The Effect of Cooling Rates on Thermal, Crystallization, Mechanical and Barrier Properties of Rotational Molding Polyamide 11 as the Liner Material for High-Capacity High-Pressure Vessels
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Analysis
2.2. Crystal Structure Analysis
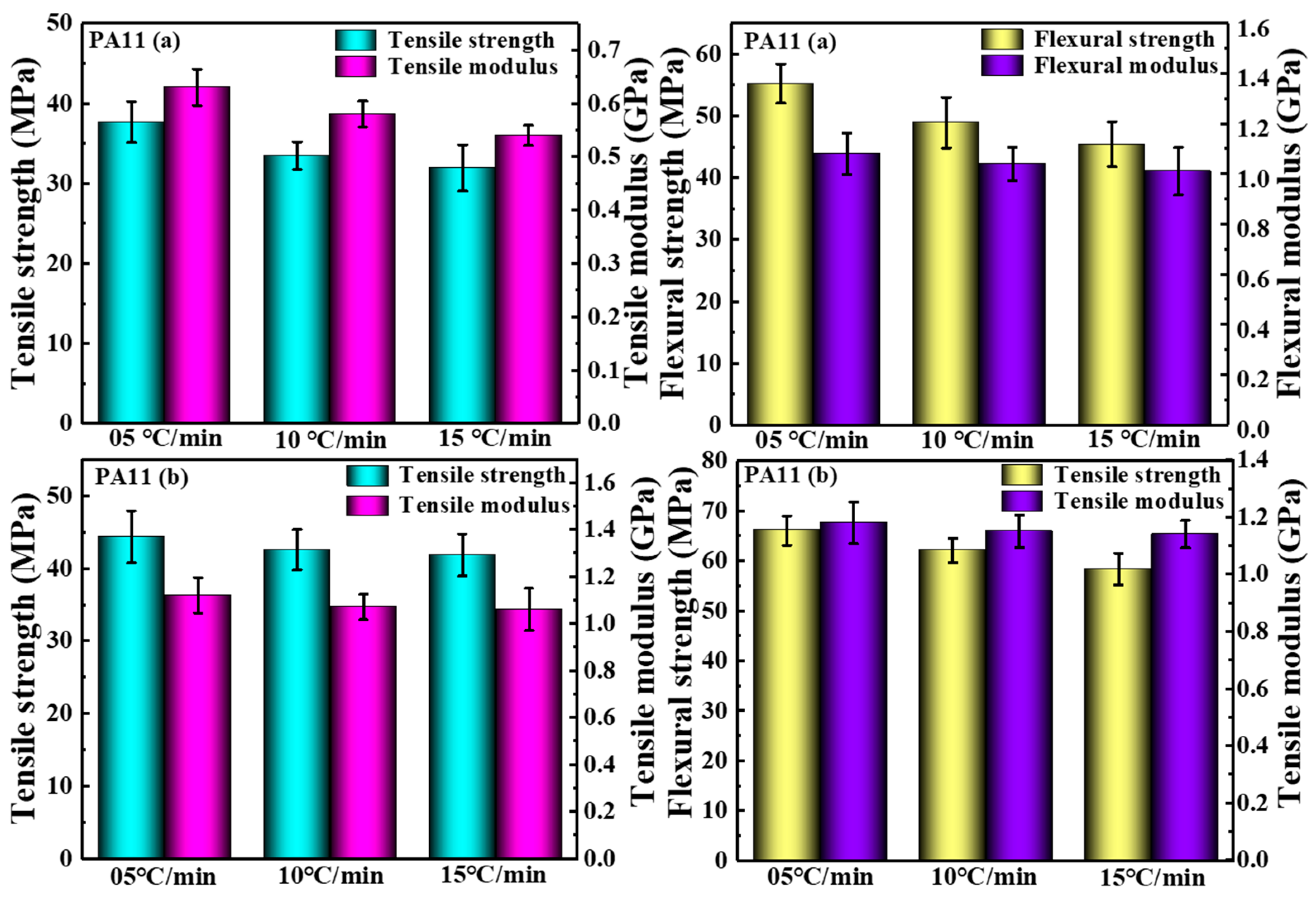
2.3. Mechanical Properties
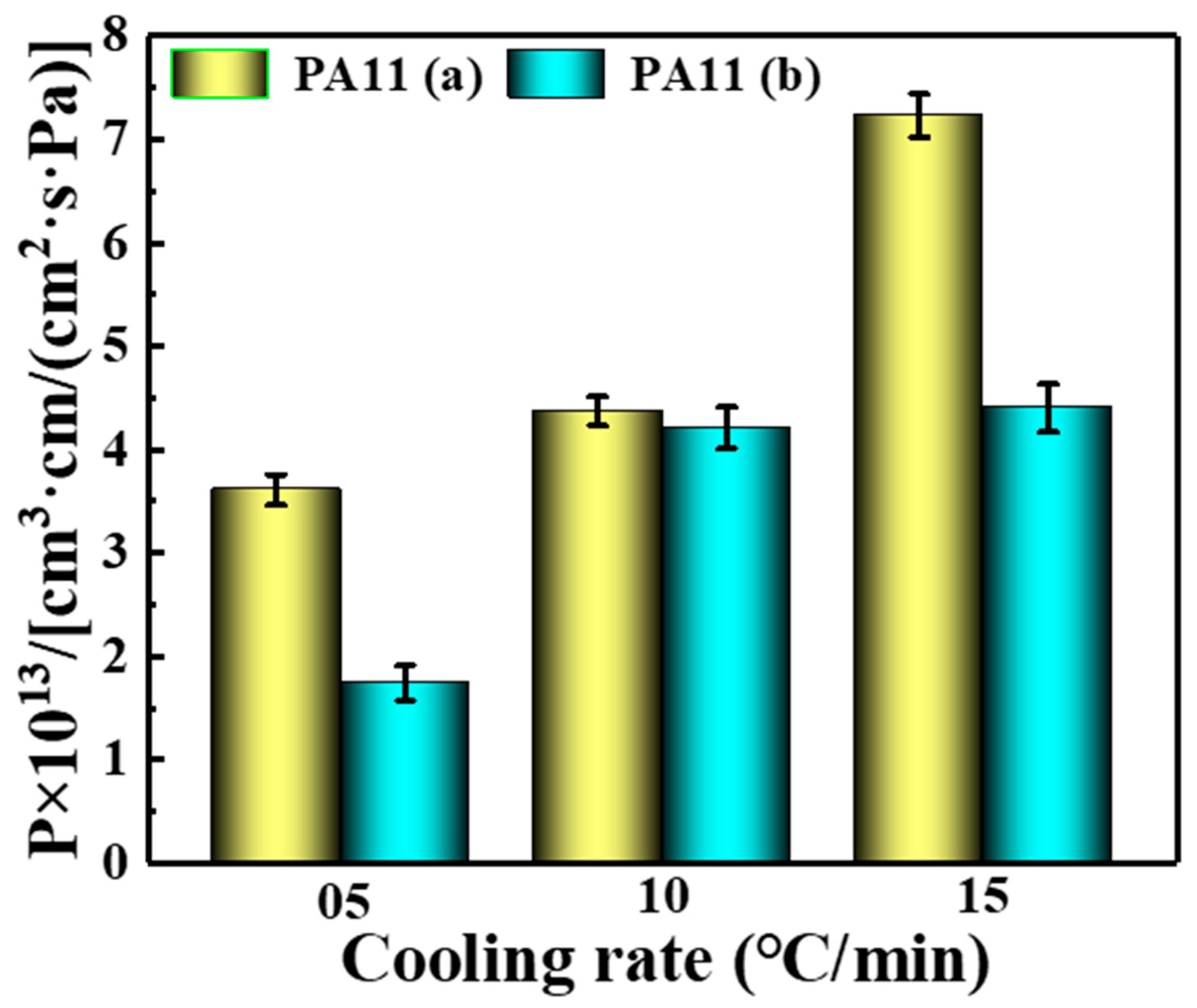
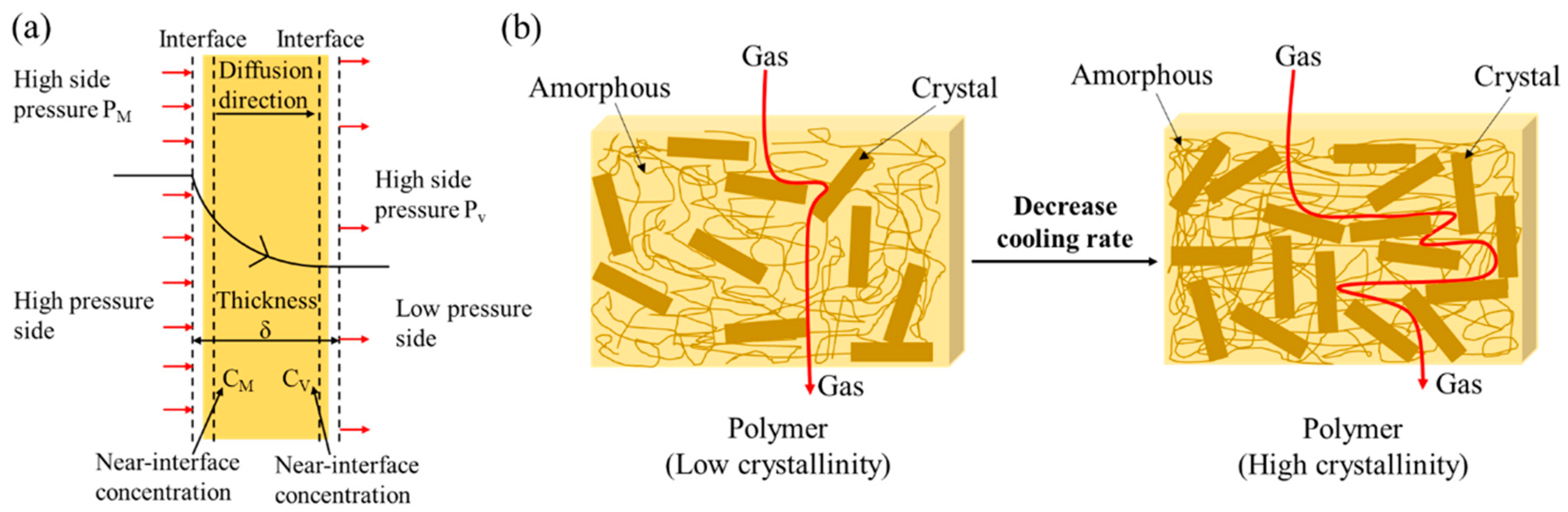
2.4. Barrier Properties
3. Experimental
3.1. Materials
3.2. Control of PA11 Liner Processing
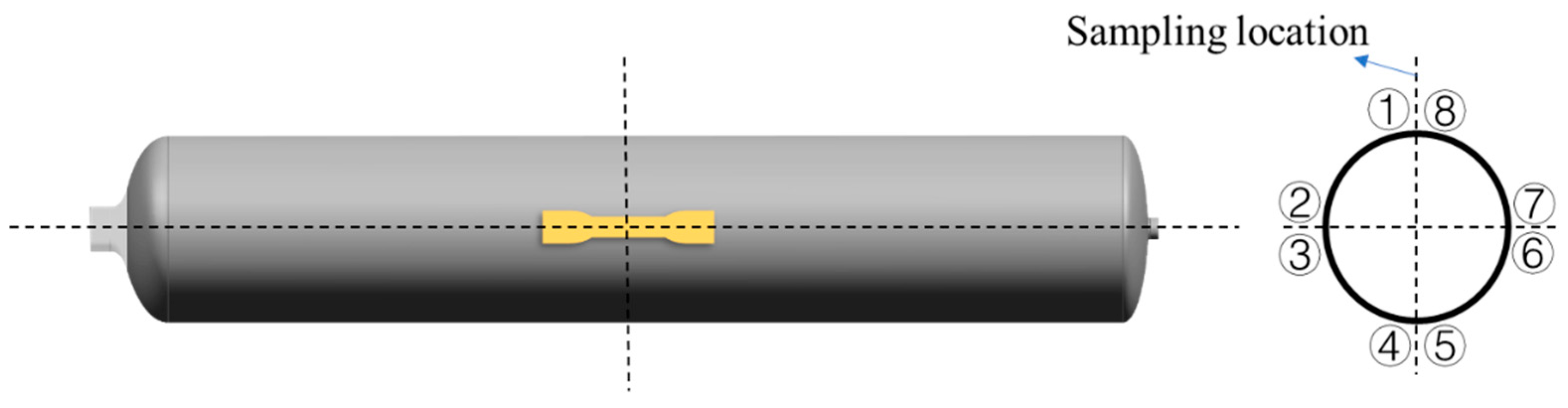
3.3. Characterization and Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Huang, K.; Liu, Z. Synthesis of High Molecular Weight Nylon 46 in Supercritical Carbon Dioxide. Macromolecules 2011, 44, 820–825. [Google Scholar] [CrossRef]
- Yuan, S.; Li, S.; Zhu, J.; Tang, Y. Additive manufacturing of polymeric composites from material processing to structural design. Compos. Part B Eng. 2021, 219, 108903. [Google Scholar] [CrossRef]
- Maïza, S.; Lefebvre, X.; Brusselle-Dupend, N.; Klopffer, M.-H.; Cangémi, L.; Castagnet, S.; Grandidier, J.-C. Physicochemical and mechanical degradation of polyamide 11 induced by hydrolysis and thermal aging. J. Appl. Polym. Sci. 2019, 136, 47628. [Google Scholar]
- Duan, Z. Research Progress of Polymers/Inorganic Nanocomposite Electrical Insulating Materials. Molecules 2022, 27, 7867. [Google Scholar]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Kang, H.; Zhou, W.; Zhang, C. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Li, M.; Zhang, Y.; Cai, P. Preparation and properties of a magnetorheological material used for pipeline and pressure vessel damage repair. Compos. Struct. 2021, 276, 114566. [Google Scholar] [CrossRef]
