Synthesis, Structure, and Electrochemical Properties of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives
2.2. Electrochemical Properties of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives
3. Materials and Methods
3.1. General
3.2. DFT Calculations
3.3. Electrochemical Measurements
3.4. ESR Measurements
3.5. Single Crystal X-ray Diffraction
3.6. Synthesis
3.6.1. Synthesis of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene (2)
3.6.2. Synthesis of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene-1-Tungstenpentacarbonyl (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Štěpnička, S. Forever young: The first seventy years of ferrocene. Dalton Trans. 2022, 51, 8085–8102. [Google Scholar] [CrossRef] [PubMed]
- Mathey, F. Phosphametallocenes: From discovery to applications. J. Organomet. Chem. 2002, 646, 15–20. [Google Scholar] [CrossRef]
- Le Floch, P. Phosphaalkene, phospholyl and phosphinine ligands: New tools in coordination chemistry and catalysis. Coord. Chem. Rev. 2006, 250, 627–681. [Google Scholar] [CrossRef]
- Bezkishko, I.A.; Zagidullin, A.A.; Milyukov, V.A.; Sinyashin, O.G. Alkali and transition metal phospholides. Russ. Chem. Rev. 2014, 83, 555–574. [Google Scholar] [CrossRef]
- Wang, Z.C.; Qiao, L.; Sun, Z.M.; Scheer, M. Inorganic Ferrocene Analogue [Fe(P4)2]2–. J. Am. Chem. Soc. 2022, 144, 6698–6702. [Google Scholar] [CrossRef] [PubMed]
- Kostic, N.M.; Fenske, R.F. Bonding in phosphaferrocenes and reactivity of the phospholyl ligand studied by molecular orbital calculations. Organometallics 1983, 2, 1008–1013. [Google Scholar] [CrossRef]
- Fu, G.C. Applications of planar-chiral heterocycles as ligands in asymmetric catalysis. Acc. Chem. Res. 2006, 39, 853–860. [Google Scholar] [CrossRef]
- Ogasawara, M.; Watanabe, S.; Nakajima, K.; Takahashi, T. Enantioselective synthesis of planar-chiral phosphaferrocenes by molybdenum-catalyzed asymmetric interannular ring-closing metathesis. J. Am. Chem. Soc. 2010, 132, 2136–2137. [Google Scholar] [CrossRef]
- Willms, H.; Frank, W.; Ganter, C. Coordination Chemistry and Catalytic Application of Bidentate Phosphaferrocene-Pyrazole and -Imidazole Based P, N-Ligands. Organometallics 2009, 28, 3049–3058. [Google Scholar] [CrossRef]
- Tian, R.; Ng, Y.; Ganguly, R.; Mathey, F. A New Type of Phosphaferrocene–Pyrrole–Phosphaferrocene PNP Pincer Ligand. Organometallics 2012, 31, 2486–2488. [Google Scholar] [CrossRef]
- Shintani, R.; Fu, G.C. Catalytic Enantioselective Synthesis of β-Lactams: Intramolecular Kinugasa Reactions and Interception of an Intermediate in the Reaction Cascade. Angew. Chem. Int. Ed. 2003, 42, 4082–4085. [Google Scholar] [CrossRef]
- Shintani, R.; Fu, G.C. A new copper-catalyzed [3 + 2] cycloaddition: Enantioselective coupling of terminal alkynes with azomethine imines to generate five-membered nitrogen heterocycles. J. Am. Chem. Soc. 2003, 125, 10778–10779. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Qiao, S.; Tobisu, M.; Lo, M.M.C.; Fu, G.C. Enantioselective isomerization of allylic alcohols catalyzed by a rhodium/phosphaferrocene complex. J. Am. Chem. Soc. 2000, 122, 9870–9871. [Google Scholar] [CrossRef]
