Integrating Untargeted and Targeted Metabolomics Coupled with Pathway Analysis Reveals Muscle Disorder in Osteoporosis on Orchiectomized Mice
Abstract
1. Introduction
2. Results
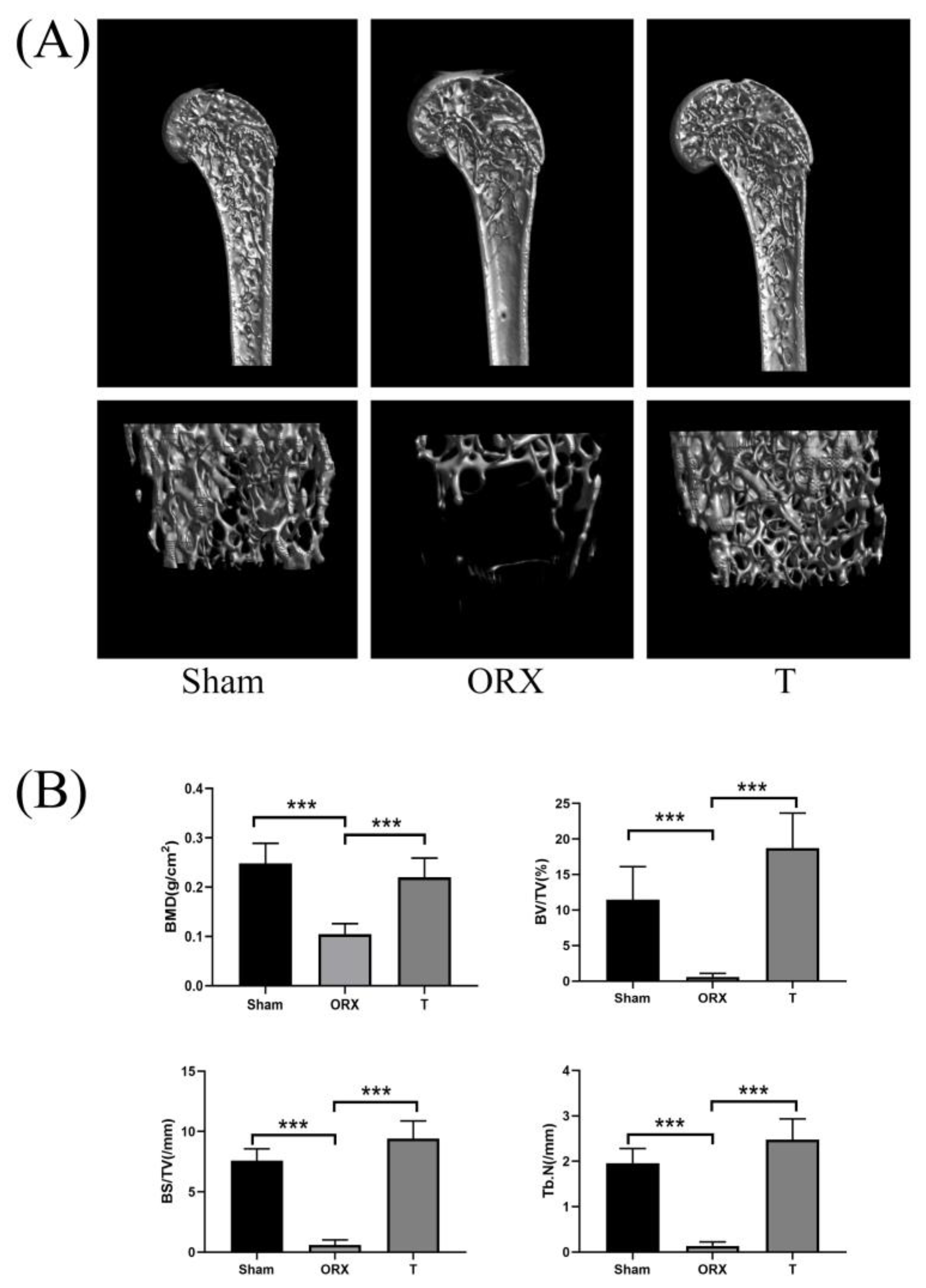
2.1. Assessment of OP Modeling
2.1.1. Micro-Computed Tomography Assessment
2.1.2. Femur Section Staining Histopathology
2.2. Body, Prostate Weights, and Clinical Observation
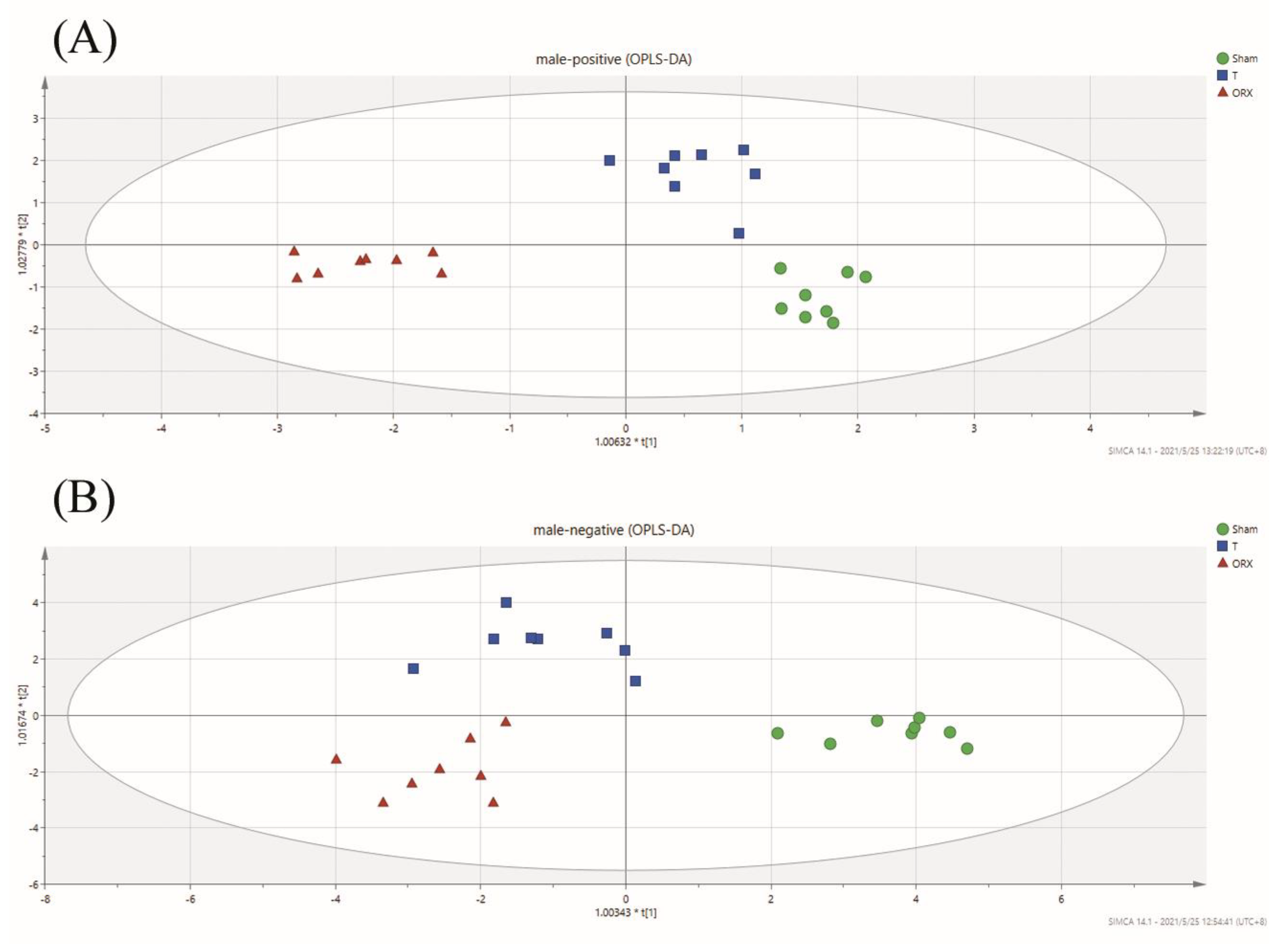
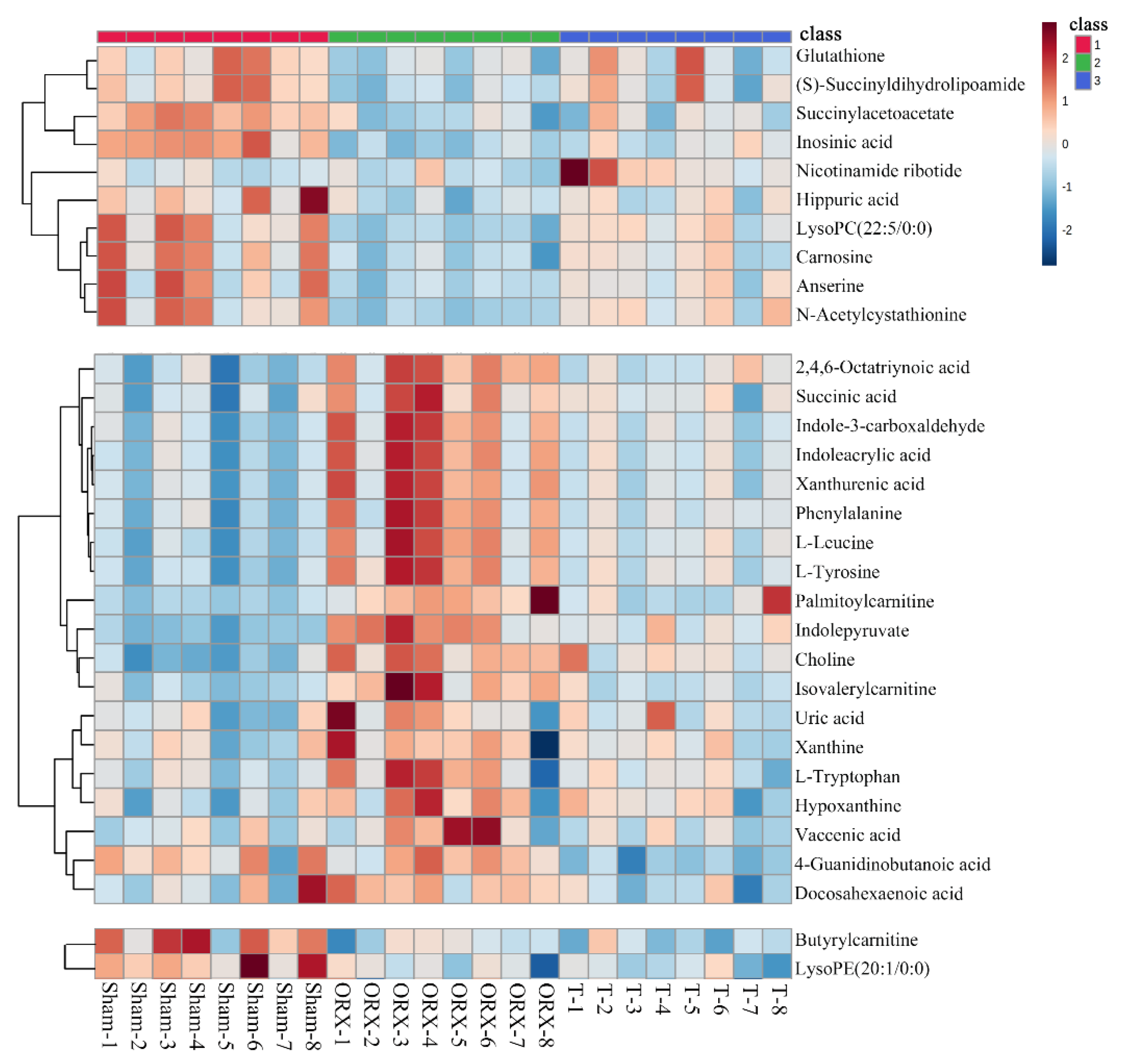
2.3. Untargeted Metabolomic Profiling Analysis
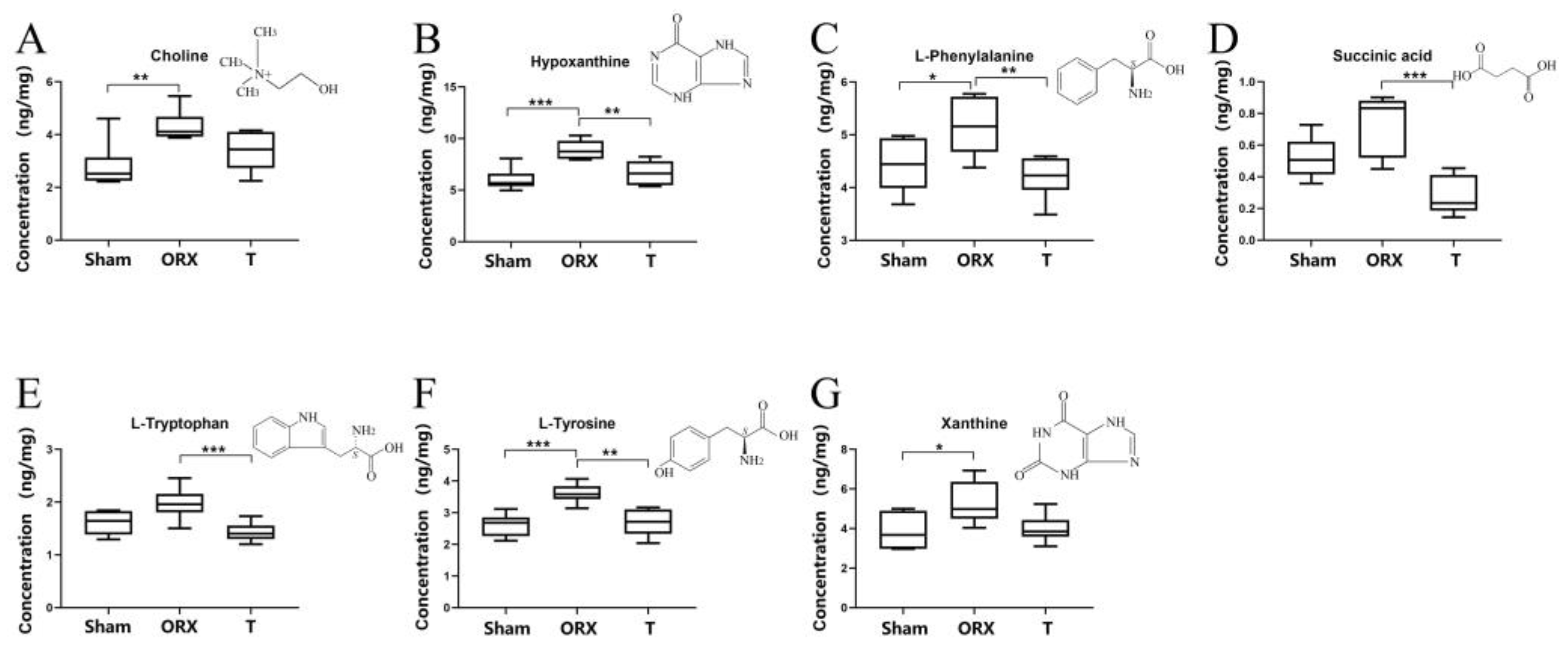
2.4. Targeted Validation of Representative Metabolites
3. Discussion
3.1. Muscle Dysfunction in OP Model
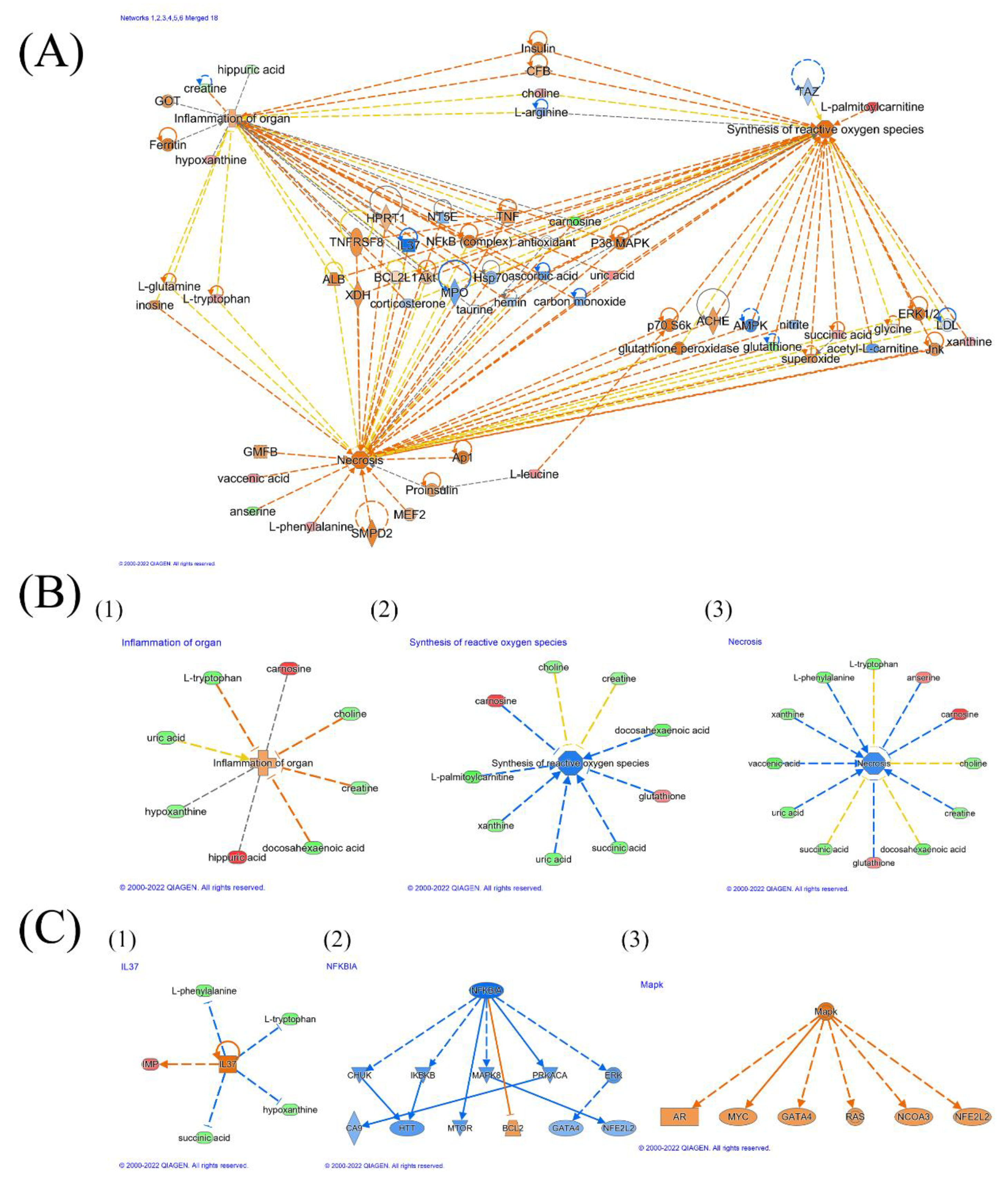
3.2. Inflammation Environment
3.3. Oxidative Stress
3.4. TNF, TNF Receptor, and Necrosis
3.5. Comparison of Muscle Disorder in OP Male Mice and in OP Female Mice
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals & Treatment
4.3. Micro-Computed Tomography and Histopathology Assessment
4.4. Untargeted Metabolomic Analysis
4.5. Targeted Metabolomic Analysis
4.6. Data Processing & Statistical Analysis
4.7. Bioinformatics Analysis of Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laurent, M.R.; Jardí, F.; Dubois, V.; Schollaert, D.; Khalil, R.; Gielen, E.; Carmeliet, G.; Claessens, F.; Vanderschueren, D. Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone 2016, 93, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C. Osteoporosis in men: A review of endogenous sex hormones and testosterone replacement therapy. J. Pharm. Pract. 2011, 24, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; PhD, A.K.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R. Primer on the metabolic bone diseases and disorders of mineral metabolism. Indian J. Med. Res. 2016, 144, 489–490. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Shahnazari, M.; Orwoll, E.S.; Lane, N.E. Osteoporosis in men: Findings from the Osteoporotic Fractures in Men Study (MrOS). Ther. Adv. Musculoskelet. Dis. 2016, 8, 15–27. [Google Scholar] [CrossRef]
- Caballero Casanoves, P.; Barbería Marcalain, E.; Suelves Joanxich, J.M.; García Sayago, F. Descriptive study of a series of deaths due to accidental falls in the elderly. Rev. Esp. Salud Publica 2021, 95, e202110179. [Google Scholar]
- Meyer, M.; Constancias, F.; Vogel, T.; Kaltenbach, G.; Schmitt, E. Gait Disorder among Elderly People, Psychomotor Disadaptation Syndrome: Post-Fall Syndrome, Risk Factors and Follow-Up–A Cohort Study of 70 Patients. Gerontology 2021, 67, 17–24. [Google Scholar] [CrossRef]
