Abstract
As a promising kind of functional material, highly reactive thermite energetic materials (tEMs) with outstanding reactive activation can release heat quickly at a high reaction rate after low-energy stimulation, which is widely used in sensors, triggers, mining, propellants, demolition, ordnance or weapons, and space technology. Thus, this review aims to provide a holistic view of the recent progress in the development of multifunctional highly reactive tEMs with controllable micro/nano-structures for various engineering applications via different fabricated techniques, including the mechanical mixing method, vapor deposition method, assembly method, sol-gel method, electrospinning method, and so on. The systematic classification of novel structured tEMs in terms of nano-structural superiority and exothermic performance are clarified, based on which, suggestions regarding possible future research directions are proposed. Their potential applications within these rapidly expanding areas are further highlighted. Notably, the prospects or challenges of current works, as well as possible innovative research ideas, are discussed in detail, providing further valuable guidelines for future study.
1. Introduction
Energetic materials (EMs), regarded as a kind of promising functional material, can generate enormous amounts of thermal energy at a high rate of heat release within a surprisingly short time (<1 s) after a small energy stimulation, and are widely used in the fields of sensors, trigger, mining, propellants, demolition, ordnance or weapons, civilian and military [1,2,3,4]. With the rapid development of nanotechnology, designing nano-energetic materials (nEMs) with nanostructured-fuel and oxidizer has attracted increasing attention, especially in the last two decades [5,6]. Compared with traditional micro-EMs, nEMs have a faster burning rate and a more intense loading process due to a larger contact area and shorter mass transfer distance among reactants during the exothermic reaction process [7,8], and their superior structure and performance advantages allow them to exhibit wider application potentials in micro-electro-mechanical systems (MEMS), lab-on-a-chip devices, miniaturized or sophisticated weapons and equipment [9,10,11,12,13,14].
As the promising subclass of nEMs, highly reactive thermite EMs (tEMs) with an outstanding heat release ability due to high reaction enthalpy have drawn increasing interest and have wide application prospects in the fields of sensors, microelectronics, propeller machinery, miniaturized detonating primers and advanced military industry [15]. Generally, the metal in tEMs acts as the high energy fuel, and the other reactants act as oxidizing agents, including metal or nonmetal (Ni [16], Ti [17], etc), oxidizer (RxOy) (R = Fe [18,19,20], Cu [21,22,23], Bi [24], Co [25,26], Ni [27], Mo [28], W [29], etc.), inorganic salt (polytetrafluoroethylene (PTFE) [30], KMnO4 [31], NaClO4 [32], etc.), organic molecule (trinitrotoluene (TNT) [33], Hexanitrohexazisowootane (CL-20) [34], trimethylenetrinitramine (RDX) [35] etc.), 3D organic framework [36], etc. For example, activated Al/Co3O4 tEM was prepared by high-energy ball milling, as reported by R. J. Yang group, and the reaction properties of the Al/Co3O4 powders with the water steam were closely related to the milling time [25]. K.S. Martirosyan et al. systematically studied eight energetic systems: nano-Al@Fe2O3, Al@Fe3O4, Al@MnO2, Al@MoO2, Al@WO3, Al@Bi2O3, and Al@CuO, demonstrating that the Al@Bi2O3 generated the highest peak pressure of ~10 MPa due to the lower boiling point of metal Bi, showing great application in gas generators [37]. Moreover, due to long-range electrostatic force and covalent interactions, carbon fiber oxidant (CFO) was introduced to design CFO/Al/Bi2O3, which can detonate RDX without primary explosives and bridge film, largely improving the reliability of the ignition device [38]. Notably, the nanostructures largely grant tEMs advantages in detonation performance and exothermic process and show wider potential applications in the fields of war industry, electronics, national defense, etc.
Designing tEMs with different novel microstructures and improving the energy-releasing capacity of tEMs have been two of the most essential research focuses, and the corresponding amount of important literature regarding tEMs is growing fast. In fact, some reviews are available for energetic materials [39,40,41]. For example, Y.M. Maximov summarized the common exothermic reactions and their main utilization of metallurgical applications, synthesis of materials, etc. [40]. The preparation methods and fundamental properties of the various kinds of core-shell structured nEMs were concluded, and the future related research challenges (e.g., not enough research on the combustion mechanisms of complex nEMs) was proposed by K.L. Zhang group [3]. However, a large quantity of reported emerging oxidizer materials have been explored to optimize the performances of tEMs, especially in the last few years. And the family system or classification of tEMs is extended accordingly. For example, in situ synthesis yielded novel metastable intermixed core-shell n-Al@annic acid@M(IO3)x (M = Fe, Cu, Bi) nanocomposites with Al as the core and annic acid as an interfacial layer, which demonstrated higher thermal reactivity, larger volume reaction heat, higher combustion efficiency, and a faster burning rate than mechanically mixed n-Al/M(IO3)x [42], showing a wide application. Moreover, several novel and high-efficiency synthetic technologies (e.g., two-step ball milling) are emerging in the latest reports. Thus, it is urgent to update research progress or review on tEMs.
This review focuses on nEMs, mainly reviewing tEMs in terms of classification, fabrication technique and breadth of application (Figure 1). Section 2 summarizes the more complete classification of tEMs. Notably, the advantages and drawbacks of different preparation methods are clarified in Section 3. Moreover, we will also highlight the recent impressive application prospects of emerging tEMs and their associated challenges in Section 4. Finally, the conclusions are elaborated in Section 5.
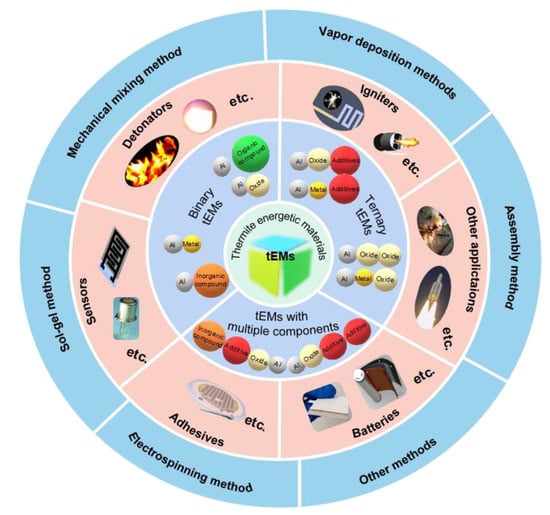
Figure 1.
The classification, application and preparation process of highly reactive tEMs.
2. Classification of Highly Reactive Thermite EMs (tEMs)
It cannot be denied that after domestic and foreign exploration in recent decades, the research of tEMs has been gradually systematized, theorized and diversified. In general, tEMs can be divided into binary and multivariate materials according to the type of components.
2.1. Binary tEMs
2.1.1. Al/Metal Oxide tEMs
Regarding the oxidizer components of tEMs, metal oxides are the most frequently used due to their diversity, easy accessibility, and high thermal reactivity with Al, which is generally regarded as the most intensively researched energetic system. They can release energy quickly because of the classic aluminothermic reaction, which provides their own oxygen supply due to the presence of oxygen and can be self-sustaining. The commonly used metal oxides include Fe2O3, MnO2, Cr2O3, WO3, Bi2O3, CuO, NiO, etc. [43]. The microstructures of Al/metal oxide composite energetic materials are adjusted by different fabrication methods, and the choice of metal oxide is mainly determined according to different application requirements.
As a classical thermite system, Al/Fe2O3 EMs can release a lot of thermitic reaction heat under the excitation of small external energy. The heat-release (Q) of the theoretical stoichiometric Al/Fe2O3 is more than 3.9 kJ/g, and its adiabatic temperature is up to 3135 K [44]. Different fabrication techniques have been explored to obtain Al/Fe2O3 EMs, including mechanical mixing, self-assembly, electrophoretic deposition, etc. [3,18,20,45]. For example, Y.J. Luo group prepared Al/Fe2O3 EMs via a self-assembly method combined with a sol-gel process method, and the nano-Al particles did not aggregate and were coated by the nano-Fe2O3 particles, providing a high effective contact area between the fuel and oxidizing agent (Figure 2a,b). Moreover, the heat release of the assembly-Al/Fe2O3 sample can reach ~2.0 kJ/g [46]. The advanced isoconversional kinetic analysis of Fe2O3-2Al thermite reaction was studied by M.J.S. de Lemos group, which is used for plug and abandonment of oil wells [19]. The Al/Fe2O3 composite with promising three-dimensional structures (Figure 2c,d) was constructed by L Hao team using a selective laser melting method [47]. N.N. Thadhani et al. reported the Al/Fe2O3 EMs using nano-Al particles and the Fe2O3 nanotubes via facile mixed self-assembled technique, and the commercially purchased nano-Al powders are relatively evenly distributed around the Fe2O3 nanotubes (Figure 2e), largely contributing to the adequate thermite reaction. The rapid reaction propagation occurs throughout the self-assembled Al/Fe2O3 EMs when nano-Al starts to melt, and reactive sites quickly spread from the melting of the Al core to the surface of the Al particles to react with the surrounding oxidizers-Fe2O3 (Figure 2f) [48], and distinct segregated agglomeration of Al clusters was observed throughout the physically solvent-mixed Fe2O3 nanotubes-Al nanoparticles sample (Figure 2g,h). S. H. Kim et al. reported nano-/micro-Al/Fe2O3 EMs using a simple ultrasonic mixing method, and successfully demonstrated that the Al/Fe2O3 EMs can act as an effective heat energy source for melting the SAC 305 (Sn: 96.5 wt%, Ag: 3.0 wt% and Cu: 0.5 wt%) powder layer and as a bonding medium to realize the alloying reaction of two metal (Al/Cu) substrates. The mechanical bonding strength of the bonded Al/Cu substrates can be controlled by adjusting the fuel-to-oxidizer ratio in the EM layer [49].
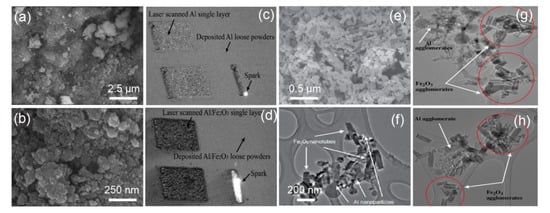
Figure 2.
Field emission scanning electron microscopy (FESEM) image (a,b) of Al/Fe2O3 nEMs by soft template self-assembly with sol-gel process [46] Copyright 2015, Journal of Solid State Chemistry, a comparative visual observation of laser melting method process on a thick layer of (c) Al powder and (d) Al/5 wt% Fe2O3 powder mixture [47] Copyright 2012, Advanced Engineering Materials, the (e) FESEM and (f) transmission electron microscope (TEM) image of self-assembled Al/Fe2O3 nEMs, and followed by the (g) and (h) TEM images of physically solvent-mixed Fe2O3 nanotubes-Al nanoparticles sample [48] Copyright 2010, Combustion and Flame.
