Synthesis and Biological Evaluation of Piperazine Hybridized Coumarin Indolylcyanoenones with Antibacterial Potential
Abstract
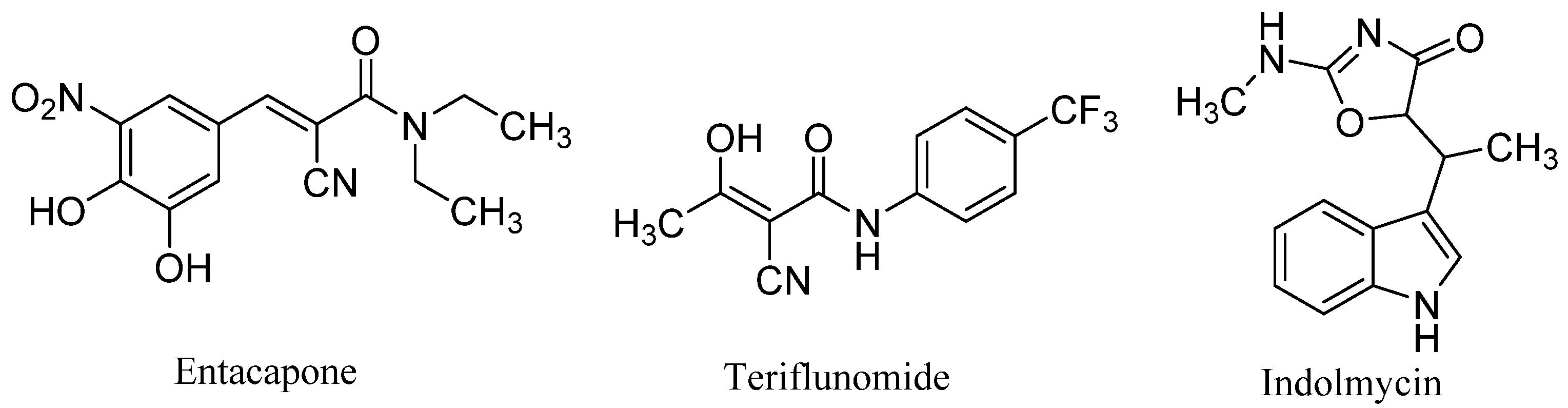
1. Introduction
2. Results and Discussion
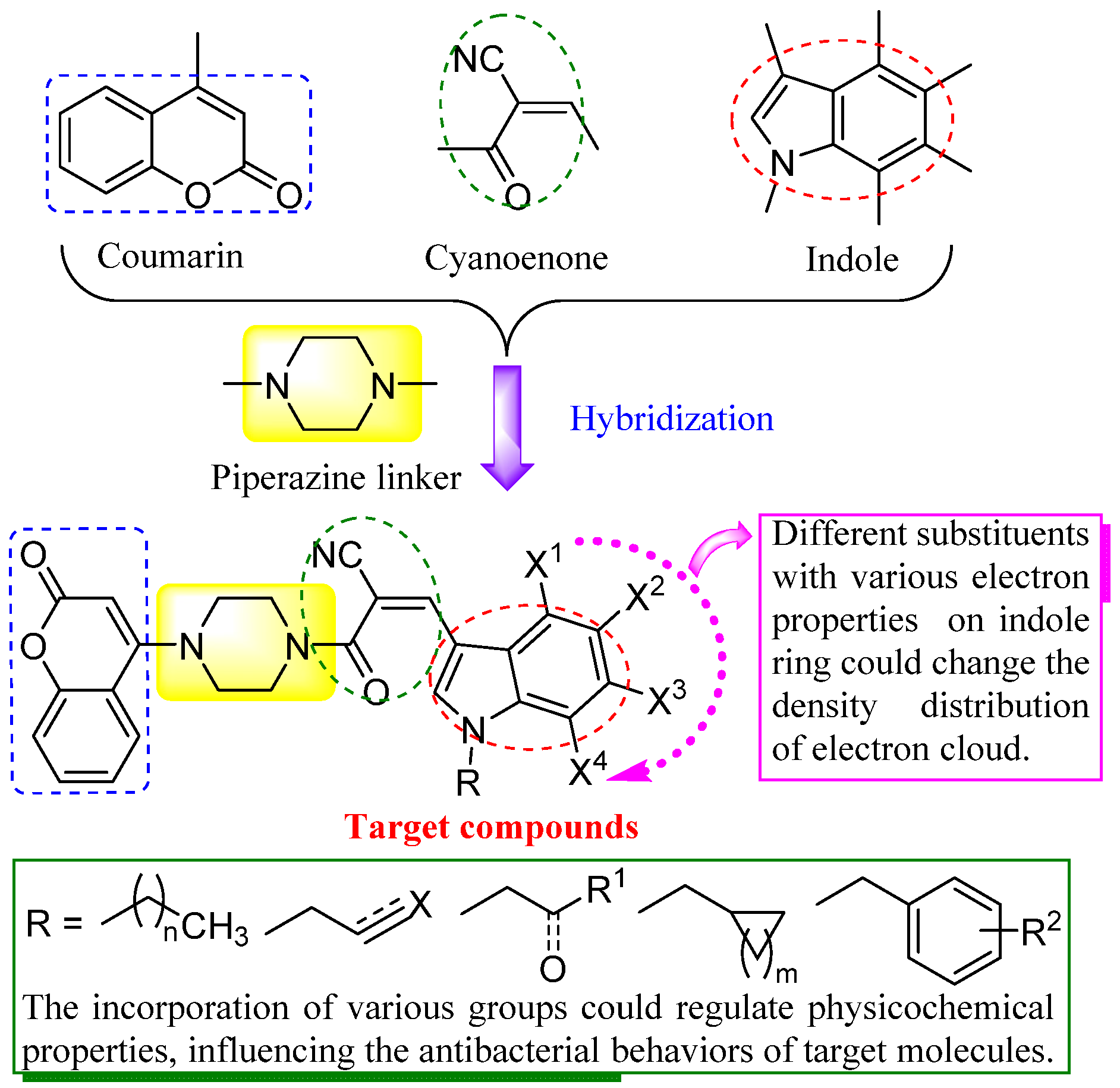
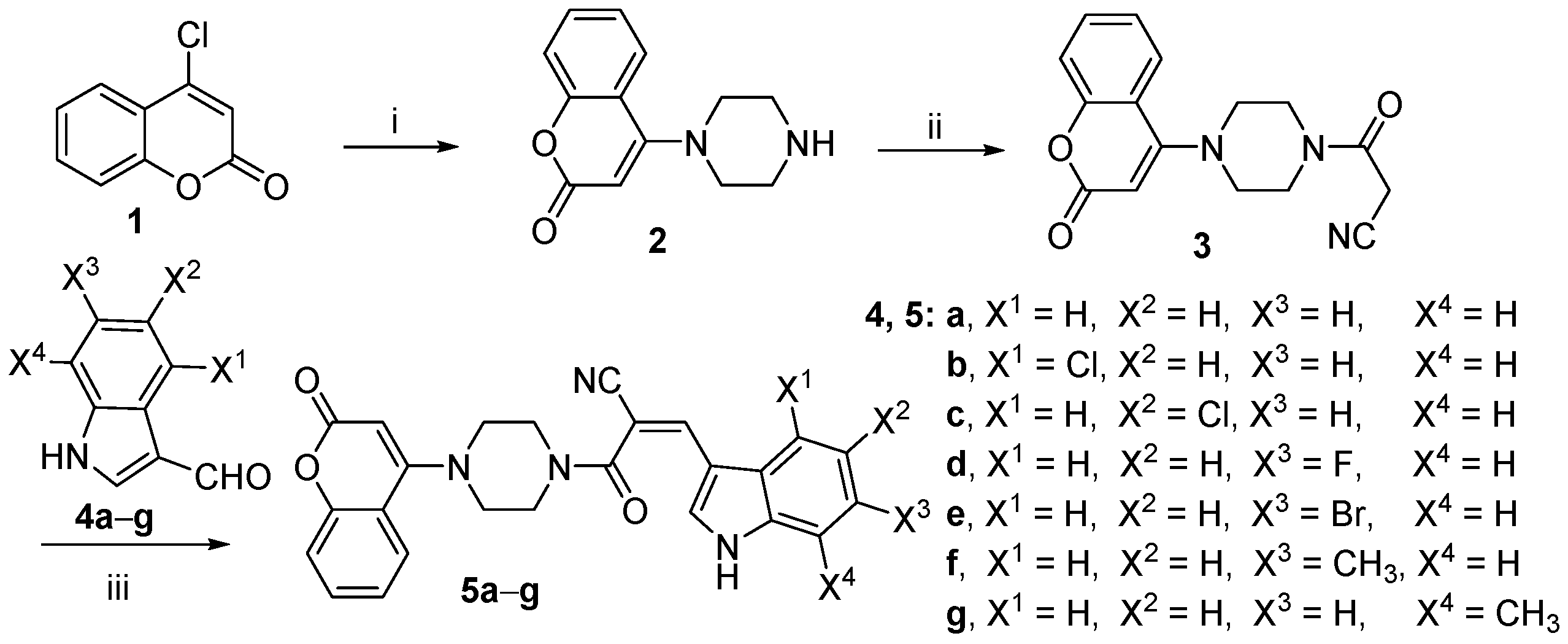
2.1. Chemistry
2.2. Antibacterial Activity
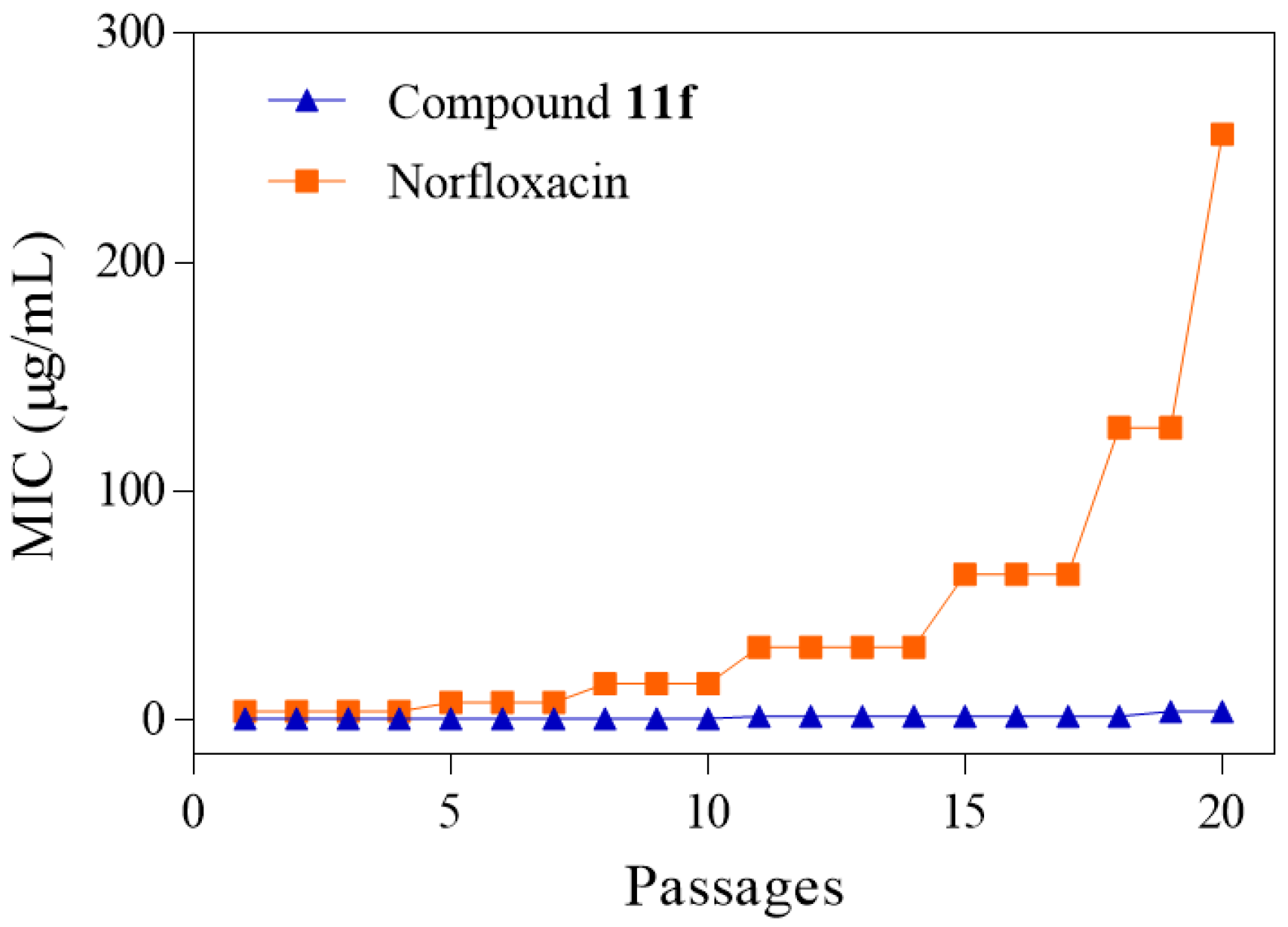
2.3. Tendency of Coumarin 11f to Induce Bacterial Resistance
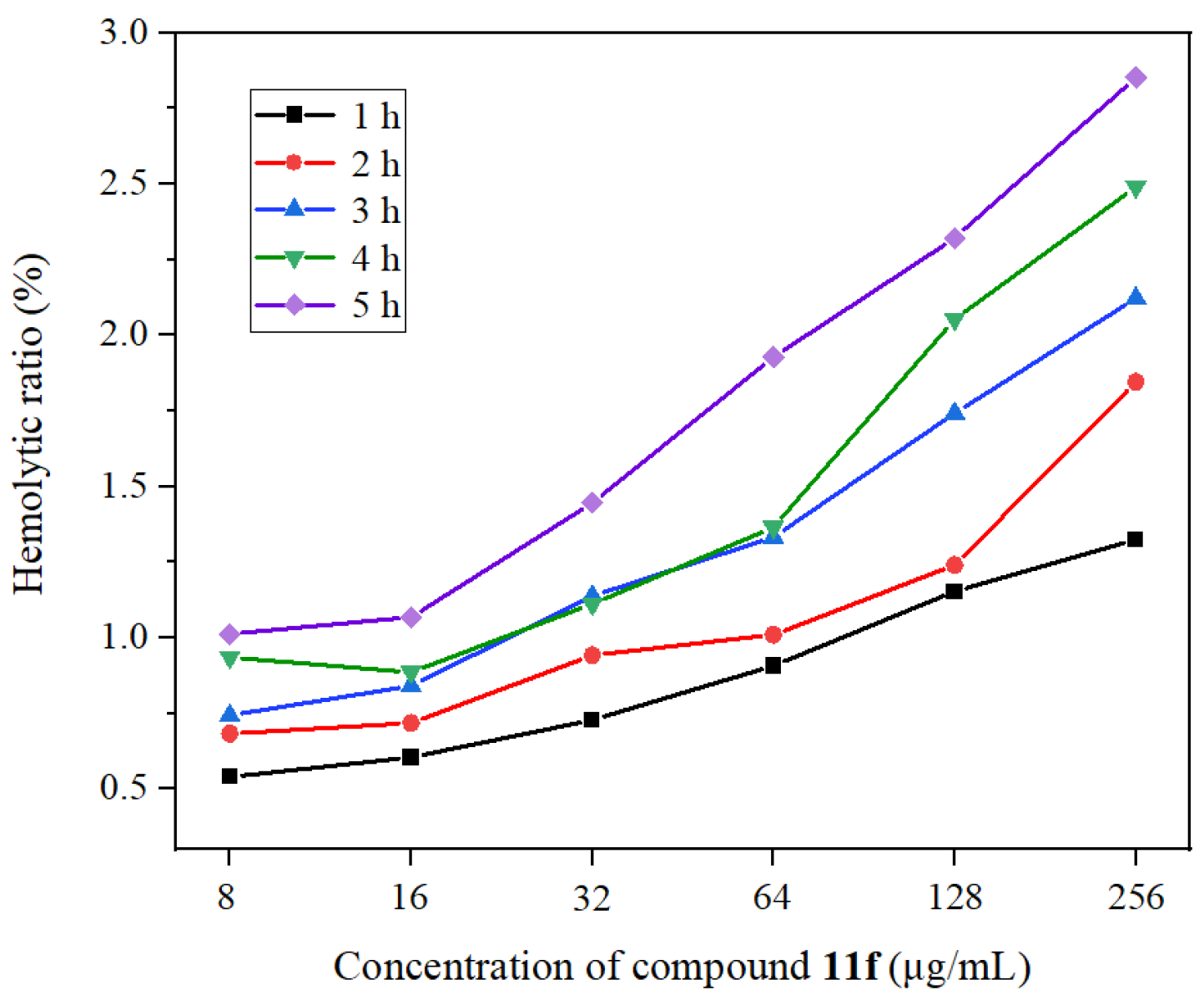
2.4. Hemolytic Activity
2.5. Membrane Disruption
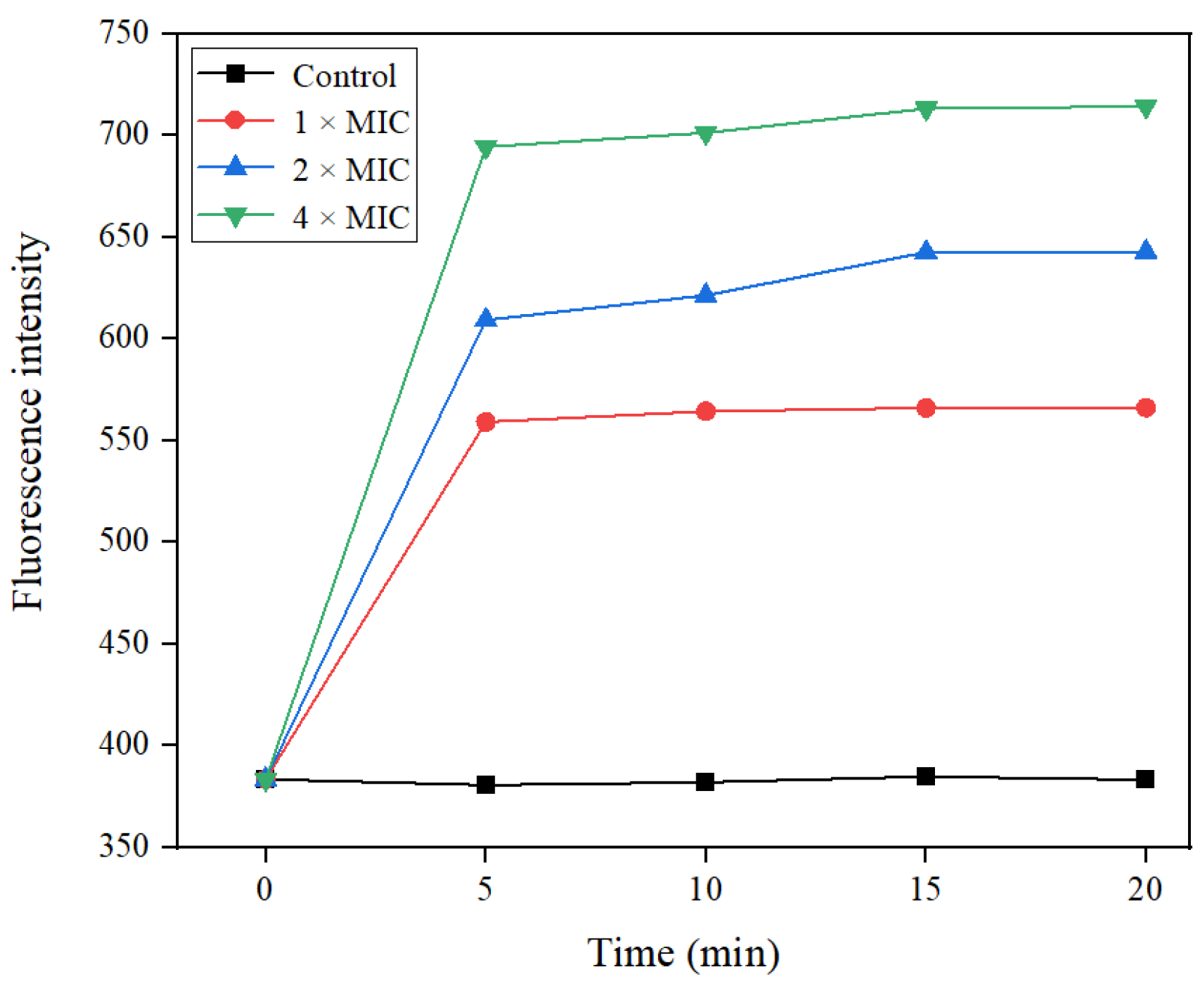
2.5.1. Outer Membrane Permeability
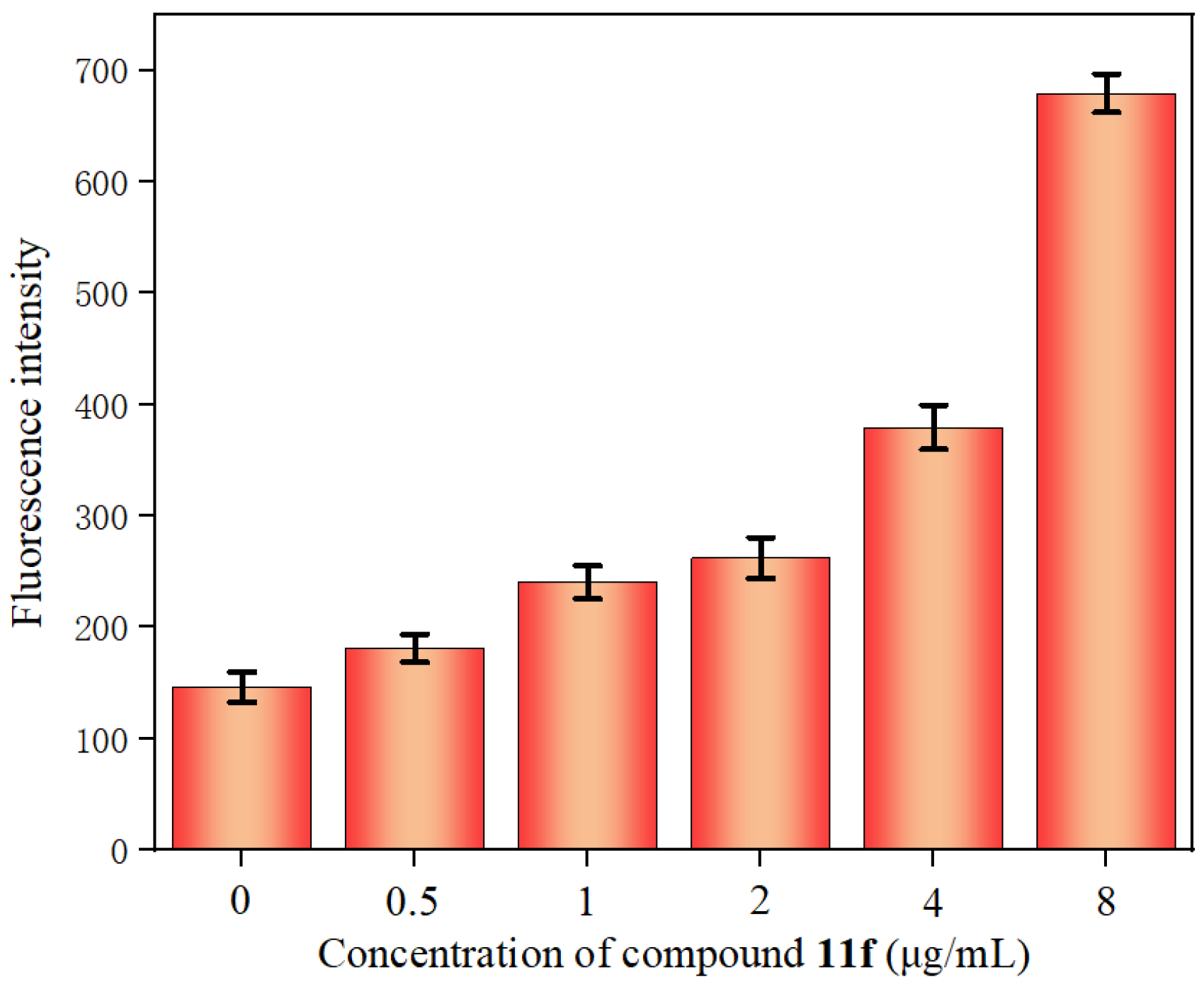
2.5.2. Inner Membrane Permeability
2.5.3. Membrane Depolarization
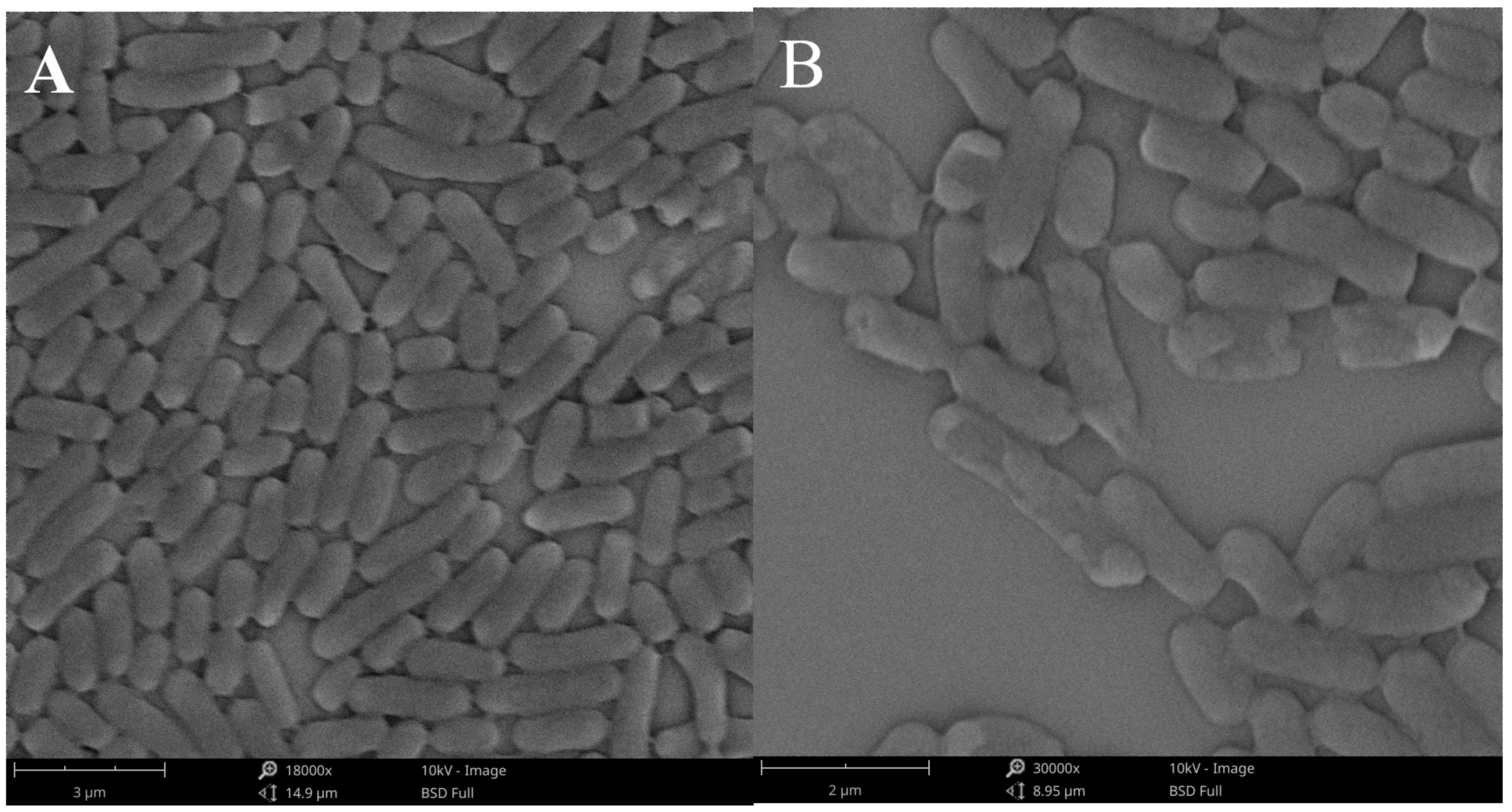
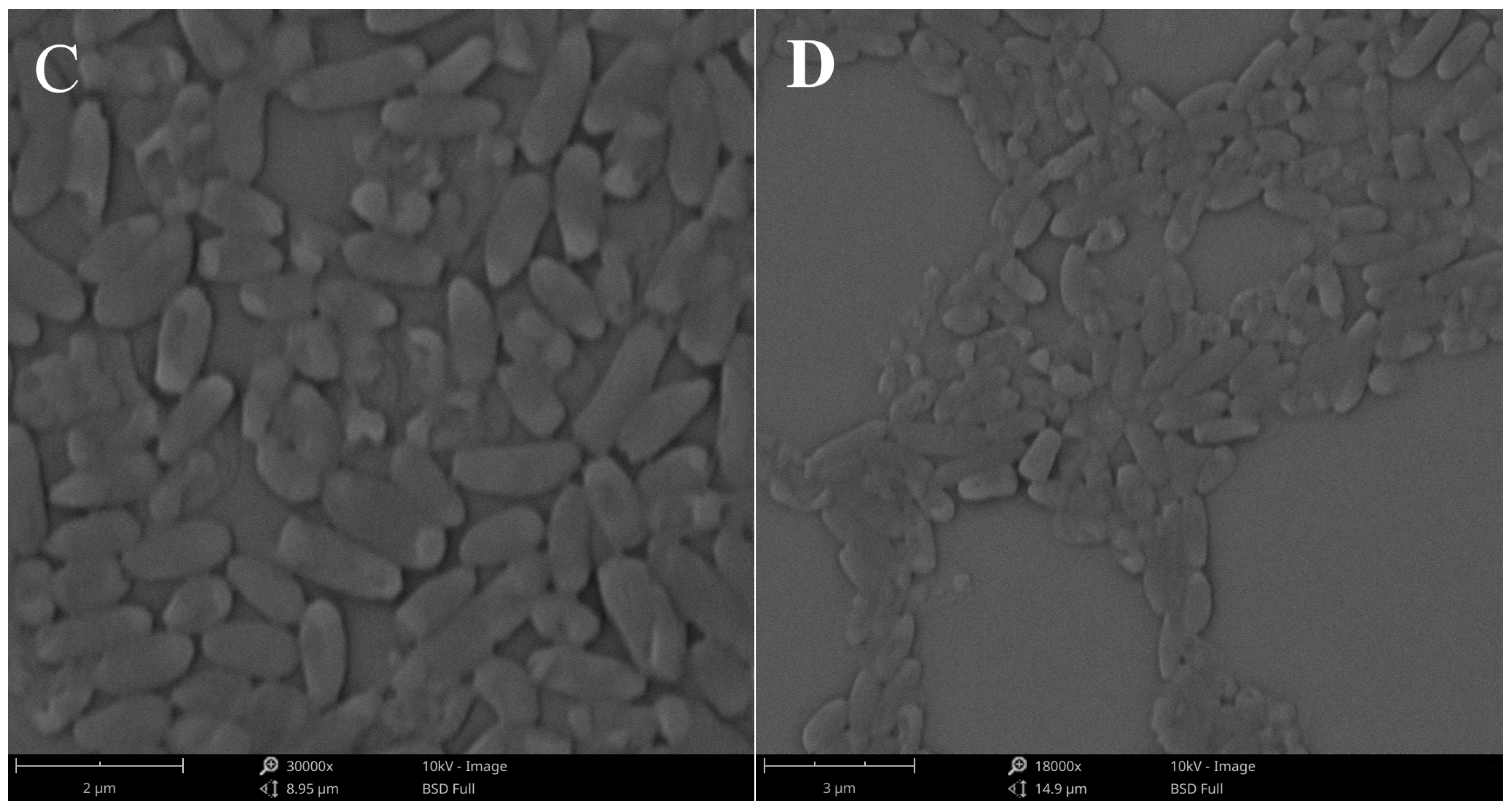
2.5.4. Cell Morphology Observation
2.6. Leakage of Intracellular Components
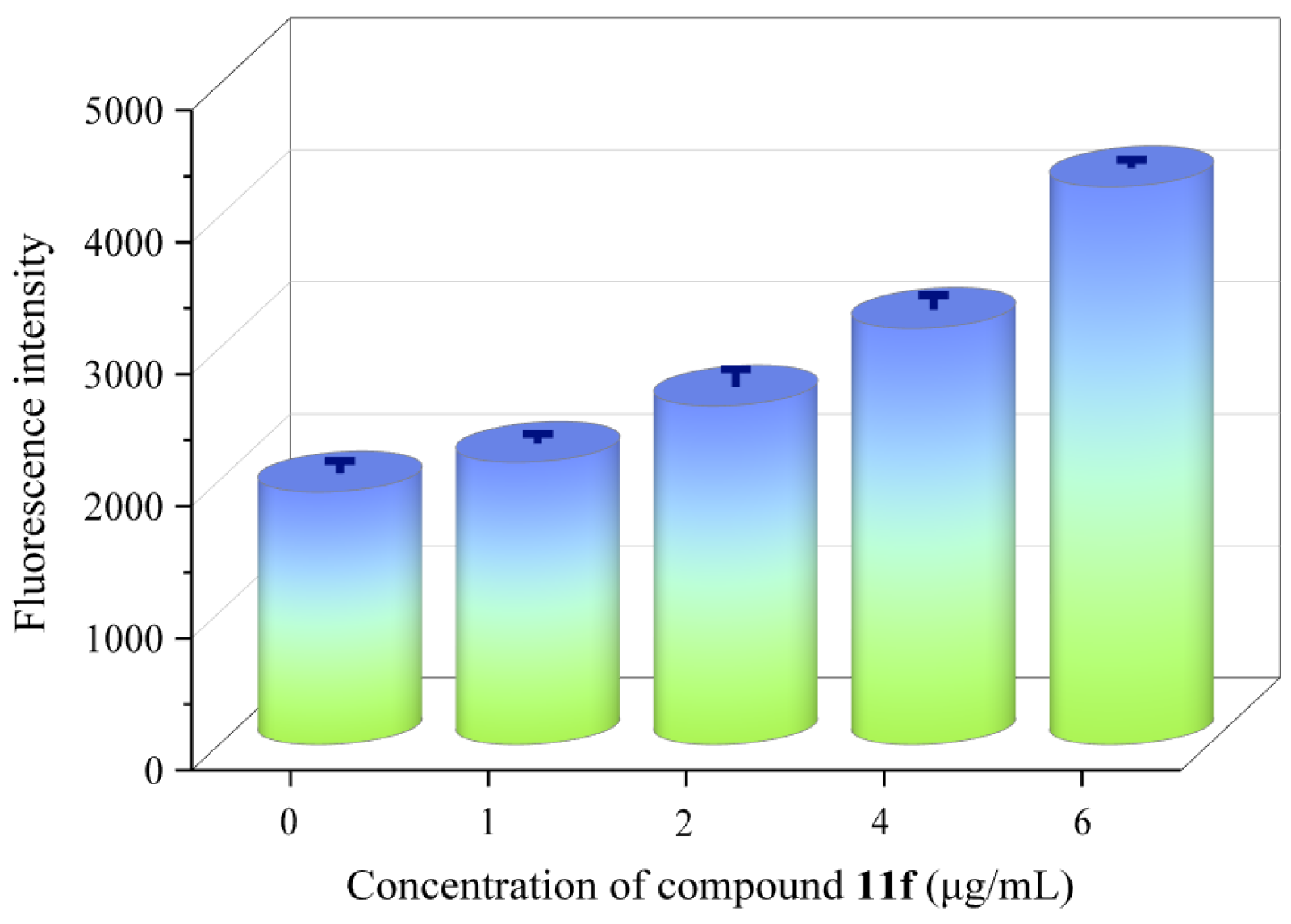
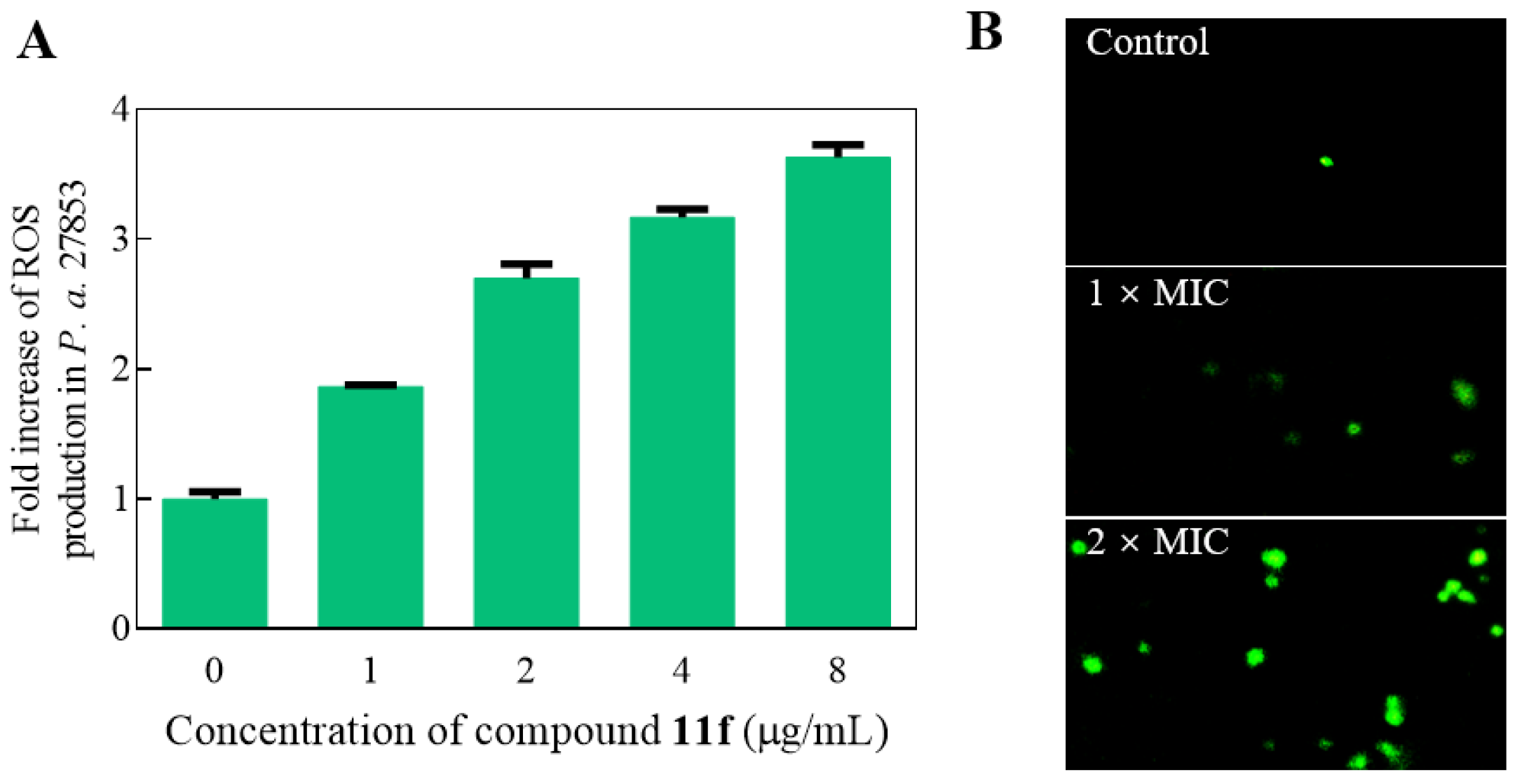
2.7. Oxidative Stress Assay
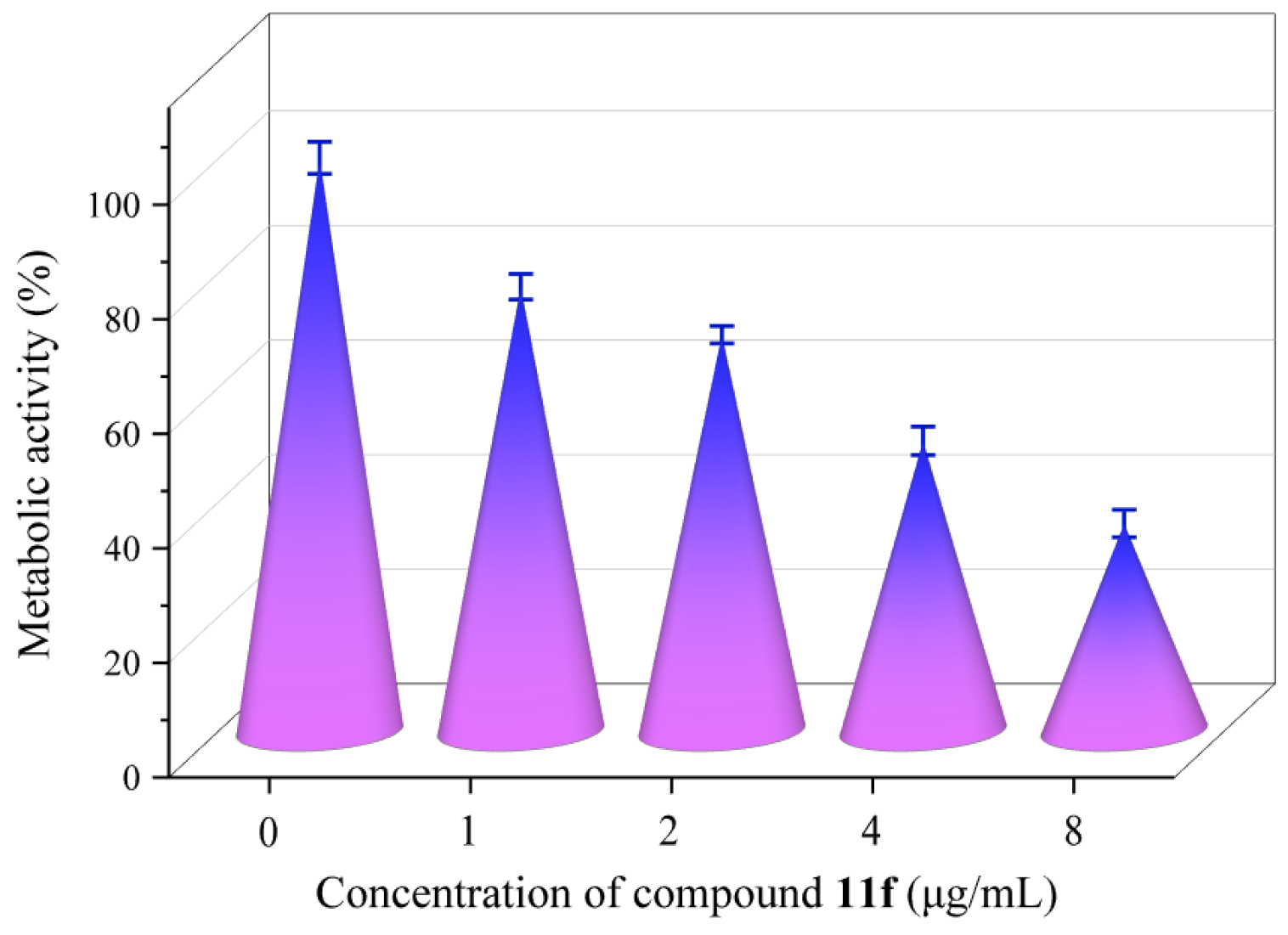
2.8. Metabolic Activity
2.9. Interactions of 11f with DNA
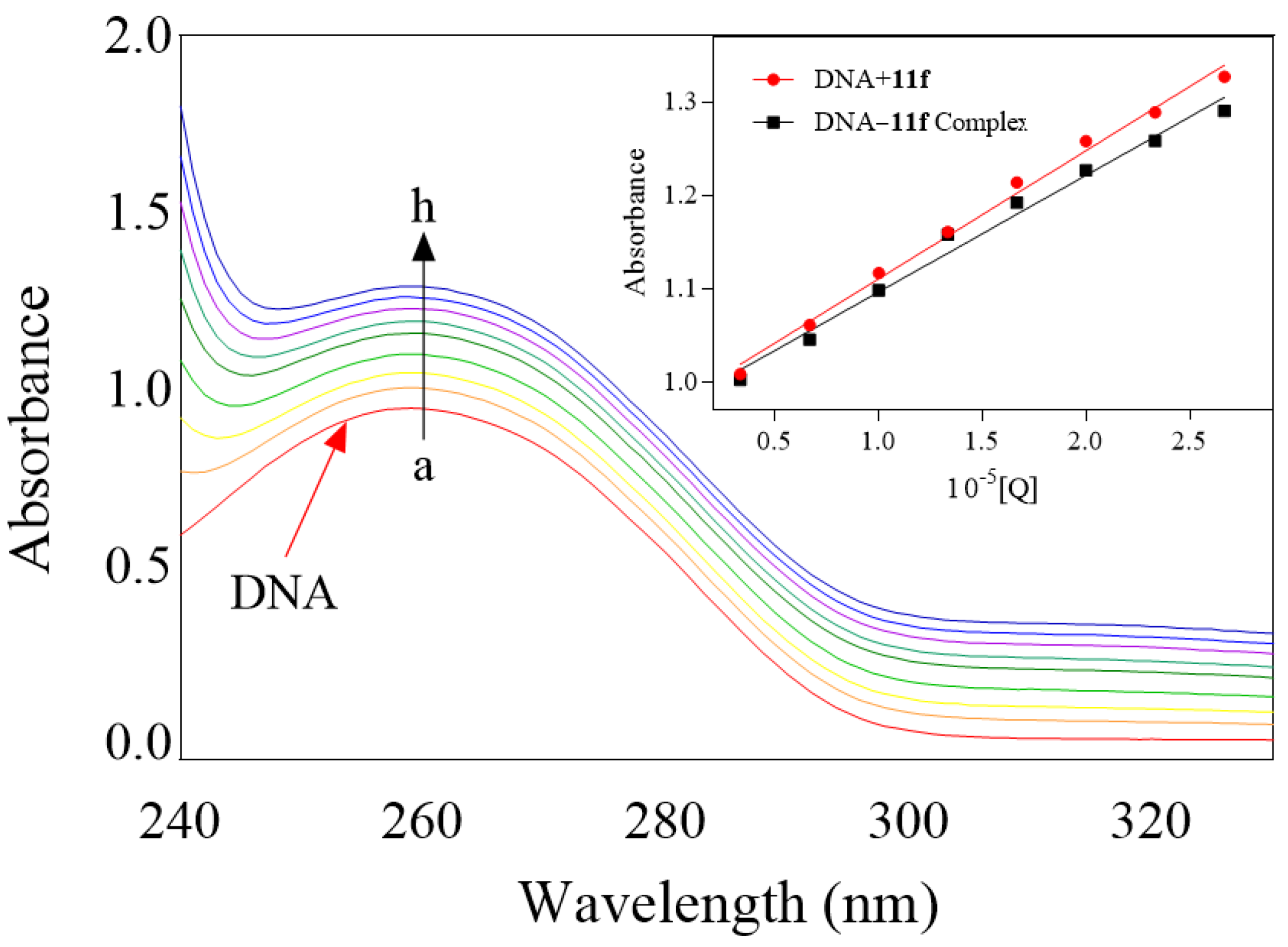
2.9.1. DNA Binding Study
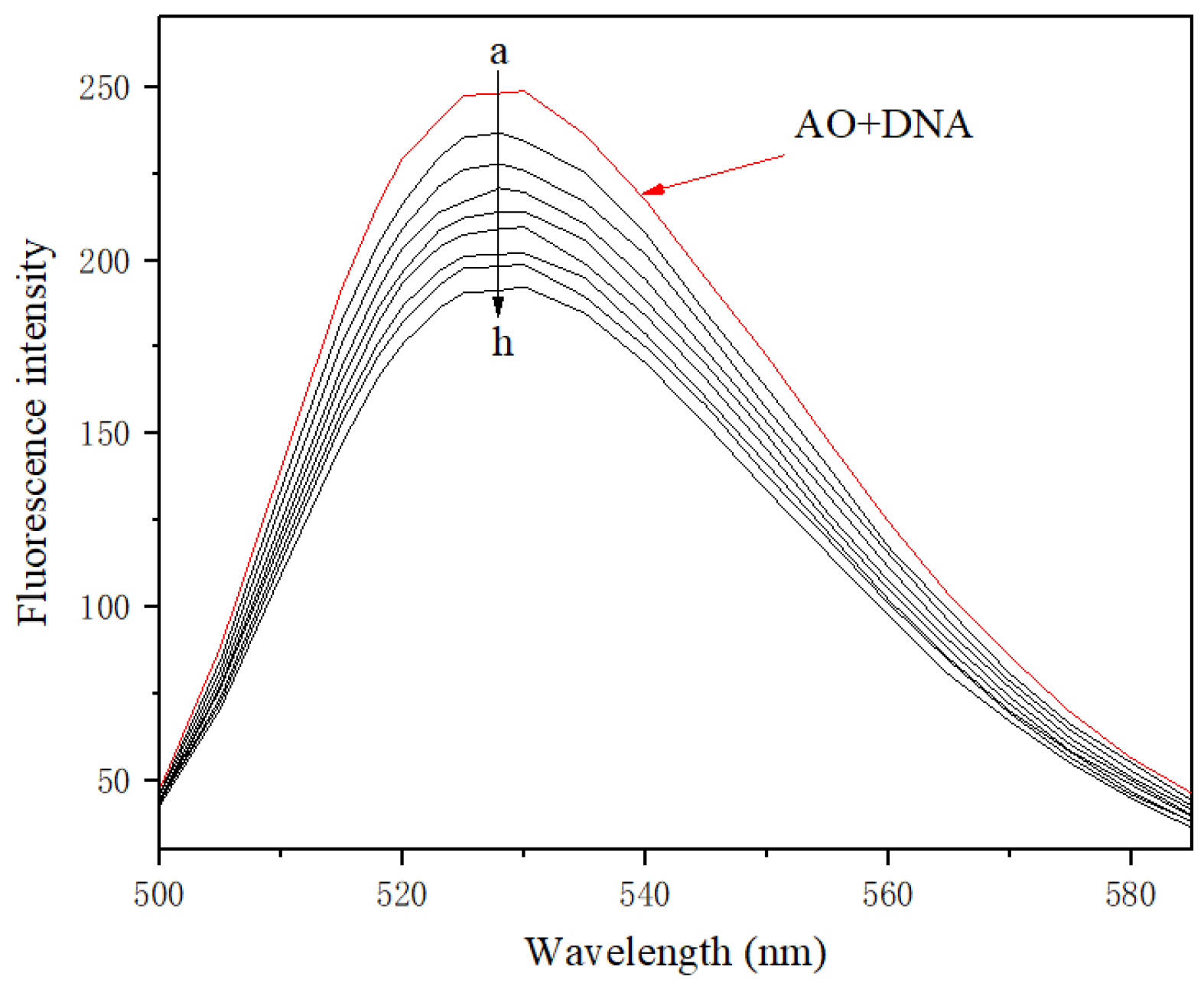
2.9.2. Binding Model between 11f and DNA
2.10. Molecular Docking
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Synthesis of Intermediate and Piperazine Hybridized Coumarin Indolylcyanoenones
3.2.1. Synthesis of Intermediate 2
3.2.2. Synthesis of 3-Oxo-3-(4-(2-oxo-2H-chromen-4-yl)piperazin-1-yl)propanenitrile (3)
3.2.3. Synthesis of (Z)-3-(1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5a)
3.2.4. Synthesis of (Z)-3-(4-chloro-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5b)
3.2.5. Synthesis of (Z)-3-(5-chloro-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5c)
3.2.6. Synthesis of (Z)-3-(6-fluoro-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5d)
3.2.7. Synthesis of (Z)-3-(6-bromo-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5e)
3.2.8. Synthesis of (Z)-3-(6-methyl-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5f)
3.2.9. Synthesis of (Z)-3-(7-methyl-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (5g)
3.2.10. Synthesis of (Z)-3-(1-ethyl-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (7a)
3.2.11. Synthesis of (Z)-3-(1-butyl-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (7b)
3.2.12. Synthesis of (Z)-3-(1-hexyl-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (7c)
3.2.13. Synthesis of (Z)-3-(1-(prop-2-yn-1-yl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (7d)
3.2.14. Synthesis of (Z)-3-(1-allyl-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (7e)
3.2.15. Synthesis of (Z)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (7f)
3.2.16. Synthesis of (Z)-3-(1-(cyclopropylmethyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (9a)
3.2.17. Synthesis of (Z)-3-(1-(cyclopentylmethyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (9b)
3.2.18. Synthesis of (Z)-3-(1-(cyclohexylmethyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (9c)