- Nowakowski, K.; El Kirat, K.; Dao, T.-T. Deep reinforcement learning coupled with musculoskeletal modelling for a better understanding of elderly falls. Med. Biol. Eng. Comput. 2022, 60, 1745–1761. [Google Scholar] [CrossRef]
- Yu, R.; Leung, J.; Woo, J. Sarcopenia Combined With FRAX Probabilities Improves Fracture Risk Prediction in Older Chinese Men. J. Am. Med. Dir. Assoc. 2014, 15, 918–923. [Google Scholar] [CrossRef]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef]
- Su, Y.; Lam, F.M.H.; Leung, J.; Cheung, W.-H.; Ho, S.C.; Kwok, T. The Predictive Value of Sarcopenia and Falls for 2-Year Major Osteoporotic Fractures in Community-Dwelling Older Adults. Calcif. Tissue Int. 2020, 107, 151–159. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, Y.; Tian, Q.; Papasian, C.J.; Hu, T.; Deng, H.W. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos. Int. 2016, 27, 473–482. [Google Scholar] [CrossRef]
- Saeki, C.; Oikawa, T.; Kanai, T.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; Saruta, M.; Tsubota, A. Relationship between osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia in patients with primary biliary cholangitis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Reginster, J.Y.; Bruyère, O. Association Between the Decline in Muscle Health and the Decline in Bone Health in Older Individuals from the SarcoPhAge Cohort. Calcif. Tissue Int. 2019, 104, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; Liu, R.; Little, D.G. The contribution of different cell lineages to bone repair: Exploring a role for muscle stem cells. Differentiation 2009, 77, 12–18. [Google Scholar] [CrossRef]
- Laurent, M.R.; Dubois, V.; Claessens, F.; Verschueren, S.M.P.; Vanderschueren, D.; Gielen, E.; Jardí, F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell. Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]
- Maurel, D.B.; Jähn, K.; Lara-Castillo, N. Muscle-Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines 2017, 5, 62. [Google Scholar] [CrossRef]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef]
- Jitprapaikulsarn, S.; Benjawongsathien, K.; Patamamongkonchai, C.; Gromprasit, A.; Thremthakanpon, W. Combined medial gastrocnemius and hemisoleus flap: A reproducible alternative for open tibial fractures complicated with large or double soft tissue defects. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 413–420. [Google Scholar] [CrossRef]
- Harry, L.E.; Sandison, A.; Paleolog, E.M.; Hansen, U.; Pearse, M.F.; Nanchahal, J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J. Orthop. Res. 2008, 26, 1238–1244. [Google Scholar] [CrossRef]
