oxidation of Raw-Cooked Meat Product (Frankfurters). Foods 2021, 10, 2957. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