3.2.19. Synthesis of (Z)-3-(1-(2-fluorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11a)
3.2.20. Synthesis of (Z)-3-(1-(2-chlorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11b)
3.2.21. Synthesis of (Z)-3-(1-(3-fluorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11c)
3.2.22. Synthesis of (Z)-3-(1-(3-chlorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11d)
3.2.23. Synthesis of (Z)-3-(1-(4-fluorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11e)
3.2.24. Synthesis of (Z)-3-(1-(4-chlorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11f)
3.2.25. Synthesis of (Z)-3-(1-(4-bromobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11g)
3.2.26. Synthesis of (Z)-3-(1-(4-iodobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11h)
3.2.27. Synthesis of (Z)-3-(1-(2,4-dichlorobenzyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (11i)
3.2.28. Synthesis of (Z)-3-(1-(cyanomethyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (13a)
3.2.29. Synthesis of (Z)-3-(1-(2-hydroxyethyl)-1H-indol-3-yl)-2-(4-(2-oxo-2H-chromen-4-yl)piperazine-1-carbonyl)acrylonitrile (13b)
3.2.30. Synthesis of ethyl (Z)-2-(3-(2-cyano-3-oxo-3-(4-(2-oxo-2H-chromen-4-yl)piperazin-1-yl) prop-1-en-1-yl)-1H-indol-1-yl)acetatee (13c)
3.3. Biological Assay
3.3.1. Antibacterial Activity
3.3.2. Resistance Study
3.3.3. Hemolytic Assay
3.3.4. Outer Membrane Permeability
3.3.5. Inner Membrane Permeability
3.3.6. Depolarization of Membrane
3.3.7. Cell Morphology Observation
3.3.8. The Leakage of Intracellular Components
3.3.9. The Oxidative Stress Assay
3.3.10. Metabolic Activity
3.3.11. Supramolecular Interaction of Indolylcyanoenone 11f with DNA
3.3.12. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Han, D.L.; Liu, X.M.; Wu, S.L. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem. Soc. Rev. 2022, 51, 7138–7169. [Google Scholar] [CrossRef] [PubMed]
- Lázár, V.; Snitser, O.; Barkan, D.; Kishony, R. Antibiotic combinations reduce Staphylococcus aureus clearance. Nature 2022, 610, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, S.Y.; Jeyakkumar, P.; Cai, G.X.; Fang, B.; Zhou, C.H. Natural berberine-derived azolyl ethanols as new structural antibacterial agents against drug-resistant Escherichia coli. J. Med. Chem. 2022, 65, 436–459. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Lavore, F.; Maity, S.; Derks, M.G.N.; Jones, C.R.; Vermeulen, B.J.A.; Melcrová, A.; Morris, M.A.; Becker, L.M.; Wang, X.; et al. Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature 2022, 608, 390–396. [Google Scholar] [CrossRef]
- Li, F.F.; Zhao, W.H.; Tangadanchu, V.K.R.; Meng, J.P.; Zhou, C.H. Discovery of novel phenylhydrazone-based oxindole-thiolazoles as potent antibacterial agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef]
- Lin, S.M.; Wade, J.D.; Liu, S.P. De novo design of flavonoid-based mimetics of cationic antimicrobial peptides: Discovery, development, and applications. Acc. Chem. Res. 2021, 54, 104–119. [Google Scholar] [CrossRef]