- Croisier, J.L.; Camus, G.; Venneman, I.; Deby-Dupont, G.; Juchmes-Ferir, A.; Lamy, M.; Crielaard, J.M.; Deby, C.; Duchateau, J. Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle Nerve 1999, 22, 8–12. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Z.-K.; Liang, C.; Li, J.; Liu, J.; Lu, A.; Zhang, B.-T.; Zhang, G. Molecular Communication from Skeletal Muscle to Bone: A Review for Muscle-Derived Myokines Regulating Bone Metabolism. Calcif. Tissue Int. 2017, 100, 184–192. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Opazo, R.; Angel, B.; Márquez, C.; Lera, L.; Cardoso Dos Santos, G.R.; Monnerat, G.; Albala, C. Sarcopenic metabolomic profile reflected a sarcopenic phenotype associated with amino acid and essential fatty acid changes. Metabolomics 2021, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, D.; Zhao, A.; Hou, X.; Zheng, X.; Chen, P.; Bao, Y.; Jia, W.; Hu, C.; Zhang, Z.L.; et al. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 2019, 30, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Marta, G.; Giuseppe, B.; Giovanni, L. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Wilson, I.D.; Theodoridis, G.; Virgiliou, C. A perspective on the standards describing mass spectrometry-based metabolic phenotyping (metabolomics/metabonomics) studies in publications. J. Chromatogr. B 2021, 1164, 122515. [Google Scholar] [CrossRef]
- Sun, J.; Pan, Y.; Li, X.; Wang, L.; Liu, M.; Tu, P.; Wu, C.; Xiao, J.; Han, Q.; Da, W.; et al. Quercetin Attenuates Osteoporosis in Orchiectomy Mice by Regulating Glucose and Lipid Metabolism via the GPRC6A/AMPK/mTOR Signaling Pathway. Front. Endocrinol (Lausanne) 2022, 13, 849544. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Lu, X.; Zheng, S.; Li, F.; Xiong, Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography–tandem mass spectrometry. J. Ethnopharmacol. 2012, 139, 1–7. [Google Scholar] [CrossRef]
- Wei, Z.; Ge, F.; Che, Y.; Wu, S.; Dong, X.; Song, D. Metabolomics Coupled with Pathway Analysis Provides Insights into Sarco-Osteoporosis Metabolic Alterations and Estrogen Therapeutic Effects in Mice. Biomolecules 2021, 12, 41. [Google Scholar] [CrossRef]