- Wang, X.; Tian, M.; Chen, X.; Xie, P.; Yang, J.; Chen, J.; Yang, W. Advances on materials design and manufacture technology of plastic liner of type Ⅳ hydrogen storage vessel. Int. J. Hydrogen Energy 2022, 47, 8382–8408. [Google Scholar] [CrossRef]
- Magneville, B.; Gentilleau, B.; Villalonga, S.; Nony, F.; Galiano, H. Modeling, parameters identification and experimental validation of composite materials behavior law used in 700 bar type IV hydrogen high pressure storage vessel. Int. J. Hydrogen Energy 2015, 40, 13193–13205. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, M.; Zu, L.; Jia, X.; Shen, A.; Yang, Q.; Xu, K. Investigation on failure behaviors of 70 MPa Type IV carbon fiber overwound hydrogen storage vessels. Compos. Struct. 2021, 259, 113387. [Google Scholar] [CrossRef]
- Wang, M. Optimization of the Laminate Structure of a Composite Cylinder Based on the Combination of Response Surface Methodology (RSM) and Finite Element Analysis (FEA). Molecules 2022, 27, 7361. [Google Scholar]
- Neto, E.B.; Chludzinski, M.; Roese, P.B.; Fonseca, J.S.O.; Amico, S.C.; Ferreira, C.A. Experimental and numerical analysis of a LLDPE/HDPE liner for a composite pressure vessel. Polym. Test. 2011, 30, 693–700. [Google Scholar] [CrossRef]
- Gentilleau, B.; Touchard, F.; Grandidier, J.C. Numerical study of influence of temperature and matrix cracking on type IV hydrogen high pressure storage vessel behavior. Compos. Struct. 2014, 111, 98–110. [Google Scholar] [CrossRef]
- Liu, G.; Yang, F.; Liu, W.; Bai, Y.; Han, C.; Jiao, W.; Wang, R. Ultra-high gas barrier composites with aligned graphene flakes and polyethylene molecules for high-pressure gas storage tanks. J. Energy Storage 2021, 40, 102692. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C. Research on hydrogen permeability of polyamide 6 as the liner material for type Ⅳ hydrogen storage tank. Int. J. Hydrogen Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Quang Dao, D.; Luche, J.; Rogaume, T.; Richard, F.; Bustamante-Valencia, L.; Ruban, S. Polyamide 6 and Polyurethane Used as Liner for Hydrogen Composite Cylinder: An Estimation of Fire Behaviours. Fire Technol. 2016, 52, 397–420. [Google Scholar] [CrossRef]
- Ramirez, J.P.B.; Halm, D.; Grandidier, J.C.; Villalonga, S.; Nony, F. 700 bar type IV high pressure hydrogen storage vessel burst—Simulation and experimental validation. Int. J. Hydrogen Energy 2015, 40, 13183–13192. [Google Scholar] [CrossRef]
- Sathees Kumar, S.; Kanagaraj, G. Investigation on Mechanical and Tribological Behaviors of PA6 and Graphite-Reinforced PA6 Polymer Composites. Arab. J. Sci. Eng. 2016, 41, 4347–4357. [Google Scholar] [CrossRef]
- Klopffer, M.H.; Berne, P.; Espuche, E. Development of Innovating Materials for Distributing Mixtures of Hydrogen and Natural Gas. Study of the Barrier Properties and Durability of Polymer Pipes. Oil Gas Sci. Technol. 2015, 70, 305–315. [Google Scholar] [CrossRef]
- Simmons, K.L.; Kuang, W.; Burton, S.D.; Arey, B.W.; Shin, Y.; Menon, N.C.; Smith, D.B. H-Mat hydrogen compatibility of polymers and elastomers. Int. J. Hydrogen Energy 2020, 46, 12300–12310. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, Z.; Liu, S.; Zhang, H. Influence of annealing on structure of Nylon 11. Macromolecules 2000, 33, 5999–6005. [Google Scholar] [CrossRef]
- Motaharinejad, V.; Delnaud, L.; Fouque, M.; Lucas, A.; Shirinbayan, M.; Fitoussi, J.; Tcharkhtchi, L.A. Enhancement of adhesion between the polymeric liner and the metallic connector of high-pressure hydrogen storage tank. Int. J. Mater. Form. 2021, 14, 249–260. [Google Scholar] [CrossRef]