In comparison, another typical energetic system, Al/CuO EMs, has been also intensively investigated because of its large energy density, high theoretical heat (~4.08 kJ/g) of reaction, and high reactivity [8,23,37,50,51]. Due to the uniqueness of nature of CuO, it can be synthesized into different structures, including rods, wires, tubes, porous, or sphere structures. Thus, Al/CuO EMs with more and more novel structures have been explored, showing promising ignition and combustion performance. For the core-shell CuO/Al EMs in Yang et al. [22] and reports, CuO nanowires as core are fabricated by electrochemical methods; subsequently, Al as shell is sputtered on CuO nanowires (Figure 3a,b), indicating an effective technique, which is also demonstrated by C.P. Yu et al. [52] and X Zhou et al. [53]. The target CuO/Al EMs showed a great heat-release process [22,52,53], a low reduction of activation energy (181.142 kJ/mol) and a lower ignition energy of only 10 mJ [22]. The superhydrophobic Al/CuO film was fabricated by electrophoretic deposition using the mixture of ethanol and acetyl acetone as the optimal dispersant. The target energetic film shows good structural superiority (e.g., even distribution, porous, nanoscale-Al or CuO, as seen in Figure 3c,d) and stable heat-release capacity for at least one year [54]. In addition, Al/CuO EMs with different microstructures, including the novel leaf or flaky-like CuO/Al EMs, were designed by hydrothermal reaction and mechanical mixing [55], and the 3D-ordered macroporous Al/CuO EMs were also fabricated by colloidal crystal template of polystyrene microspheres combined with magnetron sputtering technique, and the target film showed a three-dimensional and macroscopically ordered network with not only inter-connected, but also controllable distributed porosity (Figure 3e–h), possessing higher heat release (2541.4 J/g for CuO/Al with the deposition thickness of 200 nm) compared to that of the mechanically mixed sample, and a low activation energy (213.36 kJ/mol) and high combustion pressure/pressurization rate (24.6 MPa/647.98 GPa/s), respectively [56]. Another Al/CuO EM with even, multilayer structures were neatly obtained by the magnetron sputtering method [57]. In addition to exploring different structures, research into thermite reactions or the definition mechanisms of Al/CuO EMs are also receiving increasing attention. For example, C. Rossi group [58] reported a new 2D nonstationary model implementing both oxygen and Al diffusion and solving the differential equations for heat and mass transport and chemical reactions, and reached interesting results regarding the inverse evolution of flame front width with respect to the reaction front velocity, contributing to boosting the reaction velocity and the upfront heating after adding a metallic particle into the Al/CuO energetic system due to the high thermal conductivity of added metal. Additionally, in Al/CuO EMs, the nature of the monolayer interface between CuO and alumina/Al is the key factor in controlling the kinetics of Al diffusion, providing a theoretical reference for understanding the essence of the thermite interfacial reaction [59].
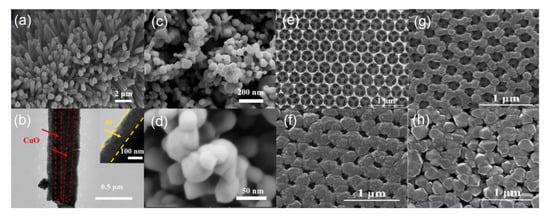
Figure 3.
Typical FESEM image (a,b) of Al/CuO EMs by electrochemical and magnetron sputtering method [22] Copyright 2021, Journal of Alloys and Compounds, typical FESEM images with low (c) and high (d) resolution of the superhydrophobic Al/CuO energetic films [54] Copyright 2018, Materials Letters, (e) the 3D porous CuO prepared bycolloidal crystal template of polystyrene microspheres [56], followed by (f) the 3D Al/CuO EMs after magnetron sputtering of Al with different thicknesses of (f) 200 nm, (g) 100 nm, and (h) 300 nm, respectively. Copyright 2020, Chemical Engineering Journal.
Moreover, there are several other representative oxidizers such as Co3O4, NiO, MnO2, MoO3, WO3, and Bi2O3 also used to design Al/metal oxide EMs. Al/Co3O4 EMs can show high output of heat (~9.6 kJ/g) and a mild detonation pressure (12.6 ± 1 to 20 ± 2 MPa) [60], and highly exothermic super-hydrophobic Al/Co3O4 EMs have been prepared by an electrophoretic assembly and surface energy treatment method [15,61]. The target energetic films show nanoscale and even mixing, and a violent deflagration process after capacitive detonation ignition, a low activation energy, and superlong hydrophobic stability, underscoring the significance of the exothermic stability in long-term humid environments. The Al/NiO EMs usually show a great advantage in the design of a low-gas trigger or igniter due to the lesser amount of gas produced during the thermitic reaction of Al and NiO. The theoretical gas production was ca. 2% of that from Al/CuO EMs [62]. As a comparison, the heat-release property of Al/MoO3 EMs was also explored due to the high theoretical stoichiometric thermite reaction of 4703 J/g [63]. Moreover, among the common EMs, the Al/Bi2O3 EMs show ultra-high deflagration and shock pressure, which are widely used as a gas sensors. For example, compared with Al/CuO, Al/MoO3, and Al/PTFE EMs, the Al/Bi2O3 with highest unconfined burning rate of 420 ms shows the maximum pressurization rate (~5762 kPa/μs) and shortest delay time (5 μs) to reach the maximal pressure, better than the others: 172, 35, and 33 kPa μs−1, and 15, 110, and 550 μs for Al/CuO, Al/MoO3, and Al/PTFE, respectively [64].
2.1.2. Al/Metal tEMs
As a relatively special branch of tEMs, Al/metal EMs, which contains different metals, can release immense energy due to the intermetallic reaction process, and can also generate high reaction temperature, showing great potential application value, especially in welding and alloy forming. Because of the growing enthusiasm for research, this kind of Al/metal EMs has high performance requirements, so there are relatively few research objects to choose from. Current studies mainly focus on Al/Ti, Al/Ni, and so on. Thompson et al. researched synthesized multilayer Al/Ni/composite films by electron beam alternating evaporation method, and the burning rate could be as high as 400 m/s after electric heating excitation [65]. M. Milosavljevic group prepared nano-scale Al/Ti multilayer composite films by magnetron sputtering [66]. Moreover, Wang Liang’s research group studied the exothermic reaction processes of Al/Ti and Al/Ni using numerical simulation method, compared with the experimental data, which provides a favorable reference for the research of other metal composite energetic materials [67]. It is worth mentioning that in our group, the Al/Ni nano-composite energetic film was designed by an electrophoretic deposition method after optimizing suspension composition [16], showing great deflagration with a heat output of 316.2 J/g, and its exothermic stability was greatly improved after fluoride surface modification treatment [68]. In addition, promising 3D porous superhydrophobic Al/Ni EMs with great application value have been fabricated via a simple two-step method combined with hydrogen bubble dynamic template and electrophoretic deposition technique after 1H, 1H, 2H, 2H-perfluorodecyltriethoxysilane treatment [69], and the schematic diagram of the synthesis procedure is displayed in Figure 4, providing a promising novel technique for designing micro/nano energy materials for longer-term storage or transportation, especially in high humidity environments.
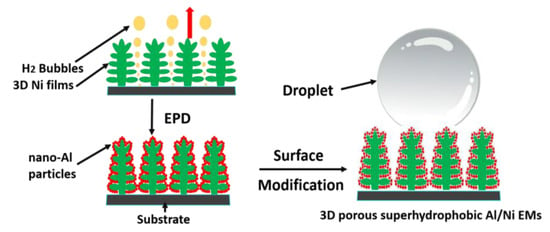
Figure 4.
The schematic diagram of the synthesis process for 3D porous superhydrophobic Al/Ni EMs [69]. Copyright 2016, Applied Surface Science.
2.1.3. Al/Organic or Macromolecule Compound tEMs
As a new style of tEMs that have widely been reported on at home and abroad in recent years, Al/organic or macromolecule compound tEMs have attracted much interest recently. The appropriate organic or macromolecule compounds should have strong oxidation property, usually including Teflon or polytetrafluoroethylene (PTFE), perfluoropolyether (PFPE), trinitrotoluene (TNT), hexanitrohexaazaisowurtzitane (CL-20), metal-organic frame (MOF), and other high-energy nitramines. For example, the reaction kinetics of Al and polytetrafluoroethylene (PTFE or Teflon) were recently analyzed using nanoparticles of both Al and Teflon [70], and the nanoparticles with smaller particle size were more beneficial to the unique pre-ignition reaction, delaying the decomposition temperature of teflon and facilitating the Al/Teflon reaction. Compared with the nano-Al/RXOY (R = Bi, Cu, Mo) EMs, the micron-sized PTFE/Al showed the lowest burning and pressurization rate and longest delay time to reach the maximal pressure [64]. Moreover, using a situ-synthesized polydopamine (PDA) binding layer can effectively improve energy release and reduce sensitivity and, more importantly, tunable reactivity in an integrated n-Al@PDA/PTFE EMs, compared with that of the traditional n-Al/PTFE EMs [71]. Moreover, N. A. Clayton reported on Al/PFPE EMs based on polystyrene fibers via an electrospinning technique, and found that the increased loading of n-Al/PFPE results in a decrease in temperature of decomposition, and the loading of the energetic blend into the fibers showed little effect on the combustion rate from flame propagation experiments [72]. Brousseau group [73] found that compared with a micron-sized Al/TNT mixture, the use of nano-Al can reduce the critical diameter of the mixture and increase the combustion and heat release. In addition, an interesting “father-son” exothermic effect in the promising Al/[Mn(BTO)(H2O)2]n (BTO = 1H,1′H-[5,5′-bitetrazole]-1,1′-bis(olate)) prepared through a simple ultrasonic dispersion method was due to the contribution of the “father” [Mn(BTO)(H2O)2]n’ thermal decomposition reaction and the “son”-metal oxide’s thermite reaction with nano-Al, showing the excellent energetic properties [74]. A novel and promising energy-storage system using energetic metal-organic frameworks (EMOFs) as the oxidizers has been proposed recently [75], and the preparation mechanism is shown in detail in Figure 5. The energetic MOF-activated Al (n-Al@EMOFs) with the multilayer core-shell structure guarding against n-Al oxidation displays the unique two-step exothermic processes (n-Al@EMOF→n-Al@PDA + CuO + Others and n-Al@PDA + CuO→Al2O3 + Cu + others) with a total heat release of ~4142 J/g), and lowers the ignition temperature to 301.5 °C, which lays the groundwork for the development of novel tEMs.
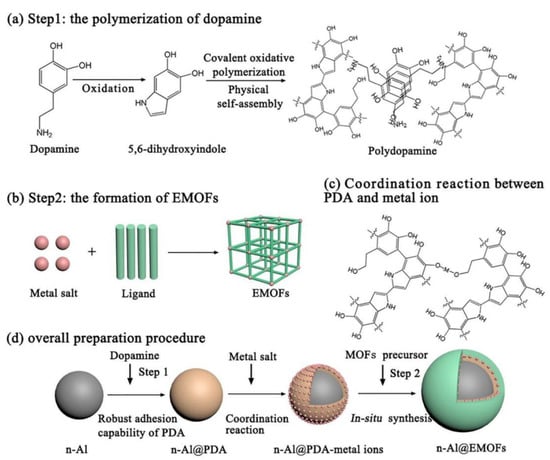
Figure 5.
