Therapeutic Effects of Coumarins with Different Substitution Patterns
Abstract
1. Introduction
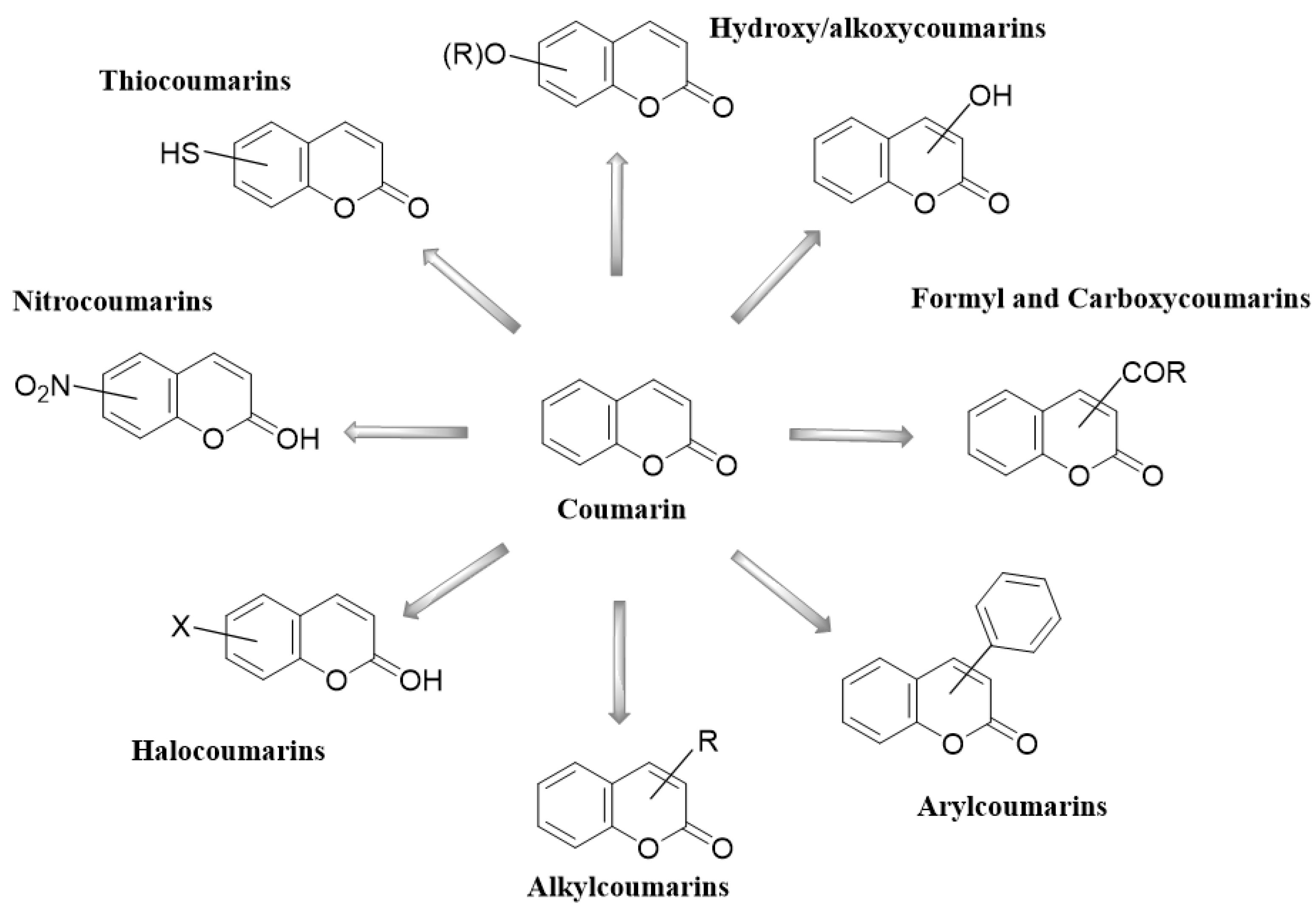
2. Coumarins
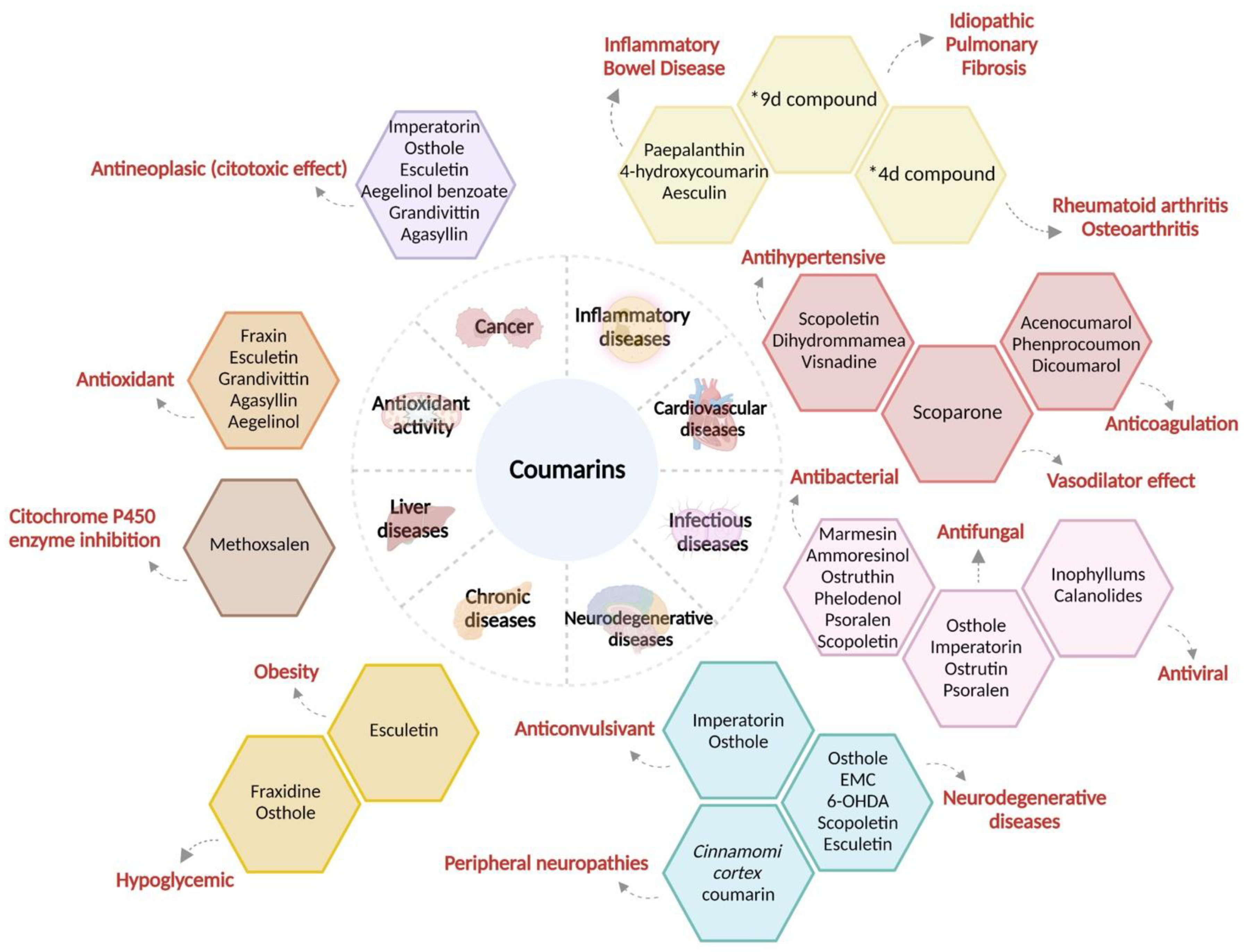
3. Coumarins and Their Pharmacological Activity
3.1. Therapeutic Use of Coumarins in Inflammatory Diseases
3.1.1. Coumarins in Inflammatory Bowel Disease
3.1.2. Coumarins in Idiopathic Pulmonary Fibrosis
3.1.3. Coumarins in Rheumatoid Arthritis and Osteoarthritis
3.2. Coumarins in Cardiovascular Disease
3.2.1. Coumarins with Antihypertensive Activity
3.2.2. Coumarins with Vasodilator Effect
3.2.3. Coumarins in Heart Failure
3.2.4. Coumarins for the Treatment of Thromboembolic Conditions
3.3. Coumarins in Infectious Diseases
3.3.1. Antibacterial Activities of Coumarins
3.3.2. Coumarins with Antifungal Activities
3.3.3. Coumarins with Antiviral Activity
3.4. Coumarins in Neurodegenerative Diseases
3.4.1. Coumarins with Neuroprotective Activity
3.4.2. Epilepsy
3.4.3. Multiple Sclerosis
3.4.4. Parkinson’s Disease
3.4.5. Amyotrophic Lateral Sclerosis
3.4.6. Alzheimer’s Disease
3.4.7. Huntington’s Disease
3.4.8. Peripheral Neuropathies
3.5. Coumarins in Chronic Degenerative Diseases
3.5.1. Obesity
3.5.2. Metabolic Syndrome
3.5.3. Coumarins with Antihyperglycemic Activity in Diabetes Mellitus
3.6. Coumarins in Liver Diseases
3.7. Coumarins with Antioxidant Activity
3.8. Coumarins as Phytoalexins
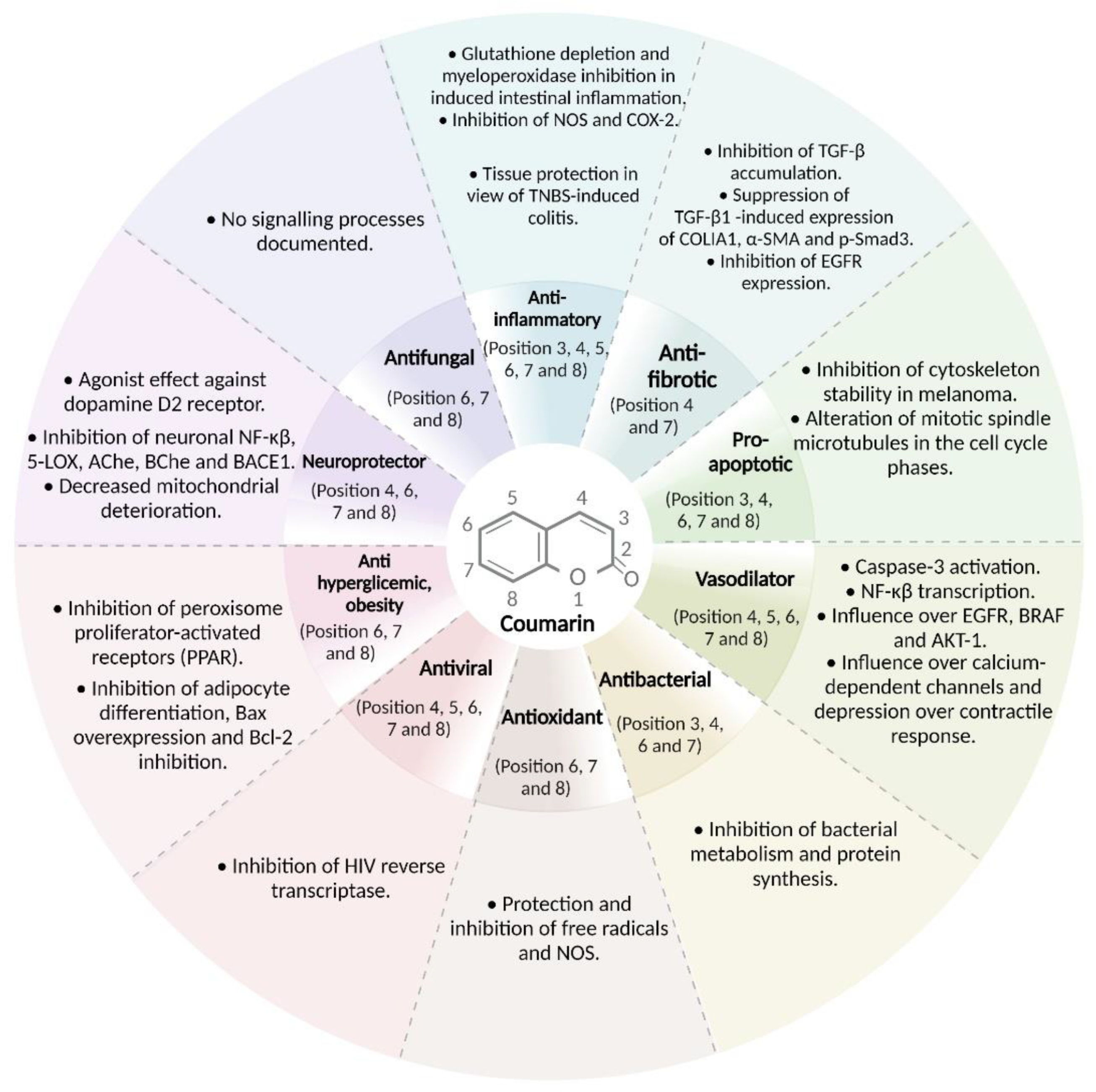
3.9. Main Biological Targets of Coumarin Derivatives According to In Vitro, In Vivo, and Structure-Activity Relationship Analyses
4. Cancer
4.1. Molecular Aspects of Cancer and Therapies
4.2. Coumarins with Antineoplastic Activity
Synthetic Derivatives of Coumarin with Antineoplastic Activity
4.3. Coumarin Derivatives and Their Effect on Cell Signaling Pathways in Cancer
4.4. Usefulness of Structure–Activity (SAR) Analyses of Coumarin Derivatives to Identify Molecular Targets against Cancer
4.5. Adjuvant Cancer Therapy: Potential Role of Coumarins
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, Z.; Rasheed, H.M.; Bashir, K.; Gurgul, A.; Wahid, F.; Che, C.-T.; Shahzadi, I.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Dryopteris ramosa (Hope) C. Chr. and evaluation of cytotoxicity against liver and prostate cancer cell lines. Heliyon 2022, 8, e11286. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mishra, N. Coumarin-based combined computational study to design novel drugs against Candida albicans . J. Microbiol. 2022, 60, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, B.R.; Khandelwal, A.; Gu, W.; Brown, D.; Liu, W.; Vielhauer, G.; Holzbeierlein, J.; Blagg, B.S.J. Synthesis and biological evaluation of coumarin replacements of novobiocin as Hsp90 inhibitors. Bioorganic Med. Chem. 2014, 22, 1441–1449. [Google Scholar] [CrossRef]
- Gao, W.; Li, Q.; Chen, J.; Wang, Z.; Hua, C. Total synthesis of six 3,4-unsubstituted coumarins. Molecules 2013, 18, 15613–15623. [Google Scholar] [CrossRef] [PubMed]
- Wörner, M.; Schreier, P. Flüchtige inhaltsstoffe aus tonkabohnen (Dipteryx odorata Willd.). Z. Lebensm. Unters. Forch. 1991, 193, 21–25. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins. Plants, structure, properties. Chem. Nat. Compd. 1998, 34, 345–409. [Google Scholar] [CrossRef]
- McAdam, K.; Enos, T.; Goss, C.; Kimpton, H.; Faizi, A.; Edwards, S.; Wright, C.; Porter, A.; Rodu, B. Analysis of coumarin and angelica lactones in smokeless tobacco products. Chem. Cent. J. 2018, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Hroboňová, K.; Brokešová, E. Comparison of different types of sorbents for extraction of coumarins. Food Chem. 2020, 332, 127404. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Wen, Q.; Hu, S.; Ma, Y.-L.; Jiang, Z.-H.; Tang, J.-Y.; Fu, Y.-H.; Qiu, S.-X. Furanocoumarins with potential antiproliferative activities from Clausena lenis . Nat. Prod. Res. 2019, 33, 2631–2637. [Google Scholar] [CrossRef]
- Appendino, G.; Bianchi, F.; Bader, A.; Campagnuolo, C.; Fattorusso, E.; Taglialatela-Scafati, O.; Blanco-Molina, M.; Macho, A.; Fiebich, B.L.; Bremner, P.; et al. Coumarins from Opopanax chironium. New dihydrofuranocoumarins and differential induction of apoptosis by imperatorin and heraclenin. J. Nat. Prod. 2004, 67, 532–536. [Google Scholar] [CrossRef]
- Xia, H.-M.; Li, C.-J.; Yang, J.-Z.; Ma, J.; Li, Y.; Li, L.; Zhang, D.M. Hepatoprotective pyranocoumarins from the stems of Clausena emarginata . Phytochemistry 2016, 130, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Era, B.; Delogu, G.L.; Pintus, F.; Fais, A.; Gatto, G.; Uriarte, E.; Borges, F.; Kumar, A.; Matos, M.J. Looking for new xanthine oxidase inhibitors: 3-Phenylcoumarins versus 2-phenylbenzofurans. Int. J. Biol. Macromol. 2020, 162, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, S.; Kannan, K.; Yamajala, R.B.R.D. Thiamine hydrochloride as a recyclable organocatalyst for the synthesis of bis(indolyl)methanes, tris(indolyl)methanes, 3,3-di(indol-3-yl)indolin-2-ones and biscoumarins. Org. Biomol. Chem. 2019, 17, 9620–9626. [Google Scholar] [CrossRef] [PubMed]
- Sadik, N.A.H.; El-Boghdady, N.A.; Omar, N.N.; Al-Hamid, H.A. Esculetin and idebenone ameliorate galactose-induced cataract in a rat model. J. Food Biochem. 2020, 44, e13230. [Google Scholar] [CrossRef]
- Joseph, A.; Thuy, T.T.T.; Thanh, L.T.; Okada, M. Antidepressive and anxiolytic effects of ostruthin, a TREK-1 channel activator. PLoS ONE 2018, 13, e0201092. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xiong, Y.; Xia, C.; Hu, X. Effects of osthol on activity, mRNA and protein expression of Cyp3a in rats in vivo. Biopharm. Drug Dispos. 2020, 41, 64–71. [Google Scholar] [CrossRef]