- Sun, H.; Ansari, M.F.; Fang, B.; Zhou, C.H. Natural berberine-hybridized benzimidazoles as novel unique bactericides against Staphylococcus aureus. J. Agric. Food Chem. 2021, 69, 7831–7840. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar]
- Yang, X.C.; Hu, C.F.; Zhang, P.L.; Li, S.; Hu, C.S.; Geng, R.X.; Zhou, C.H. Coumarin thiazoles as unique structural skeleton of potential antimicrobial agents. Bioorg. Chem. 2022, 124, 105855. [Google Scholar] [CrossRef]
- Peng, X.M.; Damu, G.L.V.; Zhou, C.H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Broeck, A.V.; McEwen, A.G.; Chebaro, Y.; Potier, N.; Lamour, V. Structural basis for DNA gyrase interaction with coumermycin A1. J. Med. Chem. 2019, 62, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.M.; Kumar, K.V.; Damu, G.L.V.; Zhou, C.H. Coumarin-derived azolyl ethanols: Synthesis, antimicrobial evaluation and preliminary action mechanism study. Sci. China Chem. 2016, 59, 878–894. [Google Scholar] [CrossRef]
- Yang, X.C.; Zeng, C.M.; Avula, S.R.; Peng, X.M.; Geng, R.X.; Zhou, C.H. Novel coumarin aminophosphonates as potential multitargeting antibacterial agents against Staphylococcus aureus. Eur. J. Med. Chem. 2023, 245, 114891. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.F.; Wu, X.H.; Tu, X.; Wang, G.X. Synthesis and biological evaluation of coumarin derivatives containing imidazole skeleton as potential antibacterial agents. Eur. J. Med. Chem. 2018, 143, 958–969. [Google Scholar] [CrossRef]
- Yang, X.C.; Zhang, P.L.; Kumar, K.V.; Li, S.; Geng, R.X.; Zhou, C.H. Discovery of unique thiazolidinone-conjugated coumarins as novel broad spectrum antibacterial agents. Eur. J. Med. Chem. 2022, 232, 114192. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.F.; Zhang, P.L.; Sui, Y.F.; Lv, J.S.; Ansari, M.F.; Battini, N.; Li, S.; Zhou, C.H.; Geng, R.X. Ethylenic conjugated coumarin thiazolidinediones as new efficient antimicrobial modulators against clinical methicillin-resistant Staphylococcus aureus. Bioorg. Chem. 2020, 94, 103434. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Identification of unique quinazolone thiazoles as novel structural scaffolds for potential gram-negative bacterial conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef]
- Tangadanchu, V.K.R.; Sui, Y.F.; Zhou, C.H. Isatin-derived azoles as new potential antimicrobial agents: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2021, 41, 128030. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Itoh, T.; Hori, T.; Kato, A.; Anami, Y.; Yoshimoto, N.; Yamamoto, K. Identification of the Histidine residue in vitamin D receptor that covalently binds to electrophilic ligands. J. Med. Chem. 2018, 61, 6339–6349. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Boon, N.; Kaur, M.; Aziz, A.; Bradnick, M.; Shibayama, K.; Eguchi, Y.; Lund, P.A. The signaling molecule indole inhibits induction of the AR2 acid resistance system in Escherichia coli. Front. Microbiol. 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Tangadanchu, V.K.R.; Battini, N.; Bheemanaboina, R.R.Y.; Zang, Z.L.; Zhang, S.L.; Zhou, C.H. Indole-nitroimidazole conjugates as efficient manipulators to decrease the genes expression of methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2019, 179, 723–735. [Google Scholar] [CrossRef]