- Badraoui, R.; Amri, N.; Zammel, N.; Chaabane, R.; Rebai, T. Corticosteroid treatment exacerbates bone osteopenia in mice with gonadal hormone deficiency-induced osteoporosis. Eur. J. Pharm. Sci. 2017, 105, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, P.R. Androgens and osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Blouin, S.; Libouban, H.; Moreau, M.F.; Chappard, D. Orchidectomy models of osteoporosis. Methods Mol. Biol. 2008, 455, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Audran, M.; Chappard, D.; Legrand, E.; Libouban, H.; Baslé, M.F. Bone microarchitecture and bone fragility in men: DXA and histomorphometry in humans and in the orchidectomized rat model. Calcif. Tissue Int. 2001, 69, 214–217. [Google Scholar] [CrossRef]
- Gunness, M.; Orwoll, E. Early induction of alterations in cancellous and cortical bone histology after orchiectomy in mature rats. J. Bone Min. Res. 1995, 10, 1735–1744. [Google Scholar] [CrossRef]
- Zhendong, M.; Xin, D.; Yu, Q.; Dun, H.; Ziang, X.; Guanfeng, Y.; An, Q.; Songyan, G.; Jianying, H.; Liming, L.; et al. Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine 2020, 62, 103111. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Bagati, A.; Moparthy, S.; Fink, E.E.; Bianchi-Smiraglia, A.; Yun, D.H.; Kolesnikova, M.; Udartseva, O.O.; Wolff, D.W.; Roll, M.V.; Lipchick, B.C.; et al. KLF9-dependent ROS regulate melanoma progression in stage-specific manner. Oncogene 2019, 38, 3585–3597. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-κB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Barbiera, A.; Sorrentino, S.; Lepore, E.; Carfì, A.; Sica, G.; Dobrowolny, G.; Scicchitano, B.M. Taurine Attenuates Catabolic Processes Related to the Onset of Sarcopenia. Int. J. Mol. Sci. 2020, 21, 8865. [Google Scholar] [CrossRef]
- Peterson, J.M.; Bakkar, N.; Guttridge, D.C. Chapter four-NF-κB Signaling in Skeletal Muscle Health and Disease. In Current Topics in Developmental Biology; Pavlath, G.k., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 96, pp. 85–119. [Google Scholar]
- La Rosa, F.; Agostini, S.; Saresella, M.; Costa, A.S.; Piancone, F.; Miglioli, R.; Trecate, F.; Clerici, M. Deregulation of IL-37 and its miRNAs modulators in sarcopenic patients after rehabilitation. J. Transl. Med. 2021, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Abuna, R.P.F.; Almeida, L.O.; Souza, A.T.P.; Fernandes, R.R.; Sverzut, T.F.V.; Rosa, A.L.; Beloti, M.M. Osteoporosis and osteoblasts cocultured with adipocytes inhibit osteoblast differentiation by downregulating histone acetylation. J. Cell Physiol. 2021, 236, 3906–3917. [Google Scholar] [CrossRef] [PubMed]
- Hipmair, G.; Böhler, N.; Maschek, W.; Soriguer, F.; Rojo-Martínez, G.; Schimetta, W.; Pichler, R. Serum leptin is correlated to high turnover in osteoporosis. Neuro Endocrinol. Lett. 2010, 31, 155–160. [Google Scholar] [PubMed]
