Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions
Abstract
1. Introduction
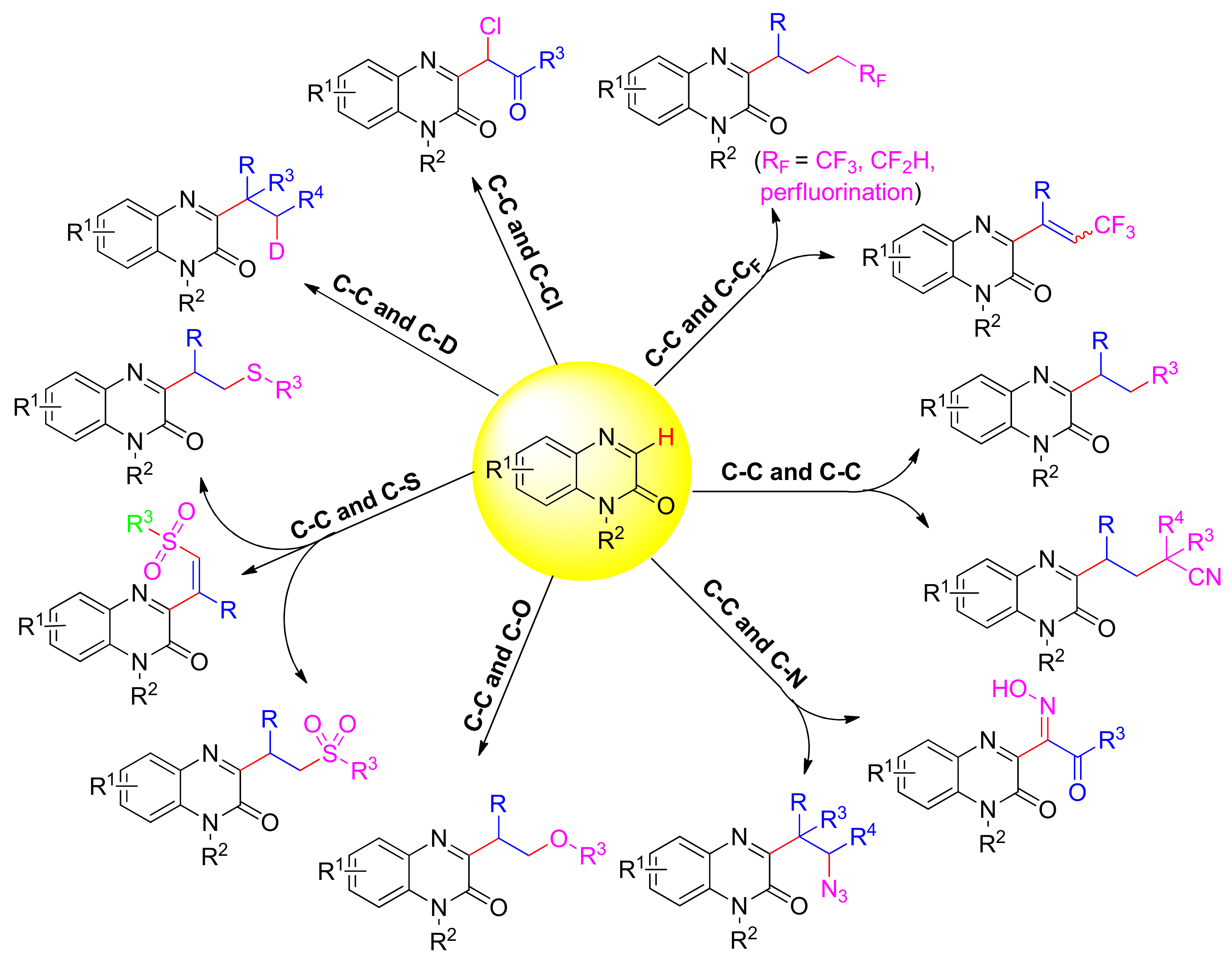
2. Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions
2.1. Simultaneous Construction of Both C–C and C–RF Bonds via Multi-Component Tandem Reactions
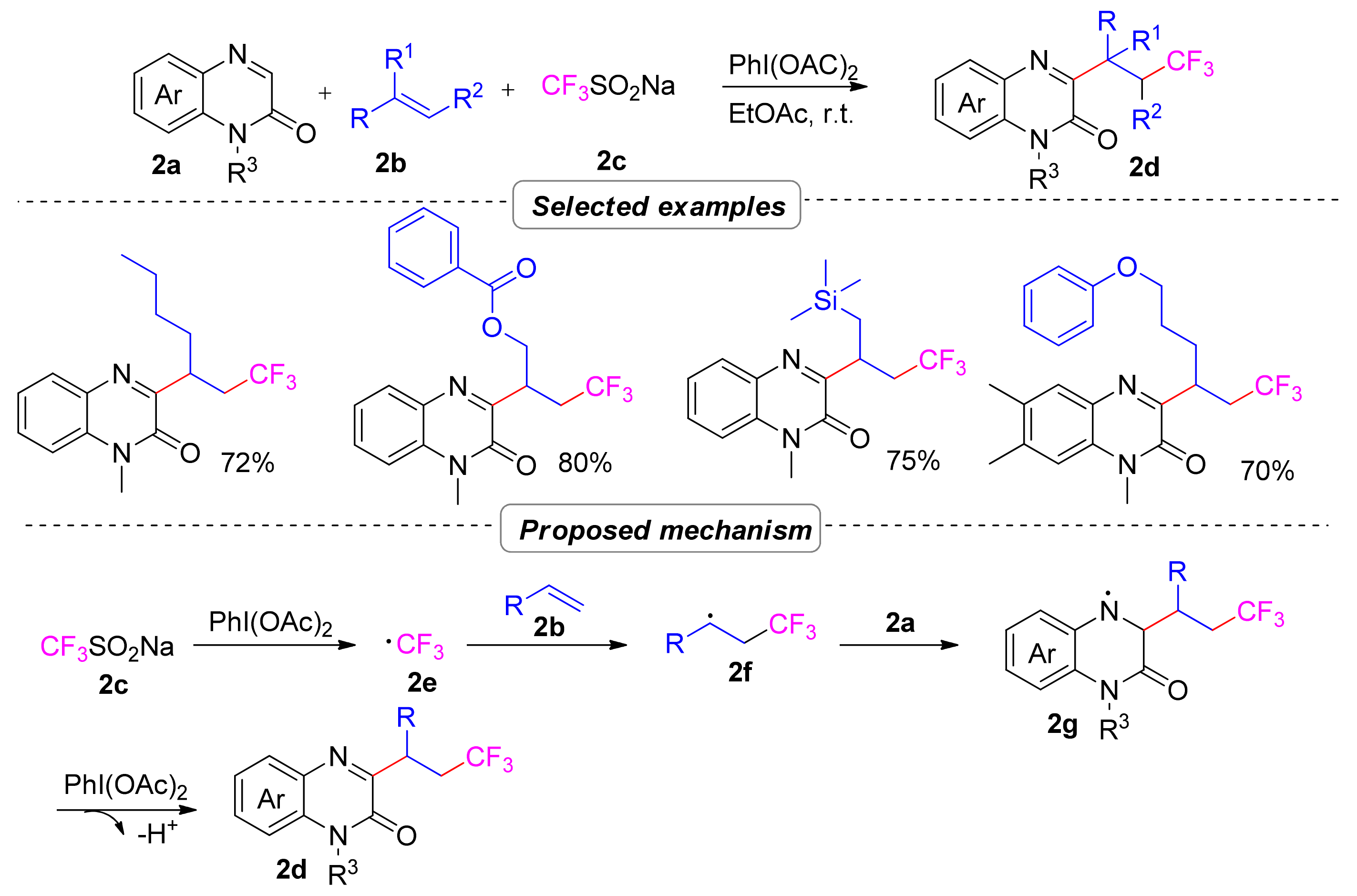
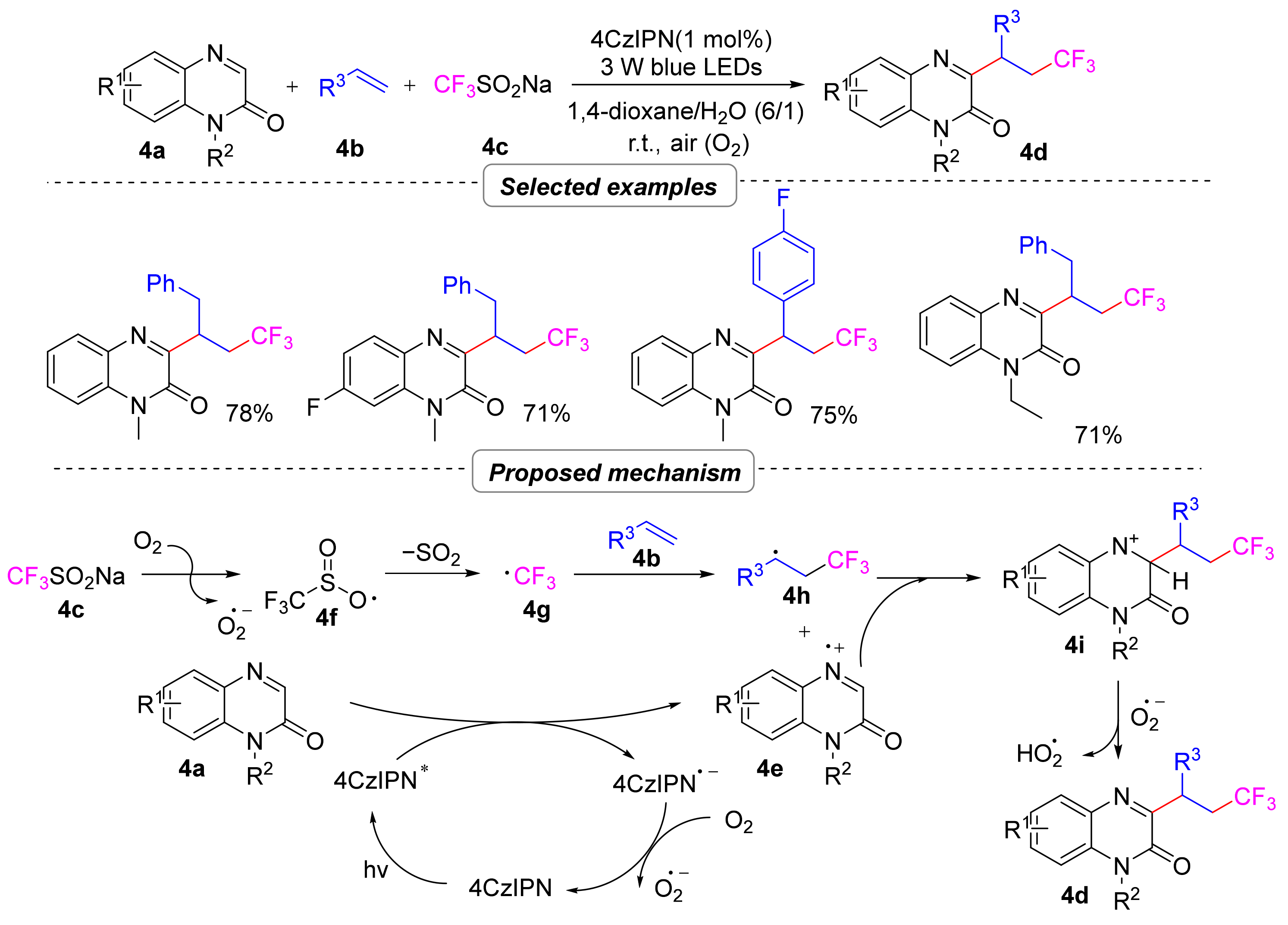
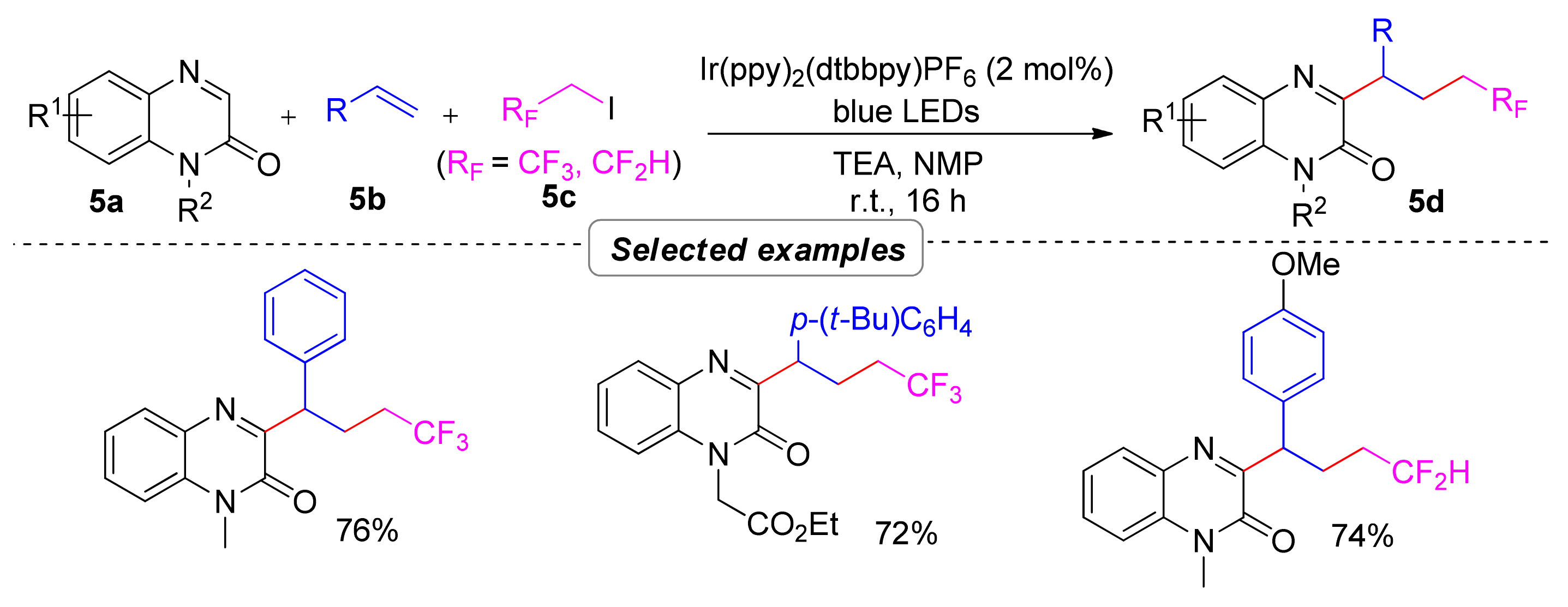
2.1.1. Trifluoroalkylation
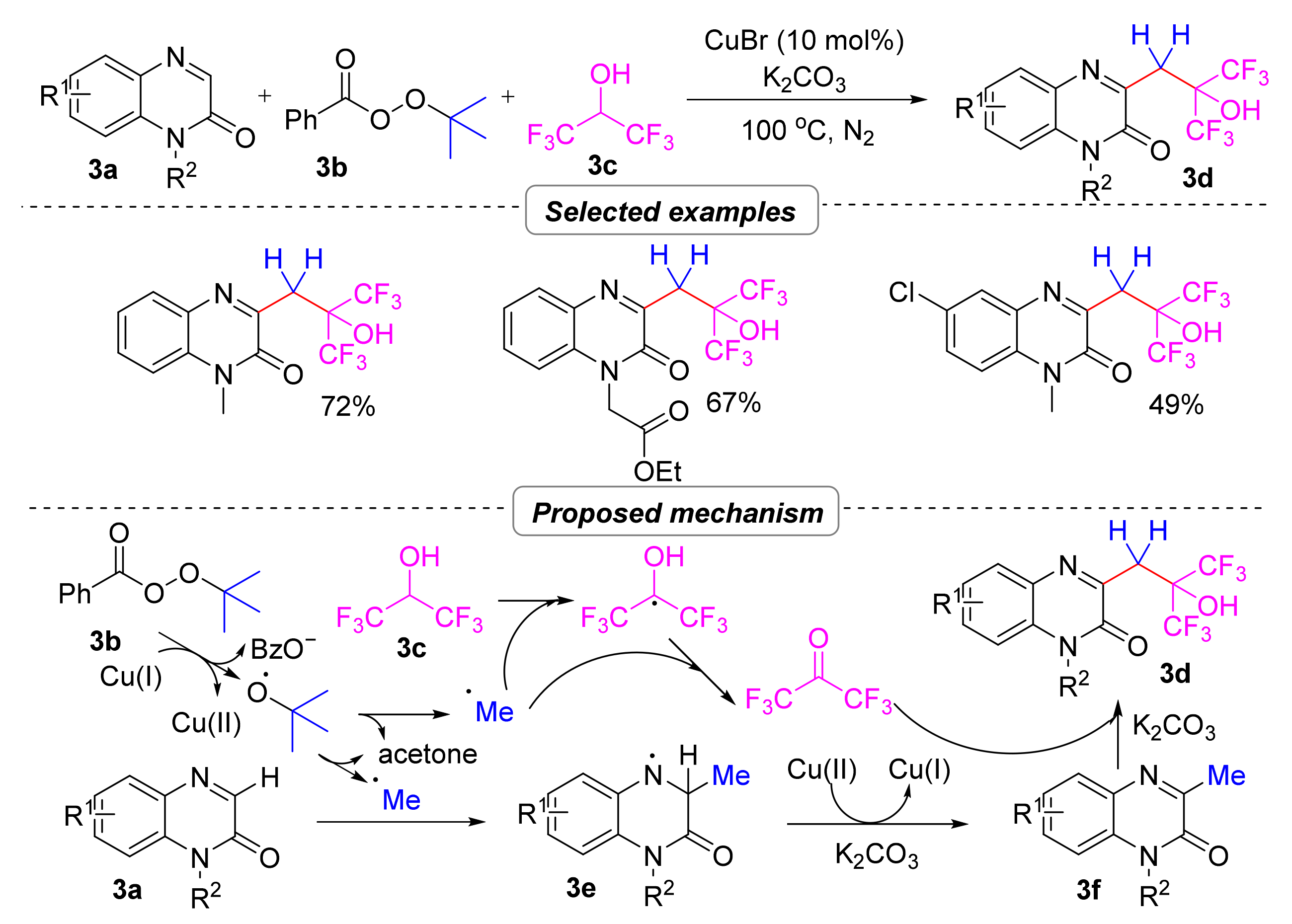
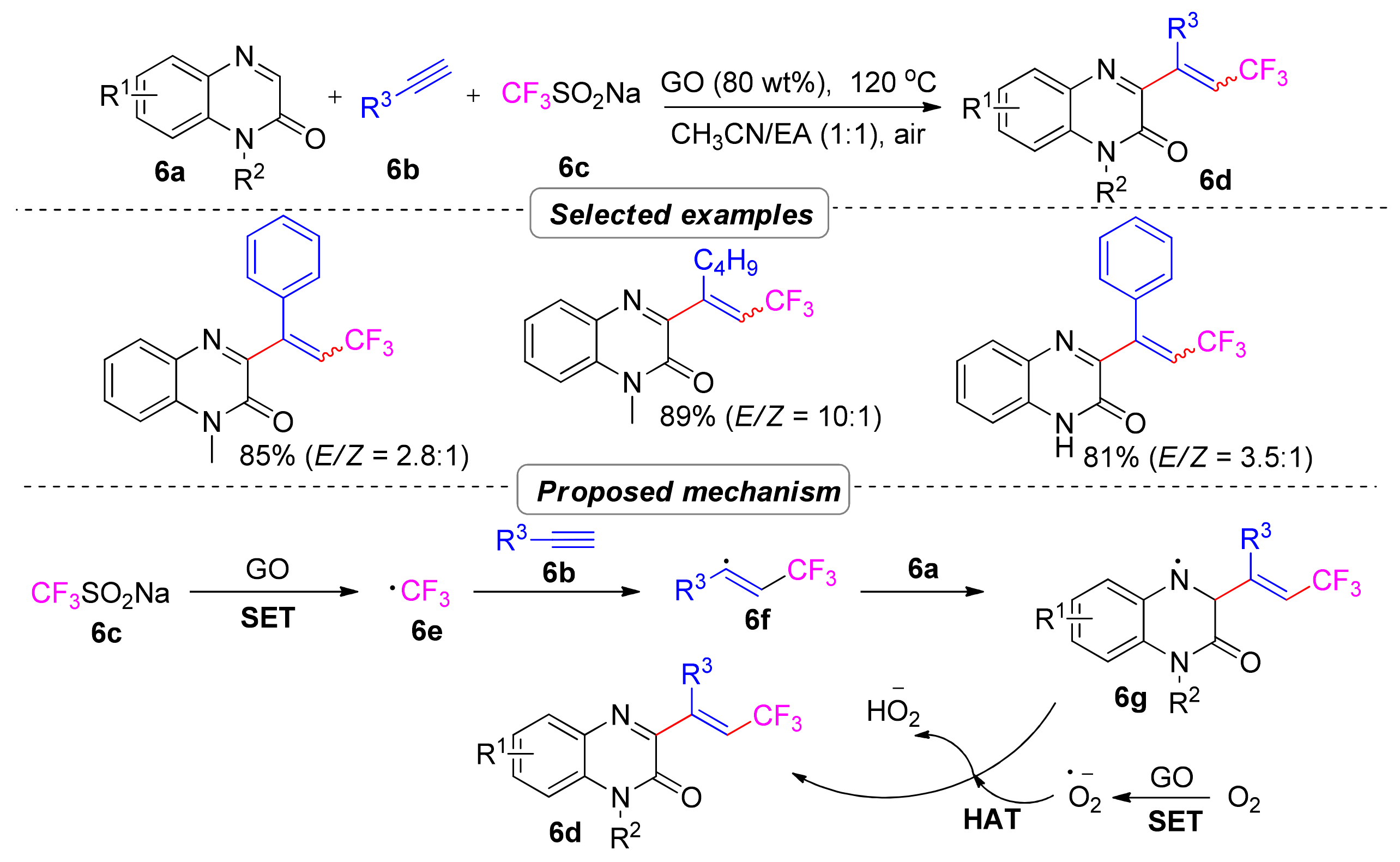
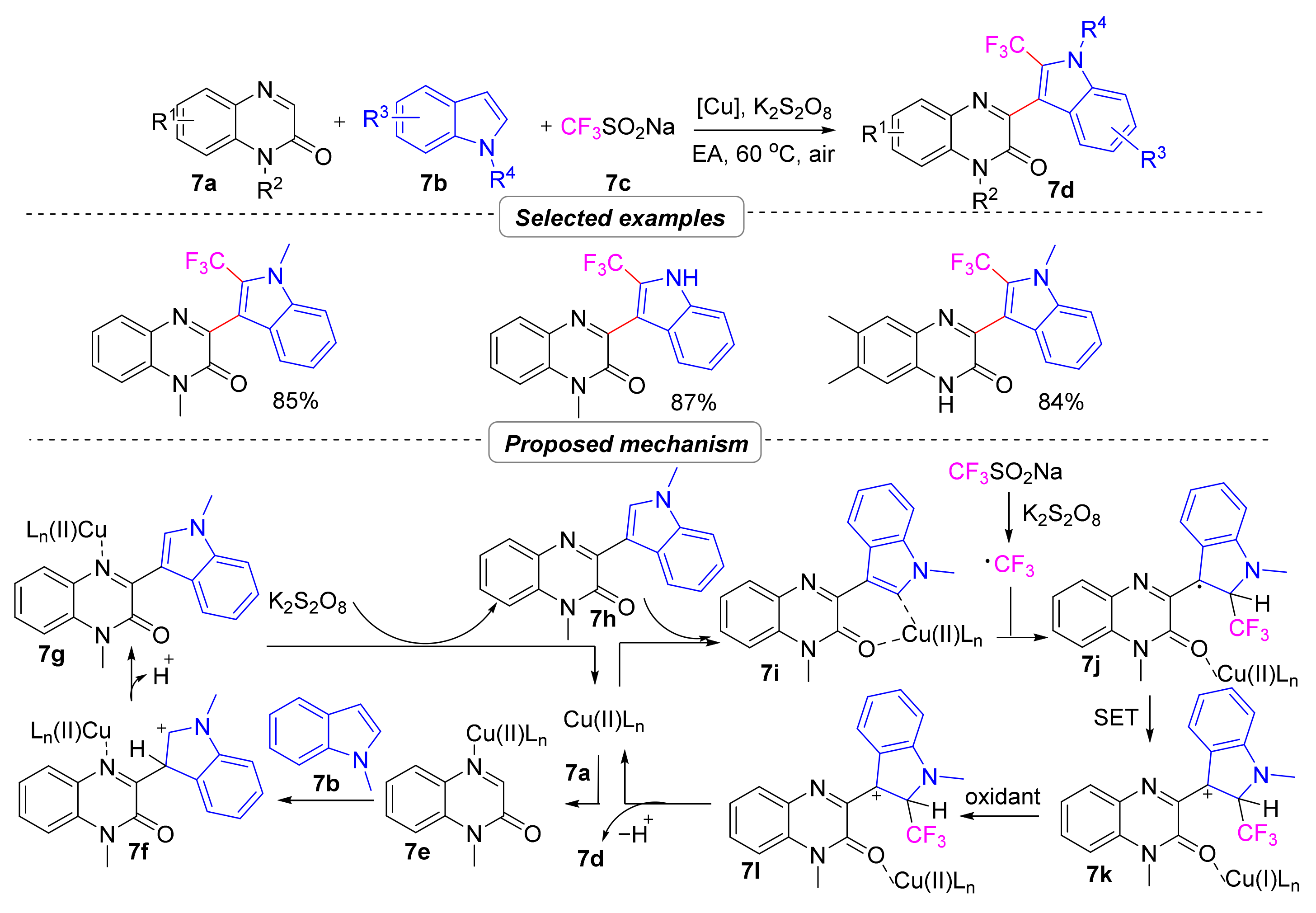
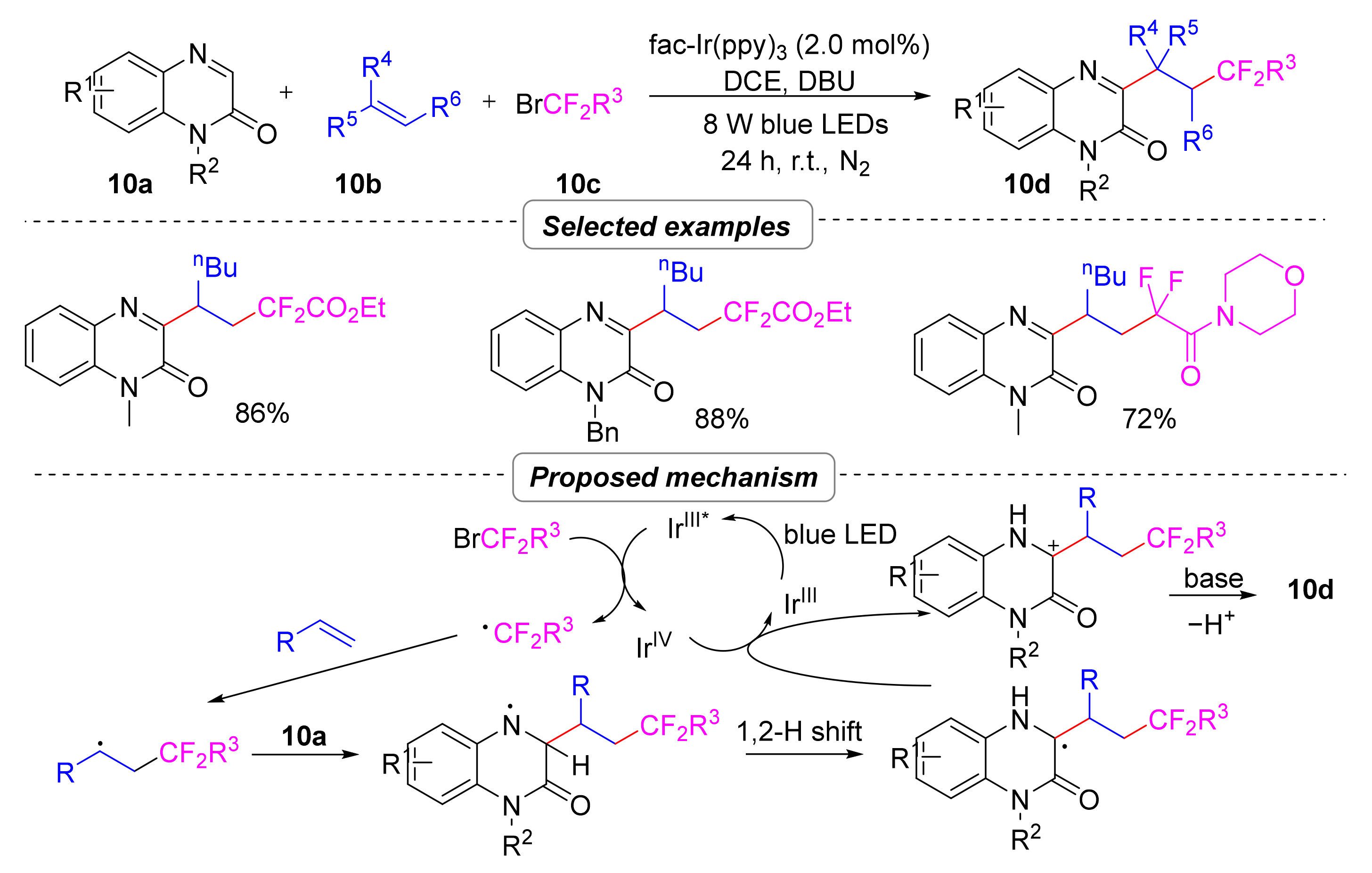
2.1.2. Perfluoroalkyl
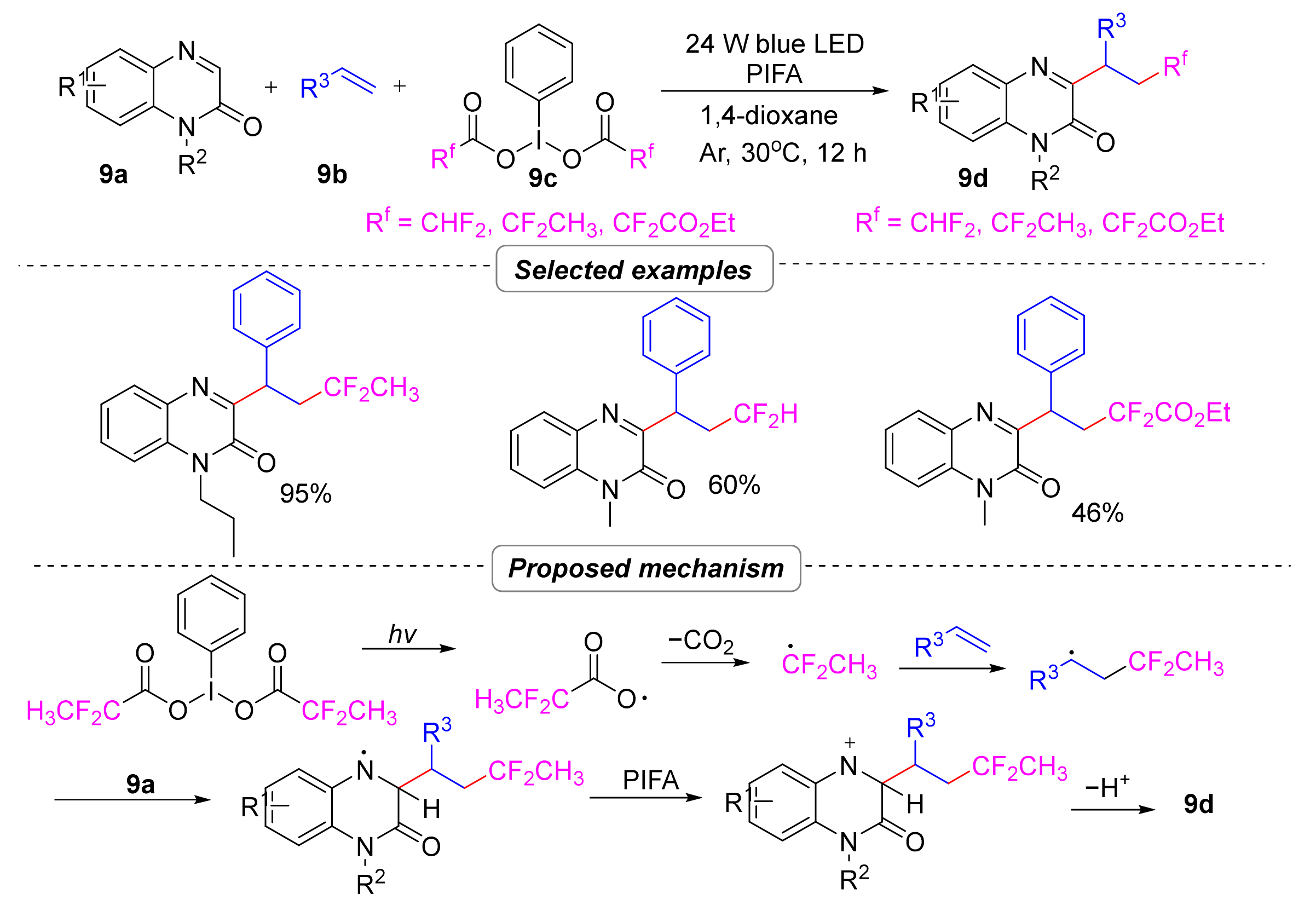
2.1.3. Difluoroalkylation
2.2. Simultaneous Construction of Double C–C Bonds via Multi-Component Tandem Reactions
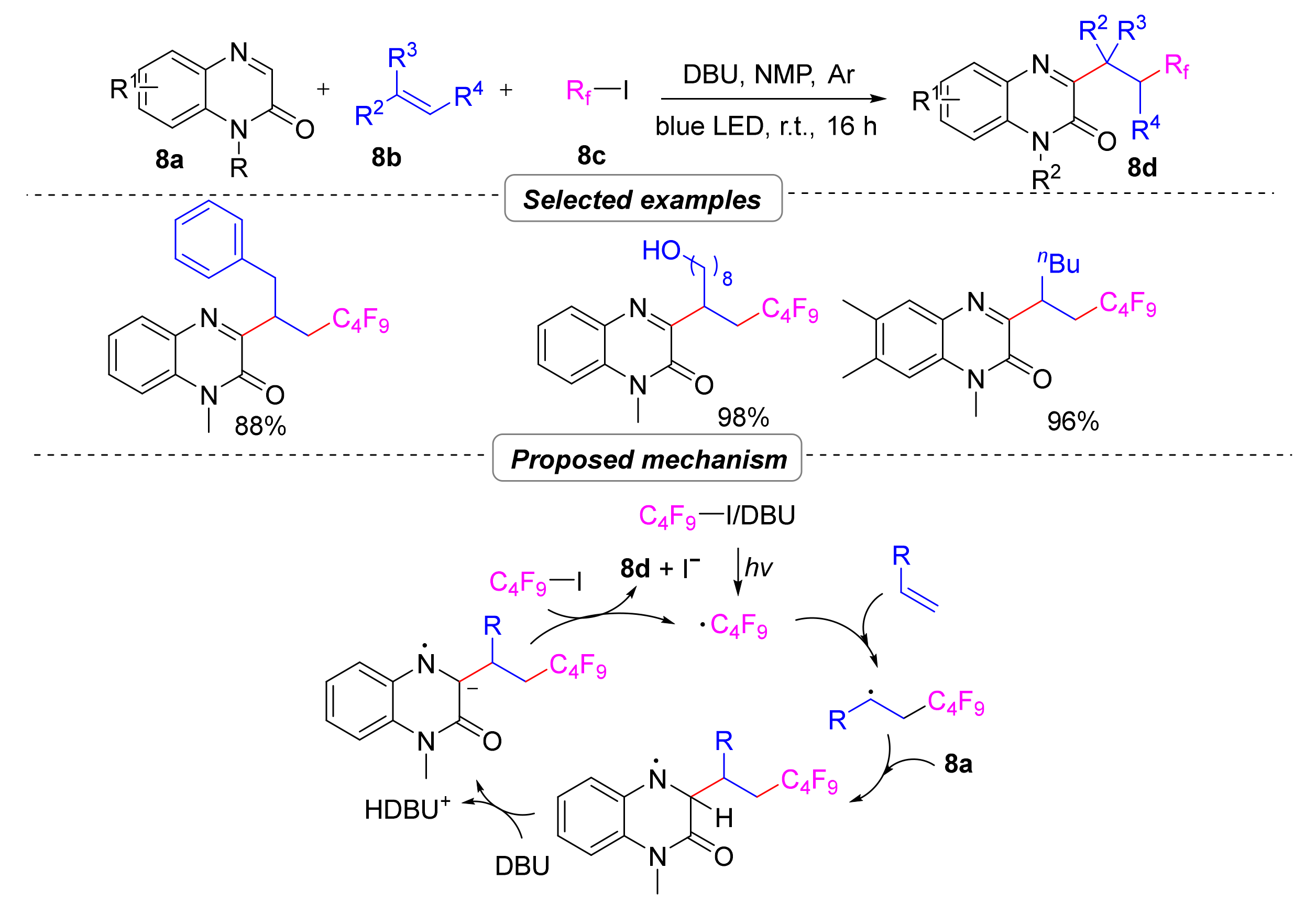
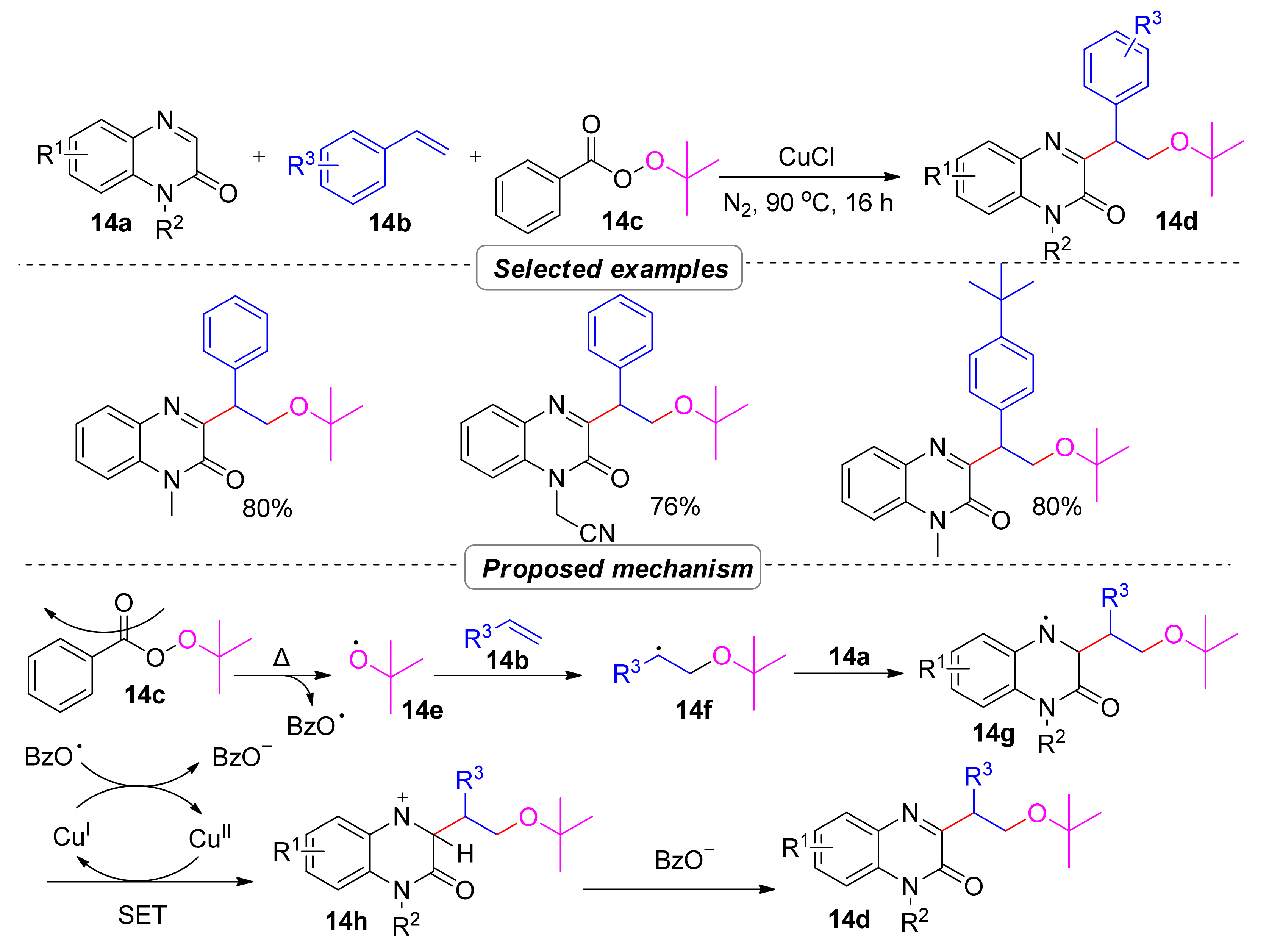
2.2.1. Alkylation
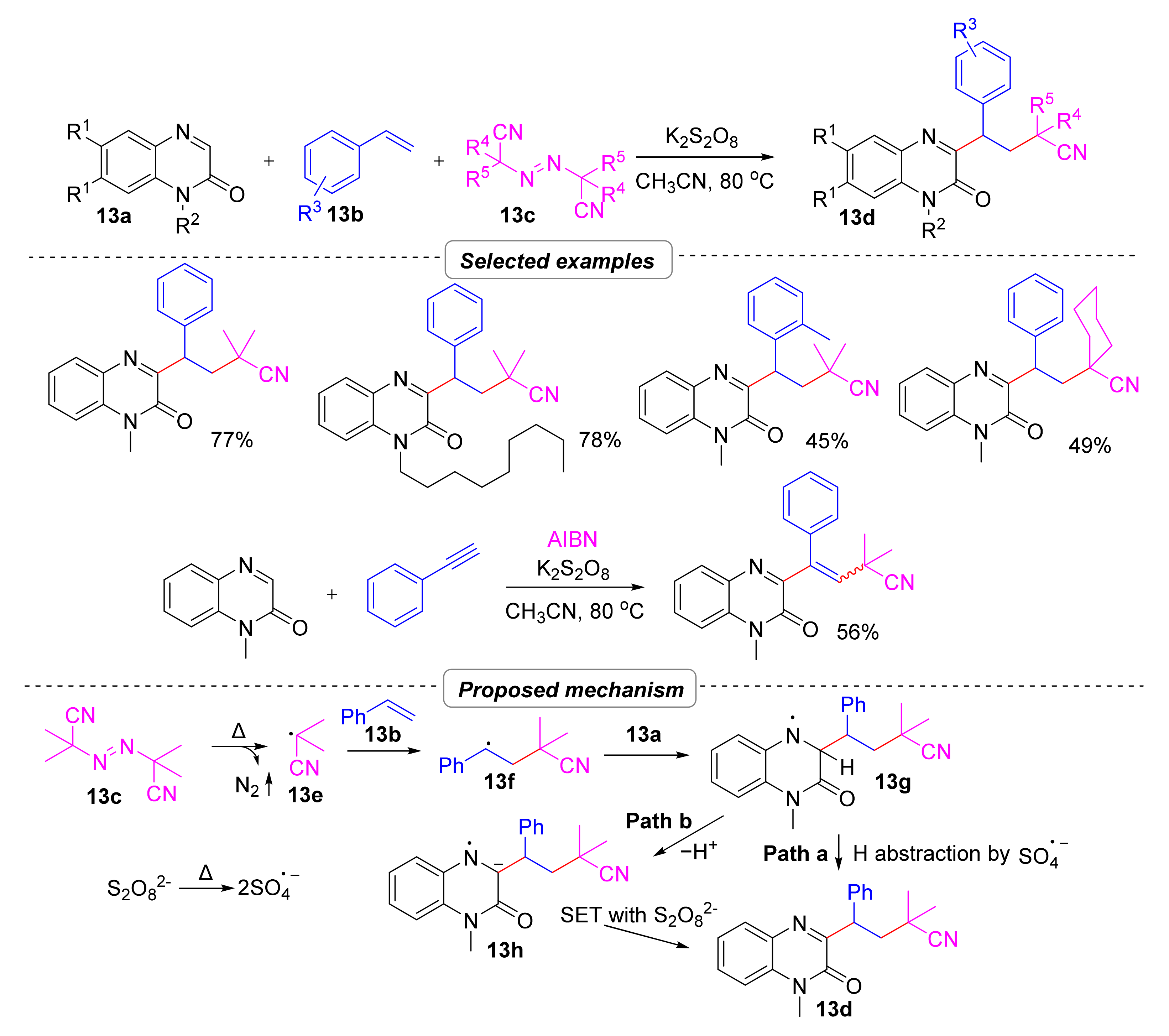
2.2.2. Cyanoalkylation
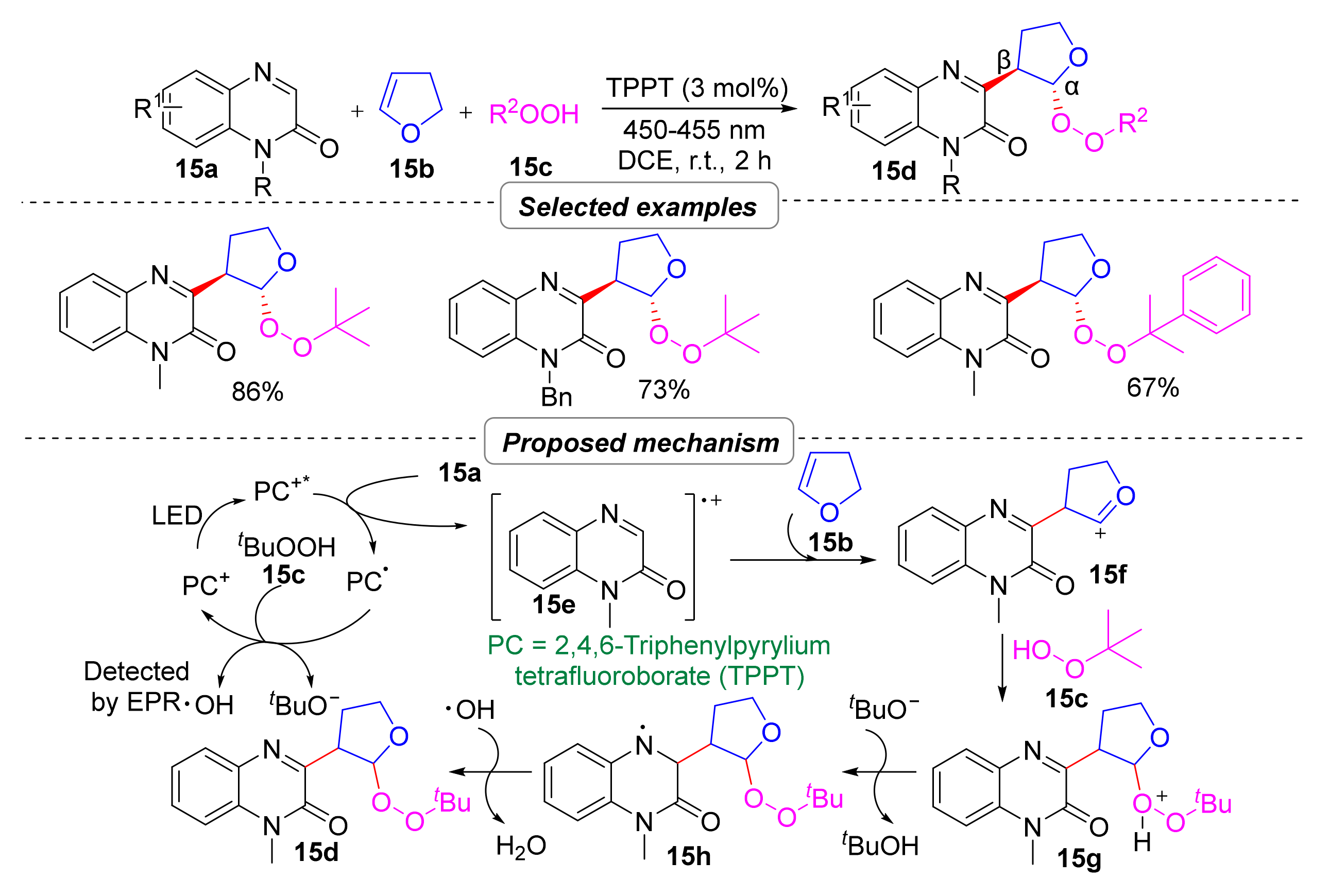
2.3. Simultaneous Construction of Both C–C and C–O Bonds via Multi-Component Tandem Reactions
2.4. Simultaneous Construction of Both C–C and C–N Bonds via Multi-Component Tandem Reactions
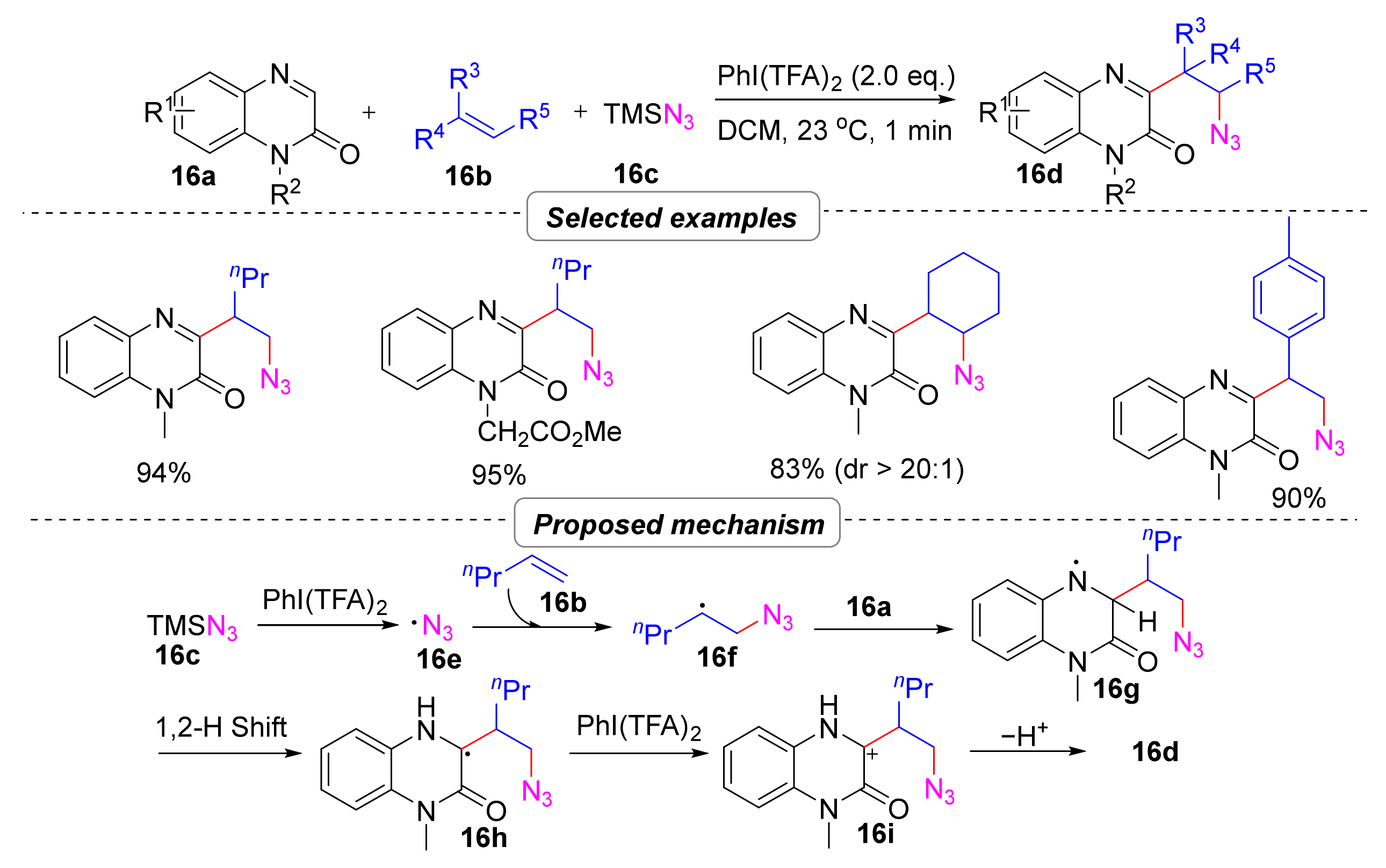
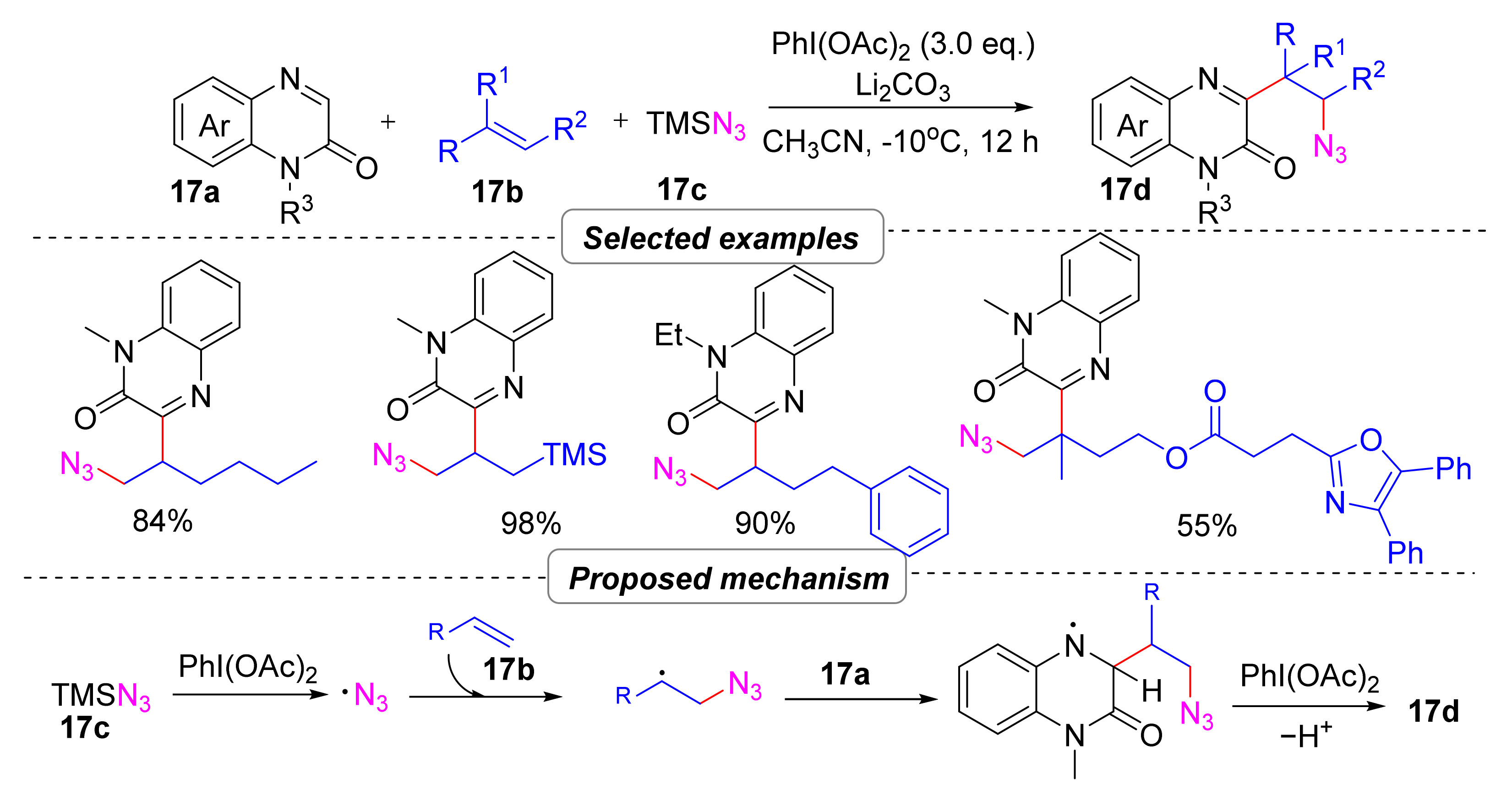
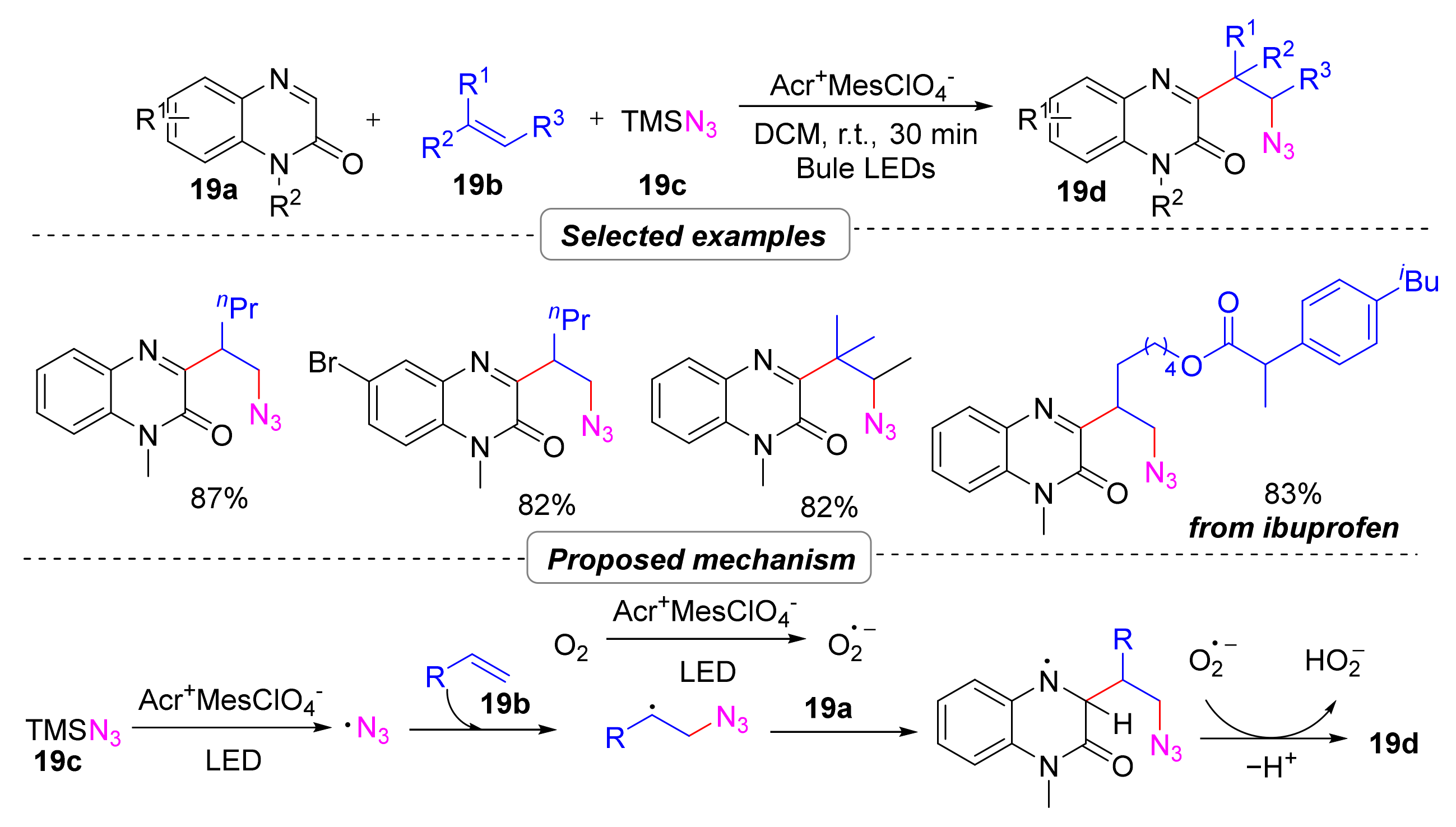
2.4.1. Azidoalkylation
2.4.2. Oxime
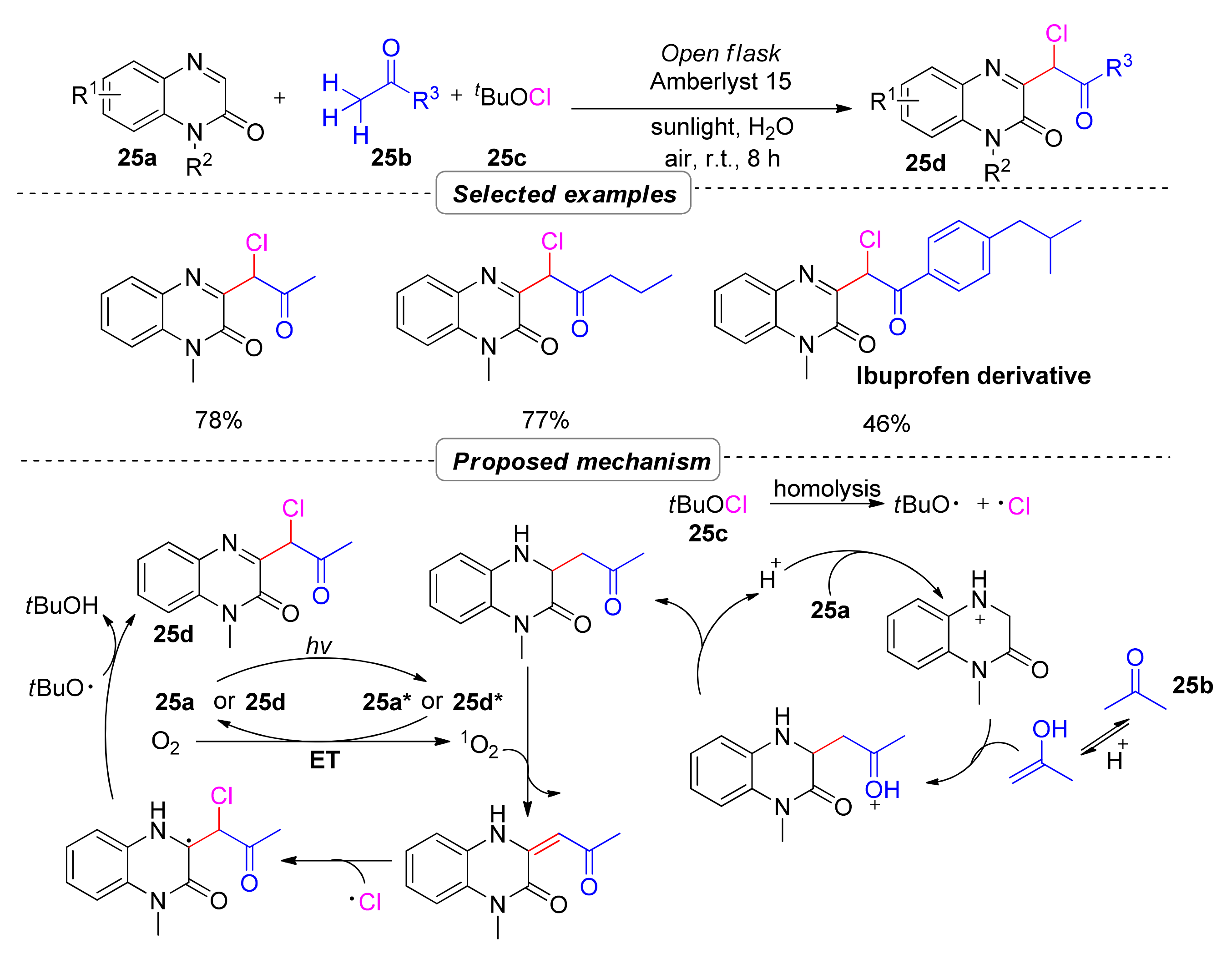
2.5. Simultaneous Construction of Both C–C and C–Cl Bonds via Multi-Component Tandem Reactions
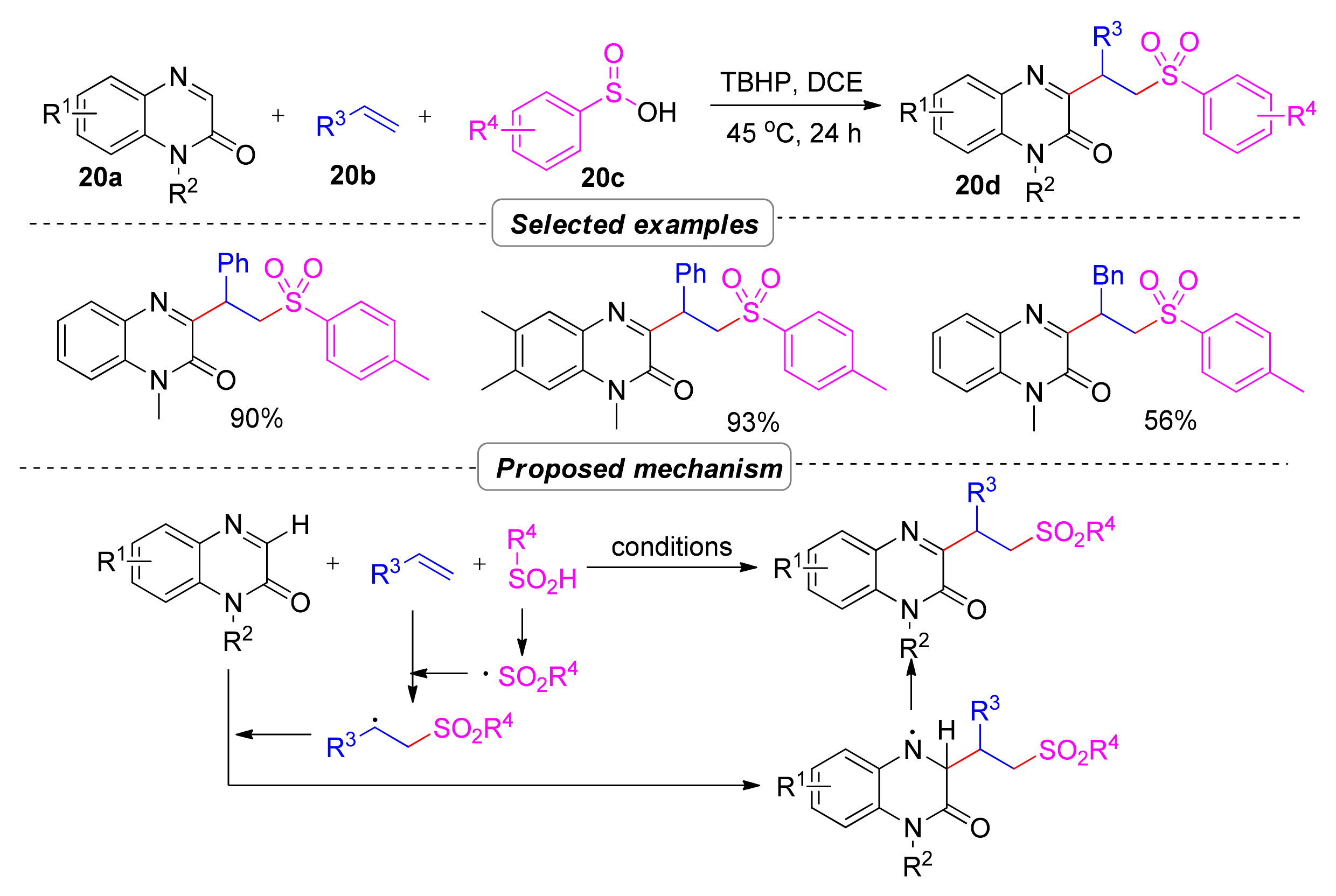
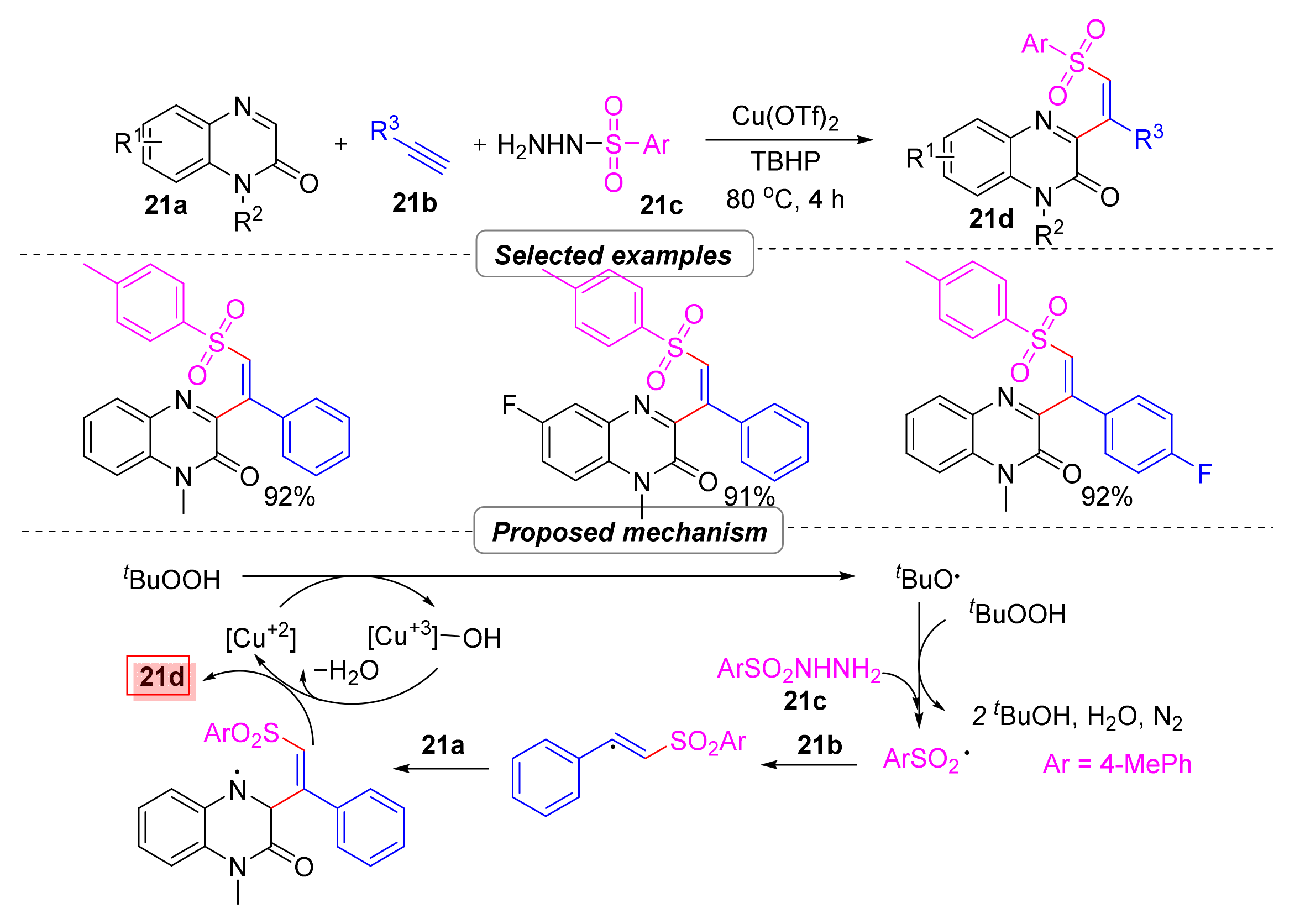
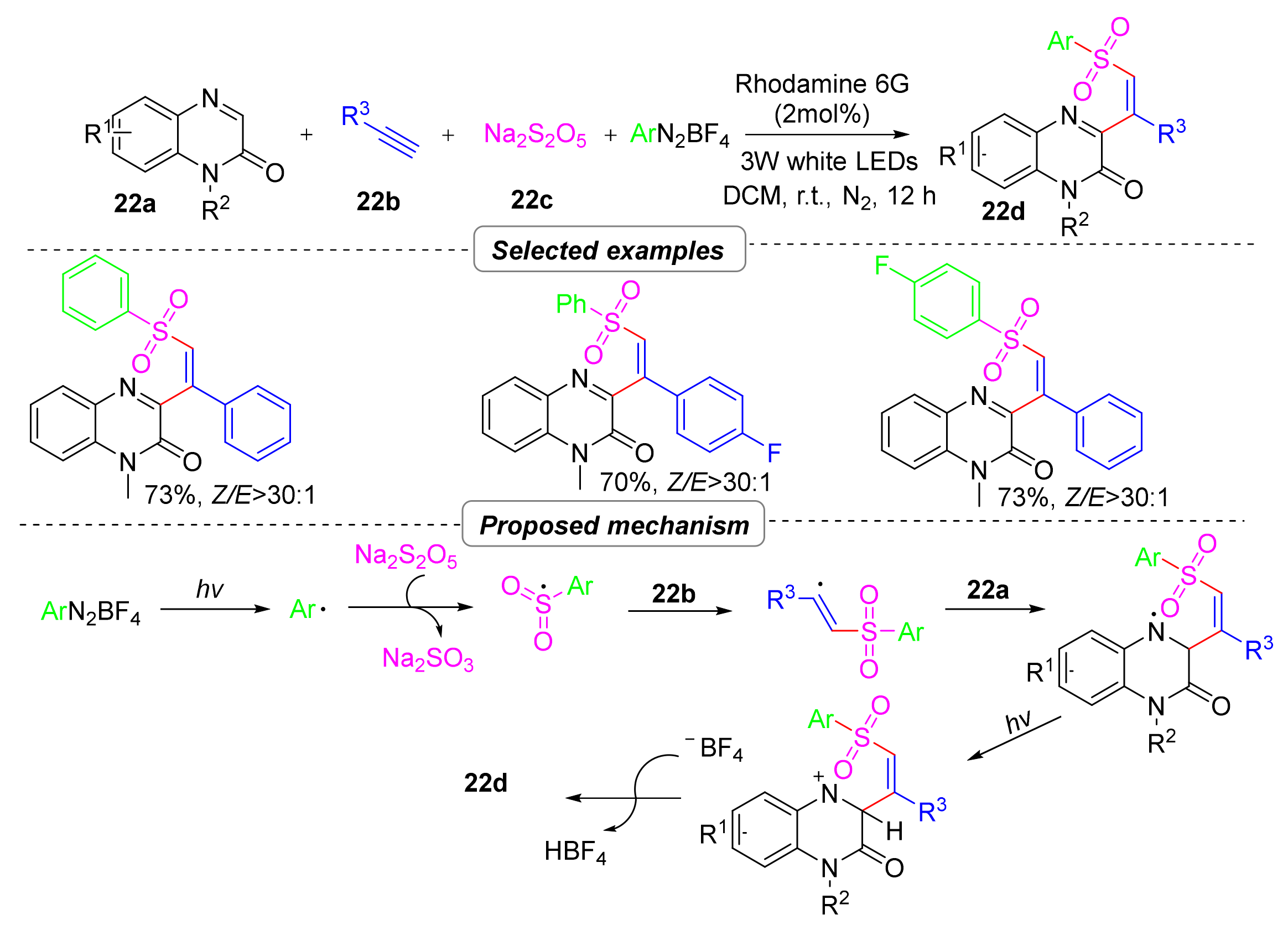
2.6. Simultaneous Construction of Both C–C and C–S Bonds via Multi-Component Tandem Reactions
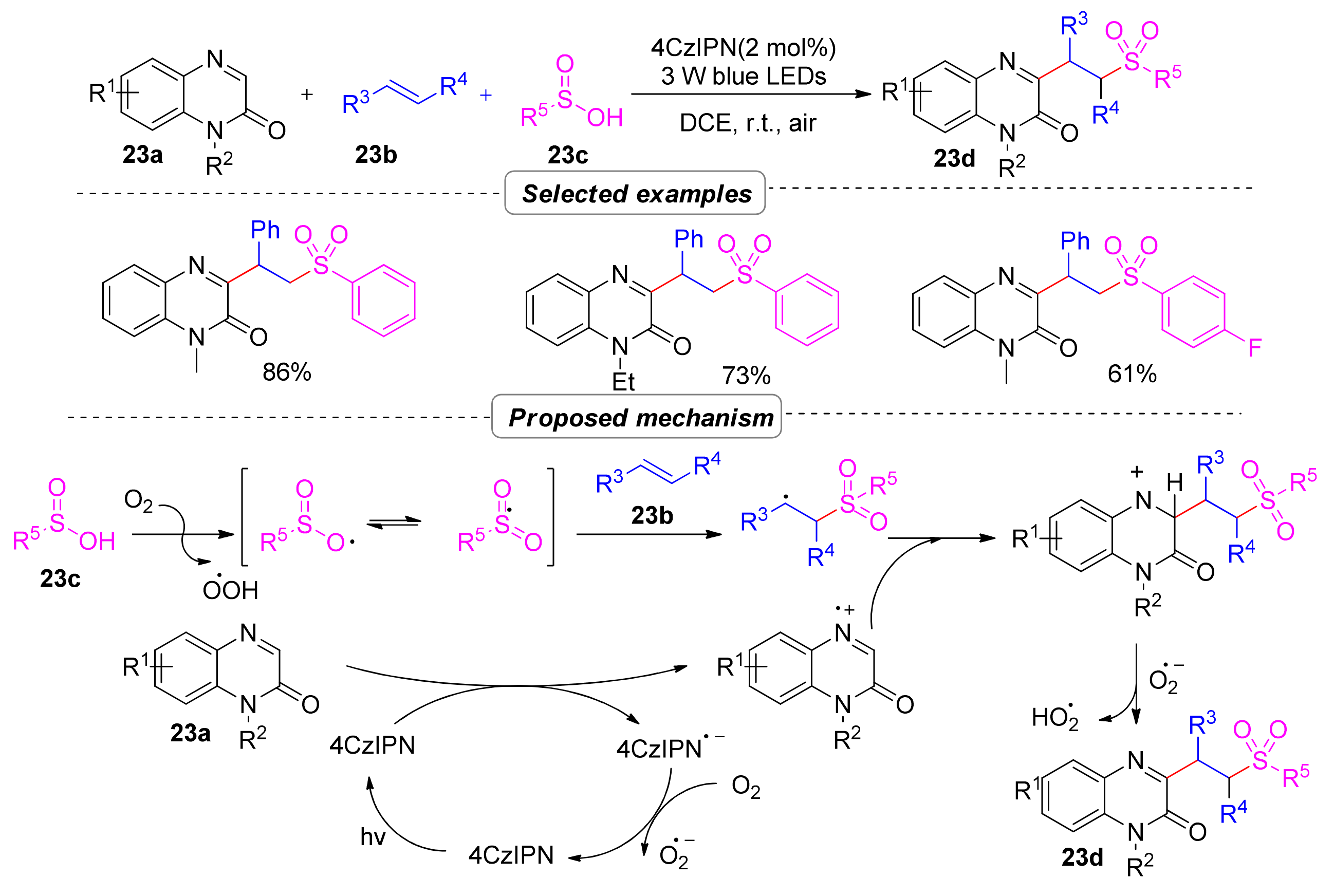
2.6.1. Sulfonyl Hydroxylation
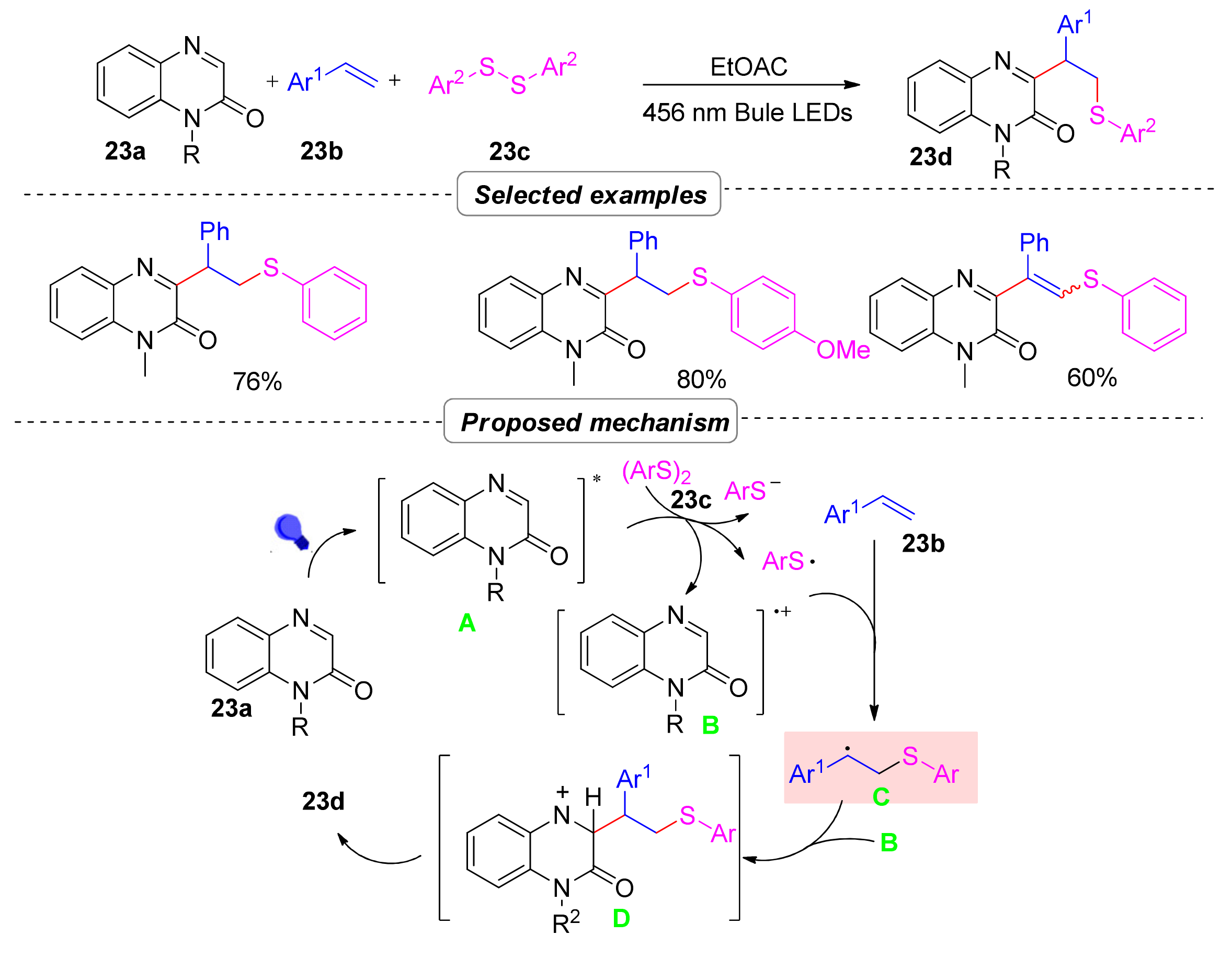
2.6.2. Thioalkylation
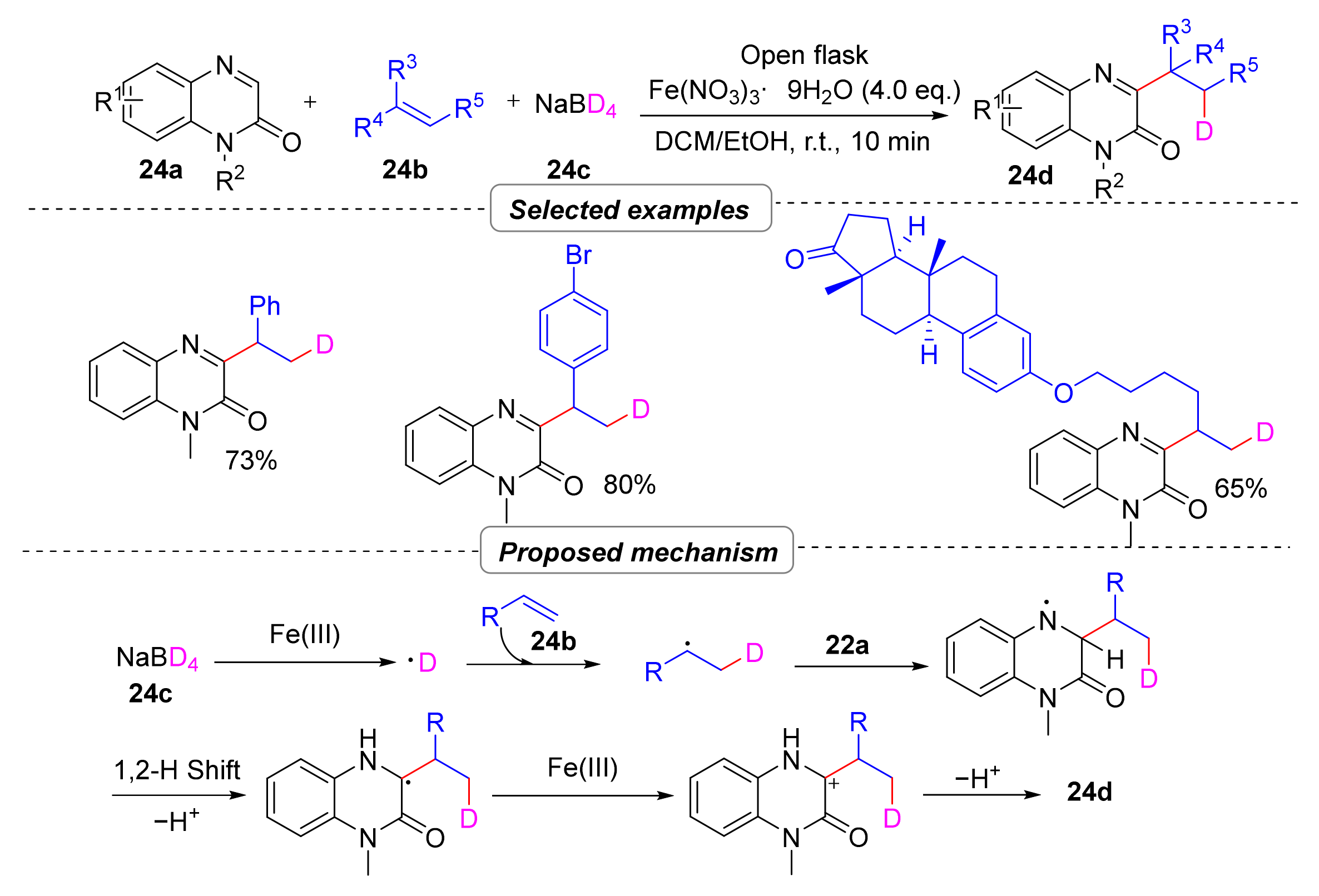
2.7. Simultaneous Construction of Both C–C and C–D Bonds via Multi-Component Tandem Reactions
3. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Müller, T.J.J. Multicomponent reactions. Beilstein J. Org. Chem. 2011, 7, 960–961. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. Front Matter. In Multicomponent Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. i–xvi. [Google Scholar]