- Li, H.D.; Wu, S.; Yang, X.; He, H.F.; Wu, Z.X.; Song, B.A.; Song, R.J. Synthesis, antibacterial activity, and mechanisms of novel indole derivatives containing pyridinium moieties. J. Agric. Food Chem. 2022, 70, 12341–12354. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Li, H.X.; Liu, J.Y.; Zhong, R.C.; Li, H.Z.; Fang, S.F.; Liu, S.P.; Lin, S.M. Synthesis and biological evaluation of indole-based peptidomimetics as antibacterial agents against Gram-positive bacteria. Eur. J. Med. Chem. 2021, 226, 113813. [Google Scholar] [CrossRef]
- Qin, R.; Zhao, Q.; Han, B.; Zhu, H.P.; Peng, C.; Zhan, G.; Huang, W. Indole-based small molecules as potential therapeutic agents for the treatment of fibrosis. Front. Pharmacol. 2022, 13, 845892. [Google Scholar] [CrossRef]
- Zhang, P.L.; Gopala, L.; Zhang, S.L.; Cai, G.X.; Zhou, C.H. An unanticipated discovery towards novel naphthalimide corbelled aminothiazoximes as potential anti-MRSA agents and allosteric modulators for PBP2a. Eur. J. Med. Chem. 2022, 229, 114050. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Bheemanaboina, R.R.Y.; Battini, N.; Zhou, C.H. Sulfonamide-derived four-component molecular hybrids as novel DNA-targeting membrane active potentiators against clinical Escherichia coli. Mol. Pharmaceutics 2019, 16, 1036–1052. [Google Scholar] [CrossRef]
- Chen, J.P.; Battini, N.; Ansari, M.F.; Zhou, C.H. Membrane active 7-thiazoxime quinolones as novel DNA binding agents to decrease the genes expression and exert potent antimethicillin-resistant Staphylococcus aureus activity. Eur. J. Med. Chem. 2021, 217, 113340. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.X.; Yu, Q.; Zheng, Z.X.; Liu, J.Y.; Cai, Q.N.; Liu, S.P.; Lin, S.M. Design and synthesis of phenyl sulfide-based cationic amphiphiles as membrane-targeting antimicrobial agents against Gram-Positive pathogens. J. Med. Chem. 2022, 65, 14221–14236. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, H.; Maddili, S.K.; Li, S.; Yang, R.G.; Zhou, C.H. Dihydropyrimidinone imidazoles as unique structural antibacterial agents for drug-resistant gram-negative pathogens. Eur. J. Med. Chem. 2022, 232, 114188. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.F.; Ansari, M.F.; Zhou, C.H. Pyrimidinetrione-imidazoles as a unique structural type of potential agents towards Candida albicans: Design, synthesis and biological evaluation. Chem. Asian J. 2021, 16, 1417–1429. [Google Scholar] [CrossRef]
- Mitcheltree, M.J.; Pisipati, A.; Syroegin, E.A.; Silvestre, K.J.; Klepacki, D.; Mason, J.D.; Terwilliger, D.W.; Testolin, G.; Pote, A.R.; Wu, K.J.Y.; et al. A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 2021, 599, 507–512. [Google Scholar] [CrossRef]