Schematic illustrations of the core-shell n-Al@EMOFs tEMs: (a) the polymerization of dopamine; (b) the formation of EMOFs; (c) metal-chelating reactions between PDA and metal ions; (d) the overall preparation procedure [75]. Copyright 2020, Chemical Engineering Journal.
2.1.4. Al/Inorganic Compound tEMs
Inorganic compounds are regarded as another attractive oxidizing agent to be applied to tEMs due to their low-cost and convenient preparation process, and the reported inorganic salts include KMnO4, Na/KIO4, NaClO4, etc. For example, Zachariah et al. [76] reported on Al/KMnO4 EMs using KMnO4 as the oxidizing agent due to its high volatility, strong oxidation nature, and low decomposition temperature (~300 °C), and the target EMs showed ultra-fast reactivity and a pressurization rate of 290 psi/μs, compared with that of Al/Fe2O3, Al/CuO, and Al/MoO3. Moreover, the same group also designed Al/periodate salts (NaIO4 and KIO4) EMs [77], whose maximal detonation pressure can reach ~40 atmospheres within a very short time (~0.01 ms). The direct gas phase oxygen release from the NaIO4 and KIO4 decomposition is key to the ignition and combustion of Al/periodate EMs, showing a great advantage in designing super-reactive nano energetic-based gas generators. AgIO3 was selected as a special oxidizer for systems designed for biocidal activity. The Al/AgIO3 EMs have multiple advantages, including a faster deflagration process, better pressurization enhancement ability due to the released gases (O2 and I2) from AgIO3 than the traditional Al/CuO and Al/Fe2O3 EMs, and a long-lasting biocidal nature, indicating a wide range of potential thermite-based biocidal applications [78].
2.2. Ternary tEMs
With the increasing improvement requirements for performance, including increasing output of heat, and adjusting the reaction velocity, energy density, or sensitivity, ternary tEMs with single or multiple fuels and oxidants have gradually entered the field of vision of researchers. For example, ammonium perchlorate (AP), as a widely used oxidizing agent in solid rocket propellant, has been added in Al/Fe2O3 EMs to obtain AP/Al/Fe2O3 ternary nano-thermites [79], showing a lower activation energy of 109.22 kJ/mol and a stronger exothermic peak, with the output of heat of ~1.82 kJ/g, which is 1.3 times that of the simply mixed sample. Several other additives of polytetrafluoroethylene (PTFE) [80], graphene oxide [81], hexogen (RDX) [82] and SiO2 [83] have been added into traditional binary Al-based EMs, forming the corresponding ternary tEMs of Al/Bi2O3/PTFE, Al/CuO/graphene oxide, Al/Fe2O3/RDX and Al/Fe2O3/SiO2, respectively. The addition of nonmetals (boron, etc.) or (Ni, etc.) can also enhance the exothermic capacity of tEMs. For example, thermodynamically, boron (B) releases more energy on both a mass and volumetric basis, thus, nano-B can enhance the reactivity of Al/CuO EMs when added as the minor component (<50% by mole) of the fuel [84]. The ternary composite AlxNiy(Bi2O3)z has also been designed and showed better heat release than that of Al/Ni EMs [16] and Al/Bi2O3 EMs, also processing excellent thermal stability property at least for nearly one year [85].
2.3. tMs with Multiple Components
Additionally, tEMs with multiple components comprise a promising branch of energetic materials. At present, there are also a few reports on adding multiple additives to design novel tEMs. For instance, the Al/CuO/(polyvinylidene fluoride) PVDF/RDX energetic microspheres assembled by an electrospray technology method (Figure 6a) display a shorter delay time, burning time, and higher pressure, pressurization rate than the physical mixtures, and the content of RDX effectively regulates the combustion performance of the product [86]. A. Fahd et al. reported on the quaternary nitrocellulose/GO/Al/KClO4 EMs (Figure 6b) using a facile electrospinning method. The addition of nitrocellulose (NC) not only contributes to improving the heat-release process, but also introduces another step to the GO/Al/KClO4 reaction of a gas–solid phase and liquid–liquid phase diffusion reaction, with the liquid–liquid phase reaction increasing with the content of NC [87]. Adding different inert materials into tEMs will be an effective strategy to solve the safety issue, and adjust exothermic performance and sensitivity according to specific requirements.
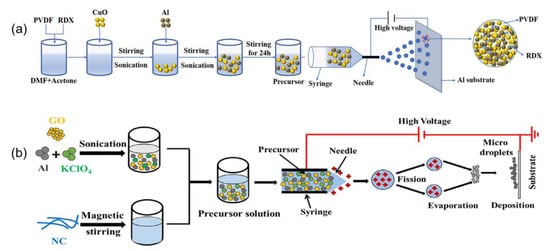
Figure 6.
Schematic illustration of (a) quaternary Al/CuO/PVDF/RDX microspheres [86] Copyright 2021, Chemical Engineering Science, and (b) quaternary NC/GO/Al/KClO4 nEMs [87], respectively. Copyright 2021, Combustion and Flame.
3. Synthesis Methods for tEMs
3.1. Mechanical Mixing Method
The mechanical mixing method is the most common and widely used technique to obtain tEMs with an appropriate reaction measurement ratio after accurate weighing. The method does not need large-scale instruments, is simple to operate, and has a wide application range. Thus, lots of researchers at home and abroad use this approach to study the properties of tEMs. For example, as one of the most frequently studied systems, the Al/CuO tEMs is conveniently prepared by the ultrasonic mixing method [55,88]. The K. Lee group proposed that the maximum pressurization rate occurs in Al-rich thermite composites due to the higher deposition rate of Cu on the surface of Al particles during thermite reaction than that of the whole heat-release reaction, rendering Al NPs relatively less reactive too the activity [88], which provides valuable reference for designing other tEMs with better exothermic performance. The heat release performance of four systems: Al/MoO3, Al/CuO, Al/Bi2O3, and Al/WO3 EMs, were systematically analyzed by the mechanical mixing method [89], and the relationships between the molar ratio of different components and heat release, explosion pressure, and burning rate were explored, respectively. In addition, K.S. Martirosyan et al. [37] investigated eight kinds of EMs: Al/Fe2O3, Al/Fe3O4, Al/MoO3, Al/MoO2, Al/CuO, Al/WO3, Al/Bi2O3, and Al/MnO2, and concluded that the explosive pressure of Al/Bi2O3 EMs was the largest, producing a ultra-high pressure pulse of nearly 100 atmospheres, and the peak pressure generally increases using smaller Al and/or bismuth oxide particles, which proved that Al/Bi2O3 tEMs has a great application prospect in gas sensors. Undeniably, the mechanical mixing method is a simple and effective method for screening valuable or superior tEMs, but it shows no advantage for film-forming combined with different devices used in various applications.
3.2. Vapor Deposition Method
Vapor deposition method is regarded as an effective technique for designing tEMs with controllable stratified structures. The key of this method is how to change solid or liquid material into gas by means of external energy, including electromagnetic energy, thermal energy, etc., and to control it precisely.
One of the most common and effective methods is magnetron sputtering, which is a method of using high-speed charged ions accelerated by the external electric field to collide with the target sputtering material, and under the control of certain appropriate parameters such as voltage, frequency, electromagnetic field, etc. The main advantage of the magnetron sputtering technique is the high degree of controllable thickness of a single layer among layered tEMs. For example, multi-layer Al/Ni tMEs with different layer thicknesses 50–200 nm were prepared by the magnetron sputtering method [90], and their exothermic performances were largely affected by the layer thickness. The highest electrical explosion temperature of 7000 K occurred when the layer thickness was 50 nm. Until now, lots of frequently reported tEMs, such as Al/Ti [66], Al/MoOx [91], and Al/CuO [92], have been designed by this method, applied in trigger and detonator with superior performances. Another common method is using thermal evaporation to fabricate tEMs with outstanding properties. For example, Al/NiO EMs based on silicon substrates have been fabricated by the thermal evaporation technique, showing the advantages of enhanced interfacial contact area, lowered ignition temperature (~400 °C), reduced impurities, tailored dimensions, and strong heat capacity (~2.2 kJ/g) [93]. The sequence of steps to prepare Al/Fe2O3 EMs via this method (Figure 7a) was proposed by L. Menon et al. [94]. Moreover, atomic-layer deposition is another approach using uninterrupted, sufficient gas-solid reactions to obtain target coatings. For example, Al/ZnO EMs with stratified or core-shell structures [95] have been successfully prepared by using the atomic-layer deposition technique, and the reaction rate of the core-shell-structured Al/ZnO EMs was several times faster than that of the simple mixed sample under laser ignition experiments.
In addition, several recent reports have focused on a combination method of vapor deposition and other techniques to design novel tEMs. For example, the Al/Co3O4 EMs were also successfully fabricated by chemically synthesizing Co3O4 nanorods as the core (Figure 7b,c) and depositing Al layers as the shell (Figure 7d,e) using thermal evaporation, and the total heat of reaction was as high as 3635 J/g, benefiting from the full contact core-shell structure [96]. The Al/NiO or Fe2O3 or Co3O4 EMs [97,98,99] with a novel ordered macroporous structure was prepared by polystyrene colloidal template technique and thermal evaporation or magnetron sputtering method, and the corresponding schematic of the synthesis procedure is displayed in Figure 7f.
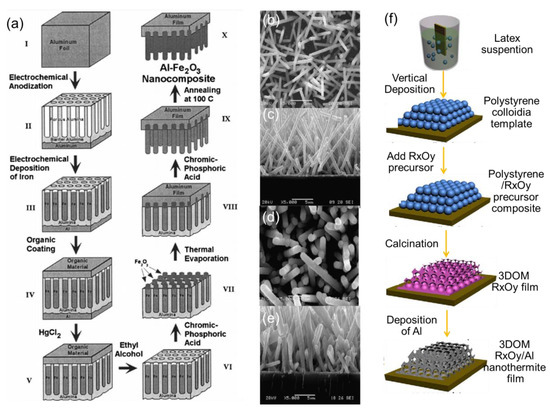
Figure 7.
(a) The steps for preparing Al/Fe2O3 energetic nanocomposites [94] Copyright 2012, Journal of Applied Physics, SEM images ((b) top view and (c) cross-section view) of the Co3O4 nanorods; SEM images ((d) top view and (e) cross-section view) of the Al/Co3O4 nEMs [96] Copyright 2012, Combustion and Flame, and (f) the schematic of the synthesis procedure for 3D ordered macroporous (3DOM) RXOY nanothermite films, (R = Fe, Co and Ni) [98] Copyright 2016, Materials and Design.