- Weber, L.; Illgen, K.; Almstetter, M. Discovery of New Multi Component Reactions with Combinatorial Methods. Synlett 1999, 1999, 366–374. [Google Scholar] [CrossRef]
- Strecker, A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Ann. Chem. 1850, 75, 27–45. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Feng, X. Asymmetric Strecker Reactions. Chem. Rev. 2011, 111, 6947–6983. [Google Scholar] [CrossRef]
- Zuend, S.J.; Coughlin, M.P.; Lalonde, M.P.; Jacobsen, E.N. Scaleable catalytic asymmetric Strecker syntheses of unnatural α-amino acids. Nature 2009, 461, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Suk Oh, J.; Lee, J.-W.; Eui Song, C. Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator. Nat. Commun. 2012, 3, 1212. [Google Scholar] [CrossRef]
- Treesa, S.G.S.; Saranya, S.; Meera, G.; Anilkumar, G. Recent Advances and Perspectives in the Silver-catalyzed Multi-component Reactions. Curr. Org. Chem. 2020, 24, 291–313. [Google Scholar] [CrossRef]
- Nia, H.R.; Mamaghani, M.; Tavakoli, F. Ag-catalyzed Multicomponent Synthesis of Heterocyclic Compounds: A Review. Curr. Org. Synth. 2022, 19, 484–506. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Habib, N.S.; Kassem, M.A. Synthesis of Some New Quinoxalines and 1,2,4-Triazolo[4,3-a]-quinoxalines for Evaluation of in vitro Antitumor and Antimicrobial Activities. Arch. Pharm. 2006, 339, 564–571. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Z.; Murray, M.G.; Guo, X.; Liu, G. Quinoxalin-2(1H)-One Derivatives As Inhibitors Against Hepatitis C Virus. J. Med. Chem. 2011, 54, 5747–5768. [Google Scholar] [CrossRef]
- Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; et al. Design and Synthesis of Potent and Multifunctional Aldose Reductase Inhibitors Based on Quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [CrossRef]