- Suzuki, K.; Taniyama, K.; Aoyama, T.; Watanabe, Y. Usefulness of novobiocin as a selective inhibitor of intestinal breast cancer resistance protein (Bcrp) in rats. Xenobiotica 2019, 50, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Vander Broeck, A.; McEwen, A.G.; Chebaro, Y.; Potier, N.; Lamour, V. Structural basis for DNA gyrase interaction with coumermycin A1. J. Med. Chem. 2019, 62, 4225–4231. [Google Scholar] [CrossRef]
- Hijazin, T.; Radwan, A.; Abouzeid, S.; Dräger, G.; Selmar, D. Uptake and modification of umbelliferone by various seedlings. Phytochemistry 2019, 157, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Osoba, O.A.; Roberts, M.F. Methyltransferase activity in Allanthm altissima cell suspension cultures. Plant Cell Rep. 1994, 13, 277–281. [Google Scholar] [CrossRef]
- Delnavazi, M.-R.; Soleimani, M.; Hadjiakhoondi, A.; Yass, N. Isolation of phenolic derivatives and essential oil analysis of Prangos ferulacea (L.) Lindl. Aerial parts. IJPR 2017, 16, 207–215. [Google Scholar] [PubMed]
- Brown, S.A.; Sampathkumar, S. The biosynthesis of isopimpinellin. Can. J. Biochem. 1977, 55, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, J.; Shen, W.; Li, X. Psoralen as an interstrand DNA crosslinker in the selection of DNA-Encoded dynamic chemical library. BBRC 2020, 533, 215–222. [Google Scholar] [CrossRef]
- Saettone, M.F.; Trambusti Et, M.; Giannaccini, B. Inhibition de l’action phototoxique du bergaptène par des filtres UV-A. Int. J. Cosmet. Sci. 1983, 5, 201–213. [Google Scholar] [CrossRef]
- Guillon, C.D.; Jan, Y.-H.; Foster, N.; Ressner, J.; Heck, D.E.; Laskin, J.D.; Heindel, N.D. Synthetically modified methoxsalen for enhanced cytotoxicity in light and dark reactions. Bioorganic Med. Chem. Lett. 2019, 29, 619–622. [Google Scholar] [CrossRef]
- Pynam, H.; Dharmesh, S.M. Antioxidant and anti-inflammatory properties of marmelosin from Bael (Aegle marmelos L.); inhibition of TNF-α mediated inflammatory/tumor markers. Biomed. Pharmacother. 2018, 106, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Shokohinia, Y.; Kiani, A.; Sadrjavadi, K.; Nowroozi, A.; Shahlaei, M. Molecular insight into the grandivitin- matrix metalloproteinase 9 interactions. J. Photochem. Photobiol. B Biol. 2016, 162, 493–499. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. Coumarin derivatives from Eryngium campestre . Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Razavi, S.M.; Imanzadeh, G.; Jahed, F.S.; Zarrini, G. Pyranocoumarins from Zosima absinthifolia (Vent) link roots. Russ. J. Bioorganic Chem. 2013, 39, 215–217. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Gonçalves-Martins, M.; Jones, G.L. Chemogeography and antimicrobial activity of essential oils from Geijera parviflora and Geijera salicifolia (Rutaceae): Two traditional Australian medicinal plants. Phytochemistry 2014, 104, 60–71. [Google Scholar] [CrossRef]
- Jebir, R.M.; Mustafa, Y.F. Natural coumarin-lead compounds: A review of their medicinal potentials. Iraqi. J. Pharm. 2021, 18, 139–161. [Google Scholar] [CrossRef]
- Galinis, D.L.; Fuller, R.W.; Mckee, T.C.; Cardellina, J.H.; Gulakowski, R.J.; McMahon, J.B.; Boyd, M.R. Structure-activity modifications of the HIV-1 inhibitors (+)-calanolide A and (−)-calanolide B. J. Med. Chem. 1996, 39, 4507–4510. [Google Scholar] [CrossRef]
- Mckee, T.C.; Fuller, R.W.; Covington, C.D.; Cardellina, J.H.; Gulakowski, R.J.; Krepps, B.L.; McMahon, J.B.; Boyd, M.R. New pyranocoumarins isolated from Calophyllum lanigerum and Calophyllum teysmannii . J. Nat. Prod. 1996, 59, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.O.; Pirbasti, F.G.; Jalalifard, Z. Recent advances in the synthesis of biscoumarin derivatives. JCCS 2018, 65, 383–394. [Google Scholar] [CrossRef]
- Hughes-Formella, B.; Wunderlich, O.; Williams, R. Anti-inflammatory and skin-hydrating properties of a dietary supplement and topical formulations containing oligomeric proanthocyanidins. Ski. Pharmacol. Physiol. 2007, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.S.; Lee, B.H.; Kim, J.-K.; Ahn, E.-K.; Ko, H.-J.; Cho, Y.-R.; Lee, S.-J.; Bae, G.-U.; Kim, Y.K.; et al. Marmesin-mediated suppression of VEGF/VEGFR and integrin β1 expression: Its implication in non-small cell lung cancer cell responses and tumor angiogenesis. Oncol. Rep. 2017, 37, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Sekiguchi, F.; Ishikawa, T. Studies on the chemical constituents of rutaceous plants-XLI: Absolute configurations of rutaretin methyl ether. Tetrahedron 1981, 37, 285–290. [Google Scholar] [CrossRef]
- Guilet, D.; Séraphin, D.; Rondeau, D.; Richomme, P.; Bruneton, J. Cytotoxic coumarins from Calophyllum dispar . Phytochemistry 2001, 58, 571–575. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Schilling, J.K.; Kingston, D.G. New cytotoxic coumarins and prenylated benzophenone derivatives from the bark of Ochrocarpos punctatus from the Madagascar rainforest. J. Nat. Prod. 2002, 65, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.T.; Gény, C.; Blanchard, P.; Rouger, C.; Tonnerre, P.; Charreau, B.; Rakolomalala, G.; Randriamboavonjy, J.I.; Loirand, G.; Pacaud, P.; et al. Advanced glycation inhibition and protection against endothelial dysfunction induced by coumarins and procyanidins from Mammea neurophylla . Fitoterapia 2014, 96, 65–75. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Govindaiah, P.; Dumala, N.; Grover, P.; Prakash, M.J. Synthesis and biological evaluation of novel 4,7-dihydroxycoumarin derivatives as anticancer agents. Bioorganic Med. Chem. Lett. 2019, 29, 1819–1824. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Rzeski, W.; Jakubowicz-Gil, J. Antiglioma potential of coumarins combined with Sorafenib. Molecules 2020, 25, 5192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.