3.3. Assembly Methods
3.3.1. Electrophoretic Assembly Method
The electrophoretic assembly method is regarded as a convenient and low-cost technique to assemble differently charged micro/nanoparticles or polymer molecules to form even target films on substrates with different structures [100,101,102], including tiny devices used in micro-electro-mechanical systems (MEMS). Recently, different kinds of tEMs with controllable structures and properties were successfully fabricated by the electrophoretic assembly method. For example, Al/CuO binary tEMs were obtained by the electrophoretic assembly technique on a simple two-dimensional surface [103] and complex 3D printing substrate with channels and hurdles (Figure 8a) [104]. The schematic illustration of the electrophoretic assembly process is shown in Figure 8b, followed by optical images of channel (Figure 8c) and hurdle architectures (Figure 8d) after deposition of the target energetic films. The tiny energetic devices with differently designed electrode spacing and direction distributions showed different orientations of the multiphase expansion event relative to the flame propagation direction (Figure 8e). The electrophoretic assembly film-forming of other common tEMs (e.g., Al/NiO [62], Al/Ni [16], Al/Fe2O3 [105], Al/Co3O4 [106], Al/MoO3 [107], Al/WO3 [108], etc.) have been also demonstrated, especially in the last decade.
Several tEMs with promising structures and stable exothermic performance can be prepared by combining electrophoretic assembly and other fabrication methods. In order to solve the problem of poor exothermic stability of energetic materials due to the hydrophilicity of Al-based materials, anti-wetting EMs (e.g., Al/Fe2O3 [109], Al/ZnO [110], Al/Ni/Bi2O3 [85], Al/Bi2O3 [111], Al/MoO3 [112], Al/NiO [113], Al/Ni [69], Al/Co3O4 [61], Al/CuO [54]) have been designed by electrophoretic assembly and surface modification process. For example, Guo et al. [111] reported on superhydrophobic Al/Bi2O3 films using the mentioned approach by per-fluoroalkyltriethoxysilane (FAS-17) modification, and the preparation mechanism is shown in Figure 8f. The product is rough but evenly distributed in nanoscale (Figure 8g,h), which improves combustion performance significantly. The superhydrophobic Al/Bi2O3 films show an outstanding anti-wetting property, with a water contact angle of ~170° and high exothermic stability for 2 years (Figure 8i).
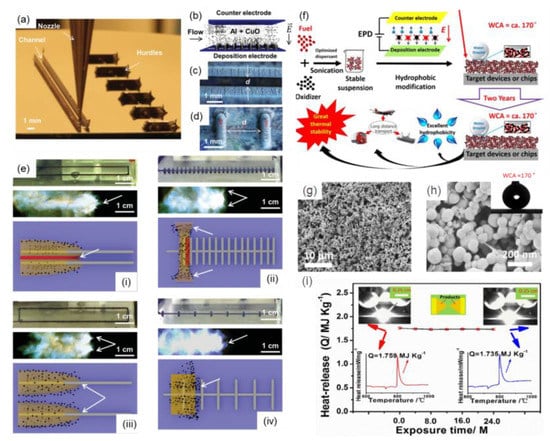
Figure 8.
(a) Optical image of 3D printing process for channels (left) and hurdles (right) composed of silver nanoparticle ink, (b) schematic illustration of the electrophoretic assembly process of Al/CuO EMs onto the electrode surfaces, (c) optical images (top view) of a channel, (d) hurdle architectures after deposition of Al/CuO film, (e) optical image of four kinds of channel and hurdle structures, followed by the corresponding combustion process and the resultant pressurization region and expansion process illustration [104] Copyright 2016, Advanced Materials; (f) the schematic diagram of the fabrication of superhydrophobic Al/Bi2O3 films, the SEM images ((g) low resolution and (h) high resolution) of product, and (i) the thermal stability property of product after different exposure time. Inset, DSC and ignition tests reveal the exothermic performance of products before and after two years exposure [111], Copyright 2018, Chemical Engineering Journal.
3.3.2. Other Assembly Method
Recently, some novel promising assembly methods have also been explored to improve the contact area and reactive activation of tEMs. The main driving forces for assembly methods include the electrostatic force, strong affinity of the functional groups, biomolecular driving force, and so on. Electrostatically enhanced nano-Al and nano-Fe2O3 particle self-assembly was realized to design reactive Al/Fe2O3 tEMs, and the burning process of the resulting product is controlled by adjusting the magnitude of the particle charge [114]. The Al/NiO tEMs are fabricated via co-assembly with poly(4-vinylpyridine) (P4VP), and the structure of the Al/NiO nanocomposites with P4VP is more regular and compact, resulting in a higher output of heat (2190 J/g), a higher maximum explosion pressure (0.35 MPa), a faster pressure rise rate (260 MPa/s) and burning rate (462 m/s), compared with those of a physically mixed sample [115]. In addition, C. Rossi et al. [116] reported a novel technique of DNA (e.g., a linker with sequence 5′ to 3′ of GAGGGATTATTGTTAd)TTAACGTACAGTATG)-directed assembly procedure to obtain highly Al/CuO tEMs, showing a total highest heat of reaction of 1800 J/g for the 80 nm Al NPs, and the onset temperature can be adjusted by changing the size of Al particles.
3.4. Sol-Gel Method
The sol-gel method is a widely used method to prepare nanometer metals, oxides, or metal/oxides. To be specific, the selected raw materials are dispersed into a solvent as a precursor, and they then form the colloidal solution (sol) with high dispersion after hydrolysis, and certain space structures (gel) are finally obtained after the polymerization process with other components added. Tillotson et al. prepared the aerogel and xerogel monoliths of Al/Fe2O3 tEMs using the sol-gel method, all releasing dazzling white light after ignition, and the aerogel composites were more sensitive to ignition than that of the aerogel sample [117]. In addition, a ternary tEMs of Al/Fe2O3/SiO2 thermite was designed by this method, and examining the influence of the mass fraction of SiO2 as an additive display showed a great effect on the exothermic performance and combustion velocity [118]. The sol-gel method is simple and can be operated in a beaker. However, there are many impurities in the product that will greatly affect the performance of the target product. In addition, similar to the high-energy ball milling technique, the microstructure of the product is difficult to control, thus, the general applicability of this method is still limited.
3.5. Electrospinning Method
As a facile and highly versatile technique, electrospinning is also used to generate multifunctional ultrathin fibers from various polymers, polymer blends, and polymer nanoparticles [87]. For example, nitrocellulose (NC) as the chief ingredient in single-base and double-base gun and rocket propellants has been introduced into Al/Fe2O3 tEMs to obtain Al/Fe2O3/NC energetic fibers by an electrospinning method [119], and the product with the increased elastic modulus of NC fibers after the addition of 5 wt% Al/Fe2O3 shows a lower onset temperature of thermite reaction compared to Al/Fe2O3/NC powders. Another similar report is the design of Al/CuO/NC tEMs by M. R. Zachariah et al. The color and morphology of the samples changed obviously compared with pure NC, NC/Al, and Al/CuO/NC tEMs, and regarding the Al/CuO being electrospun in the NC polymer matrix, the deflagration process is more complete and the flame area is larger and more dazzling (Figure 9a), showing wide applications in solid rocket propellant systems [120]. Promising n-Al@PVDF/EMOF (DHBT) highly energetic fibers were fabricated by Q.L. Yan group using the electrospinning method combined with a typical in situ synthesis process (Figure 9b), and the product shows increased heat release (3.464 kJ/g) and burning rate (2.8 m/s), and improved combustion efficiency due to the greater amount of reaction channels after the decomposition of EMOF and the etching reaction [121].
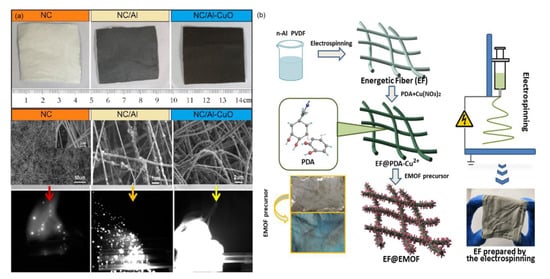
Figure 9.
(a) The optical and SEM image of pure NC, NC/Al (50 wt%) and NC/Al-CuO (50 wt%), followed by the respective burning static snapshot [120] Copyright 2012, ACS Applied Materials Interfaces, and (b) the general procedures for the preparation of n-Al@PVDF/EMOF (DHBT) [121] Copyright 2020, Chemical Engineering Journal.
3.6. Other Methods
In addition to the method mainly used for designing tEMs, researchers at home and abroad tried to explore other preparation technologies, including arrested reactive milling [122,123], reactive plasma spraying [124], electrospray deposition [125], powder metallurgy [126], the freeze-drying method [127], the hydrothermal synthesis method [128] and the brush-mixing technique [129]. Interestingly, J.M. Slocik et al. constructed energetic biothermite inks, that is, nAl@ferritin liquid tEMs using the freeze-drying technique, and demonstrated greater processability and functionality, increased energy output and performance, enhanced dispersion and oxidation stability, and lower activation temperatures (<400 °C) [127], which provides valuable reference for designing Al-based energetic biological liquids with flexible structures and shapes using different biological activities or other various capabilities.
4. Prospects and Suggestions
Until now, energetic materials, particularly tEMs, have been extensively reported and fabricated via different preparation methods, owing to their wide range of application, which include the defense industry, detonators [128,129,130,131], igniters [132,133], gas sensors [77,134,135,136,137], thin-film battery [138] and chip adhesive [139]. Beyond that, the design of an all-solid-state lithium/electrolyte interface was realized by inducing an energetic reaction between metal fuel and nitrate, showing high capacity, small overpotential, high efficiency, and a long cycle life [140]. Bacteria growth neutralized by Al/I2O5 tEMs with great biocidal property was demonstrated by B. R. Clark et al. [141]. The means by which energetic materials can be boldly and organically combined with other fields will be a hot topic that can be continuously paid attention to in the future.
There is no denying that more and more techniques are being developed for designing tEMs, including those mentioned in this review, such as the mechanical mixing method, vapor deposition methods, assembly method, sol-gel method, electrospinning method, freeze-drying method, etc. However, as the application of tEMs in the fields (e.g., national defense) becomes more and more demanding, new requirements are put forward for the development and exploration of novel technologies. For example, although the mechanical mixing method is simple and convenient, it does not allow for film formation and integration with complex micro-devices. Although they have high precision and controllability, it is still difficult for them to achieve large-scale production due to the high preparation cost and difficult operation of magnetron sputtering, electrospinning, etc. The sol-gel method has great advantages in preparing porous tEMs with good contact, but the purity of the product often requires subsequent impurity removal processes, such as heat treatment, that have increased limitations for tEMs with high thermal sensitivity, though it is relatively efficient. For arrested reactive milling, its preparation cost is low, but the size of the molding sample is mostly micron, leading to a limited heat-release performance. Moreover, the low loading rate and adhesion to the substrate using the electrophoretic assembly method needs to be further improved, though it is rather convenient and has high film-forming efficiency, being perfectly matched with devices (e.g., chips). The generality and universality of new technologies (e.g., freeze-drying method) for tEMs need further verification. Therefore, in the future, the means by which novel methods with high universality, high safety of preparation process, environmental friendliness, and a better combination of reported mature traditional technologies can be developed should be the focus of additional research.