- Wang, J.; Battini, N.; Ansari, M.F.; Zhou, C.H. Synthesis and biological evaluation of quinazolonethiazoles as new potential conquerors towards Pseudomonas aeruginosa. Chin. J. Chem. 2021, 39, 1093–1103. [Google Scholar] [CrossRef]
- Zhou, X.M.; Hu, Y.Y.; Fang, B.; Zhou, C.H. Benzenesulfonyl thiazoloimines as unique multitargeting antibacterial agents towards Enterococcus faecalis. Eur. J. Med. Chem. 2023, 248, 115088. [Google Scholar] [CrossRef]
- Zhou, C.H.; Wang, Y. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yu, R.J.; Wang, L.Q.; Huang, H.Y.; Wang, J.T.; Liao, X.W.; Duan, X.M.; Xiong, Y.S. Design, synthesis, and evaluation of aryl-thioether ruthenium polypyridine complexes: A multi-target antimicrobial agents against Gram-positive bacteria. Eur. J. Med. Chem. 2022, 240, 114562. [Google Scholar]
- Sui, Y.F.; Ansari, M.F.; Fang, B.; Zhang, S.L.; Zhou, C.H. Discovery of novel purinylthiazolylethanone derivatives as anti-Candida albicans agents through possible multifaceted mechanisms. Eur. J. Med. Chem. 2021, 221, 113557. [Google Scholar] [CrossRef]
- Cui, S.F.; Addla, D.; Zhou, C.H. Novel 3-aminothiazolquinolones: Design, synthesis, bioactive evaluation, SARs, and preliminary antibacterial mechanism. J. Med. Chem. 2016, 59, 4488–4510. [Google Scholar] [CrossRef]
- Pham, D.D.D.; Mojr, V.; Helusová, M.; Mikušová, G.; Pohl, R.; Dávidová, E.; Šanderová, H.; Vítovská, D.; Bogdanová, K.; Večeřová, R.; et al. LEGO-Lipophosphonoxins: A novel approach in designing membrane targeting antimicrobials. J. Med. Chem. 2022, 65, 10045–10078. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Zhang, P.L.; Tangadanchu, V.K.R.; Li, S.; Zhou, C.H. Novel metronidazole-derived three-component hybrids as promising broad-spectrum agents to combat oppressive bacterial resistance. Bioorg. Chem. 2022, 122, 105718. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From plants to antimicrobials: Natural products against bacterial membranes. Phytother. Res. 2022, 36, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ansari, M.F.; Lin, J.M.; Zhou, C.H. Design and synthesis of sulfanilamide aminophosphonates as novel antibacterial agents towards Escherichia coli. Chin. J. Chem. 2021, 39, 2251–2263. [Google Scholar] [CrossRef]
- Liang, X.Y.; Battini, N.; Sui, Y.F.; Ansari, M.F.; Gan, L.L.; Zhou, C.H. Aloe-emodin derived azoles as a new structural type of potential antibacterial agents: Design, synthesis, and evaluation of the action on membrane, DNA, and MRSA DNA isomerase. RSC Med. Chem. 2021, 12, 602–608. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, F.Y.; Yao, J.; Zhu, Y.W.; Zhu, N.Y.; Zhang, J.Y.; Ouyang, X.; Zhang, T.Y.; Li, B.B.; Xie, J.Q.; et al. New antimicrobial peptides with repeating unit against multidrug resistant bacteria. ACS Infect. Dis. 2021, 7, 1619–1637. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.L.; Ansari, M.F.; Li, S.; Zhou, C.H. Molecular design and preparation of 2-aminothiazole sulfanilamide oximes as membrane active antibacterial agents for drug resistant Acinetobacter baumannii. Bioorg. Chem. 2021, 113, 105039. [Google Scholar] [CrossRef]