-R.; Liu, H.-S.; Cheng, M.; Xia, P.; Qian, K.; Wu, P.-C.; Lai, C.-Y.; Xia, Y.; Yang, Z.-Y.; et al. Antitumor Agents 292. Design, synthesis and pharmacological study of S- and O-substituted 7-mercapto- or hydroxy-coumarins and chromones as potent cytotoxic agents. Eur. J. Med. Chem. 2012, 49, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Baruah, P.; Basumatary, G.; Yesylevskyy, S.O.; Aguan, K.; Bez, G.; Mitra, S. Novel coumarin derivatives as potent acetylcholinesterase inhibitors: Insight into efficacy, mode and site of inhibition. J. Biomol. Struct. Dyn. 2019, 37, 1750–1765. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Fraser, T.R. V.—On the connection between chemical constitution and physiological action. Part. I.—On the physiological action of the salts of the ammonium bases, derived from Strychnia, Brucia, Thebaia, Codeia, Morphia, and Nicotia . Earth Environ. Sci. Trans. R. Soc. Edinb. 1868, 25, 151–203. [Google Scholar] [CrossRef]
- Baune, M.; Kang, K.; Schenkeveld, W.D.C.; Kraemer, S.M.; Hayen, H.; Weber, G. Importance of oxidation products in coumarin-mediated Fe(hydr)oxide mineral dissolution. Biometals 2020, 33, 305–321. [Google Scholar] [CrossRef]
- Chen, L.Z.; Sun, W.W.; Bo, L.; Wang, J.Q.; Xiu, C.; Tang, W.J.; Shi, J.B.; Zhou, H.P.; Liu, X.H. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 138, 170–181. [Google Scholar] [CrossRef]
- Huang, G.-J.; Deng, J.-S.; Liao, J.-C.; Hou, W.-C.; Wang, S.-Y.; Sung, P.-J.; Kuo, Y.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis . J. Agric. Food Chem. 2012, 60, 1673–1681. [Google Scholar] [CrossRef]
- Rabe, S.Z.T.; Iranshahi, M.; Mahmoudi, M. In vitro anti-inflammatory and immunomodulatory properties of umbelliprenin and methyl galbanate. J. Immunotoxicol. 2016, 13, 209–216. [Google Scholar] [CrossRef]
- Yildirim, M.; Unal, Z.N.; Ersatir, M.; Yetkin, D.; Degirmenci, U.; Giray, E.S. Anti-inflammatory effects of coumarin–selenophene derivatives on LPS-stimulated RAW 264.7 macrophage cells. Russ. J. Bioorganic Chem. 2022, 48, 1209–1214. [Google Scholar] [CrossRef]
- Ghosh, S.; Pariente, B.; Diane, R.M.; Schreiber, S.; Petersson, J.; Hommes, D. New tools and approaches for improved management of inflammatory bowel diseases. JCC 2014, 8, 1246–1253. [Google Scholar] [CrossRef]
- Di Stasi, L.C.; Camuesco, D.; Nieto, A.; Vilegas, W.; Zarzuelo, A.; Galvez, J. Intestinal anti-inflammatory activity of paepalantine, an isocoumarin isolated from the capitula of Paepalanthus bromelioides, in the trinitrobenzenesulphonic acid model of rat colitis. Planta Medica 2004, 70, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Seito, L.N.; Witaicenis, A.; Pellizzon, C.H.; Di Stasi, L.C. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzenesulphonic acid model of rat colitis. Biol. Pharm. Bull. 2008, 31, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Peng, Z.; Luo, S.; Zhang, S.; Li, B.; Zhou, C.; Fan, H. Aesculin protects against DSS-induced colitis though activating PPARγ and inhibiting NF-кB pathway. Eur. J. Pharmacol. 2019, 857, 172453. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. NEJM 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.J.; Heckel, A.; Colbatzky, F.; Handschuh, S.; Kley, J.; Lehmann-Lintz, T.; Lotz, R.; Tontsch-Grunt, U.; Walter, R.; Hilberg, F. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120). J. Med. Chem. 2009, 52, 4466–4480. [Google Scholar] [CrossRef]
- Deng, D.; Pei, H.; Lan, T.; Zhu, J.; Tang, M.; Xue, L.; Yang, Z.; Zheng, S.; Ye, H.; Chen, L. Synthesis and discovery of new compounds bearing coumarin scaffold for the treatment of pulmonary fibrosis. Eur. J. Med. Chem. 2019, 185, 111790. [Google Scholar] [CrossRef]
- Lanza, F.L.; Rack, M.F.; Simon, T.J.; Quan, H.; Bolognese, J.A.; Hoover, M.E.; Wilson, F.R.; Harper, S.E. Specific inhibition of cyclooxygenase-2 with MK-0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Aliment. Pharmacol. Ther. 1999, 13, 761–767. [Google Scholar] [CrossRef]
- El-Nezhawy, A.O.H.; Biuomy, A.R.; Hassan, F.S.; Ismaiel, A.K.; Omar, H.A. Design, synthesis and pharmacological evaluation of omeprazole-like agents with anti-inflammatory activity. Bioorganic Med. Chem. 2013, 21, 1661–1670. [Google Scholar] [CrossRef]
- Arora, R.K.; Kaur, N.; Bansal, Y.; Bansal, G. Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B 2014, 4, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, G.R.; Balraju, V.; Mallesham, B.; Chakrabarti, R.; Lohray, V.B. Novel coumarin derivatives of heterocyclic compounds as lipid-Lowering agents. Bioorganic Med. Chem. Lett. 2003, 13, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Yuce, B.; Danis, O.; Ogan, A.; Sener, G.; Bulut, M.; Yarat, A. Antioxidative and lipid lowering effects of 7,8-dihydroxy-3- (4-methylphenyl) coumarin in hyperlipidemic rats. Arzneimittelforschung 2009, 59, 129–134. [Google Scholar] [CrossRef]
- Crichton, E.G.; Waterman, P.G. Dihydromammea C/OB: A new coumarin from the seed of Mammea africana . Phytochemistry 1978, 17, 1783–1786. [Google Scholar] [CrossRef]
- Mead, J.A.R.; Smith, J.N.; Williams, R.T. Studies in detoxication. 72. The metabolism of coumarin and of o-coumaric acid. Biochem. J. 1958, 68, 67–74. [Google Scholar] [CrossRef]
- Duarte, J.; Vallejo, I.; Pérez-Vizcaino, F.; Jiménez, R.; Zarzuelo, A.; Tamargo, J. Effects of visnadine on rat isolated vascular smooth muscles. Planta Medica 1997, 63, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chu, S.-H.; Chao, P.-D.L. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur. J. Pharmacol. 1991, 198, 211–213. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lee, C.-R.; Weng, Y.-I.; Lee, M.-C.; Lee, Y.-T. Vasodilator effect of scoparone (6,7-dimethoxycoumarin) from a Chinese herb. Eur. J. Pharmacol. 1992, 218, 123–128. [Google Scholar] [CrossRef]