- Quiroga Cortés, L.; Caussé, N.; Dantras, E.; Lonjon, A.; Lacabanne, C. Morphology and dynamical mechanical properties of poly ether ketone ketone (PEKK) with meta phenyl links. J. Appl. Polym. Sci. 2016, 133, 43396. [Google Scholar] [CrossRef]
- Ebnesajjad, S. 12—Rotational Molding and Linings. In Fluoroplastics, 2nd ed.; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 348–369. [Google Scholar]
- Yang, Z.; Huang, S.; Liu, T. Crystallization behavior of polyamide 11/multiwalled carbon nanotube composites. J. Appl. Polym. Sci. 2011, 122, 551–560. [Google Scholar] [CrossRef]
- Yu, F.; Xiao, L. Non-isothermal crystallization kinetics of poly(ether sulfone) functionalized graphene reinforced poly(ether ether ketone) composites. Polym. Test. 2021, 97, 107150. [Google Scholar] [CrossRef]
- Hafsaoui, S.L.; Mahmoud, R.; Farzaneh, S.; Tcharkhtchi, A. Study of polyamide 12 crystallization behavior within rotational molding process. Iran. Polym. J. 2013, 22, 187–197. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, S.H.; Hirai, T. Crystallization kinetics and nucleation activity of silica nanoparticle-filled poly(ethylene 2,6-naphthalate). Polymer 2003, 44, 5625–5634. [Google Scholar] [CrossRef]
- Regis, M.; Zanetti, M.; Pressacco, M.; Bracco, P. Opposite role of different carbon fiber reinforcements on the non-isothermal crystallization behavior of poly(etheretherketone). Mater. Chem. Phys. 2016, 179, 223–231. [Google Scholar] [CrossRef]