- Carmichael, D.; Goldet, G.; Klankermayer, J.; Ricard, L.; Seeboth, N.; Stankevič, M. Comparison of Phosphaferrocene and Phospharuthenocene Ligands in Rh+-Catalysed Enamide Hydrogenation Reactions: Superior Performance of the Phospharuthenocene. Chem. Eur. J. 2007, 13, 5492–5502. [Google Scholar] [CrossRef]
- Carmichael, D.; Escalle-Lewis, A.; Frison, G.; Le Goff, X.; Muller, E. Stepwise syntheses of tri-and tetraphosphaporphyrinogens. Chem. Commun. 2012, 48, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Escobar, A.; Mathey, F. A New P,N-Chelating Ligand Combining Phosphaferrocene and Azacymantrene Units. Organometallics 2011, 30, 1738–1740. [Google Scholar] [CrossRef]
- Tian, R.; Mathey, F. Phosphaferrocene Analogues of Calixpyrroles. Organometallics 2011, 30, 3472–3474. [Google Scholar] [CrossRef]
- Carmichael, D.; Le Goff, X.-F.; Muller, E. Oligo(metallocene)s Containing Keto-Bridged Phospholyl Rings. Eur. J. Inorg. Chem. 2014, 1610–1614. [Google Scholar] [CrossRef]
- Liebscher, M.; Bruhn, C.; Siemeling, U.; Baio, J.; Lu, H.; Weidner, T. The Interaction of 1, 1′-Diphosphaferrocenes with Gold: Molecular Coordination Chemistry and Adsorption on Solid Substrates. Eur. J. Inorg. Chem. 2017, 2, 351–359. [Google Scholar] [CrossRef]
- Komath Mallissery, S.; Gudat, D. On the Immobilization of a Monophosphaferrocene on a Silica Support. Z. Anorg. Allg. Chem. 2012, 638, 1141–1145. [Google Scholar] [CrossRef]
- Mathey, F.; Mitschler, A.; Weiss, R. Phosphaferrocene. J. Am. Chem. Soc. 1977, 99, 3537–3538. [Google Scholar] [CrossRef]
- Bitta, J.; Fassbender, S.; Reiss, G.; Ganter, C. Mechanistic insight into the formation of phosphaferrocene. Organometallics 2006, 25, 2394–2397. [Google Scholar] [CrossRef]
- Sava, X.; Marinetti, A.; Ricard, L.; Mathey, F. Optically active phospholes: Synthesis and use as chiral precursors for phosphinidene and phosphaferrocene complexes. Eur. J. Inorg. Chem. 2002, 2002, 1657–1665. [Google Scholar] [CrossRef]
- Roberts, R.M.G.; Wells, A.S. A new synthetic route to monophosphaferrocenes. Inorg. Chim. Acta 1986, 112, 171–175. [Google Scholar] [CrossRef]
- Masaoka, K.; Ohkubo, M.; Taue, H.; Wakioka, M.; Ohki, Y.; Ogasawara, M. Synthesis of Monophosphaferrocenes Revisited. Chem. Select. 2022, 7, e202104472. [Google Scholar] [CrossRef]
- Deschamps, B.; Fischer, J.; Mathey, F.; Mitschler, A. Reaction of lithium alkyls and aryls with 1,1′-diphosphaferrocenes. Synthesis and structure of a stable bis(diene)iron(-I) species. Inorg. Chem. 1981, 20, 3252–3259. [Google Scholar] [CrossRef]
- Deschamps, B.; Fischer, J.; Mathey, F.; Mitschler, A.; Ricard, L. Reaction of 1-alkyl-1, 1′-diphosphaferrocene monoanions with acyl chlorides. Synthesis and zwitterionic structure of stable 3,5-diphosphaferrocenes. Organometallics 1982, 1, 312–316. [Google Scholar] [CrossRef]