- Visser, L.E.; Bleumink, G.S.; Trienekens, P.H.; Vulto, A.G.; Hofman, A.; Stricker, B.H.C. The risk of overanticoagulation in patients with heart failure on coumarin anticoagulants. Bri. J. Haematol. 2004, 127, 85–89. [Google Scholar] [CrossRef]
- Cannegieter, S.C.; Rosendaal, F.R.; Wintzen, A.R.; van der Meer, F.J.M.; Vandenbroucke, J.P.; Briet, E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. NEJM 1995, 333, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, W.; Wang, X.; Sun, Y.; Chen, X. A pharmacological review of dicoumarol: An old natural anticoagulant agent. Pharmacol. Res. 2020, 160, 105193. [Google Scholar] [CrossRef] [PubMed]
- Dadák, V.; Hoďák, K. Some relations between the structure and the antibacterial activity of natural coumarins. Experientia 1966, 22, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Magiatis, P.; Mitaku, S.; Skaltsounis, A.-L.; Chinou, E.; Chinou, I. Natural and synthetic 2,2-dimethylpyranocoumarins with antibacterial activity. J. Nat. Prod. 2005, 68, 78–82. [Google Scholar] [CrossRef]
- Soares, V.; Marini, M.B.; de Paula, L.A.; Gabry, P.S.; Amaral, A.C.F.; Malafaia, C.A.; Leal, I.C.R. Umbelliferone esters with antibacterial activity produced by lipase-mediated biocatalytic pathway. Biotechnol. Lett. 2021, 43, 469–477. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Cheng, M.-J.; Peng, C.-F.; Huang, H.-Y.; Chen, I.-S. A novel dimeric coumarin analog and antimycobacterial constituents from Fatoua . pilosa. Chem. Biodivers. 2010, 7, 1728–1736. [Google Scholar] [CrossRef]
- Cunha, S.; Iunes, C.E.M.; Oliveira, C.C.; de Santana, L.L.B. Síntese de ácidos cumarino-3-carboxílicos e sua aplicação na síntese total da aiapina, cumarina e umbeliferona. Quim. Nova 2015, 38, 1125–1131. [Google Scholar] [CrossRef]
- Wang, C.-M.; Zhou, W.; Li, C.-X.; Chen, H.; Shi, Z.-Q.; Fan, Y.-J. Efficacy of osthol, a potent coumarin compound, in controlling powdery mildew caused by Sphaerotheca fuliginea . J. Asian. Nat. Prod. Res. 2009, 11, 783–791. [Google Scholar] [CrossRef]
- Montagner, C.; de Souza, S.M.; Groposo, C.; Delle Monache, F.; Smânia, E.F.A.; Smânia, A., Jr. Antifungal activity of coumarins. Z Naturforsch C J Biosci 2008, 63, 21–28. [Google Scholar] [CrossRef]
- Kashman, Y.; Gustafson, K.R.; Fuller, R.W.; Cardellina, J.H., II; McMahon, J.B.; Currens, M.J.; Buckheit, R.W., Jr.; Hughes, S.H.; Cragg, G.M.; Boyd, M.R. HIV inhibitory natural products. Part 7. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum . J. Med. Chem. 1992, 35, 2735–2743. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R.; Johnson, R.K.; et al. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, F.S.S.; Salmas, R.E.; Irmak, S.; Acar, O.O.; Turgut, G.C.; Sen, A.; Zbancioc, A.-M.; Luca, S.V.; Skiba, A.; et al. Evaluation of anti-Alzheimer activity of synthetic coumarins by combination of in vitro and in silico approaches. Chem. Biodivers. 2022, 19, e202200315. [Google Scholar] [CrossRef]
- Wang, C.; Pei, A.; Chen, J.; Yu, H.; Sun, M.-L.; Liu, C.-F.; Xu, X. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J. Neurochem. 2012, 121, 1007–1013. [Google Scholar] [CrossRef]
- Baek, N.I.; Ahn, E.M.; Kim, H.Y.; Park, Y.D. Furanocoumarins from the root of Angelica dahurica . Arch. Pharm. Res. 2000, 23, 467–470. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Berenbaum, M.R. Furanocoumarin induction in wild parsnip: Genetics and population variation. Ecology 1990, 71, 1933–1940. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Wojda, E.; Andres-Mach, M.; Cisowski, W.; Glensk, M.; Glowniak, K.; Czuczwar, S.J. Anticonvulsant and acute neurotoxic effects of imperatorin, osthole and valproate in the maximal electroshock seizure and chimney tests in mice: A comparative study. Epilepsy Res. 2009, 85, 293–299. [Google Scholar] [CrossRef]
- Chen, X.; Pi, R.; Zou, Y.; Liu, M.; Ma, X.; Jiang, Y.; Mao, X.; Hu, X. Attenuation of experimental autoimmune encephalomyelitis in C57 BL/6 mice by osthole, a natural coumarin. Eur. J. Pharmacol. 2010, 629, 40–46. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, F.; Costas-Lago, M.C.; Besada, P.; Alonso-Pena, M.; Torres-Terán, I.; Viña, D.; Fontenla, J.Á.; Sturlese, M.; Moro, S.; Quezada, E.; et al. Novel coumarin-pyridazine hybrids as selective MAO-B inhibitors for the Parkinson’s disease therapy. Bioorganic Chem. 2020, 104, 104203. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Chen, Z.; Jiang, X.; Zhou, Z.; Yuan, J.; Li, F.; Zhang, Y.; Huang, X.; Fan, S.; et al. Pepper component 7-ethoxy-4-methylcoumarin, a novel dopamine D2 receptor agonist, ameliorates experimental Parkinson’s disease in mice and Caenorhabditis elegans . Pharmacol. Res. 2021, 163, 105220. [Google Scholar] [CrossRef]
- Gowland, A.; Opie-Martin, S.; Scott, K.M.; Jones, A.R.; Mehta, P.R.; Batts, C.J.; Ellis, C.M.; Leigh, P.N.; Shaw, C.E.; Sreedharan, J.; et al. A Predicting the future of ALS: The impact of demographic change and potential new treatments on the prevalence of ALS in the United Kingdom, 2020–2116. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 264–274. [Google Scholar] [CrossRef]
- Mogana, R.; Teng-Jin, K.; Wiart, C. Anti-Inflammatory, Anticholinesterase, and antioxidant potential of scopoletin isolated from Canarium patentinervium Miq. (Burseraceae Kunth). eCAM 2013, 2013, 734824. [Google Scholar] [CrossRef]
- Barber, S.C.; Higginbottom, A.; Mead, R.J.; Barber, S.; Shaw, P.J. An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free. Radic. Biol. Med. 2009, 46, 1127–1138. [Google Scholar] [CrossRef]
- Orhan, G.; Orhan, I.; Sener, B. Recent developments in natural and synthetic drug research for Alzheimers Disease. Lett. Drug Des. Discov. 2006, 3, 268–274. [Google Scholar] [CrossRef]