- Jana, R.N.; Cho, J.W. Non-isothermal crystallization of poly(ε-caprolactone)-grafted multi-walled carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1524–1530. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, S.I.; Kim, D.K.; Kim, S.H. Mechanical reinforcement and crystallization behavior of poly(ethylene 2,6-naphthalate) nanocomposites induced by modified carbon nanotube. Compos. Part A Appl. Sci. Manuf. 2009, 40, 45–53. [Google Scholar] [CrossRef]
- Kuo, M.C.; Kuo, J.S.; Yang, M.H.; Huang, J.C. On the crystallization behavior of the nano-silica filled PEEK composites. Mater. Chem. Phys. 2010, 123, 471–480. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Silvestre, C. Non-isothermal crystallization of polymers. Prog. Polym. Sci. 1999, 24, 917–950. [Google Scholar] [CrossRef]
- Patel, G.N. 8. Chemical Methods in Polymer Physics. Methods Exp. Phys. 1980, 16, 237–286. [Google Scholar]
- Guo, X.; Xu, X.; Bai, Z.; Chen, X.; Qin, J. Non-isothermal crystallization kinetics of polypropylene/layered double hydroxide composites. Polym. Adv. Technol. 2022, 33, 1257–1268. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly (aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Samantaray, S.K.; Satapathy, B.K. On the crystal growth kinetics of ultra-toughened biobased polyamide 410: New insights on dynamic crystallization. J. Appl. Polym. Sci. 2022, 139, 51494. [Google Scholar] [CrossRef]
- Song, J.; Ren, M.; Chen, Q.; Sun, X.; Zhang, H.; Song, C.; Zhang, H.; Mo, Z. Isothermal and nonisothermal crystallization kinetics of irradiated nylon 1212. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2326–2333. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Nair, S.S.; Ramesh, C.; Tashiro, K. Crystalline Phases in Nylon-11: Studies Using HTWAXS and HTFTIR. Macromolecules 2006, 39, 2841–2848. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Mallardo, S.; Cerruti, P.; Di Lorenzo, M.L.; Boldt, R.; Rhoades, A.M.; Androsch, R. Shear-induced crystallization of polyamide 11. Rheol. Acta 2021, 60, 231–240. [Google Scholar] [CrossRef]