- Li, H.X.; Liu, J.Y.; Liu, C.F.; Li, H.Z.; Luo, J.C.; Fang, S.F.; Chen, Y.Z.; Zhong, R.C.; Liu, S.P.; Lin, S.M. Design, synthesis, and biological evaluation of membrane-active bakuchiol derivatives as effective broad-spectrum antibacterial agents. J. Med. Chem. 2021, 64, 5603–5619. [Google Scholar] [CrossRef]
- Xie, Y.P.; Sangaraiah, N.; Meng, J.P.; Zhou, C.H. Unique Carbazole-oxadiazole derivatives as new potential antibiotics for combating gram-positive and -negative bacteria. J. Med. Chem. 2022, 65, 6171–6190. [Google Scholar] [CrossRef]
- Xing, M.; Liu, S.; Yu, Y.P.; Guo, L.; Wang, Y.; Feng, Y.G.; Fei, P.; Kang, H.B.; Ali, M.A. Antibacterial mode of Eucommia ulmoides male flower extract against Staphylococcus aureus and its application as a natural preservative in cooked beef. Front. Microbiol. 2022, 13, 846622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.L.; Lv, J.S.; Ansari, M.F.; Battini, N.; Cai, G.X.; Zhou, C.H. Synthesis of naphthalimide triazoles with a novel structural framework and their anti-Aspergillus fumigatus effects. Sci. Sin. Chim. 2021, 51, 1094–1103. [Google Scholar] [CrossRef]
- Zhang, E.; Bai, P.Y.; Cui, D.Y.; Chu, W.C.; Hua, Y.G.; Liu, Q.; Yin, H.Y.; Zhang, Y.J.; Qin, S.S.; Liu, H.M. Synthesis and bioactivities study of new antibacterial peptide mimics: The dialkyl cationic amphiphiles. Eur. J. Med. Chem. 2018, 143, 1489–1509. [Google Scholar] [CrossRef]
- Pasero, C.; D’Agostino, I.; Luca, F.D.; Zamperini, C.; Deodato, D.; Truglio, G.I.; Sannio, F.; Prete, R.D.; Ferraro, T.; Visaggio, D.; et al. Alkyl-guanidine compounds as potent broad-spectrum antibacterial agents: Chemical library extension and biological characterization. J. Med. Chem. 2018, 61, 9162–9176. [Google Scholar] [CrossRef]
- Kumar, P.; Shaikh, A.A.; Kumar, P.; Gupta, V.K.; Dhyani, R.; Sharma, T.K.; Hussain, A.; Gangele, K.; Poluri, K.M.; Rao, K.N.; et al. Double-edged nanobiotic platform with protean functionality: Leveraging the synergistic antibacterial activity of a food-grade peptide to mitigate multidrug-resistant bacterial pathogens. ACS Appl. Mater. Interfaces 2022, 14, 20652–20668. [Google Scholar] [CrossRef]
- Deng, Z.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Aloe emodin-conjugated sulfonyl hydrazones as novel type of antibacterial modulators against S. aureus 25923 through multifaceted synergistic effects. Bioorg. Chem. 2022, 127, 106035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Zhang, Z.C.; Shu, Q.M.; Xu, C.; Zheng, Q.Q.; Guo, Z.; Wang, C.; Hao, Z.X.; Liu, X.; Wang, G.Q.; et al. Copper clusters: An effective antibacterial for eradicating multidrug-resistant bacterial infection in vitro and in vivo. Adv. Funct. Mater. 2021, 31, 2008720. [Google Scholar] [CrossRef]
- Guo, F.Y.; Chen, Q.P.; Liang, Q.; Zhang, M.; Chen, W.X.; Chen, H.M.; Yun, Y.H.; Zhong, Q.P.; Chen, W.J. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Yang, X.; Syed, R.; Fang, B.; Zhou, C.H. A new discovery towards novel skeleton of benzimidazole-conjugated pyrimidinones as unique effective antibacterial agents. Chin. J. Chem. 2022, 40, 2642–2654. [Google Scholar] [CrossRef]