- Ali, Y.; Jannat, S.; Jung, H.A.; Choi, R.J.; Roy, A.; Choi, J.S. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac. J. Trop. Med. 2016, 9, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Van Roon-Mom, W.M.C.; Pepers, B.A.; C’t Hoen, P.A.; Verwijmeren, C.A.C.M.; den Dunnen, J.T.; Dorsman, J.C.; van Ommen, G.B. Mutant huntingtin activates Nrf2-responsive genes and impairs dopamine synthesis in a PC12 model of Huntington’s disease. BMC Mol. Biol. 2008, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Pruccoli, L.; Breda, C.; Teti, G.; Falconi, M.; Giorgini, F.; Tarozzi, A. Esculetin provides neuroprotection against mutant huntingtin-induced toxicity in Huntington’s disease models. Pharmaceuticals 2021, 14, 1044. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Kim, W.; Li, D.; Kim, Y.; Lee, K.; Kim, S.K. The suppressive effects of cinnamomi cortex and its phytocompound coumarin on oxaliplatin-induced neuropathic cold allodynia in Rats. Molecules 2016, 21, 1253. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Choi, K.-M.; Yoo, H.-S.; Lee, C.-K.; Hwang, B.Y.; Lee, M.K. Inhibitory effects of coumarins from the stem barks of Fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells. Biol. Pharm. Bull. 2010, 33, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Girman, C.J.; Rhodes, T.; Mercuri, M.; Pyörälä, K.; Kjekshus, J.; Pedersen, T.R.; Beere, P.A.; Gotto, A.M. Clearfield, M.; the 4S Group, the AFCAPS/TexCAPS Research Group: The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am. J. Cardiol. 2004, 93, 136–141. [Google Scholar] [CrossRef]
- Trischitta, V.; Brunetti, A.; Chiavetta, A.; Benzi, L.; Papa, V.; Vigneri, R. Defects in insulin-receptor internalization and processing in monocytes of obese subjects and obese NIDDM patients. Diabetes 1989, 38, 1579–1584. [Google Scholar] [CrossRef]
- Fort, D.M.; Rao, K.; Jolad, S.D.; Luo, J.; Carlson, T.J.; King, S.R. Antihyperglycemic activity of Teramnus labialis (Fabaceae). Phytomedicine 2000, 6, 465–467. [Google Scholar] [CrossRef]
- Liang, H.-J.; Suk, F.-M.; Wang, C.-K.; Hung, L.F.; Liu, D.-Z.; Che, N.-Q.; Chen, Y.-C.; Chang, C.-C.; Liang, Y.-C. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem. Biol. Interact. 2009, 181, 309–315. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Hankins, D.C.; Taraday, J.K. Single-dose methoxsalen effects on human cytochrome P-450 2A6 activity. Drug Metab. Dispos. 2000, 28, 28–33. [Google Scholar] [PubMed]
- Tinel, M.; Belghiti, J.; Descatoire, V.; Amouyal, G.; Letteron, P.; Geneve, J.; Larrey, D.; Pessayre, D. Inactivation of human liver cytochrome P-450 by the drug methoxsalen and other psoralen derivatives. Biochem. Pharmacol. 1987, 36, 951–955. [Google Scholar] [CrossRef]
- Pitaro, M.; Croce, N.; Gallo, V.; Arienzo, A.; Salvatore, G.; Antonini, G. Coumarin-induced hepatotoxicity: A narrative review. Molecules 2022, 27, 9063. [Google Scholar] [CrossRef]
- Whang, W.K.; Park, H.S.; Ham, I.H.; Oh, M.; Namkoong, H.; Kim, H.K.; Hwang, D.W.; Hur, S.Y.; Kim, T.E.; Park, Y.G.; et al. Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp. Mol. Med. 2005, 37, 436–446. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta. Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- García, M.R.; Pérez, L.R. Fitoalexinas: Mecanismo de defensa de las plantas. Revista. Chapingo. 2003, 9, 5–10. Available online: https://www.redalyc.org/articulo.oa?id=62990101 (accessed on 25 November 2022).
- Lake, B. Coumarin metabolism, toxicity, and carcinogenicity: Relevance for human risk assessment. FTC 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Gouda, M.A.; Hussein, B.H.; El-Demerdash, A.; Ibrahim, M.E.; Salem, M.A.; Helal, M.H.; Hamama, W.S. A review: Synthesis and medicinal importance of coumarins and their analogues (Part II). Curr. Bioact. Compd. 2020, 16, 993–1008. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Fitriati, F.F.; Tjahjandarie, T.S. Antimalarial and antioxidant activities of isoprenylated coumarins from the stem bark of Mesua borneensis L. JBAPN 2016, 6, 95–100. [Google Scholar] [CrossRef]
- Prashanth, T.; Avin, B.V.; Thirusangu, P.; Ranganatha, V.L.; Prabhakar, B.T.; Chandra, J.N.S.; Khanum, S.A. Synthesis of coumarin analogs appended with quinoline and thiazole moiety and their apoptogenic role against murine ascitic carcinoma. Biomed. Pharmacother. 2019, 112, 108707. [Google Scholar] [CrossRef]
- Khode, S.; Maddi, V.; Aragade, P.; Palkar, M.; Ronad, P.K.; Mamle Desai, S.; Thippeswamy, A.H.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar] [CrossRef]
- Reddy, C.P.K.; Goud, V.M.; Sreenivasulu, N.; Prasad, R. Design, synthesis and chemical characterization of some novel coumarin compounds and evaluation of their biological activity. Int. J. Pharm. World Res. 2010, 1, 1–19. [Google Scholar] [CrossRef]
- Lin, C.M.; Huang, S.T.; Lee, F.W.; Kuo, H.S.; Lin, M.H. 6-Acyl-4-aryl/alkyl-5,7-dihydroxycoumarins as anti-inflammatory agents. Bioorganic Med. Chem. 2016, 14, 4402–4409. [Google Scholar] [CrossRef]
- Nofal, Z.M.; El-Zahar, M.I.; Abd El-Karim, S.S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. JNCI 2008, 100, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations: Acquired genetic lability permits stepwise selection of variant sublines and underlies tumor prograssion. Science 1950, 194, 23–28. [Google Scholar] [CrossRef]
- Dantas da Silva, B. Acción de Compuestos Bioactivos de los Aceites de Oliva Vírgenes en la Expresión del CD36 en un Modelo celular Humano de Áncer de Mama. Master’s Thesis, Universidad de Jaén, Jaén, España, 2017. Available online: https://tauja.ujaen.es/handle/10953.1/6153 (accessed on 15 November 2022).