- Slichter, W. Crystal structures in polyamides made from ω-amino acids. J. Polym. Sci. 1959, 36, 259–266. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Ikawa, T.; Fujiwara, Y.; Tabuchi, M.; Monobe, K. Polymorphism in lamellar single crystals of nylon 11. J. Macromol. Sci. Part B Phys. 1981, 20, 1–20. [Google Scholar] [CrossRef]
- Salame, M. Prediction of gas barrier properties of high polymers. Polym. Eng. Sci. 1986, 26, 1543–1546. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Ohyama, K.; Kasai, M.; Kaneko, F.; Nishimura, S. Hydrogen permeation under high pressure conditions and the destruction of exposed polyethylene-property of polymeric materials for high-pressure hydrogen devices (2). Int. J. Hydrogen Energy 2021, 46, 11832–11848. [Google Scholar] [CrossRef]
- Macher, J.; Hausberger, A.; Macher, A.E.; Morak, M.; Schrittesser, B. Critical review of models for H2-permeation through polymers with focus on the differential pressure method. Int. J. Hydrogen Energy 2021, 46, 22574–22590. [Google Scholar] [CrossRef]
- Castagnet, S.; Ono, H.; Benoit, G.; Fujiwara, H.; Nishimura, S. Swelling measurement during sorption and decompression in a NBR exposed to high-pressure hydrogen. Int. J. Hydrogen Energy 2017, 42, 19359–19366. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Choi, K.S.; Kuang, W.; Menon, N.; Mills, B.; Soulami, A.; Simmons, K. Damage evolution in polymer due to exposure to high-pressure hydrogen gas. Int. J. Hydrogen Energy 2021, 46, 19001–19022. [Google Scholar] [CrossRef]