Exothermic performance optimization is an essential index to evaluate the application of tEMs. The key to thermal energy release is the design of microstructures, which mainly are stratified, nucleated, or granular. It is rather urgent and necessary to design novel structures to further shorten the mass transfer distance between fuel and oxidizer, improve the heat transfer efficiency, and meet the requirements of ignition and heat release under complex conditions, such as acid, alkali, wet environments, underwater environments, etc.
Additionally, the analysis of mechanism is a long-term research focus, which plays a significant role in optimizing the heat-release property of tEMs. The main research has focused on the improvement of their performance and structure optimization. Admittedly, several researchers have explored the reaction mechanism of tEMs. For example, M. R. Zachariah proposed possible mechanisms of the burning process of Al/CuO/NC nEMs fabricated by two different physical mixing and electrospray techniques (Figure 10a), and found that adding additional NC (>6.5 wt%,) apparently offers diminishing returns, leading to its disintegration into smaller structures and its pressure and the pressurization rate becoming smaller, respectively [142]. Heat-release schematic illustrations of Al/Fe2O3, Al/CuO, and Al/NiO nEMs with different microstructures (Figure 10b–d) have also been analyzed recently [48,88,131]. However, for the complex system of tEMs, the mechanism research is far from enough. Therefore, the relative mechanisms and simulation (via COSMOL, DSC, etc.) analysis of preparation, exothermic reaction and modification process, etc. also need to be focused on for tEMs, especially multi-component tEMs.
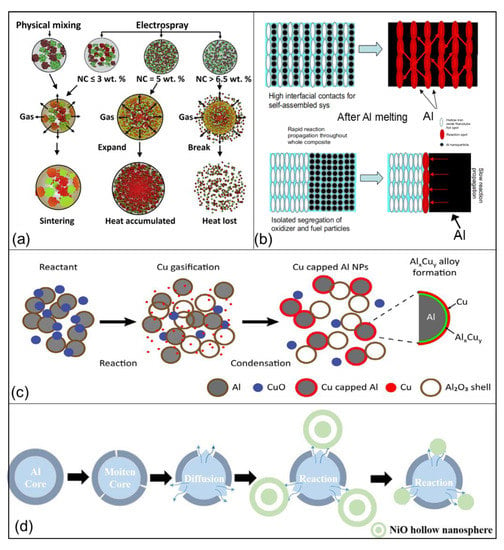
Figure 10.
(a) Possible mechanisms of the burning of Al/CuO/NC nEMs made from physical mixing and electrospray. Note: Al (red); CuO (green); nitrocellulose (light blue) [142] Copyright 2014, Combustion and Flame, (b) the schematic illustration showing an exaggerated perspective that demonstrates random versus ordered assembly [48] Copyright 2010, Combustion and Flame, (c) schematic illustration of Al/CuO thermite reaction steps. Condensation of gasified Cu species on Al NPs hamper’s reaction of Al NPs with neighboring CuO NPs [88] Copyright 2015, Combustion and Flame, and (d) combustion process of Al/NiO nEMs [131] Copyright 2020, Chemical Engineering Journal.
5. Conclusions
In brief, this review systematically introduces the classification, various synthesis methods, and recent research achievements of highly reactive tEMs with broad and promising applications. Until now, a great deal of promising work has been reported to prepare tEMs for optimizing their performance. Due to the particularity of tEMs, the development of new methods with high universality and environmental friendliness needs to be focused on in future research, even though there are many technologies reported at present. The family and application breadth of tEMs needs to be further expanded and applied in fields such as intelligence, biology, information technology, etc. Additionally, the nanostructures of tEMs need to be further designed to improve the adequacy and stability of the reaction. A combination of microstructure design and exploration of technology is used to finally achieve efficient mass production. Additionally, further study is needed to explore the relative mechanism for providing a solid foundation for optimized performance. In brief, the subject of this review belongs to a youthful and promising research area, and significant and meaningful work can be explored to expand its scope.
Author Contributions
Writing—original draft preparation, X.G.; writing-review and editing, T.L. and M.L.I.; format modification, X.C. and Z.W., supervision, X.G. funding acquisition, X.G. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21805014 and No. 82102635), and Chongqing Municipal Education Commission (No. KJQN202201408).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the financial support from National Natural Science Foundation of China, and Chongqing Municipal Education Commission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, C.; Sun, C.G.; Hu, B.C.; Yu, C.M.; Lu, M. Synthesis and characterization of the pentazolate anion cyclo-N5 in (N5)6 (H3O)3(NH4)4Cl. Science 2017, 355, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.G.; Wang, Q.; Shen, C.; Lin, Q.H.; Wang, P.C.; Lu, M. A series of energetic metal pentazolate hydrates. Nature 2017, 549, 78–81. [Google Scholar] [CrossRef]
- Ma, X.X.; Li, Y.X.; Hussain, I.; Shen, R.Q.; Yang, G.C.; Zhang, K.L. Core-shell structured nanoenergetic materials: Preparation and fundamental properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wang, Y.; Qi, C.; Zhao, X.X.; Zhang, J.C.; Zhang, S.W.; Pang, S.P. 3D energetic metal-organic frameworks: Synthesis and properties of high energy materials. Angew. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef]
- Becker, C.R.; Apperson, S.; Morris, C.J.; Gangopadhyay, S.; Currano, L.J.; Churaman, W.A.; Stoldt, C.R. Galvanic poroussilicon composites for high-velocity nanoenergetics. Nano Lett. 2011, 11, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Song, S.W.; Wang, K.C.; Wang, Y.; Zhang, Q.H. Modifying polynitro benzene and pyrazine skeletons with flexible nitratoethyl substituents towards new energetic melt-castable materials. Chem. Eng. J. 2022, 435, 135053. [Google Scholar] [CrossRef]
- Comet, M.; Vidick, G.; Schnell, F.; Suma, Y.B.; Spitzer, D. Sulfates-based nanothermites: An expanding horizon for metastable interstitial composites. Angew. Chem. Int. Ed. 2015, 54, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Nanayakkara, C.E.; Veyan, J.; Warot-Fonrose, B.; Joulie, S.; Estève, A.; Tenailleau, C.; Chabal, Y.J.; Rossi, C. Enhancing the reactivity of Al/CuO nanolaminates by Cu incorporation at the interfaces. ACS Appl. Mater. Interfaces 2015, 7, 11713–11718. [Google Scholar] [CrossRef]
- Yu, C.P.; Zheng, Z.L.; Zhang, W.C.; Hu, B.; Chen, Y.J.; Chen, J.H.; Ma, K.F.; Ye, J.H.; Zhu, J.W. Sustainable electrosynthesis of porous cun3 films for functional energetic chips. ACS Sustain. Chem. Eng. 2020, 8, 3969–3975. [Google Scholar] [CrossRef]
- Wang, J.; Qu, Y.Y.; Gong, F.Y.; Shen, J.P.; Zhang, L. A promising strategy to obtain high energy output and combustion properties by self-activation of nano-Al. Combust. Flame 2019, 204, 220–226. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.G.; Yang, G.C.; Zhang, Q.B.; Shen, J.P.; Lu, J.; Zhang, K.L. Highly exothermic and superhydrophobic Mg/fluorocarbon core/shell nanoenergetic arrays. ACS Appl. Mater. Interfaces 2014, 6, 10497–10505. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.; Kuznetsov, V.; Joyner, T.; Shapter, J.; Voelcker, N.H. The burning rate of energetic films of nanostructured porous silicon. Small 2011, 7, 3392–3398. [Google Scholar] [CrossRef]
- Zhong, K.; Bu, R.P.; Jiao, F.B.; Liu, G.R.; Zhang, C.Y. Toward the defect engineering of energetic materials: A review of the effect of crystal defects on the sensitivity. Chem. Eng. J. 2022, 429, 132310. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.G.; Lu, J.; Zhang, K.L. CuO/Mg/fluorocarbon sandwich-structure superhydrophobic nanoenergetic composite with anti-humidity property. Chem. Eng. J. 2015, 266, 163–170. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Huang, H.H.; Yuan, B.F.; Wang, J. Additive-free super-reactive metastale intermixed C-doped Al/Co3O4 coating with excellent structural, exothermic and hydrophobic stability for a transient-chip. Appl. Surf. Sci. 2022, 581, 152324. [Google Scholar] [CrossRef]
- Guo, X.G.; Li, X.M.; Li, H.R.; Zhang, D.X.; Lai, C.; Li, W.L. A comprehensive investigation on the electrophoretic deposition (EPD) of nano-Al/Ni energetic composite coatings for the combustion application. Surf. Coat. Tech. 2015, 265, 83–91. [Google Scholar] [CrossRef]
- Kostov, A.; Zivkovic, D. Thermodynamic analysis of alloys Ti-Al, Ti-V, Al-V and Ti-Al-V. J. Alloy. Compd. 2008, 460, 164–171. [Google Scholar] [CrossRef]
- Grapes, M.D.; Reeves, R.V.; Fezzaa, K.; Sun, T.; Densmore, J.M.; Sullivan, K.T. In situ observations of reacting Al/Fe2O3 thermite: Relating dynamic particle size to macroscopic burn time. Combust. Flame 2019, 201, 252–263. [Google Scholar] [CrossRef]
- De Souza, K.M.; de Lemos, M.J.S.; Ribeiro, R.R.; Marin, A.M.G. Advanced isoconversional kinetic analysis of Fe2O3-2Al thermite reaction for plug and abandonment of oil wells. Chem. Eng. J. 2023, 455, 140725. [Google Scholar] [CrossRef]
- Zhao, N.N.; He, C.C.; Liu, J.B.; Gong, H.J.; An, T.; Xu, H.X.; Zhao, F.Q.; Hu, R.Z.; Ma, H.X.; Zhang, J.Z. Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions. J. Solid State Chem. 2014, 219, 67–73. [Google Scholar] [CrossRef]
- Wang, Y.T.; Dai, J.; Xu, J.B.; Shen, Y.; Wang, C.-A.; Ye, Y.H.; Shen, R.Q. Experimental and numerical investigations of the effect of charge density and scale on the heat transfer behavior of Al/CuO nano-thermite. Vacuum 2021, 184, 109878. [Google Scholar] [CrossRef]