- Zagidullin, A.A.; Petrov, A.V.; Bezkishko, I.A.; Miluykov, V.A. Alkali metal polyphosphides as intermediates in the synthesis of organophosphorus compounds from elemental phosphorus. Russ. Chem. Bull. 2021, 70, 1260–1268. [Google Scholar] [CrossRef]
- Bezkishko, I.A.; Zagidullin, A.A.; Khrizanforov, M.N.; Gerasimova, T.P.; Ivshin, K.A.; Kataeva, O.N.; Ganushevich, Y.S.; Miluykov, V.A.; Lönnecke, P.; Hey-Hawkins, E. Synthesis, structure and electrochemical properties of 3,4,5-triaryl-1,2-diphosphaferrocenes. Inorg. Chem. Front. 2022, 9, 2608–2616. [Google Scholar] [CrossRef]
- Zagidullin, A.A.; Akhmatkhanova, F.F.; Khrizanforov, M.N.; Fayzullin, R.R.; Gerasimova, T.P.; Bezkishko, I.A.; Miluykov, V.A. Synthesis of 3,4,5-tris(chlorophenyl)-1,2-diphosphaferrocenes and their electrochemical properties. Beilstein. J. Org. Chem. 2022, 18, 1338–1345. [Google Scholar] [CrossRef]
- Petrov, A.V.; Zagidullin, A.A.; Bezkishko, I.A.; Khrizanforov, M.N.; Kholin, K.V.; Gerasimova, T.P.; Miluykov, V.A.; Ivshin, K.A.; Shekurov, R.P.; Katsyuba, S.B.; et al. Synthesis, structure, and electrochemical properties of 4, 5-diaryl-1, 2, 3-triphosphaferrocenes and the first example of multi(phosphaferrocene). Dalton Trans. 2020, 49, 17252–17262. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.V.; Conrad, L.; Coles, N.T.; Weber, M.; Andrae, D.; Zagidullin, A.A.; Miluykov, V.A.; Müller, C. Reactivity of Sodium Pentaphospholide Na[cyclo-P5] towards C≡E (E=C, N, P) Triple Bonds. Chem. Eur. J. 2022, 28, e2022030. [Google Scholar] [CrossRef]
- Oshchepkova, E.; Zagidullin, A.; Burganov, T.; Katsyuba, S.; Miluykov, V.; Lodochnikova, O. Novel enantiopure monophospholes: Synthesis, spatial and electronic structure, photophysical characteristics and conjugation effects. Dalton Trans. 2018, 47, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Zagidullin, A.; Grigoreva, E.; Burganov, T.; Katsyuba, S.; Li, Y.; Leung, P.H.; Miluykov, V. A rational synthetic approach to 2,3,4,5-tetraphenyl-1-monophosphole and its derivatives. Inorg. Chem. Commun. 2021, 134, 108949. [Google Scholar] [CrossRef]
- Sava, X.; Ricard, L.; Mathey, F.; Le Floch, P. Octaethyldiphosphaferrocene: An Efficient Ligand in the Palladium-Catalyzed Suzuki Cross-Coupling Reaction. Organometallics 2000, 19, 4899–4903. [Google Scholar] [CrossRef]
- Ogasawara, M.; Arae, S.; Watanabe, S.; Subbarayan, V.; Sato, H.; Takahashi, T. Synthesis and Characterization of Benzo[b] phosphaferrocene Derivatives. Organometallics 2013, 32, 4997–5000. [Google Scholar] [CrossRef]
- De Lauzon, G.; Deschamps, B.; Fischer, J.; Mathey, F.; Mitschler, A. 1,1′-Diphosphaferrocenes. Synthesis, basic chemistry, and structure. J. Am. Chem. Soc. 1980, 102, 994–1000. [Google Scholar] [CrossRef]
- Zhang, L.; Hissler, M.; Bu, H.B.; Bäuerle, P.; Lescop, C.; Réau, R. A Study of Mono-and 1,1‘-Diphosphaferrocenes as Building Blocks for π-Conjugated Systems. Organometallics 2005, 24, 5369–5376. [Google Scholar] [CrossRef]