- Burganov, T.I.; Katsyuba, S.A.; Islamova, L.N.; Fazleeva, G.M.; Sharipova, S.M.; Kalinin, A.A.; Monari, A.; Assfeld, X. To what extent are the photophysical properties of quinoxaline- and quinoxalinone-based chromophores predictable? Dyes Pigm. 2019, 170, 107580. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Burganov, T.I.; Katsyuba, S.A.; Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M.; Ahmadeev, B.S.; Mustafina, A.R.; Monari, A.; Assfeld, X.; et al. Halochromic luminescent quinoxalinones as a basis for pH-sensing in organic and aqueous solutions. Dyes Pigm. 2021, 186, 108958. [Google Scholar] [CrossRef]
- Mao, P.; Zhu, J.; Yuan, J.; Yang, L.; Xiao, Y.; Zhang, C. Recent Advances on the Catalytic Functionalization of Quinoxalin-2(1H)-ones via C-H Bond Activation. Chinese J. Org. Chem. 2019, 39, 1529–1547. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, S. Recent advances and perspectives on the synthesis and C-H bond functionalization of quinoxalin-2(1H)-one. Synth. Commun. 2020, 50, 2266–2312. [Google Scholar] [CrossRef]
- Ke, Q.; Yan, G.; Yu, J.; Wu, X. Recent advances in the direct functionalization of quinoxalin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5863–5881. [Google Scholar] [CrossRef]
- Monika, M.; Selvakumar, S. Recent Developments in Direct C-H Functionalization of Quinoxalin-2(1H)-ones via Radical Addition Processes. Synthesis 2019, 51, 4113–4136. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; Zhang, H.-Y.; Zhang, Y.; Zhao, J. The C3-H Bond Functionalization of Quinoxalin-2(1H)-Ones With Hypervalent Iodine(III) Reagents. Front. Chem. 2020, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Rostoll-Berenguer, J.; Blay, G.; Pedro, J.R.; Vila, C. Recent Advances in Photocatalytic Functionalization of Quinoxalin-2-ones. Eur. J. Org. Chem. 2020, 2020, 6148–6172. [Google Scholar] [CrossRef]
- Kiran; Rani, P.; Chahal, S.; Sindhu, J.; Kumar, S.; Varma, R.S.; Singh, R. Transition metal-free C-3 functionalization of quinoxalin-2(1H)-ones: Recent advances and sanguine future. New J. Chem. 2021, 45, 18722–18763. [Google Scholar] [CrossRef]
- Xiang, L.; Wen, L.; Canzhan, Z.; Hua, C. Application of Photochemical/Electrochemical Synthesis in C-H Functionalization of Quinoxalin-2(1H)-one. Chinese J. Org. Chem. 2021, 41, 3459–3481. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Photo-/electrocatalytic functionalization of quinoxalin-2(1H)-ones. Chinese J. Catal. 2021, 42, 1921–1943. [Google Scholar] [CrossRef]