- Liu, Y.; Yang, Y.; Li, L.; Liu, Y.; Geng, P.; Li, G.; Song, H. LncRNA SNHG1 enhances cell proliferation, migration, and invasion in cervical cancer. Biochem. Cell Biol. 2018, 96, 38–43. [Google Scholar] [CrossRef]
- Al-Mehdi, A.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R.J. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef]
- Wada, K.; Goto, M.; Lee, K.H.; Yamashita, H. Antiproliferative effect of N-heterocyclo-coumarin derivatives against multidrug-resistant cells. Chem. Pharm. Bull. 2023, 71, 52–57. [Google Scholar] [CrossRef]
- Dettori, T.; Sanna, G.; Cocco, A.; Serreli, G.; Deiana, M.; Palmas, V.; Onnis, V.; Pilia, L.; Melis, N.; Moi, D.; et al. Synthesis and antiproliferative effect of halogenated coumarin derivatives. Molecules 2022, 27, 8897. [Google Scholar] [CrossRef] [PubMed]
- Pestrin, M.; Bessi, S.; Galardi, F.; Truglia, M.; Biggeri, A.; Biagioni, C.; Cappadona, S.; Biganzoli, L.; Giannini, A.; Di Leo, A. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res. Treat. 2009, 118, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Carr, A.J.; Hulley, P.A. Cell proliferation is a key determinant of the outcome of FOXO3a activation. BBRC 2015, 462, 78–84. [Google Scholar] [CrossRef]
- Rahmani, M.; Nkwocha, J.; Hawkins, E.; Pei, X.; Parker, R.E.; Kmieciak, M.; Leverson, J.D.; Sampath, D.; Ferreira-Gonzalez, A.; Grant, S. Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML Cells. Cancer Res. 2018, 78, 3075–3086. [Google Scholar] [CrossRef]
- Santos, M.; Río, P.; Ruiz, S.; Martínez-Palacio, J.; Segrelles, C.; Lara, M.F.; Segovia, J.C.; Paramio, J.M. Altered T cell differentiation and Notch signaling induced by the ectopic expression of keratin K10 in the epithelial cells of the thymus. J. Cell. Biochem. 2005, 95, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Cadoret, A.; Ovejero, C.; Terris, B.; Souil, E.; Lévy, L.; Lamers, W.H.; Kitajewski, J.; Kahn, A.; Perret, C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 2002, 21, 8293–8301. [Google Scholar] [CrossRef]
- Mroz, E.A.; Tward, A.M.; Hammon, R.J.; Ren, Y.; Rocco, J.W. Intra-tumor genetic heterogeneity and mortality in head and neck cancer: Analysis of data from the Cancer Genome Atlas. PLoS Med. 2015, 12, e1001786. [Google Scholar] [CrossRef]
- Dairkee, S.H.; Nicolau, M.; Sayeed, A.; Champion, S.; Ji, Y.; Moore, D.H.; Yong, B.; Meng, Z.; Jeffrey, S.S. Oxidative stress pathways highlighted in tumor cell immortalization: Association with breast cancer outcome. Oncogene 2007, 26, 6269–6279. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Le, S.; Jin, X.; Liu, G.; Chen, J.; Hu, J. CSN5 promotes the invasion and metastasis of pancreatic cancer by stabilization of FOXM1. Exp. Cell Res. 2019, 374, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Moattari, M.; Jaafari, B.; Talei, A.; Piroozi, S.; Tahmasebi, S.; Zakeri, Z. The effect of combined decongestive therapy and pneumatic compression pump on lymphedema indicators in patients with breast cancer related lymphedema. Iran. Red Crescent Med. J. 2012, 14, 210–221. [Google Scholar]
- Mamone, L.A. Búsqueda de Nuevos Foto Sensibilizantes Para el Tratamiento del cáNcer, Inactivación Bacteriana y de Principios Activos Antineoplásicos a Partir de Especies Vegetales de Argentina. Doctor Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2014. Available online: https://bibliotecadigital.exactas.uba.ar/collection/tesis/document/tesis_n5514_Mamone (accessed on 20 November 2022).
- Chaki, S.P.; Barhoumi, R.; Rivera, G.M. Nck adapter proteins promote podosome biogenesis facilitating extracellular matrix degradation and cancer invasion. Cancer Med. 2019, 8, 7385–7398. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, A.; Blyuss, O.; Metodieva, G.; Gentry-Maharaj, A.; Ryan, A.; Kiseleva, E.M.; Prytomanova, O.M.; Jacobs, I.J.; Widschwendter, M.; Menon, U.; et al. Testing breast cancer serum biomarkers for early detection and prognosis in pre-diagnosis samples. Bri. J. Cancer 2017, 116, 501–508. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Bozic, I.; Reiter, J.G.; Allen, B.; Antal, T.; Chatterjee, K.; Shah, P.; Moon, Y.S.; Yaqubie, A.; Kelly, N.; Le, D.T.; et al. Evolutionary dynamics of cancer in response to targeted combination therapy. eLife 2013, 2, e00747. [Google Scholar] [CrossRef]
- Park, S.; Cho, D.H.; Andera, L.; Suh, N.; Kim, I. Curcumin enhances TRAIL-induced apoptosis of breast cancer cells by regulating apoptosis-related proteins. Mol. Cell. Biochem. 2013, 383, 39–48. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Y.; Xia, X.; Shao, Z.; Huang, C.; He, J.; Jiang, L.; Tang, D.; Liu, J.; Huang, H. Targeting GRP78-dependent AR-V7 protein degradation overcomes castration-resistance in prostate cancer therapy. Theranostics 2020, 10, 3366–3381. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Cao, J.; Liu, X.; Yang, Y.; Wei, B.; Li, Q.; Mao, G.; He, Y.; Li, Y.; Zheng, L.; Zhang, Q.; et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/BAI1 signaling pathway. Angiogenesis 2020, 23, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Stahlberg, C.; Pedersen, A.T.; Lynge, E.; Ottesen, B. Hormone replacement therapy and risk of breast cancer: The role of progestins. Acta. Obstet. Gynecol. Scand. 2003, 82, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Lew, E.D.; Yuzawa, S.; Tomé, F.; Lax, I.; Schlessinger, J. The selectivity of receptor tyrosine kinase signaling is controlled by a secondary SH2 domain binding site. Cell 2009, 138, 514–524. [Google Scholar] [CrossRef]
- Kirson, E.D.; Schneiderman, R.S.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 2009, 9, 1. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Waryoba, C.; Ugochukwu, N. In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res. 2010, 30, 4613–4617. [Google Scholar] [PubMed]
- Yang, D.; Gu, T.; Wang, T.; Tang, Q.; Ma, C. Effects of osthole on migration and invasion in breast cancer cells. Biosci. Biotechnol. Biochem. 2010, 74, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Shin, E.J.; Kim, H.C.; Choi, Y.S.; Shin, T.; Wie, M.B. Esculetin inhibits N-methyl-D-aspartate neurotoxicity via glutathione preservation in primary cortical cultures. Lab. Anim. Res. 2011, 27, 259–263. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.M.; Faraone, N.; Spadaro, V.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.H.; Bruno, M. The cytotoxic properties of natural coumarins isolated from roots of Ferulago. campestris (apiaceae) and of synthetic ester derivatives of aegelinol. Nat. Prod. Commun. 2009, 4, 1701–1706. [Google Scholar] [CrossRef]
- Jarząb, A.; Grabarska, A.; Skalicka-Woźniak, K.; Stepulak, A. Pharmacological features of osthole. Adv. Hyg. Exp. Med. 2017, 71, 411–421. [Google Scholar] [CrossRef]
- Velasco-Velázquez, M.A.; Salinas-Jazmín, N.; Mendoza-Patiño, N.; Mandoki, J.J. Reduced paxillin expression contributes to the antimetastatic effect of 4-hydroxycoumarin on B16-F10 melanoma cells. Cancer Cell Int. 2008, 8, 8. [Google Scholar] [CrossRef]
- Mandoki, J.J.; López, G.J.S.; Mandoki, J.J.; Aguilar, C.D.; Medina, M.F.; Rivera, R.R.M.; García, H.V.; Cervera, M.I. Efecto citostático de la cumarina y la 7-hidroxicumarina en tres líneas celulares de adenocarcinoma pulmonar humano. Revista. del. Instituto. Nacional. de. Enfermedades. Respiratorias 1998, 11, 261–267. Available online: https://www.imbiomed.com.mx/articulo.php?id=11147 (accessed on 25 November 2022).