- Zhao, H.; Du, H.; Liu, M.; Gao, S.; Li, N.; Chao, Y.; Li, R.; Chen, W.; Lou, Z.; Dong, X. Integrative Proteomics–Metabolomics Strategy for Pathological Mechanism of Vascular Depression Mouse Model. J. Proteome Res. 2018, 17, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Psihogios, N.G.; Gazi, I.F.; Elisaf, M.S.; Seferiadis, K.I.; Bairaktari, E.T. Gender-related and age-related urinalysis of healthy subjects by NMR-based metabonomics. NMR Biomed. 2008, 21, 195–207. [Google Scholar] [CrossRef]
- Aleidi, S.M.; Alnehmi, E.A.; Alshaker, M.; Masood, A.; Benabdelkamel, H.; Al-Ansari, M.M.; Abdel Rahman, A.M. A Distinctive Human Metabolomics Alteration Associated with Osteopenic and Osteoporotic Patients. Metabolites 2021, 11, 628. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, D.; Chen, H.; Chao, Y.; Wu, K.; Dong, X.; Su, J. Integrative Bone Metabolomics-Lipidomics Strategy for Pathological Mechanism of Postmenopausal Osteoporosis Mouse Model. Sci. Rep. 2018, 8, 16456. [Google Scholar] [CrossRef]
- Shan, L.; Jiang, T.; Ci, L.; Liu, Z.; Lv, X.; Li, J. Purine signaling regulating HSCs inflammatory cytokines secretion, activation, and proliferation plays a critical role in alcoholic liver disease. Mol. Cell Biochem. 2020, 466, 91–102. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Tondravi, M.M. NF-κB and bone: The breaking point. Nat. Med. 1997, 3, 1189–1190. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, X.; Liu, S.; Wang, S.; Nold-Petry, C.A.; Nold, M.F.; Bufler, P.; Norris, D.; Dinarello, C.A.; Fujita, M. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Evans, W.J.; Anker, S.D. Myopenia-a new universal term for muscle wasting. J. Cachexia Sarcopenia Muscle 2011, 2, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. A role for myokines in muscle-bone interactions. Exerc. Sport Sci. Rev. 2011, 39, 43–47. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous Metabolite, Dietary, and Therapeutic Supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef]
- Wim, D.; Barbora, D.C.; Shahid, B. An update on carnosine and anserine research. Amino Acids 2019, 51, 1–4. [Google Scholar] [CrossRef]
- Everaert, I.; Mooyaart, A.; Baguet, A.; Zutinic, A.; Baelde, H.; Achten, E.; Taes, Y.; Heer, E.D.; Derave, W. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids 2011, 40, 1221–1229. [Google Scholar] [CrossRef]