- Yang, H.F.; Yang, G.C.; Li, X.D.; Bao, H.B.; Yang, Y.J.; Guo, X.G.; Qiao, Z.Q.; Li, X.M.; Li, X. Facile synthesis of high tightly ordered Al/CuO core-shell nanowire arrays and the effect of surface density on combustion. J. Alloy. Compd. 2021, 877, 160025. [Google Scholar] [CrossRef]
- Yin, Y.J.; Li, X.M.; Shu, Y.J.; Guo, X.G.; Bao, H.B.; Li, W.L.; Zhu, Y.H.; Li, Y.; Huang, X.Y. Fabrication of electrophoretically deposited, self-assembled three-dimensional porous Al/CuO nanothermite films for highly enhanced energy output. Mater. Chem. Phys. 2017, 194, 182–187. [Google Scholar] [CrossRef]
- Nellums, R.R.; Terry, B.C.; Tappan, B.C.; Son, S.F.; Groven, L.J. Effect of solids loading on resonant mixed Al-Bi2O3 nanothermite powders. Propellants Explos. Pyrotech. 2013, 38, 605–610. [Google Scholar] [CrossRef]
- Xiao, F.; Li, J.M.; Zhou, X.Y.; Yang, R.J. Preparation of mechanically activated aluminum-rich Al-Co3O4 powders and their thermal properties and reactivity with water steam at high temperature. Combust. Sci. Technol. 2018, 190, 1935–1949. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z.Q.; Shen, J.P.; Li, R.; Yang, Y.T.; Yang, G.C. Large-scale synthesis of a porous Co3O4 nanostructure and its application in metastable intermolecular composites. Propellants Explos. Pyrotech. 2015, 40, 514–517. [Google Scholar] [CrossRef]
- Sui, H.T.; Li, B.Y.; Wen, J.Z. Interaction between single-walled carbon nanotubes and reactive nanoparticle constituents in multilayered Al/NiO nanocomposite. ACS Appl. Energy Mater. 2018, 1, 5245–5256. [Google Scholar] [CrossRef]
- Liu, J.; Shao, S.Y.; Fang, G.; Meng, B.; Xie, Z.Y.; Wang, L.X. High-efficiency inverted polymer solar cells with transparent and work-function tunable MoO3-Al composite film as cathode buffer layer. Adv. Mater. 2012, 24, 2774–2779. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermite foams: From nanopowder to object. Chem. Eng. J. 2017, 316, 807–812. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Mao, Y.F.; Gong, F.Y. An effective way to enhance energy output and combustion characteristics of Al/PTFE. Combust. Flame 2020, 214, 419–425. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, W.; Li, P.Y.; Li, L.; Chen, B.H.; Dai, J.J.; Wang, L.X.; Yuan, Y.; Li, F.S. Ignition and combustion of super-reactive thermites of AlMg/KMnO4. Rare Metal Mat. Eng. 2013, 42, 2458–2461. [Google Scholar]
- Fahd, A.; Dubois, C.; Chaouki, J.; Wen, J.Z.; Youssef, E. Synthesis and characterization of tertiary nanothermite CNMs/Al/KClO4 with enhanced combustion characteristics. Propellants Explos. Pyrotech. 2021, 46, 995–1005. [Google Scholar] [CrossRef]
- Zaky, M.G.; Abdalla, A.M.; Sahu, R.P.; Puri, I.K.; Radwan, M.; Elbasuney, S. Nanothermite colloids: A new prospective for enhanced performance. Def. Technol. 2019, 15, 319–325. [Google Scholar] [CrossRef]
- Mao, X.X.; Li, Y.C.; Li, Y.F.; Jiang, L.F.; Wang, X.M. Thermal properties of decomposition and explosion for CL-20 and CL-20/n-Al. J. Energ. Mater. 2020, 38, 98–110. [Google Scholar] [CrossRef]
- Yan, T.G.; Yang, C.; Ma, J.C.; Cheng, G.B.; Yang, H.W. Intramolecular integration of multiple heterocyclic skeletons for energetic materials with enhanced energy & safety. Chem. Eng. J. 2022, 428, 131400. [Google Scholar]
- Li, P.; Moon, S.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal-organic framework engenders thermal and long-term stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, K.S.; Wang, L.Z.; Vicent, A.; Luss, D. Nanoenergetic gas-generators: Design and performance. Propellants Explos. Pyrotech. 2009, 34, 532–538. [Google Scholar] [CrossRef]
- Yi, Z.X.; Cao, Y.Q.; Yuan, J.W.; Mary, C.; Wan, Z.Y.; Li, Y.; Zhu, C.G.; Zhang, L.; Zhu, S.G. Functionalization carbon fibers assemble with Al/Bi2O3: A new strategy for high-reliability ignition. Chem. Eng. J. 2020, 389, 124254. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Meshalkin, V.P.; Butusov, O.B.; Chistyakova, T.B.; Ferretti, M.; Cardinale, A.M.; Fabiano, B. Organic and inorganic biocidal energetic materials for agent defeat weapons: An overview and research perspectives. Energies 2023, 16, 675. [Google Scholar] [CrossRef]
- Polis, M.; Stolarczyk, A.; Glosz, K.; Jarosz, T. Quo Vadis, Nanothermite? A review of recent progress. Materials 2022, 15, 3215. [Google Scholar] [CrossRef]
- Kabra, S.; Gharde, S.; Gore, P.M.; Jain, S.; Khire, V.H.; Kandasubramanian, B. Recent trends in nanothermites: Fabrication, characteristics and applications. Nano Express 2020, 1, 032001. [Google Scholar] [CrossRef]
- Tang, D.Y.; Lyu, J.; He, W.; Chen, J.; Yang, G.; Liu, P.J.; Yan, Q.L. Metastable intermixed core-shell Al@M(IO3)x nanocomposites with improved combustion efficiency by using tannic acid as a functional interfacial layer. Chem. Eng. J. 2020, 384, 123369. [Google Scholar] [CrossRef]
- Fischer, S.H.; Grubelich, M.C. Theoretical energy release of thermites, intermetallics, and combustible metals. In Proceedings of the 24th International Pyrotechnics Seminar, Monterey, CA, USA, 27–31 July 1998. [Google Scholar]
- Cheng, J.L.; Hng, H.H.; Ng, H.Y.; Soon, P.C.; Lee, Y.W. Synthesis and characterization of self-assembled nanoenergetic Al-Fe2O3 thermite system. J. Phys. Chem. Solids. 2010, 71, 90–94. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Ke, X.; Zhang, K.L. Exploring the solid-state interfacial reaction of Al/Fe2O3 nanothermites by thermal analysis. J Mater. Sci. 2019, 54, 4115–4123. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wang, Z.; Li, G.P.; Luo, Y.J. Tuning the reactivity of Al/Fe2O3 nanoenergetic materials via an approach combining soft template self-assembly with sol-gel pro-cess process. J. Solid State Chem. 2015, 230, 1–7. [Google Scholar] [CrossRef]
- Dadbakhsh, S.S.; Hao, L. In situ formation of particle reinforced al matrix composite by selective laser melting of Al/Fe2O3 powder mixture. Adv. Eng. Mater. 2012, 14, 45–48. [Google Scholar] [CrossRef]
- Cheng, J.L.; Hng, H.H.; Lee, Y.W.; Du, S.W.; Thadhani, N.N. Kinetic study of thermal- and impact-initiated reactions in Al-Fe2O3 nanothermite. Combust. Flame 2010, 157, 2241–2249. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, M.H.; Shim, H.M.; Kim, S.H. Fabrication and thermal behavior of Al/Fe2O3 energetic composites for effective interfacial bonding between dissimilar metallic substrates. J. Ind. Eng. Chem. 2019, 78, 84–89. [Google Scholar] [CrossRef]
- Monk, I.; Schoenitz, M.; Jacob, R.J.; Dreizin, E.L.; Zachariah, M.R. Combustion characteristics of stoichiometric Al-CuO nanocomposite thermites prepared by different methods. Combust. Sci. Technol. 2017, 189, 555–574. [Google Scholar] [CrossRef]
- Yu, C.P.; Zhang, W.C.; Hu, B.; Ni, D.B.; Zheng, Z.L.; Liu, J.P.; Ma, K.F.; Ren, W. Core/shell CuO/Al nanorod thermite film based on electrochemical anodization. Nanotechnology 2018, 29, 36LT02. [Google Scholar] [CrossRef]
- Rossi, C. Engineering of Al/CuO reactive multilayer thin films for tunable initiation and actuation. Propellants Explos. Pyrotech. 2019, 44, 94–108. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.J.; Cheng, Z.P.; Ke, X.; Jiang, W. Facile preparation and energetic characteristics of core-shell Al/CuO metastable intermolecular composite thin film on a silicon substrate. Chem. Eng. J. 2017, 328, 585–590. [Google Scholar] [CrossRef]
- Guo, X.G.; Yuan, B.F.; Lin, Y.H.; Cui, X.; Gao, F.; Mi, W.H.; Lu, C.H.; Rager, M.; Li, X.M. Acile preparation of superhydrophobic nano-aluminum/copper(II) oxide composite films with their exposure and heat-release stability. Mater. Lett. 2018, 213, 294–297. [Google Scholar] [CrossRef]
- Yao, E.G.; Zhao, N.N.; Qin, Z.; Ma, H.X.; Li, H.J.; Xu, S.Y.; An, T.; Yi, J.H.; Zhao, F.Q. Thermal decomposition behavior and thermal safety of nitrocellulose with different shape CuO and Al/CuO nanothermites. Nanomaterials 2020, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Ren, W.; Zheng, Z.L.; Wu, G.G.; Hu, B.; Chen, J.H.; Wang, J.X.; Yu, C.P.; Ma, K.F.; Zhou, X.L.; et al. Reactivity adjustment from the contact extent between CuO and Al phases in nanothermites. Chem. Eng. J. 2020, 402, 126288. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, R.Q.; Ye, Y.H.; Zhu, P.; Hu, Y.; Wu, L.Z. Influence of Al/CuO reactive multilayer films additives on exploding foil initiator. J. Appl. Phys. 2011, 110, 094505. [Google Scholar] [CrossRef]
- Tichtchenko, E.; Estève, A.; Rossi, C. Modeling the self-propagation reaction in heterogeneous and dense media: Application to Al/CuO thermite. Combust. Flame 2021, 228, 173–183. [Google Scholar] [CrossRef]
- Kwon, J.; Ducéré, J.M.; Alphonse, P.; Bahrami, M.; Petrantoni, M.; Veyan, J.F.; Tenailleau, C.; Estève, A.; Rossi, C.; Chabal, Y.J. Interfacial chemistry in Al/CuO reactive nanomaterial and its role in exothermic reaction. ACS Appl. Mater. Interfaces 2013, 5, 605–613. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly reactive metastable intermixed composites (MICs): Preparation and characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Liu, G.X.; He, Y.Z.; Kong, S.Y.; Islam, M.L.; Yuan, B.F. Conveniently controllable design of nano-Al-doped@Co3O4 energetic composite with enhanced exothermic property via exploring electrophoretic assembly dynamics. J. Mater. Sci. Mater. Electron. 2022, 33, 6262–6272. [Google Scholar] [CrossRef]