- Dandan, L.; Xiaochen, W.; Shanshan, L.; Chenyu, F.; Qianqian, L.; Dongtao, X.; Yingying, M. Recent Advances in Electrochemical C(3)-H Functionalization of Quinoxalin-2(1H)-ones. Chinese J. Org. Chem. 2021, 41, 4610–4622. [Google Scholar] [CrossRef]
- Azev, Y.A.; Kodess, M.I.; Ezhikova, M.A.; Ol’ga, S.E.; Berseneva, V.S.; Bakulev, V.A. Reactions of quinoxalin-2-one with β-diketones: A new approach to 6a,7-dihydro-5H-pyrido[1,2-a]quinoxaline-6,8-diones. Mendeleev Commun. 2017, 27, 97–98. [Google Scholar] [CrossRef]
- Azev, Y.A.; Koptyaeva, O.S.; Mkrtchan, A.A.; Pospelova, T.A. Features of -C-C- coupling of quinoxaline-2-one with ethyl acetoacetate under acid catalysis. Chimica Technol. Acta 2022, 9, 20229103. [Google Scholar] [CrossRef]
- Rusinov, G.L.; Slepukhin, P.A.; Charushin, V.N.; Dyachenko, O.A.; Kazheva, O.N.; Chekhlov, A.N.; Verbitsky, E.V.; Kodess, M.I.; Chupakhin, O.N. Chemistry of O- and C-adducts derived from 1,4-diazinium salts: The use of tetrahydropyrazines in the synthesis of condensed systems. Mendeleev Commun. 2006, 16, 26–29. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Trestsova, M.A.; Musikhina, A.A.; Kucheryavaya, D.A.; Charushin, V.N.; Rempel, A.A.; Kozhevnikova, N.S.; Valeeva, A.A.; Mikhaleva, A.I.; et al. Direct functionalization of the C-H bond in (hetero)arenes: Aerobic photoinduced oxidative coupling of azines with aromatic nucleophiles (SNH-reactions) in the presence of a CdS/TiO2 photocatalyst. Russ. Chem. Bull. 2016, 65, 445–450. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Utepova, I.A.; Trestsova, M.A.; Anisimov, A.S.; Charushin, V.N.; Chupakhin, O.N.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Synthesis and approbation of new neuroprotective chemicals of pyrrolyl- and indolylazine classes in a cell model of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 222, 113577. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Sun, Q.; Shang, K.; Zhang, H.-Y.; Zhao, J. [3+2] Cyclization of Azidotrimethylsilane with Quinoxalin-2(1H)-Ones to Synthesize Tetrazolo[1,5-a]quinoxalin-4(5H)-Ones. Adv. Synth. Catal. 2018, 360, 4509–4514. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, Z.; Tan, Y.; Zhao, J.; Sun, Q.; Zhang, H.-Y.; Zhang, Y. Direct C(sp2)−H Amination to Synthesize Primary 3-aminoquinoxalin-2(1H)-ones under Simple and Mild Conditions. Adv. Synth. Catal. 2019, 361, 1662–1667. [Google Scholar] [CrossRef]