- Young, K.M.; Hua, L.G.; Aee, K.J.; Seung, C.S.; Shinku, C.; Hyo, S.S. Oxidized Low-density Lipoprotein- and Lysophosphatidylcholine-induced Ca Mobilization in Human Endothelial Cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2009, 13, 27–32. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Rolo, A.P.; Palmeira, C.M.; Moreno, A.J. Carvedilol reduces mitochondrial damage induced by hypoxanthine/xanthine oxidase: Relevance to hypoxia/reoxygenation injury. Cardiovasc. Toxicol. 2001, 1, 205–213. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 73–97. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Liu, Y.; Gorospe, M.; Xu, Q.; Holbrook, N.J. Activation of Mitogen-activated Protein Kinase by H2O2: ROLE IN CELL SURVIVAL FOLLOWING OXIDANT INJURY (∗). J. Biol. Chem. 1996, 271, 4138–4142. [Google Scholar] [CrossRef] [PubMed]
- Baghdiguian, S.; Richard, I.; Martin, M.; Coopman, P.; Beckmann, J.S.; Mangeat, P.; Lefranc, G. Pathophysiology of limb girdle muscular dystrophy type 2A: Hypothesis and new insights into the IκBα/NF-κB survival pathway in skeletal muscle. J. Mol. Med. 2001, 79, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Boriek, A.M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: A possible role in Duchenne muscular dystrophy. FASEB J. 2003, 17, 386–396. [Google Scholar] [CrossRef] [PubMed]
| m/z | RT | Name | Formula | Ion | VIP | Fold Change | Pathway | Variation in Testosterone Loss-and-Regain Process | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [CON-ORX] | [ORX—T] | [CON/ORX] | [ORX/T] | ||||||||
| 1 | 308.0970 | 0.71 | (S)-Succinyldihydrolipoamide△ | C12H21NO4S2 | [M+H]+ | 2.13 | 1.27 | 0.52 ** | 1.47 | - | ↓-↑ |
| 2 | 239.1164 | 0.59 | Anserine△ | C10H16N4O3 | [M−H]− | 3.73 | 2.82 | 0.51 *** | 1.42 | Histidine metabolism | ↓-↑ |
| 3 | 225.1004 | 0.58 | Carnosine△ | C9H14N4O3 | [M−H]− | 3.62 | 3.39 | 0.37 *** | 2.09 * | Histidine metabolism | ↓-↑ |
| 4 | 306.0735 | 0.99 | Glutathione△ | C10H17N3O6S | [M−H]− | 1.44 | 1.19 | 0.62 * | 1.39 | Cysteine and methionine metabolism | ↓-↑ |
| 5 | 178.0512 | 4.24 | Hippuric acid△ | C9H9NO3 | [M−H]− | 0.17 | 1.05 | 0.97 | 2.28 * | Phenylalanine metabolism | ↓-↑ |
| 6 | 349.0559 | 1.00 | Inosinic acid△ | C10H13N4O8P | [M+H]+ | 3.49 | 1.69 | 0.30 *** | 1.78 * | Purine metabolism | ↓-↑ |
| 7 | 571.3491 | 8.63 | LysoPC (22:5/0:0) △ | C30H52NO7P | [M+H]+ | 1.33 | 0.59 | 0.63 * | 1.15 | Glycerophospholipid metabolism | ↓-↑ |
| 8 | 265.0829 | 0.60 | N-Acetylcystathionine△ | C9H16N2O5S | [M+H]+ | 1.52 | 1.04 | 0.42 ** | 1.57 | - | ↓-↑ |
| 9 | 198.0863 | 0.57 | N-Acetylhistidine△ | C8H11N3O3 | [M+H]+ | 1.45 | 1.19 | 0.53 *** | 1.51 * | - | ↓-↑ |
| 10 | 201.0392 | 1.03 | Succinylacetoacetate△ | C8H10O6 | [M−H]+ | 2.92 | 0.83 | 0.77 ** | 1.12 | - | ↓-↑ |