- Enrique, R.; Leiva, A.M.; Casasempere, A.M.; Charrier, C.; Mathey, F.; Garland, M.T.; Le Marouille, J. Nouvell préparation, propriétés electrochimiques et etude structurale des phosphaferrocenes η5-C5Me5Fe-η5-PC4(R4). J. Organomet. Chem. 1986, 309, 323–332. [Google Scholar] [CrossRef]
- Rigo, M.W.; Sklorz, J.A.; Hatje, N.; Noack, F.; Weber, M.; Wiecko, J.; Müller, C. 2,4,6-triphenylphosphinine and 2,4,6-triphenylposphabarrelene revisited: Synthesis, reactivity and coordination chemistry. Dalton Trans. 2016, 45, 2218–2226. [Google Scholar] [CrossRef]
- Lemoine, P.; Gross, M.; Braunstein, P.; Mathey, F.; Deschamps, B.; Nelson, J.H. Electrochemistry of phosphaferrocenes. 1. Comparison of the redox properties of ferrocene, diphosphaferrocene, 3,4-dimethyl-1-phosphaferrocene and 3,3′,4,4′-tetramethyl-1,1′-diphosphaferrocene. Organometallics 1984, 3, 1303–1307. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Strekalova, S.; Kholin, K.; Khrizanforova, V.; Grinenko, V.; Gryaznova, T.; Budnikova, Y. One-stage synthesis of FcP(O)(OC2H5)2 from ferrocene and α-hydroxyethylphosphonate. RSC Adv. 2016, 6, 42701–42707. [Google Scholar] [CrossRef]
- Burney, C.; Carmichael, D.; Forissier, K.; Green, J.C.; Mathey, F.; Ricard, L. Synthesis and Properties of [NiCp*(2,5-tBu2PC4H2)], a 20-Valence-Electron Phosphanickelocene. Chem. Eur. J. 2005, 11, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Prins, R.; Reinders, F. Electron spin resonance of the cation of ferrocene. J. Am. Chem. Soc. 1969, 91, 4929–4931. [Google Scholar] [CrossRef]
- Simonneaux, G.; Schünemann, V.; Morice, C.; Carel, L.; Toupet, L.; Winkler, H.; Trautwein, A.X.; Walker, F.A. Structural, Magnetic, and Dynamic Characterization of the (d xz, dyz)4 (dxy) 1 Ground-State Low-Spin Iron (III) Tetraphenylporphyrinate Complex [(p-TTP)Fe(2,6-XylylNC)2]CF3SO3. J. Am. Chem. Soc. 2000, 122, 4366–4377. [Google Scholar] [CrossRef]
- Kannappan, R.; Tanase, S.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Low-spin iron (III) Schiff-base complexes with symmetric hexadentate ligands: Synthesis, crystal structure, spectroscopic and magnetic properties. Polyhedron 2006, 25, 1646–1654. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Clentsmith, G.K.; Gibson, V.C.; Goodgame, D.M.; McTavish, S.J.; Pankhurst, Q.A. The nature of the active site in bis(imino) pyridine iron ethylene polymerisation catalysts. Catal. Commun. 2002, 3, 207–211. [Google Scholar] [CrossRef]
- Casella, L.; Gullotti, M.; Pintar, A.; Messori, L.; Rockenbauer, A.; Gyor, M. Iron(III) Tyrosinate Models. Synthesis and Spectroscopic and Stereochemical Studies of Iron(III) Complexes of N-Salicylidene-L-amino Acids. Inorg. Chem. 1987, 26, 1031–1038. [Google Scholar] [CrossRef]
- Lemoine, P.; Gross, M.; Braunstein, P.; Mathey, F.; Deschamps, B.; Nelson, J.H. Electrochemistry of phosphaferrocenes: II. Electrochemical behavior of 3,3′,4,4′-tetramethyl-1,1′-diphosphaferrocene bonded to M(CO)5 (M = Cr, Mo, W) fragments forming heterometallic complexes with multiple redox centers. J. Organomet. Chem. 1985, 295, 189–197. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/g09citation/ (accessed on 30 January 2023).