- Yang, Q.; Han, X.; Zhao, J.; Zhang, H.-Y.; Zhang, Y. Direct C3 Alkoxylation of Quinoxalin-2(1H)-ones with Alcohols via Cross-Dehydrogenative Coupling under Catalyst-Free Conditions. J. Org. Chem. 2019, 84, 11417–11424. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
- Meng, N.; Wang, L.; Liu, Q.; Li, Q.; Lv, Y.; Yue, H.; Wang, X.; Wei, W. Metal-Free Trifluoroalkylation of Quinoxalin-2(1H)-ones with Unactivated Alkenes and Langlois’ Reagent. J. Org. Chem. 2020, 85, 6888–6896. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, S.; Chen, Y.; Liu, Y.-L.; Tang, R.-Y.; Li, Z. Transition-metal-free, three-component trifluoromethylative heteroarylation of unactivated alkenes: Efficient access to β-trifluoromethylated quinoxalinones and preliminary antifungal evaluation against Magnaporthe grisea. Tetrahedron 2020, 76, 131199. [Google Scholar] [CrossRef]
- Su, H.-Y.; Zhu, X.-L.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Copper-catalyzed chemoselective C–H functionalization of quinoxalin-2(1H)-ones with hexafluoroisopropanol. Chem. Commun. 2020, 56, 12805–12808. [Google Scholar] [CrossRef]
- Meng, N.; Lv, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Visible-light-induced three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na leading to 3-trifluoroalkylated quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2021, 32, 258–262. [Google Scholar] [CrossRef]
- Yang, X.; Meng, W.-D.; Xu, X.-H.; Huang, Y. Photoredox-catalyzed 2,2,2-trifluoroethylation and 2,2-difluoroethylation of alkenes with concomitant introduction of a quinoxalin-2(1H)-one moiety. Org. Chem. Front. 2021, 8, 6597–6602. [Google Scholar] [CrossRef]
- Li, H.; Peng, X.; Nie, L.; Zhou, L.; Yang, M.; Li, F.; Hu, J.; Yao, Z.; Liu, L. Graphene oxide-catalyzed trifluoromethylation of alkynes with quinoxalinones and Langlois’ reagent. RSC Adv. 2021, 11, 38667–38673. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Zhang, P.; Chen, C.; Shen, C. Copper-Catalyzed Multicomponent Reaction to Construct Fluorinated Indole-quinoxalin-2(1H)-ones and Their Biological Evaluation. Eur. J. Org. Chem. 2022, 2022, e202200696. [Google Scholar] [CrossRef]