- Lee, J.K.; Yao, S.X.; Li, G.; Jun, M.B.; Lee, P.C. Measurement methods for solubility and diffusivity of gases and supercritical fluids in polymers and its applications. Polym. Rev. 2017, 57, 695–747. [Google Scholar] [CrossRef]
- Kanesugi, H.; Ohyama, K.; Fujiwara, H.; Nishimura, S. High-pressure hydrogen permeability model for crystalline polymers. Int. J. Hydrogen Energy 2023, 48, 723–739. [Google Scholar] [CrossRef]
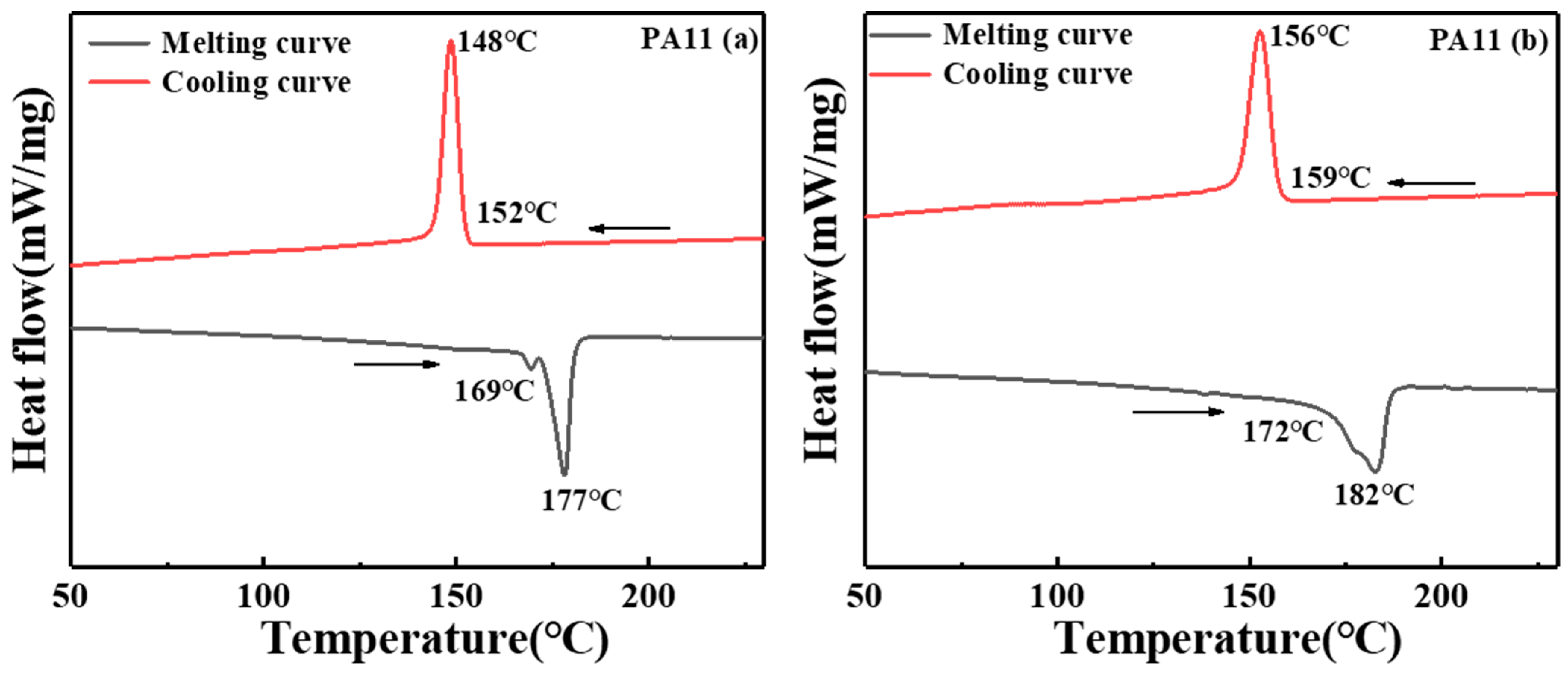
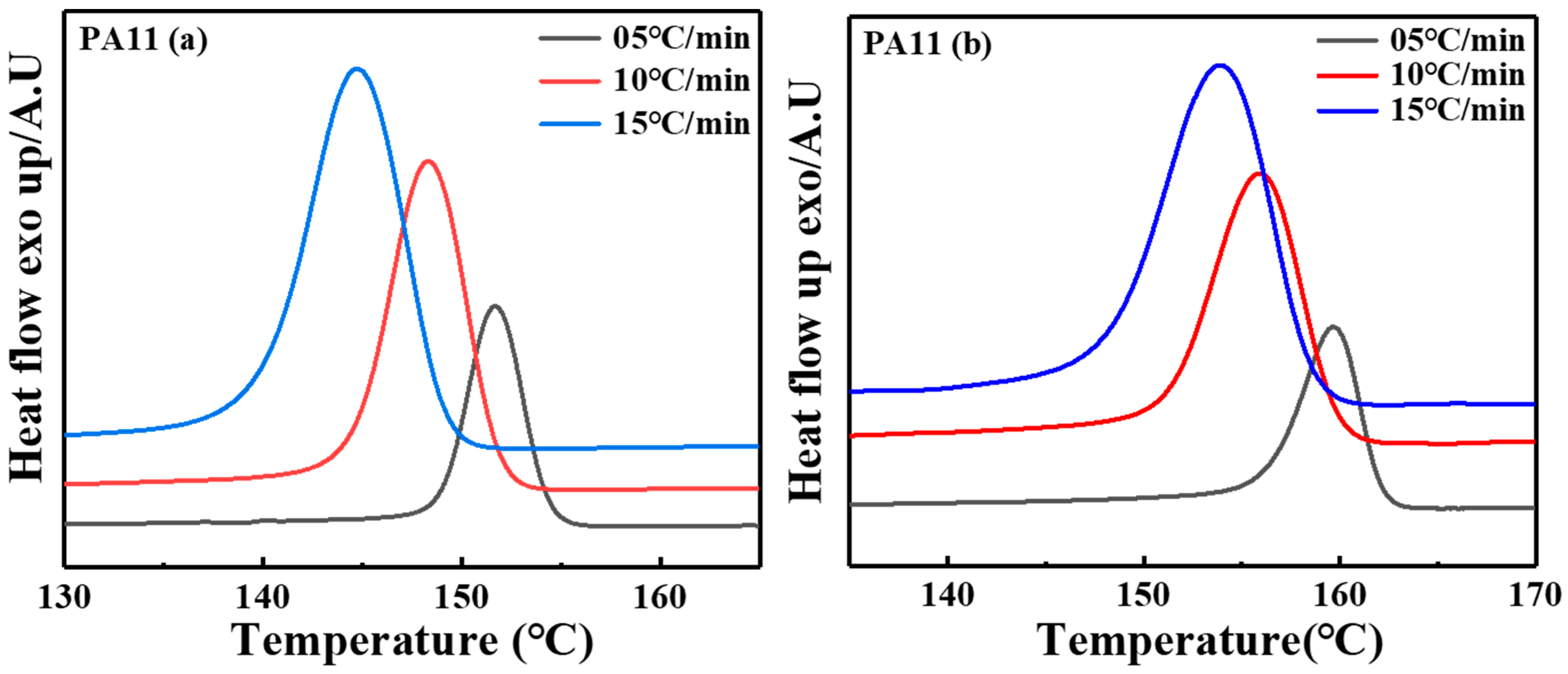
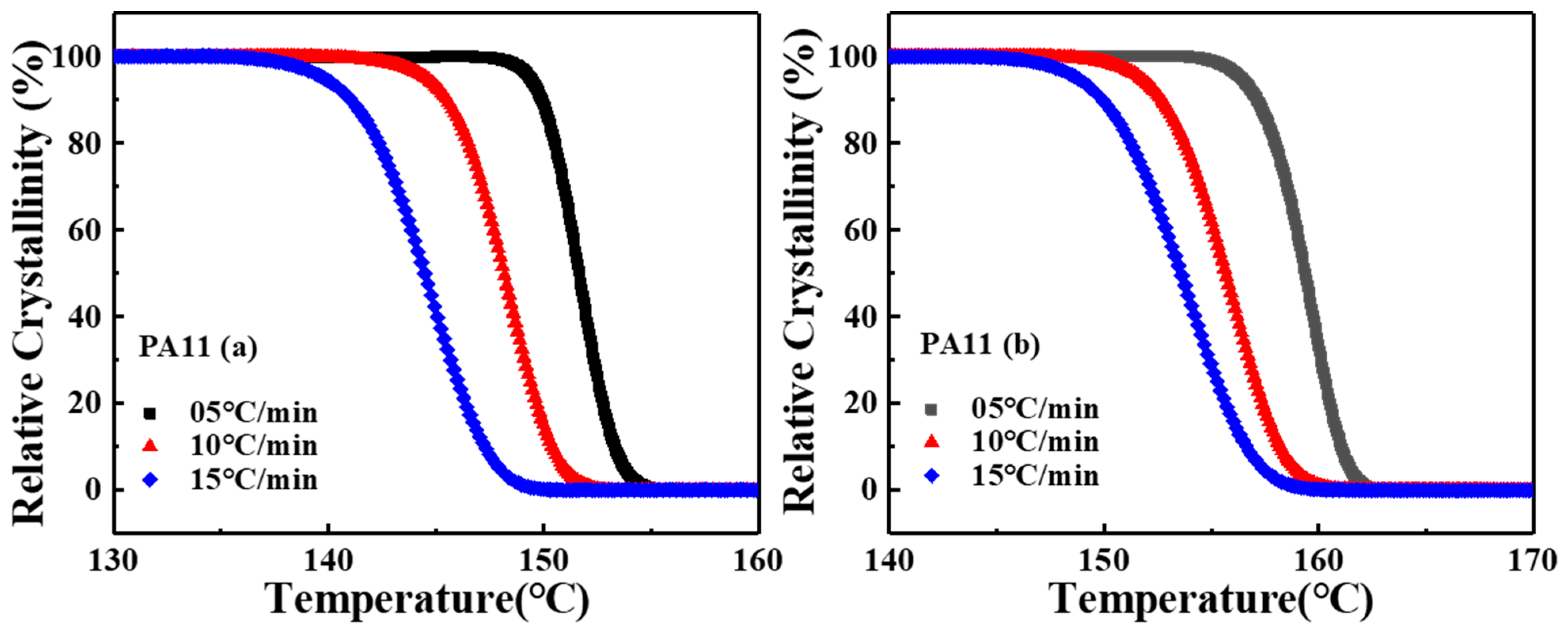
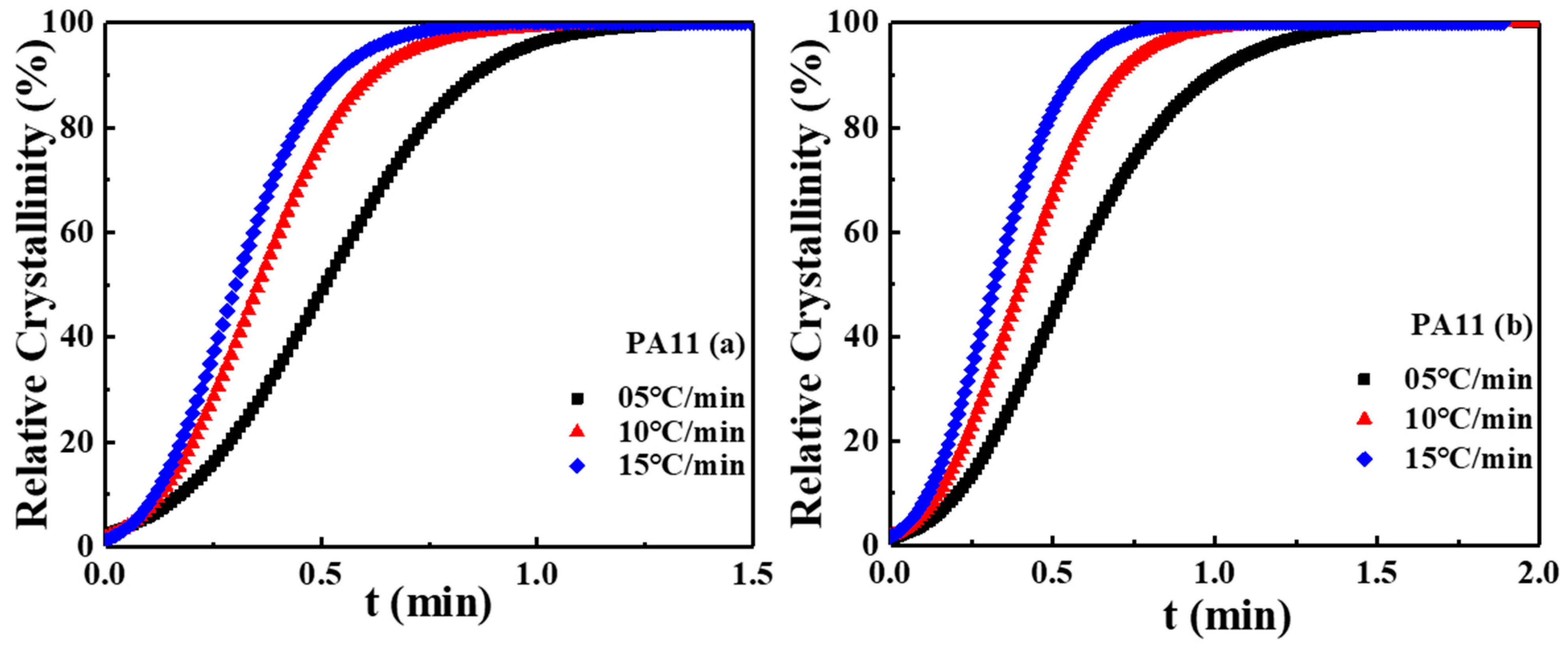
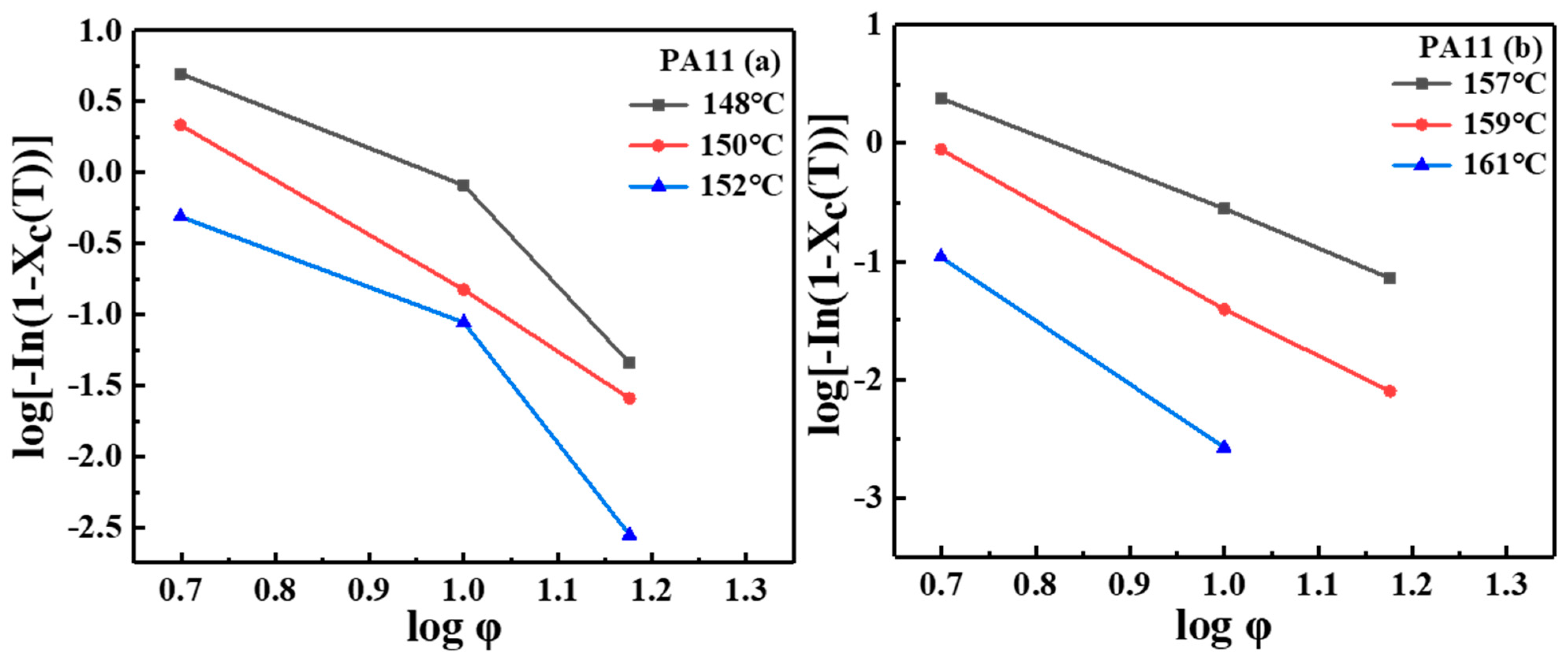

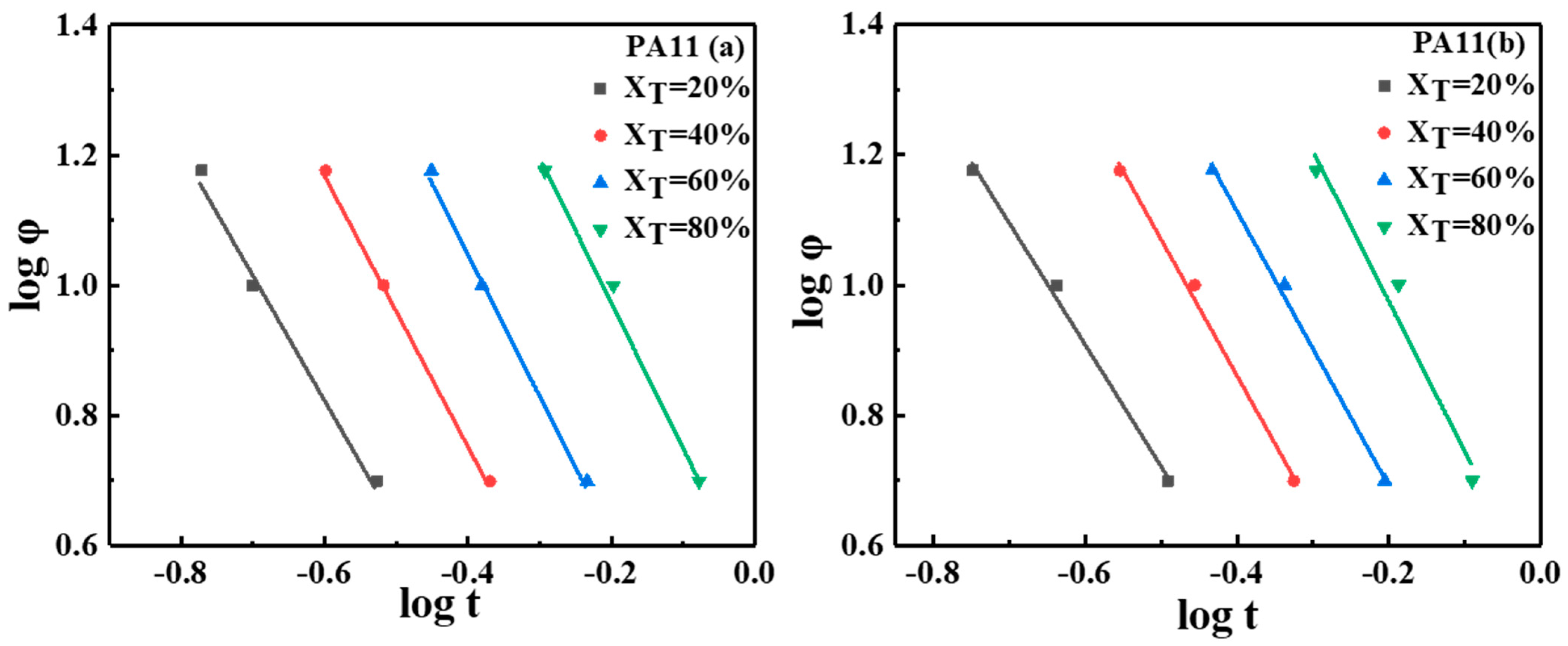
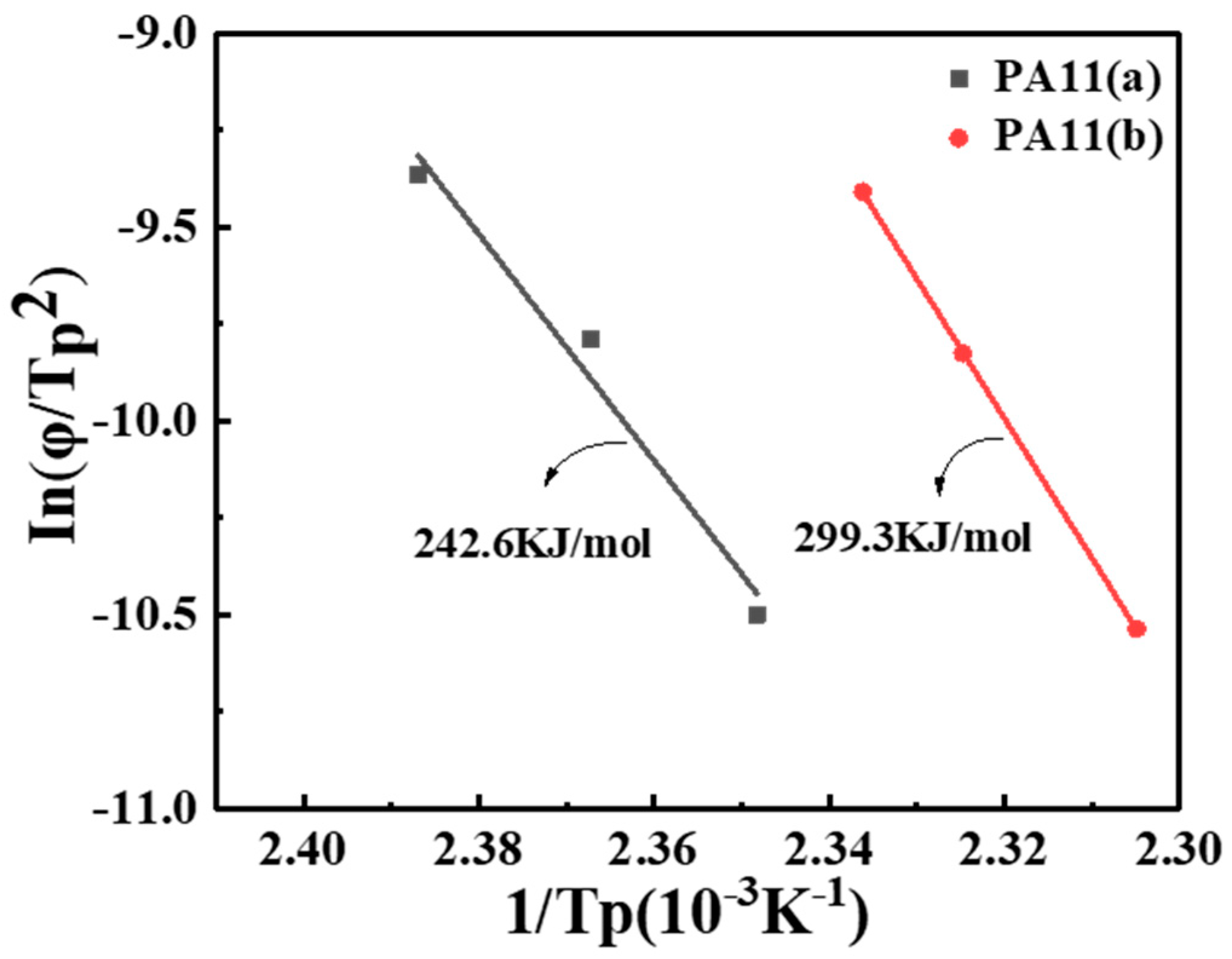
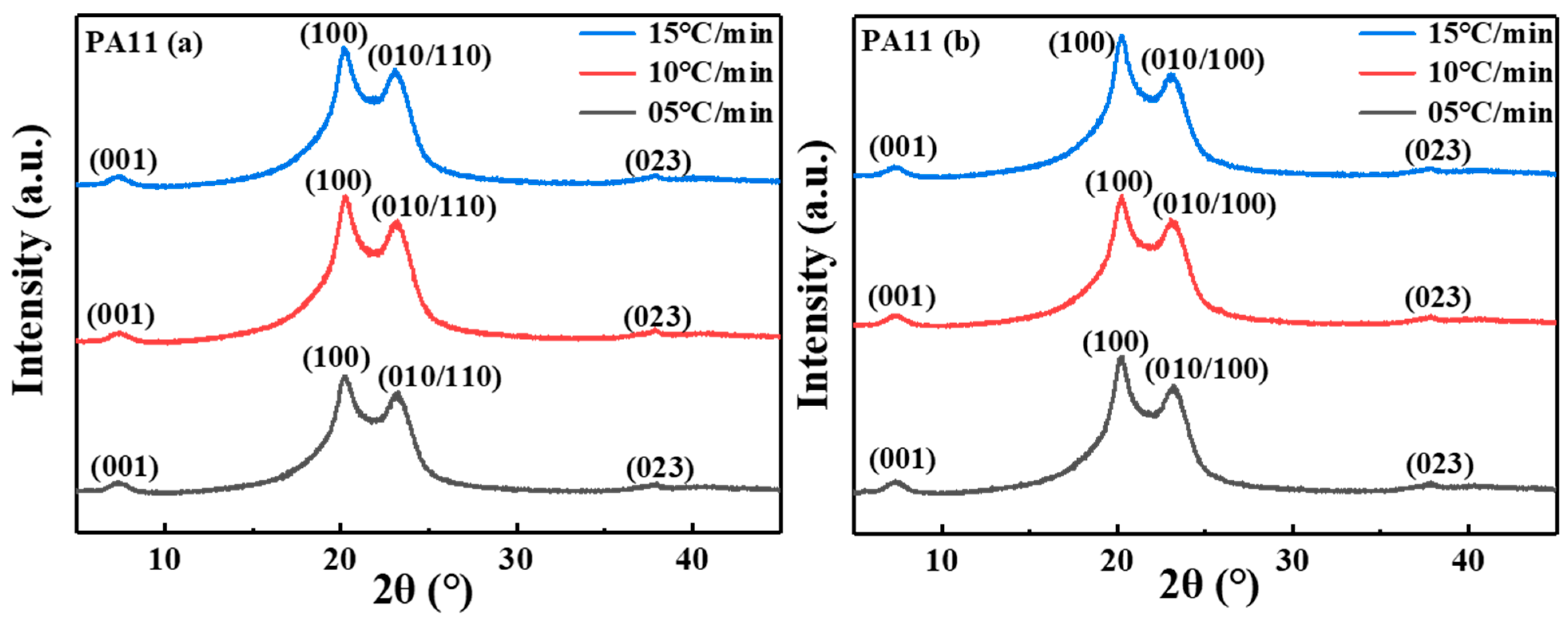



| Sample | |||||
|---|---|---|---|---|---|
| PA11 (a) | 05 | 154.2 | 151.7 | 149.1 | 1.02 |
| 10 | 151.7 | 148.3 | 144.8 | 0.69 | |
| 15 | 149.0 | 144.8 | 140.3 | 0.58 | |
| PA11 (b) | 05 | 162.1 | 159.7 | 156.4 | 1.14 |
| 10 | 159.7 | 156.0 | 151.7 | 0.8 | |
| 15 | 158.4 | 153.9 | 148.5 | 0.66 |
| PA11 (a) | PA11 (b) | |||||||
|---|---|---|---|---|---|---|---|---|
| Primary Crystallization | Secondary Crystallization | Primary Crystallization | Secondary Crystallization | |||||
| n1 | Zt1 | n2 | Zt2 | n1 | Zt1 | n2 | Zt2 | |
| 05 | 1.81 | 0.37 | 0.68 | 0.26 | 1.80 | 0.37 | 0.70 | 0.28 |
| 10 | 1.92 | 0.69 | 0.83 | 0.36 | 2.02 | 0.76 | 0.97 | 0.41 |
| 15 | 1.94 | 0.87 | 1.06 | 0.48 | 2.01 | 0.90 | 0.99 | 0.51 |
| PA11(a) | PA11(b) | |||
|---|---|---|---|---|
| 20% | 1.91 | 0.47 | 1.86 | 0.61 |
| 40% | 2.07 | 0.85 | 2.09 | 1.06 |
| 60% | 2.17 | 1.53 | 2.11 | 1.87 |
| 80% | 2.22 | 3.34 | 2.30 | 3.29 |
| PA11 (a) | PA11 (b) | |||||||
|---|---|---|---|---|---|---|---|---|
| 05 | 2.34 | 3.63 | 3.58 | 2.45 | 2.83 | 3.94 | 3.51 | 2.61 |
| 10 | 2.22 | 3.32 | 3.04 | 2.31 | 2.44 | 3.87 | 3.69 | 2.53 |