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Abel, E.W.; Wilkinson, G. Carbonyl halides of manganese and some related compounds. J. Chem. Soc. 1959, 1501–1505. [Google Scholar] [CrossRef]







| Compound | Fe–Cnt(PC4) | Fe–Cnt(C5) | Turning Angle b | ∠(PC4)(C5) c | Fe–P | Reference |
|---|---|---|---|---|---|---|
 | 1.6336(2) | 1.6600(2) | 12.38(6) | 3.14(4) | 2.2761(3) | this work |
 | 1.6393(10) | 1.6609(14) | –15.34(12) | 2.60(9) | 2.2805(11) | [38] |
 | 1.6433(9)/1.6467(9) | 1.6584(11)/1.6614(11) | 8.30(15)/–5.35(15) | 3.42(8)/3.17(8) | 2.2858(6)/2.2895(6) | [25] d |
 | 1.6440(16) | 1.660(2) | –1.4(3) | 3.83(16) | 2.2864(12) | [25] |
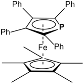 | 1.666(2) | 1.690(3) | 16.2(3) | 3.1(2) | 2.2821(16) | [39] |
| Compound | Eox1(1/2Eox1), V vs. FcH/FcH+; {Ia/Ic} | 1/2Ered1, V vs. FcH/FcH+ | 1EHOMO, eV | 1ELUMO, eV | Gap, eV |
|---|---|---|---|---|---|
| Ferrocene [42] | 0.03 (0 *); {1} | −3.18 * | −4.79 * | −1.61 * | 3.18 * |
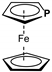 | 0.15 (0.17 **); {1} | −3.04 ** | −4.97 | −1.76 | 3.21 |
 | 0.55 (0.44—semidiffE); {0.6} | −2.25 | −5.24 | −2.55 | 2.69 |
 | 0.06 (0.1 ***); {››1} | n.a. | n.a. | n.a. | n.a. |
 | n.a. (0.07 **); {≈1} | −3.06 ** | −4.87 ** | −1.74 ** | 3.13 |
 | 0.01 (−0.03 ***); {≈1} | n.a. | n.a. | n.a. | n.a. |
 | 0.40 (0.35); {irrev} | −1.34 1/2Ered2 = −2.06V | −5.15 | −3.46 | 1.69 |
| Compound 2 | Compound 3 | |||||
|---|---|---|---|---|---|---|
| Neutral | Cation (+1) | Neutral | Cation (+1) | |||
| S = 1/2 | S = 5/2 | S = 1/2 | S = 5/2 | |||
 |  | 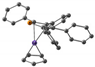 |  |  | 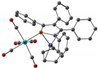 | |
| Fe-P, Å | 2.30 | 2.30 | 2.38 | 2.28 | 2.33 | 2.55 |
| Fe-C(PC4), Å | 2.06–2.08 | 2.10 | 2.30–2.68 | 2.06–2.08 | 2.08–2.11 | 2.22–2.63 |
| Fe-C(C5), Å | 2.05 | 2.08 | 2.27–2.40 | 2.07–2.09 | 2.07–2.09 | 2.22–2.32 |
| ΔE, kcal mol−1 | 0 | 12 | 0 | 7.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagidullin, A.A.; Lakomkina, A.R.; Khrizanforov, M.N.; Fayzullin, R.R.; Kholin, K.V.; Gerasimova, T.P.; Shekurov, R.P.; Bezkishko, I.A.; Miluykov, V.A. Synthesis, Structure, and Electrochemical Properties of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives. Molecules 2023, 28, 2481. https://doi.org/10.3390/molecules28062481
Zagidullin AA, Lakomkina AR, Khrizanforov MN, Fayzullin RR, Kholin KV, Gerasimova TP, Shekurov RP, Bezkishko IA, Miluykov VA. Synthesis, Structure, and Electrochemical Properties of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives. Molecules. 2023; 28(6):2481. https://doi.org/10.3390/molecules28062481
Chicago/Turabian StyleZagidullin, Almaz A., Alena R. Lakomkina, Mikhail N. Khrizanforov, Robert R. Fayzullin, Kirill V. Kholin, Tatiana P. Gerasimova, Ruslan P. Shekurov, Ilya A. Bezkishko, and Vasili A. Miluykov. 2023. "Synthesis, Structure, and Electrochemical Properties of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives" Molecules 28, no. 6: 2481. https://doi.org/10.3390/molecules28062481
APA StyleZagidullin, A. A., Lakomkina, A. R., Khrizanforov, M. N., Fayzullin, R. R., Kholin, K. V., Gerasimova, T. P., Shekurov, R. P., Bezkishko, I. A., & Miluykov, V. A. (2023). Synthesis, Structure, and Electrochemical Properties of 2,3,4,5-Tetraphenyl-1-Monophosphaferrocene Derivatives. Molecules, 28(6), 2481. https://doi.org/10.3390/molecules28062481