- Zheng, D.; Studer, A. Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis. Org. Lett. 2019, 21, 325–329. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, R.; Zhang, C.; Wang, K.; Feng, J.; Zhao, X.; Lu, K. Photoinduced Three-Component Difluoroalkylation of Quinoxalinones with Alkenes via Difluoroiodane(III) Reagents. Org. Lett. 2022, 24, 3576–3581. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-R.; Li, L.; Bu, X.; Wang, X.; Sun, R.; Zhou, M.-D.; Wang, H. Visible-Light Photoredox-Catalyzed Three-Component Difluoromethylative Heteroarylation of Unactivated Alkenes. Asian J. Org. Chem. 2022, 11, e202200139. [Google Scholar] [CrossRef]
- Alonso, N.; Miller, L.Z.; de Muñoz, J.M.; Alcázar, J.; McQuade, D.T. Cover Picture: Continuous Synthesis of Organozinc Halides Coupled to Negishi Reactions. Adv. Synth. Catal. 2014, 356, 3685. [Google Scholar] [CrossRef]
- Zhong, X.; Yao, H.; Wang, B.; Yan, Z.; Xiong, F.; Lin, S. H2O2-Promoted Alkylation of Quinoxalin-2(1H)-ones with Styrenes and Dimethyl Sulfoxide. Synlett 2021, 32, 1213–1218. [Google Scholar] [CrossRef]
- Sharma, S.; Bhuyan, M.; Baishya, G. K2S2O8 Mediated Three-component Radical Cascade C3 Alkylation of Quinoxalin-2(1H)-ones with Vinylarenes and 4-Hydroxycoumarins/4-Hydroxy-6-methyl-2-pyrone. ChemistrySelect 2022, 7, e202201541. [Google Scholar] [CrossRef]
- Bhuyan, M.; Sharma, S.; Baishya, G. Metal-free three-component cyanoalkylation of quinoxalin-2(1H)-ones with vinylarenes and azobis(alkylcarbonitrile)s. Org. Biomol. Chem. 2022, 20, 1462–1474. [Google Scholar] [CrossRef]
- Nakata, T. Total Synthesis of Marine Polycyclic Ethers. Chem. Rev. 2005, 105, 4314–4347. [Google Scholar] [CrossRef]
- Zhong, X.; Li, X.; Yao, H.; Yan, Z.; Guo, H.; Min, L.; Lin, S. Copper-Catalyzed Alkylation of Quinoxalin-2(1H)-ones with Styrenes and tert-Butyl Peroxybenzoate. Synlett 2022, 33, 998–1002. [Google Scholar] [CrossRef]