- Zhang, D.X.; Li, X.M. Fabrication and kinetics study of nano-Al/NiO thermite film by electrophoretic deposition. J. Phys. Chem. 2015, 119, 4688–4694. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Li, X.M.; Zhang, D.X.; Bao, H.B.; Shu, Y.J.; Guo, X.G.; Yin, Y.J. Tuning the surface charges of MoO3 by adsorption of polyethylenimineto realize the electrophoretic deposition of high-exothermic Al/MoO3 nanoenergetic films. Mater. Design 2016, 109, 652–658. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducéré, J.M.; Baijot, V.; Pinon, S.; Calais, T.; Estève, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Ma, E.; Thompson, C.V.; Clevenger, L.A.; Tu, K.N. Self-propagating explosive reactions in Al/Ni multilayer thin films. Appl. Phys. Lett. 1990, 57, 1262–1264. [Google Scholar] [CrossRef]
- Milosavljevi, M.; Stojanovi, N.; Perusko, D.; Timotijevi, B.; Toprek, D.; Kova, J.; Drazi, G.; Jeynes, C. Ion irradiation induced Al-Ti interaction in nano-scaled Al/Ti multilayers. Appl. Surf. Sci. 2012, 258, 2043–2046. [Google Scholar] [CrossRef]
- Wang, L.; He, B.; Jiang, X.H.; Fu, Q.B.; Wang, L.L. Modeling the propagationvelocity of reaction waves in Al/Ni multilayer films. Chin. J. Energ. Mater. 2009, 2, 233–235. [Google Scholar]
- Guo, X.G.; Lu, C.H.; Huang, H.S.; Cui, X.; Liang, T.T.; Yuan, B.F.; Wang, J.; Li, X.M. Facilely controllable synthesis of multi-functional aluminum/nickel/perfluorosilane composites for enhancing the thermal energy release stability and enhancing anti-wetting properties. Compos. Sci. Technol. 2020, 199, 108351. [Google Scholar] [CrossRef]
- Guo, X.G.; Li, X.M.; Wei, Z.B.; Li, X.L.; Niu, L.D. Rapid fabrication and characterization of superhydrophobic tri-dimensional Ni/Al coatings. Appl. Surf. Sci. 2016, 387, 8–15. [Google Scholar] [CrossRef]
- Pantoya, M.L.; Dean, S.W. The influence of alumina passivation on nano-Al/Teflon reactions. Thermochim. Acta 2009, 493, 109–110. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; Gong, F.Y.; Tao, B.; Gu, J.; Yang, Z.J.; Yan, Q.L. Tuning the reactivity of metastable intermixed composite n-Al/PTFE by polydopamine interfacial control. ACS Appl. Mater. Interfaces 2018, 10, 32849–32858. [Google Scholar] [CrossRef]
- Clayton, N.A.; Kappagantula, K.S.; Pantoya, M.L.; Kettwich, S.C.; Iacono, S.T. Fabrication, characterization, and energetic properties of metallized fibers. ACS Appl. Mater. Interfaces 2014, 6, 6049–6053. [Google Scholar] [CrossRef] [PubMed]
- Brousseau, P.; Anderson, C.J. Nanometric aluminum in explosives. Propellants Explos. Pyrotech. 2002, 27, 300–306. [Google Scholar] [CrossRef]
- Ma, X.X.; Zhu, Y.; Cheng, S.X.; Zheng, H.X.; Liu, Y.S.; Qiao, Z.Q.; Yang, G.C.; Zhang, K.L. Energetic composites based on nano-Al and energetic coordination polymers (ECPs): The “father-son” effect of ECPs. Chem. Eng. J. 2020, 392, 123719. [Google Scholar] [CrossRef]
- He, W.; Ao, W.; Yang, G.C.; Yang, Z.J.; Guo, Z.Q.; Liu, P.J.; Yan, Q.L. Metastable energetic nanocomposites of mof-activated aluminum featured with multi-level energy releases. Chem. Eng. J. 2020, 381, 122623. [Google Scholar] [CrossRef]
- Prakash, A.; McCormick, A.V.; Zachariah, M.R. Synthesis and reactivity of a super-reactive metastable intermolecular composite formulation of Al/KMnO4. Adv. Mater. 2005, 17, 900–903. [Google Scholar] [CrossRef]
- Jian, G.Q.; Feng, J.Y.; Jacob, R.J.; Egan, G.C.; Zachariah, M.R. Super-reactive nanoenergetic gas generators based on periodate salts. Angew. Chem. Int. Ed. 2013, 52, 9743–9746. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Piekiel, N.W.; Chowdhury, S.; Wu, C.; Zachariah, M.R.; Johnson, C.E. Ignition and combustion characteristics of nanoscale Al/AgIO3: A potential energetic biocidal system. Combust. Sci. Technol. 2011, 183, 285–302. [Google Scholar] [CrossRef]
- Gao, K.; Li, G.P.; Luo, Y.J.; Wang, L.; Shen, L.H.; Wang, G. Preparation and characterization of the AP/Al/Fe2O3 ternary nano-thermites. J. Therm. Anal. Calorim. 2014, 118, 43–49. [Google Scholar] [CrossRef]
- Yuan, Y.; Geng, B.Q.; Sun, T.; Yu, Q.B.; Wang, H.F. Impact-induced reaction characteristic and the enhanced sensitivity of PTFE/Al/Bi2O3 composites. Polymers 2019, 11, 2049. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xia, S.Y.; Ren, W.; Zheng, Z.L.; Chen, J.H.; Ma, K.F.; Yu, C.P.; Zhou, X.L.; Zhang, W.C. A favorable improvement in reactivity between n-Al and sheet-like porous CuO as a nanoenergetic composite by graphene oxide additives. Ind. Eng. Chem. Res. 2020, 59, 12934–12942. [Google Scholar] [CrossRef]
- Luo, Q.P.; Liu, G.X.; Zhu, M.S.; Jiang, X.H. Constant volume combustion properties of Al/Fe2O3/RDX nanocomposite: The effects of its particle size and chemical constituents. Combust. Flame 2022, 238, 111938. [Google Scholar] [CrossRef]
- Prentice, D.; Pantoya, M.L.; Clapsaddle, B.J. Synthesis and performance characterization of a nanocomposite ternary thermite: Al/Fe2O3/SiO2. J. Phys. Chem. B. 2005, 43, UCRL-JRNL-209471. [Google Scholar]
- Sullivan, K.; Young, G.; Zachariah, M.R. Enhanced reactivity of nano-B/Al/CuO MIC’s. Combust. Flame 2009, 156, 302–309. [Google Scholar] [CrossRef]
- Guo, X.G.; Li, X.M.; Lai, C.; Jiang, X.; Li, X.L.; Shu, Y.J. Facile approach to the green synthesis of novel ternary composites with excellent superhydrophobic and thermal stability property: An expanding horizon. Chem. Eng. J. 2017, 309, 240–248. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, L.J.; Ke, X.; Zhang, T.Y.; Hao, G.Z.; Hu, Y.B.; Zhang, G.P.; Guo, H.; Jiang, W. Energetic metastable Al/CuO/PVDF/RDX microspheres with enhanced combustion performance. Chem. Eng. Sci. 2021, 231, 116302. [Google Scholar] [CrossRef]
- Fahd, A.; Baranovsky, A.; Dubois, C.; Chaouki, J.; Wen, J.Z. Superior performance of quaternary NC/GO/Al/KClO4 nanothermite for high speed impulse small-scale propulsion applications. Combust. Flame 2021, 232, 111527. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Shim, J.; Bae, S.; Shin, D.J.; Treml, B.E.; Yoo, J.; Hanrath, T.; Kim, W.D.; Lee, D.C. Formation of Cu layer on Al nanoparticles during thermite reaction in Al/CuO nanoparticle composites: Investigation of off-stoichiometry ratio of Al and CuO nanoparticles for maximum pressure change. Combust. Flame 2015, 162, 3823–3828. [Google Scholar] [CrossRef]
- Sanders, V.E.; Asay, B.W.; Foley, T.J.; Tappan, B.C.; Pacheco, A.N.; Son, S.F. Reaction Propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO, and Bi2O3). J. Propul. Power 2007, 23, 707–714. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Y.; Shen, R.Q.; Ye, Y.H.; Wang, S.X.; Hua, T.L. Fabrication and performance characterization of Al/Ni multilayer energetic films. Appl. Phys. A 2014, 114, 459–464. [Google Scholar] [CrossRef]
- Zhu, P.; Jiao, J.S.; Shen, R.Q.; Ye, Y.H.; Fu, S.; Li, D. Energetic semiconductor bridge device incorporating Al/MoOx multilayer nanofilms and negative temperature coefficient thermistor chip. J. Appl. Phys. 2014, 115, 194502. [Google Scholar] [CrossRef]
- Zapata, J.; Nicollet, A.; Julien, B.; Lahiner, G.; Esteve, A.; Rossi, C. Self-propagating combustion of sputter-deposited Al/CuO nanolaminates. Combust. Flame 2019, 205, 389–396. [Google Scholar] [CrossRef]
- Zhang, K.L.; Rossi, C.; Alphonse, P.; Tenailleau, C.; Cayez, S.; Chane-Ching, J.Y. Integrating Al with NiO nano honeycomb to realize an energetic material on silicon substrate. Appl. Phys. A 2009, 94, 957–962. [Google Scholar] [CrossRef]
- Menon, L.; Patibandla, S.; Ram, K.B.; Shkuratov, S.I.; Aurongzeb, D.; Yun, M.H.B.; Temkin, H. Ignition studies of Al/Fe2O3 energetic nanocomposites. Appl. Phys. Lett. 2004, 84, 4735. [Google Scholar] [CrossRef]
- Qin, L.J.; Gong, T.; Hao, H.X.; Wang, K.Y.; Feng, H. Core-shell-structured nanothermites synthesized by atomic layer deposition. J. Nanopart. Res. 2013, 15, 2150. [Google Scholar] [CrossRef]
- Xu, D.G.; Yang, Y.; Cheng, H.; Li, Y.Y.; Zhang, K.L. Integration of nano-Al with Co3O4 nanorods to realize high-exothermic core-shell nanoenergetic materials on a silicon substrate. Combust. Flame 2012, 159, 2202–2209. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Zhang, W.C.; Yu, C.P.; Zheng, G.Q.; Ma, K.F.; Qin, Z.C.; Ye, J.H.; Chao, Y.M. Integration of the 3DOM Al/Co3O4 nanothermite film with a semiconductor bridge to realize a high-output micro-energetic igniter. RSC Adv. 2018, 8, 2552. [Google Scholar] [CrossRef]
- Yu, C.P.; Zhang, W.C.; Shen, R.Q.; Xu, X.; Cheng, J.; Ye, J.H.; Qin, Z.C.; Chao, Y.M. 3D ordered macroporous NiO/Al nanothermite film with significantly improved higher heat output, lower ignition temperature and less gas production. Mater. Design 2016, 110, 304–310. [Google Scholar] [CrossRef]
- Zhang, W.C.; Yin, B.Q.; Shen, R.Q.; Ye, J.H.; Thomas, J.A.; Chao, Y.M. Significantly enhanced energy output from 3d ordered macroporous structured Fe2O3/Al nanothermite film. ACS Appl. Mater. Interfaces 2013, 5, 239–242. [Google Scholar] [CrossRef]
- Ammam, M. Electrophoretic deposition under modulated electric fields: A review. RSC Adv. 2012, 2, 7633–7646. [Google Scholar] [CrossRef]
- Rousta, A.; Dorranian, D.; Elahi, M. Electrophoretic deposition of cobalt oxide nanoparticles on aluminium substrate. Surf. Eng. 2020, 36, 919–928. [Google Scholar] [CrossRef]