| 11 | 133.0288 | 1.01 | 2,4,6-Octatriynoic acid△ | C8H4O2 | [M+H]+ | 1.66 | 1.50 | 1.44 *** | 0.79 ** | - | ↑-↓ |
| 12 | 104.1085 | 0.63 | Choline▲ | C5H14NO | [M+H]+ | 5.54 | 3.56 | 1.30 *** | 0.89 * | Glycine, serine, and threonine metabolism | ↑-↓ |
| 13 | 163.1183 | 0.65 | 4-Guanidinobutanoic acid△ | C5H11N3O2 | [M+H]+ | 0.14 | 1.10 | 1.01 | 0.76 *** | Arginine and proline metabolism | ↑-↓ |
| 14 | 329.2441 | 13.06 | Docosahexaenoic acid△ | C22H32O2 | [M+H]+ | 0.99 | 1.89 | 1.15 | 0.73 * | Biosynthesis of unsaturated fatty acids | ↑-↓ |
| 15 | 135.0315 | 1.01 | Hypoxanthine▲ | C5H4N4O | [M−H]− | 2.85 | 2.40 | 1.31 * | 0.89 | Purine metabolism | ↑-↓ |
| 16 | 146.0593 | 3.76 | Indole-3-carboxaldehyde△ | C9H7NO | [M+H]+ | 1.19 | 1.19 | 1.45 *** | 0.74 ** | Purine metabolism | ↑-↓ |
| 17 | 188.0697 | 3.76 | Indoleacrylic acid△ | C11H9NO2 | [M+Na]+ | 3.46 | 3.43 | 1.48 *** | 0.73 *** | - | ↑-↓ |
| 18 | 238.0265 | 1.09 | Indolepyruvate△ | C11H9NO3 | [M+Cl]− | 0.67 | 0.55 | 1.64 *** | 0.86 | Tryptophan metabolism | ↑-↓ |
| 19 | 246.1686 | 4.08 | Isovalerylcarnitine△ | C12H23NO4 | [M+H]+ | 3.17 | 2.90 | 2.49 *** | 0.49 *** | - | ↑-↓ |
| 20 | 132.1007 | 1.21 | L-Leucine△ | C6H13NO2 | [M+H]+ | 13.91 | 12.68 | 1.55 *** | 0.75 ** | Valine, leucine, and isoleucine degradation | ↑-↓ |
| 130.0876 | [M−H]− | 2.40 | 5.04 | 0.62 *** | 1.32 ** | ||||||
| 21 | 203.0838 | 3.80 | L-Tryptophan▲ | C11H12N2O2 | [M−H]− | 1.52 | 1.42 | 1.48 *** | 0.74 ** | Glycine, serine, and threonine metabolism | ↑-↓ |
| 22 | 182.0772 | 1.06 | L-Tyrosine▲ | C9H11NO3 | [M−H]− | 1.74 | 1.46 | 4.62 *** | 0.81 | Phenylalanine metabolism | ↑-↓ |
| 23 | 401.3446 | 9.13 | Palmitoylcarnitine△ | C27H44O2 | [M+H]+ | 2.76 | 2.27 | 2.73 ** | 0.60 | Fatty acid degradation | ↑-↓ |
| 24 | 166.0851 | 2.01 | Phenylalanine▲ | C9H11NO2 | [M+H]+ | 8.48 | 8.30 | 1.45 *** | 0.76 ** | Phenylalanine metabolism | ↑-↓ |
| 25 | 119.0328 | 1.02 | Succinic acid▲ | C4H6O4 | [M+H]+ | 1.18 | 1.08 | 1.36 ** | 0.81 * | Citrate cycle (TCA cycle) | ↑-↓ |
| 26 | 167.0199 | 0.85 | Uric acid△ | C5H4N4O3 | [M−H]− | 2.19 | 1.71 | 1.55 * | 0.82 | Purine metabolism | ↑-↓ |
| 27 | 281.2512 | 14.64 | Vaccenic acid△ | C18H34O2 | [M−H]− | 0.89 | 1.07 | 1.56 * | 0.64 * | - | ↑-↓ |
| 28 | 151.0247 | 0.72 | Xanthine▲ | C5H4N4O2 | [M−H]− | 2.08 | 2.12 | 1.28 * | 0.90 | Purine metabolism | ↑-↓ |
| 29 | 206.0993 | 3.76 | Xanthurenic acid△ | C10H7NO4 | [M+H]+ | 1.31 | 1.32 | 1.47 *** | 0.73 ** | Tryptophan metabolism | ↑-↓ |
| 30 | 232.1530 | 3.16 | Butyrylcarnitine△ | C11H21NO4 | [M+H]+ | 1.08 | 0.29 | 0.67 ** | 0.92 | - | ↓-↓ |