- Goud, N.S.; Kanth Makani, V.K.; Pranay, J.; Alvala, R.; Qureshi, I.A.; Kumar, P.; Bharath, R.D.; Nagaraj, C.; Yerramsetty, S.; Pal-Bhadra, M.; et al. Synthesis, 18F-radiolabeling and apoptosis inducing studies of novel 4, 7-disubstituted coumarins. Bioorganic Chem. 2020, 97, 103663. [Google Scholar] [CrossRef]
- Saboormaleki, S.; Sadeghian, H.; Bahrami, A.R.; Orafaie, A.; Matin, M.M. 7-Farnesyloxycoumarin exerts anti-cancer effects on a prostate cancer cell line by 15-LOX-1 inhibition. Arch. Iran. Med. 2018, 21, 251–259. [Google Scholar] [PubMed]
- Saidu, N.E.B.; Valente, S.; Bana, E.; Kirsch, G.; Bagrel, D.; Montenarh, M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioorganic Med. Chem. 2012, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; Fontana, G.; Bo, L.D.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorganic Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Y.; Li, G.; Chen, L.; Nie, M.; Wang, Z.; Ji, H. Coumarin sulfonamides and amides derivatives: Design, synthesis, and antitumor activity in vitro. Molecules 2021, 26, 786. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, M.O.; El-Karim, S.S.A.; Anwar, M.M.; Gouda, R.H.; Zaghary, W.A.; Khedr, M.A. Discovery of new coumarin-based lead with potential anticancer, CDK4 inhibition and selective radiotheranostic effect: Synthesis, 2D & 3D QSAR, molecular dynamics, in vitro cytotoxicity, radioiodination, and biodistribution studies. Molecules 2021, 26, 2273. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Bua, S.; Bozdag, M.; Hadjipavlou-Litina, D.; Supuran, C.T. Novel 6- and 7-substituted coumarins with inhibitory action against lipoxygenase and tumor-associated carbonic anhydrase IX. Molecules 2018, 23, 153. [Google Scholar] [CrossRef]
- Madari, H.; Panda, D.; Wilson, L.; Jacobs, R.S. Dicoumarol: A unique microtubule stabilizing natural product that is synergistic with Taxol. Cancer Res. 2003, 63, 1214–1220. [Google Scholar]
- Haghighi, F.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Rassouli, F.B.; Haghighitalab, A. The cytotoxic activities of 7-isopentenyloxycoumarin on 5637 cells via induction of apoptosis and cell cycle arrest in G2/M stage. DARU J. Pharm. Sci. 2014, 22, 3. [Google Scholar] [CrossRef]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. 7,8-Dihydroxy-4-methylcoumarin induces apoptosis of human lung adenocarcinoma cells by ROS-independent mitochondrial pathway through partial inhibition of ERK/MAPK signaling. FEBS Lett. 2007, 581, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Luo, J.; Wu, F.; Hou, X.Y.; Yan, G.; Zhou, M.; Zhang, M.; Pu, C.; Li, R. Synthesis and biological evaluation of novel 2,3-dihydrochromeno[3,4-d]imidazol-4(1H)-one derivatives as potent anticancer cell proliferation and migration agents. Eur. J. Med. Chem. 2016, 114, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Q.Q.; Gong, J.-H.; Ma, J.-P. Anticancer effects of isofraxidin against A549 human lung cancer cells via the EGFR signaling pathway. Mol. Med. Rep. 2018, 18, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Benci, K.; Mandić, L.; Suhina, T.; Sedić, M.; Klobučar, M.; Pavelić, S.K.; Pavelić, K.; Wittine, K.; Mintas, M. Novel coumarin derivatives containing 1,2,4-triazole, 4,5-dicyanoimidazole and purine moieties: Synthesis and evaluation of their cytostatic activity. Molecules 2012, 17, 11010–11025. [Google Scholar] [CrossRef] [PubMed]
- Mirunalini, S.; Deepalakshmi, K.; Manimozhi, J. Antiproliferative effect of coumarin by modulating oxidant/antioxidant status and inducing apoptosis in Hep2 cells. Biomed. Aging Pathol. 2014, 4, 131–135. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Huang, Y.F.; Lu, H.F.; Ho, H.C.; Yang, J.S.; Li, T.M.; Chang, N.W.; Chung, J.G. Coumarin induces cell cycle arrest and apoptosis in human cervical cancer HeLa cells through a mitochondria- and caspase-3 dependent mechanism and NF-ΚB down-regulation. In Vivo 2007, 21, 1003–1009. [Google Scholar]
- Khan, S.; Shehzad, O.; Cheng, M.S.; Li, R.J.; Kim, Y.S. Pharmacological mechanism underlying anti-inflammatory properties of two structurally divergent coumarins through the inhibition of pro-inflammatory enzymes and cytokines. J. Inflamm. 2015, 12, 47. [Google Scholar] [CrossRef]
- Paiva, A.M.; Pinto, R.A.; Teixeira, M.; Barbosa, C.M.; Lima, R.T.; Vasconcelos, M.H.; Sousa, E.; Pinto, M. Development of noncytotoxic PLGA nanoparticles to improve the effect of a new inhibitor of p53–MDM2 interaction. Int. J. Pharm. 2013, 454, 394–402. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Avula, S.R.; Sharma, K.; Palnati, G.R.; Bathula, S.R. Discovery of coumarin–monastrol hybrid as potential antibreast tumor-specific agent. Eur. J. Med. Chem. 2013, 60, 120–127. [Google Scholar] [CrossRef]
- Jamier, V.; Marut, W.; Valente, S.; Chereau, C.; Chouzenoux, S.; Nicco, C.; Lemarechal, H.; Weill, B.; Kirsch, G.; Jacob, C.; et al. Chalcone-coumarin derivatives as potential anti-cancer drugs: An in vitro and in vivo investigation. Anti-Cancer Agents Med. Chem. 2014, 14, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Schnekenburger, M.; Zwergel, C.; Gaascht, F.; Mai, A.; Dicato, M.; Kirsch, G.; Valente, S.; Diederich, M. Novel inhibitors of human histone deacetylases: Design, synthesis and bioactivity of 3-alkenoylcoumarines. Bioorganic Med. Chem. Lett. 2014, 24, 3797–3801. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Della-Fera, M.A.; Baile, C.A. Esculetin induces mitochondria-mediated apoptosis in 3T3-L1 adipocytes. Apoptosis 2006, 11, 1371–1378. [Google Scholar] [CrossRef]
- Kuo, H.C.; Lee, H.J.; Hu, C.C.; Shun, H.I.; Tseng, T.H. Enhancement of esculetin on Taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol. Appl. Pharmacol. 2006, 210, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Finn, G.J.; Kenealy, E.; Creaven, B.S.; Egan, D.A. In vitro cytotoxic potential and mechanism of action of selected coumarins, using human renal cell lines. Cancer Lett. 2002, 183, 61–68. [Google Scholar] [CrossRef]
- Egan, D.; James, P.; Cooke, D.; O’Kennedy, R. Studies on the cytostatic and cytotoxic effects and mode of action of 8-nitro-7-hydroxycoumarin. Cancer Lett. 1997, 118, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Finn, G.; Creaven, B.; Egan, D. Modulation of mitogen-activated protein kinases by 6-nitro-7-hydroxycoumarin mediates apoptosis in renal carcinoma cells. Eur. J.Pharmacol. 2003, 48, 159–167. [Google Scholar] [CrossRef]
- Finn, G.J.; Creaven, B.; Egan, D.A. Study of the in vitro cytotoxic potential of natural and synthetic coumarin derivatives using human normal and neoplastic skin cell lines. Melanoma. Res. 2001, 11, 461–467. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, K.B.; Shin, B.C.; Seo, E.A.; Lee, Y.R.; Kim, J.S.; Park, J.W.; Park, B.H.; Ryu, D.G. Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor κB and caspase-3. Life Sci. 2005, 77, 824–836. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, L.; Fu, X.L.; Chen, K.; Qian, B.C. Effect of scopoletin on PC3 cell proliferation and apoptosis. Acta Pharmacol. Sin. 2001, 22, 929–933. [Google Scholar]