- Fayette, M.; Nelson, A.; Robinson, R.D. Electrophoretic deposition improves catalytic performance of Co3O4 nanoparticles for oxygen reduction/oxygen evolution reactions. J. Mater. Chem. A 2015, 3, 4274. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Worsley, M.A.; Kuntz, J.D.; Gash, A.E. Electrophoretic deposition of binary energetic composites. Combust. Flame 2012, 159, 2210–2218. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Zhu, C.; Duoss, E.B.; Gash, A.E.; Kolesky, D.B.; Kuntz, J.D.; Lewis, J.A.; Spadaccini, C.M. Controlling material reactivity using architecture. Adv. Mater. 2016, 28, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Li, X.M.; Qin, B.; Lai, C.; Guo, X.G. Electrophoretic deposition and characterization of nano-Al/Fe2O3 thermites. Mater. Lett. 2014, 120, 224–227. [Google Scholar] [CrossRef]
- Zhang, D.X.; Xiang, Q. Electrophoretic fabrication of an Al-Co3O4 reactive nanocomposite coating and its application in a microignitor. Ind. Eng. Chem. Res. 2016, 55, 8243–8247. [Google Scholar] [CrossRef]
- Guo, X.G.; Sun, Q.; Liang, T.T.; Giwa, A.S. Controllable electrically guided nano-Al/MoO3 energetic-film formation on a semiconductor bridge with high reactivity and combustion performance. Nanomaterials 2020, 10, 955. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Bao, H.B.; Yuan, C.S.; Lai, C.; Tang, C.Y.; Giwa, A.S. Novel electrophoretic assembly design of nano-aluminum@tungsten trioxide (nano-Al@WO3) energeticcoating with controllable exothermic performance. J. Mater. Sci. Mater. Electron. 2021, 32, 15242–15250. [Google Scholar] [CrossRef]
- Yu, C.P.; Zhang, W.C.; Gao, Y.; Ni, D.B.; Ye, J.H.; Zhu, C.G.; Ma, K.F. The super-hydrophobic thermite film of the Co3O4/Al core/shell nanowires for an underwater ignition with a favorable aging-resistance. Chem. Eng. J. 2018, 338, 99–106. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Wang, J.; Li, X.M. Facilely electrophoretic derived aluminum/zinc (II) oxide nanocomposite with superhydrophobicity and thermostability. Ceram. Int. 2020, 46, 1052–1058. [Google Scholar] [CrossRef]
- Guo, X.G.; Lai, C.; Jiang, X.; Mi, W.H.; Yin, Y.J.; Li, X.M.; Shu, Y.J. Remarkably facile fabrication of extremely superhydrophobic high-energy binary composite with ultralong lifespan. Chem. Eng. J. 2018, 335, 843–854. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Yuan, B.F.; Wang, J. Nano-Al doped-MoO3 high-energy composite films with excellent hydrophobicity and thermal stability. Ceram. Int. 2021, 47, 24039–24046. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Giwa, A.S. Remarkably convenient construction of self-protected nano-aluminum/nickel oxide/perfluorosilane energetic composite to largely enhance structural, anti-wetting and exothermic stability. J. Alloy. Compd. 2022, 903, 164017. [Google Scholar] [CrossRef]
- Kim, S.H.; Zachariah, M.R. Enhancing the rate of energy release from nanoennergy materials by electrostatically enhanced assembly. Adv. Mater. 2004, 16, 1821–1825. [Google Scholar] [CrossRef]
- Dong, H.X.; Xia, M.; Wang, C.X.; Li, G.P.; Luo, Y.J. Al/NiO nanocomposites for enhanced energetic properties: Preparation by polymer assembly method. Mater. Design 2019, 183, 108111. [Google Scholar] [CrossRef]
- Séverac, A.; Alphonse, P.; Estève, A.; Bancaud, A.; Rossi, C. High-energy Al/CuO nanocomposites obtained by DNA-directed assembly. Adv. Funct. Mater. 2012, 22, 323–329. [Google Scholar] [CrossRef]
- Tillotson, T.M.; Gash, A.E.; Simpson, R.L.; Hrubesh, L.W.; Satcher, J.H.; Poco, J.F. Nanostructured energetic materials using sol-gel methodologies. J. Non-Cryst. Solids. 2001, 285, 338–345. [Google Scholar] [CrossRef]
- Prentice, D.; Pantoya, M.L.; Clapsaddle, B.J. Effect of nanocomposite synthesis on the combustion performance of a ternary thermite. J. Phys. Chem. B 2005, 109, 20180–20185. [Google Scholar] [CrossRef]
- Li, R.; Xu, H.M.; Hu, H.L.; Yang, G.C.; Wang, J.; Shen, J.P. Microstructured Al/Fe2O3/nitrocellulose energetic fibers realized by electrospinning. J. Energ. Mater. 2014, 32, 50–59. [Google Scholar] [CrossRef]
- Yan, S.; Jian, G.Q.; Zachariah, M.R. Electrospun nanofiber-based thermite textiles and their reactive properties. ACS Appl. Mater. Interfaces 2012, 4, 6432–6435. [Google Scholar] [CrossRef]
- He, W.; Li, Z.H.; Chen, S.W.; Yang, G.C.; Yang, Z.J.; Liu, P.J.; Yan, Q.L. Energetic metastable n-Al@pvdf/emof composite nanofibers with improved combustion performances. Chem. Eng. J. 2020, 383, 123146. [Google Scholar] [CrossRef]
- Schoenitz, M.; Ward, T.S.; Dreizin, E.L. Fully dense nano-composite energetic powders prepared by arrested reactive milling. P. Combust. Inst. 2005, 30, 2071–2078. [Google Scholar] [CrossRef]
- Williams, R.A.; Patel, J.V.; Ermoline, A.; Schoenitz, M.; Dreizin, E.L. Correlation of optical emission and pressure generated upon ignition of fully-dense nanocomposite thermite powders. Combust. Flame 2013, 160, 734–741. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, D.R.; Dong, Y.C.; Wang, L.; Chen, X.G.; Zhang, J.X.; He, J.N.; Li, X.Z. In situ nanostructured ceramic matrix composite coating prepared by reactive plasma spraying micro-sized Al-Fe2O3 composite powders. J. Alloy. Compd. 2011, 509, L90–L94. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Z.J.; Li, Y.C.; Zheng, B.H.; Yan, Q.L.; Guan, L.F.; Luo, G.; Li, S.B.; Nie, F. Incorporation of high explosives into nano-aluminum based microspheres to improve reactivity. Chem. Eng. J. 2020, 383, 123110. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Ahmadian, H. Effect of residual structural strain caused by the addition of Co3O4 nanoparticles on the structural, hardness and magnetic properties of an Al/Co3O4 nanocomposite produced by powder metallurgy. Int. J. Min. Met. Mater. 2020, 27, 384–390. [Google Scholar] [CrossRef]
- Slocik, J.M.; McKenzie, R.; Dennis, P.B.; Naik, R.R. Creation of energetic biothermite inks using ferritin liquid protein. Nat. Commun. 2017, 8, 15156. [Google Scholar] [CrossRef]
- Shi, K.W.; Guo, X.D.; Chen, L.; Huang, S.S.; Zhao, L.L.; Ji, J.; Zhou, X. Alcohol-thermal synthesis of approximately core-shell structured Al@CuO nanothermite with improved heat-release and combustion characteristics. Combust. Flame 2021, 228, 331–339. [Google Scholar] [CrossRef]
- Guo, S.S.; Focke, W.W.; Tichapondwa, S.M. Al-Ni-NiO pyrotechnic time-delays. Propellants Explos. Pyrotech. 2020, 45, 665–670. [Google Scholar] [CrossRef]
- Fan, R.H.; Lü, H.L.; Sun, K.N.; Wang, W.X.; Yi, X.B. Kinetics of thermite reaction in Al-Fe2O3 system. Thermochimi. Acta 2006, 440, 129–131. [Google Scholar] [CrossRef]
- Wang, N.; Hu, Y.B.; Ke, X.; Xiao, L.; Zhou, X.; Peng, S.S.; Hao, G.Z.; Jiang, W. Enhanced-absorption template method for preparation of double-shell NiO hollow nanospheres with controllable particle size for nanothermite application. Chem. Eng. J. 2020, 379, 122330. [Google Scholar] [CrossRef]
- Yin, Y.J.; Li, X.M.; Shu, Y.J.; Guo, X.G.; Zhu, Y.H.; Huang, X.Y.; Bao, H.B.; Xu, K. Highly-reactive Al/CuO nanoenergetic materials with a tubular structure. Mater. Design 2017, 117, 104–110. [Google Scholar] [CrossRef]
- Xu, J.Y.; Chen, Y.J.; Zhang, W.C.; Zheng, Z.L.; Yu, C.P.; Wang, J.X.; Song, C.K.; Chen, J.H.; Lei, X.T.; Ma, K.F. Direct ink writing of nAl/pCuO/HPMC with outstanding combustion performance and ignition performance. Combust. Flame 2022, 236, 111747. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Vignoli, V.; Hjiri, M.; Aida, M.S.; Mugnaini, M.; Addabbo, T. Co3O4/Al-ZnO nano-composites: Gas sensing properties. Sensors 2019, 19, 760. [Google Scholar] [CrossRef] [PubMed]
- Dolgoborodov, A.; Yankovsky, B.; Ananev, S.; Valyano, G.; Vakorina, G. Explosive burning of a mechanically activated Al and CuO thermite mixture. Energies 2022, 15, 489. [Google Scholar] [CrossRef]
- Martirosyan, K.S.; Wang, L.; Vicent, A.; Luss, D. Synthesis and performance of bismuth trioxide nanoparticles for high energy gas generator use. Nanotechnology 2009, 20, 405609. [Google Scholar] [CrossRef]
- Gagaoudakis, E.; Michail, G.; Katerinopoulou, D.; Moschovis, K.; Iliopoulos, E.; Kiriakidis, G.; Binas, V.; Aperathitis, E. Transparent p-type NiO:Al thin films as room temperature hydrogen and methane gas sensors. Mat. Sci. Semicon. Proc. 2020, 109, 104922. [Google Scholar] [CrossRef]
- Burrola, H.; Horii, M.; Gonzalez-Guerrero, M.J.; Bachman, J.C.; Gomez, F.A. Production of a NiO/Al primary battery employing powder-based electrodes. Electrophoresis 2020, 41, 131–136. [Google Scholar] [CrossRef]
- Sui, H.T.; Huda, N.; Shen, Z.K.; Wen, J.Z. Al-NiO energetic composites as heat source for joining silicon wafer. J. Mater. Process. Tech. 2020, 279, 116572. [Google Scholar] [CrossRef]
- Zhong, Y.; Xie, Y.J.; Hwang, S.; Wang, Q.; Cha, J.J.; Su, D.; Wang, H.L. A highly efficient all-solid-state lithium/electrolyte interface induced by an energetic reaction. Angew. Chem. 2020, 132, 14007–14112. [Google Scholar] [CrossRef]
- Clark, B.R.; Pantoya, M.L. The aluminium and iodine pentoxide reaction for the destruction of spore forming bacteria. Phys. Chem. Chem. Phys. 2010, 12, 12653–12657. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jian, G.Q.; Egan, G.C.; Zachariah, M.R. Assembly and reactive properties of Al/CuO based nanothermite microparticles. Combust. Flame 2014, 161, 2203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).