- Yu, J.H.; Xu, X.F.; Hou, W.; Meng, Y.; Huang, M.Y.; Lin, J.; Chen, W.M. Synthetic cajaninstilbene acid derivatives eradicate methicillinresistant Staphylococcus aureus persisters and biofilms. Eur. J. Med. Chem. 2021, 224, 113691. [Google Scholar] [CrossRef]
- Zhang, P.L.; Laiche, M.H.; Li, Y.L.; Gao, W.W.; Lin, J.M.; Zhou, C.H. An unanticipated discovery of novel naphthalimidopropanediols as potential broad-spectrum antibacterial members. Eur. J. Med. Chem. 2022, 241, 114657. [Google Scholar] [CrossRef]
- Vigyasa, S.; Anirban, P.; Mahendra, P.D. A polyphenolic flavonoid glabridin: Oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radical Bio. Med. 2015, 87, 48–57. [Google Scholar]
- Zhang, P.L.; Gopala, L.; Yu, Y.; Fang, B.; Zhou, C.H. Identification of a novel antifungal backbone of naphthalimide thiazoles with synergistic potential for chemical and dynamic treatment. Future Med. Chem. 2021, 13, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Yagya, P.S.; Paul, R.; Michelle, G.; Jon, Y.T.; Cheng-Wei, T.C. Development of fungal selective amphiphilic kanamycin: Cost-effective synthesis and use of fluorescent analogs for mode of action investigation. ACS Infect. Dis. 2019, 5, 473–483. [Google Scholar]
- Deng, Z.; Sun, H.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Natural aloe emodin-hybridized sulfonamide aminophosphates as novel potential membrane-perturbing and DNA-intercalating agents against Enterococcus faecalis. Bioorg. Med. Chem. Lett. 2022, 64, 128695. [Google Scholar] [CrossRef] [PubMed]
- Shounak, R.; Anupam, M.; Varnika, Y.; Ankita, S.; Ruptanu, B.; Pallab, S.; Amit, J. Mechanistic insight into the antibacterial activity of chitosan exfoliated MOS2 nanosheets: Membrane damage, metabolic inactivation, and oxidative stress. ACS Appl. Bio Mater. 2019, 2, 2738–2755. [Google Scholar]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Unique para-aminobenzenesulfonyl oxadiazoles as novel structural potential membrane active antibacterial agents towards drug-resistant methicillin resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2021, 41, 127995. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.Z.; Jeyakkumar, P.; Zang, Z.L.; Fang, B.; Zhou, C.H. A new discovery of unique 13-(benzimidazolylmethyl)berberines as promising broad-spectrum antibacterial agents. J. Agric. Food Chem. 2022, 70, 12320–12329. [Google Scholar] [CrossRef]
- Zhang, J.; Battini, N.; Ou, J.M.; Zhang, S.L.; Zhang, L.; Zhou, C.H. New efforts toward aminothiazolylquinolones with multitargeting antibacterial potential. J. Agric. Food Chem. 2023, 71, 2322–2332. [Google Scholar] [CrossRef]
- Zhang, P.L.; Tangadanchu, V.K.R.; Zhou, C.H. Identification of novel antifungal skeleton of hydroxyethy naphthalimides with synergistic potential for chemical and dynamic treatments. Molecules 2022, 27, 8453. [Google Scholar]