- Li, L.; Gao, Y.; Wang, L.; Sun, M.; Liu, J.; Li, P. Visible-Light-Induced Site-Selective Difunctionalization of 2,3-Dihydrofuran with Quinoxalin-2(1H)-ones and Peroxides. Eur. J. Org. Chem. 2022, 2022, e202200373. [Google Scholar] [CrossRef]
- Shen, J.; Xu, J.; Huang, L.; Zhu, Q.; Zhang, P. Hypervalent Iodine(III)-Promoted Rapid Cascade Reaction of Quinoxalinones with Unactivated Alkenes and TMSN3. Adv. Synth. Catal. 2020, 362, 230–241. [Google Scholar] [CrossRef]
- Du, Y.; Chen, Y.; Liu, Y.-L.; Qin, W.; Li, Z. Transition-Metal-Free, Intermolecular Azidoheteroarylation of Alkenes: Efficient Access to β-Azidoalkylated Quinoxalinones and Preliminary Antifungal Evaluation Against Magnaporthe grisea. Synthesis 2020, 52, 2395–2409. [Google Scholar] [CrossRef]
- Shen, J.; He, L.; Liang, C.; Ouyang, Y.; Yue, X.; Wu, H.; Xu, J.; Liu, X.; Zhu, Q.; Zhang, P. Photoinitiated multicomponent cascade reaction of Nheteroarenes with unactivated alkenes and trimethylsilyl azide. Mol. Catal. 2022, 524, 112330. [Google Scholar] [CrossRef]
- Carreira, E.M.; Chiu, P. Challenges and More Challenges. Org. Lett. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, L.; Liang, C.; Yue, X.; Ouyang, Y.; Zhang, P. Multicomponent Bifunctionalization of Methyl Ketones Enabled by Heterogeneous Catalysis and Solar Photocatalysis in Water. ACS Sustain. Chem. Eng. 2021, 9, 13663–13671. [Google Scholar] [CrossRef]
- Yang, G.; Wang, S.; Nie, H.; Xiong, Z.; Li, X.; Ji, F.; Jiang, G. An efficient transition metal-free difunctionalization of alkenes in water for the green preparation of sulfone compounds. Appl. Organomet. Chem. 2022, 36, e6470. [Google Scholar] [CrossRef]
- Sekhar Dutta, H.; Ahmad, A.; Khan, A.A.; Kumar, M.; Raziullah; Koley, D. Metal Free Benzylation and Alkylation of Quinoxalin-2(1H)-ones with Alkenes Triggered by Sulfonyl Radical Generated from Sulfinic Acids. Adv. Synth. Catal. 2019, 361, 5534–5539. [Google Scholar] [CrossRef]