| 31 | 506.3314 | 9.48 | LysoPE (20:1/0:0) △ | C25H50NO7P | [M−H]− | 0.75 | 0.34 | 0.68 ** | 0.91 | - | ↓-↓ |
| ID | RT (min) | Compound | FC | p Value | Ionization Mode | PRE | PRO | CE (eV) | Fragmentor (eV) | Liner R | Accuracy | Precision (LC) | Precision (HC) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ORX/Sham) | (T/ORX) | (ORX/Sham) | (T/ORX) | ||||||||||||
| HMDB0000292 | 3.79 | Xanthine | 1.37 | 0.75 | 0.0372 | 0.0603 | positive | 153.0 | 110.0 | 18 | 85 | 0.9997 | 102.51% | 0.63% | 0.46% |
| HMDB0000159 | 4.45 | L-Phenylalanine | 1.17 | 0.81 | 0.0461 | 0.0092 | positive | 166.1 | 105.1 | 9 | 70 | 0.9999 | 102.51% | 1.1% | 0.63% |
| HMDB0000097 | 4.17 | Choline | 1.55 | 0.78 | 0.0086 | 0.1128 | positive | 105.1 | 61.2 | 18 | 80 | 0.9996 | 102.51% | 0.63% | 0.46% |
| HMDB0000157 | 3.56 | Hypoxanthine | 1.48 | 0.75 | 0.0008 | 0.0069 | positive | 137.0 | 55.3 | 34 | 120 | 0.9983 | 100.57% | 4.1% | 1.5% |
| HMDB0000929 | 4.46 | L-Tryptophan | 1.22 | 0.72 | 0.0508 | 0.0038 | positive | 205.1 | 187.9 | 5 | 65 | 0.9991 | 99.16% | 0.69% | 4.2% |
| HMDB0000254 | 1.50 | Succinic acid | 1.42 | 0.37 | 0.0585 | 0.0003 | negative | 116.7 | 73.0 | 9 | 70 | 0.9994 | 106.29% | 7.8% | 3.5% |
| HMDB0000158 | 4.93 | L-Tyrosine | 1.38 | 0.75 | 0.0007 | 0.0015 | positive | 182.1 | 136.0 | 10 | 65 | 0.9996 | 105.57% | 1.5% | 0.68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, F.; Wei, Z.; Che, Y.; Qian, Q.; Song, J.; Zhao, H.; Wu, S.; Dong, X. Integrating Untargeted and Targeted Metabolomics Coupled with Pathway Analysis Reveals Muscle Disorder in Osteoporosis on Orchiectomized Mice. Molecules 2023, 28, 2512. https://doi.org/10.3390/molecules28062512
Ge F, Wei Z, Che Y, Qian Q, Song J, Zhao H, Wu S, Dong X. Integrating Untargeted and Targeted Metabolomics Coupled with Pathway Analysis Reveals Muscle Disorder in Osteoporosis on Orchiectomized Mice. Molecules. 2023; 28(6):2512. https://doi.org/10.3390/molecules28062512
Chicago/Turabian StyleGe, Fei, Ziheng Wei, Yanting Che, Qingqing Qian, Jinfei Song, Hongxia Zhao, Si Wu, and Xin Dong. 2023. "Integrating Untargeted and Targeted Metabolomics Coupled with Pathway Analysis Reveals Muscle Disorder in Osteoporosis on Orchiectomized Mice" Molecules 28, no. 6: 2512. https://doi.org/10.3390/molecules28062512
APA StyleGe, F., Wei, Z., Che, Y., Qian, Q., Song, J., Zhao, H., Wu, S., & Dong, X. (2023). Integrating Untargeted and Targeted Metabolomics Coupled with Pathway Analysis Reveals Muscle Disorder in Osteoporosis on Orchiectomized Mice. Molecules, 28(6), 2512. https://doi.org/10.3390/molecules28062512