| 15 | 2.09 | 3.18 | 2.85 | 2.20 | 2.28 | 3.91 | 3.52 | 2.39 |
| Parameters | Values |
|---|---|
| Heating time | 1800 s |
| Oven temperature | 320 °C |
| Inner surface temperature | 230 °C |
| Major axes | 3RPM |
| Minor axes | 1RPM |
| Cooling method | Free cooling 1, air cooling 2, water cooling 3 |
| Cooling time | 1500 s 1, 800 s 2, 500 s 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Qi, L.; Cheng, L.; Min, W.; Mei, Z.; Gao, R.; Sun, Z. The Effect of Cooling Rates on Thermal, Crystallization, Mechanical and Barrier Properties of Rotational Molding Polyamide 11 as the Liner Material for High-Capacity High-Pressure Vessels. Molecules 2023, 28, 2425. https://doi.org/10.3390/molecules28062425
Yu M, Qi L, Cheng L, Min W, Mei Z, Gao R, Sun Z. The Effect of Cooling Rates on Thermal, Crystallization, Mechanical and Barrier Properties of Rotational Molding Polyamide 11 as the Liner Material for High-Capacity High-Pressure Vessels. Molecules. 2023; 28(6):2425. https://doi.org/10.3390/molecules28062425
Chicago/Turabian StyleYu, Muhuo, Liangliang Qi, Lele Cheng, Wei Min, Zhonghao Mei, Ruize Gao, and Zeyu Sun. 2023. "The Effect of Cooling Rates on Thermal, Crystallization, Mechanical and Barrier Properties of Rotational Molding Polyamide 11 as the Liner Material for High-Capacity High-Pressure Vessels" Molecules 28, no. 6: 2425. https://doi.org/10.3390/molecules28062425
APA StyleYu, M., Qi, L., Cheng, L., Min, W., Mei, Z., Gao, R., & Sun, Z. (2023). The Effect of Cooling Rates on Thermal, Crystallization, Mechanical and Barrier Properties of Rotational Molding Polyamide 11 as the Liner Material for High-Capacity High-Pressure Vessels. Molecules, 28(6), 2425. https://doi.org/10.3390/molecules28062425