- Clinton, C.D.; Prasad, C.D.; Thombal, R.S.; Lee, Y.R. Copper-Catalyzed Regio- and Stereoselective Oxidative Vinylsulfonylation of Quinoxalinones with Alkynes and Sulfonyl Hydrazides. Adv. Synth. Catal. 2021, 363, 776–784. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, J.; Lin, M.; He, L.; Yue, H.; Liu, R.; Wei, W. Metal-Free Multi-Component Sulfur Dioxide Insertion Reaction Leading to Quinoxalin-2-One-Containing Vinyl Sulfones under Visible-Light Photoredox Catalysis. Adv. Synth. Catal. 2021, 363, 5122–5128. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, J.; Lin, M.; Yue, H.; Dai, B.; He, L. A visible-light photoredox-catalyzed four-component reaction for the construction of sulfone-containing quinoxalin-2(1H)-ones. Org. Chem. Front. 2021, 8, 5403–5409. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Visible-light-initiated 4CzIPN catalyzed multi-component tandem reactions to assemble sulfonated quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2022, 33, 1479–1482. [Google Scholar] [CrossRef]
- Singh, S.; Dagar, N.; Pal, G.; Raha Roy, S. Photoinduced radical cascade reactions for the thioalkylation of quinoxalin-2(1H)-ones: An access to β-heteroaryl thioethers under metal- and catalyst-free conditions. Green Chem. 2022, 24, 8460–8465. [Google Scholar] [CrossRef]
- Li, W.; Cai, H.; Huang, L.; He, L.; Zhang, Y.; Xu, J.; Zhang, P. Iron(III)-Mediated Rapid Radical-Type Three-Component Deuteration of Quinoxalinones With Olefins and NaBD4. Front. Chem. 2020, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhao, W.; Li, S.; Liu, H.; Yu, L.; Niu, W.; He, H.; Wu, L. Quinohemanine, a quinoxalinone-bohemamine hybrid compound from Streptomyces sp. CPCC 200497. J. Antibiot. 2018, 71, 965–967. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Wang, B.; Wu, M.; Lei, Y.-Z. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules 2023, 28, 2513. https://doi.org/10.3390/molecules28062513
Yang Q, Wang B, Wu M, Lei Y-Z. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules. 2023; 28(6):2513. https://doi.org/10.3390/molecules28062513
Chicago/Turabian StyleYang, Qiming, Biao Wang, Mian Wu, and Yi-Zhu Lei. 2023. "Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions" Molecules 28, no. 6: 2513. https://doi.org/10.3390/molecules28062513
APA StyleYang, Q., Wang, B., Wu, M., & Lei, Y.-Z. (2023). Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules, 28(6), 2513. https://doi.org/10.3390/molecules28062513