- Zhang, W.J.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.Y.; Luo, H.; Liu, H.; Han, X.; Yan, G.Y.; Ding, Z.Y.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef] [PubMed]
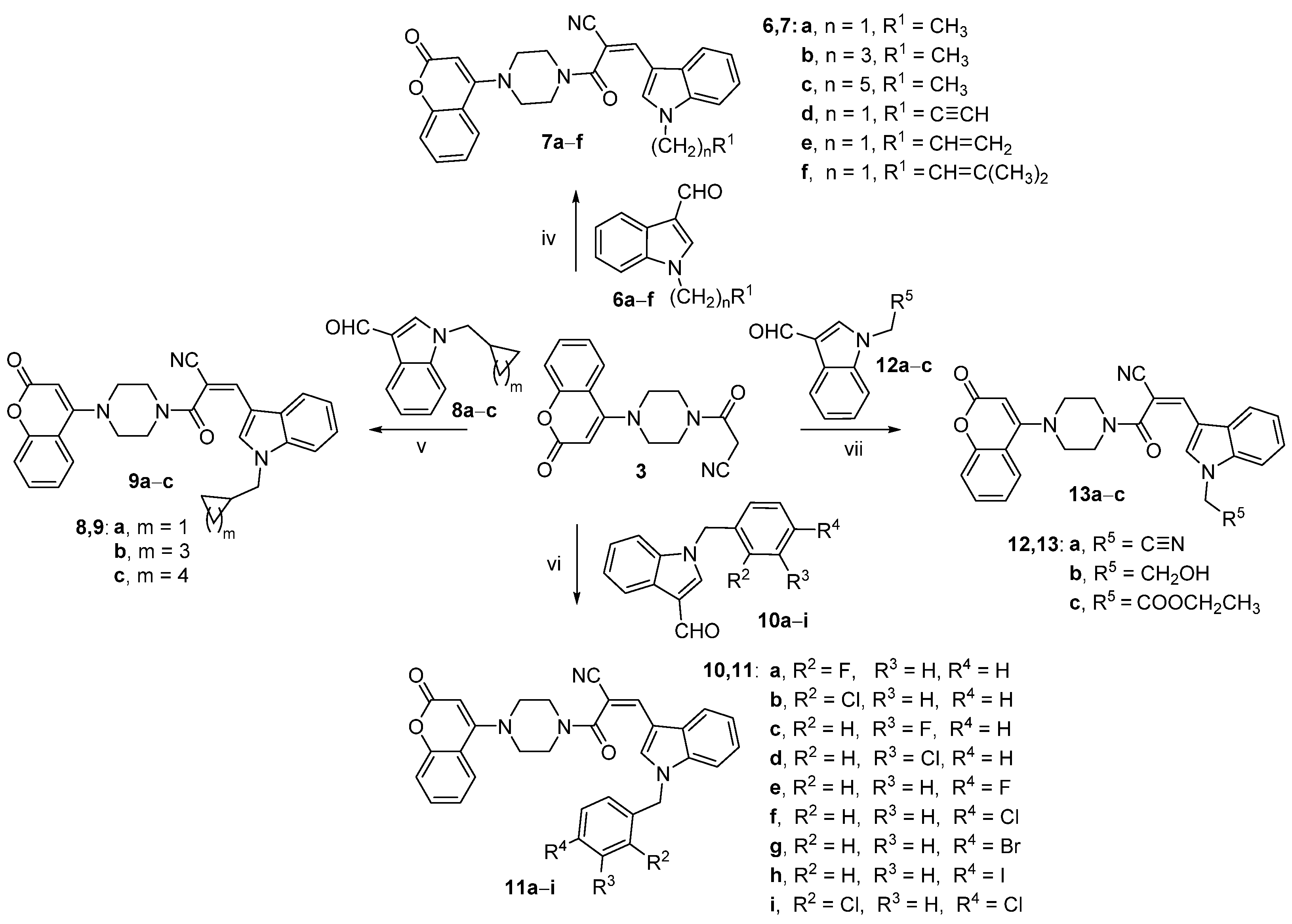


| Compounds | Gram-Positive Bacteria a | Gram-Negative Bacteria b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. a. | S. a. 26003 | S. a. 25923 | S. a. 6538 | E. f. | E. c. 9027 | E. c. 25922 | P. a. | P. a. 27853 | A. b. | |
| 5a | 128 | 16 | 16 | 16 | 64 | 64 | 32 | 4 | 8 | 16 |
| 5b | 64 | 128 | 64 | 4 | 32 | 32 | 128 | 16 | 16 | 64 |
| 5c | 64 | 128 | 128 | 128 | 64 | 64 | 16 | 4 | 4 | 64 |
| 5d | 32 | 32 | 64 | 64 | 16 | 64 | 32 | 16 | 4 | 64 |
| 5e | 16 | 4 | 2 | 32 | 32 | 64 | 64 | 4 | 4 | 16 |
| 5f | 64 | 4 | 16 | 64 | 64 | 64 | 64 | 2 | 8 | 32 |
| 5g | 64 | 16 | 128 | 16 | 64 | 32 | 128 | 16 | 4 | 64 |
| 7a | 64 | 32 | 256 | 128 | 32 | 64 | 64 | 8 | 8 | 8 |
| 7b | 64 | 32 | 16 | 64 | 64 | 128 | 8 | 8 | 4 | 64 |
| 7c | 64 | 32 | 128 | 32 | 32 | 64 | 256 | 16 | 8 | 64 |
| 7d | 32 | 16 | 32 | 64 | 64 | 64 | 32 | 4 | 4 | 64 |
| 7e | 128 | 32 | 128 | 64 | 64 | 32 | 16 | 8 | 4 | 64 |
| 7f | 128 | 8 | 4 | 32 | 32 | 128 | 128 | 4 | 4 | 32 |
| 9a | 64 | 16 | 128 | 8 | 32 | 64 | 32 | 16 | 4 | 64 |
| 9b | 64 | 64 | 32 | 16 | 64 | 64 | 16 | 8 | 4 | 64 |
| 9c | 64 | 16 | 64 | 4 | 32 | 64 | 64 | 8 | 8 | 32 |
| 11a | 8 | 32 | 128 | 32 | 64 | 32 | 64 | 4 | 8 | 64 |
| 11b | 16 | 32 | 128 | 64 | 64 | 32 | 128 | 8 | 8 | 64 |
| 11c | 4 | 32 | 128 | 4 | 64 | 32 | 128 | 4 | 8 | 64 |
| 11d | 4 | 64 | 128 | 16 | 32 | 32 | 64 | 4 | 8 | 64 |
| 11e | 128 | 32 | 16 | 64 | 8 | 32 | 64 | 8 | 2 | 32 |
| 11f | 64 | 32 | 128 | 32 | 32 | 64 | 128 | 4 | 1 | 64 |
| 11g | 8 | 32 | 32 | 64 | 16 | 32 | 64 | 4 | 2 | 64 |
| 11h | 32 | 16 | 16 | 64 | 16 | 32 | 64 | 8 | 8 | 32 |
| 11i | 16 | 16 | 16 | 64 | 64 | 32 | 16 | 2 | 2 | 64 |
| 13a | 4 | 16 | 32 | 8 | 64 | 32 | 64 | 4 | 8 | 16 |
| 13b | 128 | 16 | 8 | 32 | 128 | 64 | 64 | 4 | 8 | 64 |
| 13c | 64 | 32 | 8 | 32 | 64 | 32 | 128 | 4 | 16 | 64 |
| Norfloxacin | 2 | 2 | 1 | 2 | 2 | 8 | 2 | 4 | 4 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Avula, S.R.; Meng, J.; Zhou, C. Synthesis and Biological Evaluation of Piperazine Hybridized Coumarin Indolylcyanoenones with Antibacterial Potential. Molecules 2023, 28, 2511. https://doi.org/10.3390/molecules28062511
Zeng C, Avula SR, Meng J, Zhou C. Synthesis and Biological Evaluation of Piperazine Hybridized Coumarin Indolylcyanoenones with Antibacterial Potential. Molecules. 2023; 28(6):2511. https://doi.org/10.3390/molecules28062511
Chicago/Turabian StyleZeng, Chunmei, Srinivasa Rao Avula, Jiangping Meng, and Chenghe Zhou. 2023. "Synthesis and Biological Evaluation of Piperazine Hybridized Coumarin Indolylcyanoenones with Antibacterial Potential" Molecules 28, no. 6: 2511. https://doi.org/10.3390/molecules28062511
APA StyleZeng, C., Avula, S. R., Meng, J., & Zhou, C. (2023). Synthesis and Biological Evaluation of Piperazine Hybridized Coumarin Indolylcyanoenones with Antibacterial Potential. Molecules, 28(6), 2511. https://doi.org/10.3390/molecules28062511