- Riveiro, M.E.; Vazquez, R.; Moglioni, A.; Gomez, N.; Baldi, A.; Davio, C.; Shayo, C. Biochemical mechanisms underlying the pro-apoptotic activity of 7,8-dihydroxy-4-methylcoumarin in human leukemic cells. Biochem. Pharmacol. 2008, 75, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, C.; Genovese, S.; Lallemand, B.; Ionescu-Motatu, A.; Curini, M.; Kiss, R.; Epifano, F. Growth inhibitory activities of oxyprenylated and non-prenylated naturally occurring phenylpropanoids in cancer cell lines. Bioorganic Med. Chem. Lett. 2011, 21, 4174–4179. [Google Scholar] [CrossRef] [PubMed]
- Valiahdi, S.M.; Iranshahi, M.; Sahebkar, A. Cytotoxic activities of phytochemicals from Ferula species. DARU J. Pharm. Sci. 2013, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, M.E.; Moglioni, A.; Vazquez, R.; Gomez, N.; Facorro, G.; Piehl, L.; de Celis, E.R.; Shayo, C.; Davio, C. Structural insights into hydroxycoumarin-induced apoptosis in U-937 cells. Bioorganic Med. Chem. 2008, 16, 2665–2675. [Google Scholar] [CrossRef]
- Kawase, M.; Sakagami, H.; Hashimoto, K.; Tani, S.; Hauer, H.; Chatterjee, S.S. Structure-cytotoxic activity relationships of simple hydroxylated coumarins. Anticancer Res. 2003, 23, 3243–3246. [Google Scholar]
- Abd El-Karim, S.S.; Syam, Y.M.; El Kerdawy, A.M.; Abdelghany, T.M. New thiazol-hydrazono-coumarin hybrids targeting human cervical cancer cells: Synthesis, CDK2 inhibition, QSAR and molecular docking studies. Bioorganic Chem. 2019, 86, 80–96. [Google Scholar] [CrossRef]
- Ragab, F.A.; Eissa, A.A.M.; Fahim, S.H.; Salem, M.A.; Gamal, M.A.; Nissan, Y.M. Synthesis and biological evaluation of new coumarin derivatives as cytotoxic agents. Arch. Pharm. 2021, 354, 2100029. [Google Scholar] [CrossRef]
- Phutdhawong, W.; Chuenchid, A.; Taechowisan, T.; Sirirak, J.; Phutdhawong, W.S. Synthesis and biological activity evaluation of coumarin-3- carboxamide derivatives. Molecules 2021, 26, 1653. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, M.H.; Wang, F.; Chen, P.Y.; Ke, X.G.; Yu, B.; Yang, Y.F.; You, P.T.; Wu, H.Z. Network pharmacology and molecular docking reveal the mechanism of scopoletin against non-small cell lung cancer. Life Sci. 2021, 270, 119105. [Google Scholar] [CrossRef]
- Foo, J.; Michor, F. Evolution of resistance to targeted anti-cancer therapies during continuous and pulsed administration strategies. PLoS Comput. Biol. 2009, 5, e1000557. [Google Scholar] [CrossRef]
- Björn, N.; Badam, T.V.S.; Spalinskas, R.; Brandén, E.; Koyi, H.; Lewensohn, R.; De Petris, L.; Lubovac-Pilav, Z.; Sahlén, P.; Lundeberg, J.; et al. Whole-genome sequencing and gene network modules predict gemcitabine/carboplatin-induced myelosuppression in non-small cell lung cancer patients. npj Syst. Biol. Appl. 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Takizawa, S.; Shimada, S.; Tokudome, T.; Shindo, T.; Matsumoto, K. Effects of adrenomedullin on doxorubicin-induced cardiac damage in mice. Biol. Pharm. Bull. 2016, 39, 737–746. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Li, Y.; Tong, L.; Gu, X.; Meng, J.; Zhu, Y.; Wu, L.; Feng, M.; Tian, N.; et al. Transketolase deficiency protects the liver from DNA damage by increasing levels of ribose 5-phosphate and nucleotides. Cancer Res. 2019, 79, 3689–3701. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Lynch, R.M.; Rusyn, I.; Threadgill, D.W. Long-term combinatorial exposure to trichloroethylene and inorganic arsenic in genetically heterogeneous mice results in renal tubular damage and cancer-associated molecular changes. G3 2019, 9, 1729–1737. [Google Scholar] [CrossRef]
- Colli, L.M.; Machiela, M.J.; Zhang, H.; Myers, T.A.; Jessop, L.; Delattre, O.; Yu, K.; Chanock, S.J. Landscape of combination immunotherapy and targeted therapy to improve cancer management. Cancer Res. 2017, 77, 3666–3671. [Google Scholar] [CrossRef]
- Neelgundmath, M.; Dinesh, K.R.; Mohan, C.D.; Li, F.; Dai, X.; Siveen, K.S.; Paricharak, S.; Mason, D.J.; Fuchs, J.E.; Sethi, G.; et al. Novel synthetic coumarins that targets NF-κB in Hepatocellular carcinoma. Bioorg. Med. Chem. Lett. 2015, 25, 893–897. [Google Scholar] [CrossRef]
- Casley-Smith, J.R.; Morgan, R.G.; Piller, N.B. Treatment of lymphedema of the arms and legs with 5,6-benzo-[alpha]-pyrone. NEJM 1993, 329, 1158–1163. [Google Scholar] [CrossRef]
- Hernandez, A.V.; Bradley, G.; Khan, M.; Fratoni, A.; Gasparini, A.; Roman, Y.M.; Bunz, T.J.; Eriksson, D.; Meinecke, A.K.; Coleman, C.I. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. EHJ-QCCO 2020, 6, 301–307. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014, 5, 2371–2377. [Google Scholar] [CrossRef]
- Costas-Lago, M.C.; Besada, P.; Rodríguez-Enríquez, F.; Viña, D.; Vilar, S.; Uriarte, E.; Borges, F.; Terán, C. Synthesis and structure-activity relationship study of novel 3-heteroarylcoumarins based on pyridazine scaffold as selective MAO-B inhibitors. Eur. J. Med. Chem. 2017, 139, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Sun, Z.; Lu, Y.; Li, Y.Z.; Xu, H.R.; Zeng, C.Q. Osthole inhibits proliferation and induces apoptosis in BV-2 microglia cells in kainic acid-induced epilepsy via modulating PI3K/AKt/mTOR signalling way. Pharm. Biol. 2019, 57, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a bifunctional antioxidant prevents and counteracts the oxidative stress and neuronal death induced by amyloid protein in Sh-SY5Y cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, K.; Solnier, J.; Alperth, F.; Kunert, O.; Smole Možina, S.; Bucar, F. Efflux pump inhibition and resistance modulation in Mycobacterium smegmatis by Peucedanum ostruthium and its coumarins. Antibiotics 2021, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Palacharla, R.C.; Molgara, P.; Panthangi, H.R.; Boggavarapu, R.K.; Manoharan, A.K.; Ponnamaneni, R.K.; Ajjala, D.R.; Nirogi, R. Methoxsalen as an in vitro phenotyping tool in comparison with 1-aminobenzotriazole. Xenobiotica 2019, 49, 169–176. [Google Scholar] [CrossRef]
- Jantamat, P.; Weerapreeyakul, N.; Puthongking, P. Cytotoxicity and apoptosis induction of coumarins and carbazole alkaloids from Clausena harmandiana . Molecules 2019, 24, 3385. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Grabarska, A.; Florek-Łuszczki, M.; Plewa, Z.; Łuszczki, J.J. Synergy, additivity, and antagonism between cisplatin and selected coumarins in human melanoma cells. Int. J. Mol. Sci. 2021, 22, 537. [Google Scholar] [CrossRef] [PubMed]
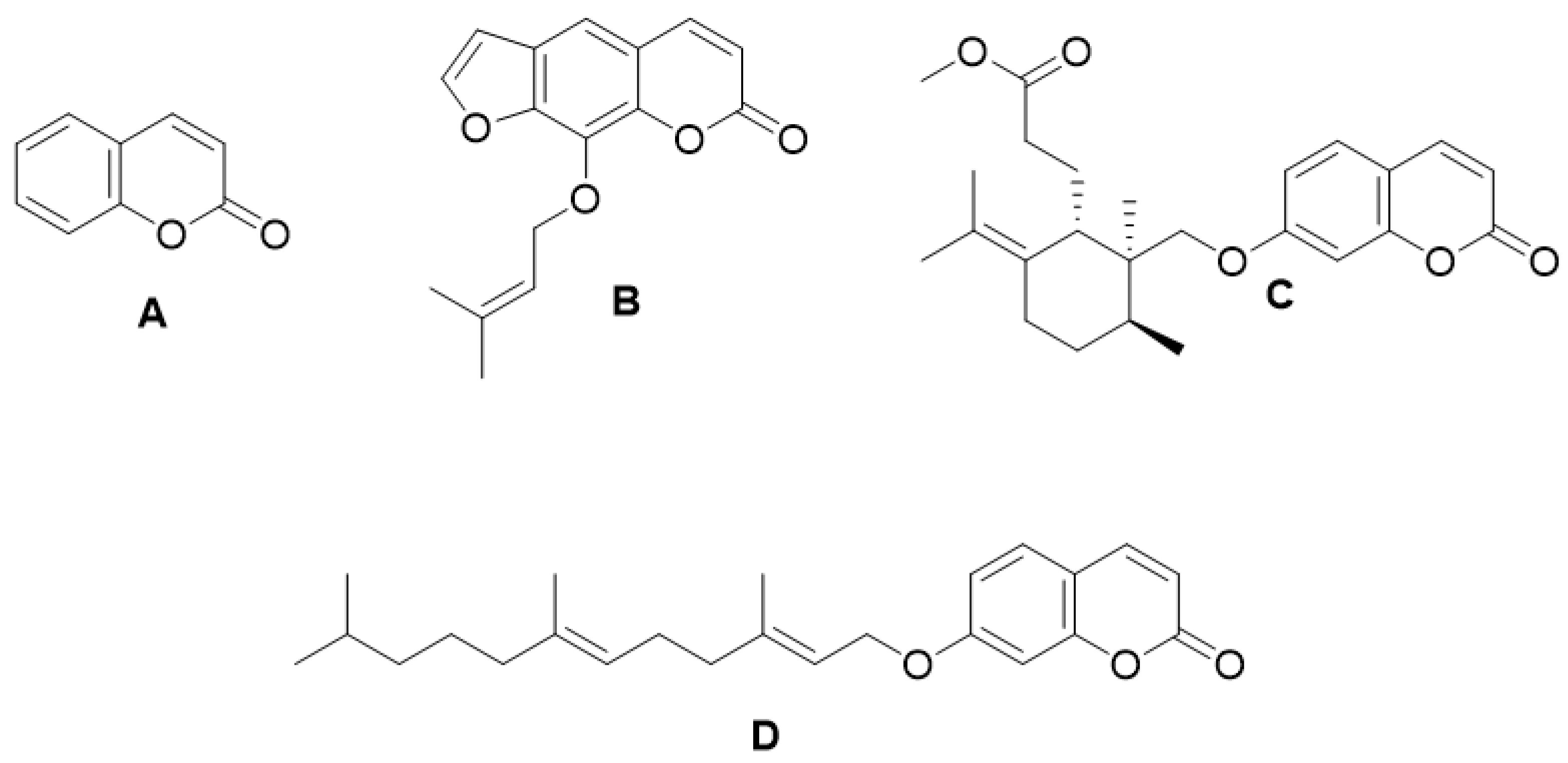
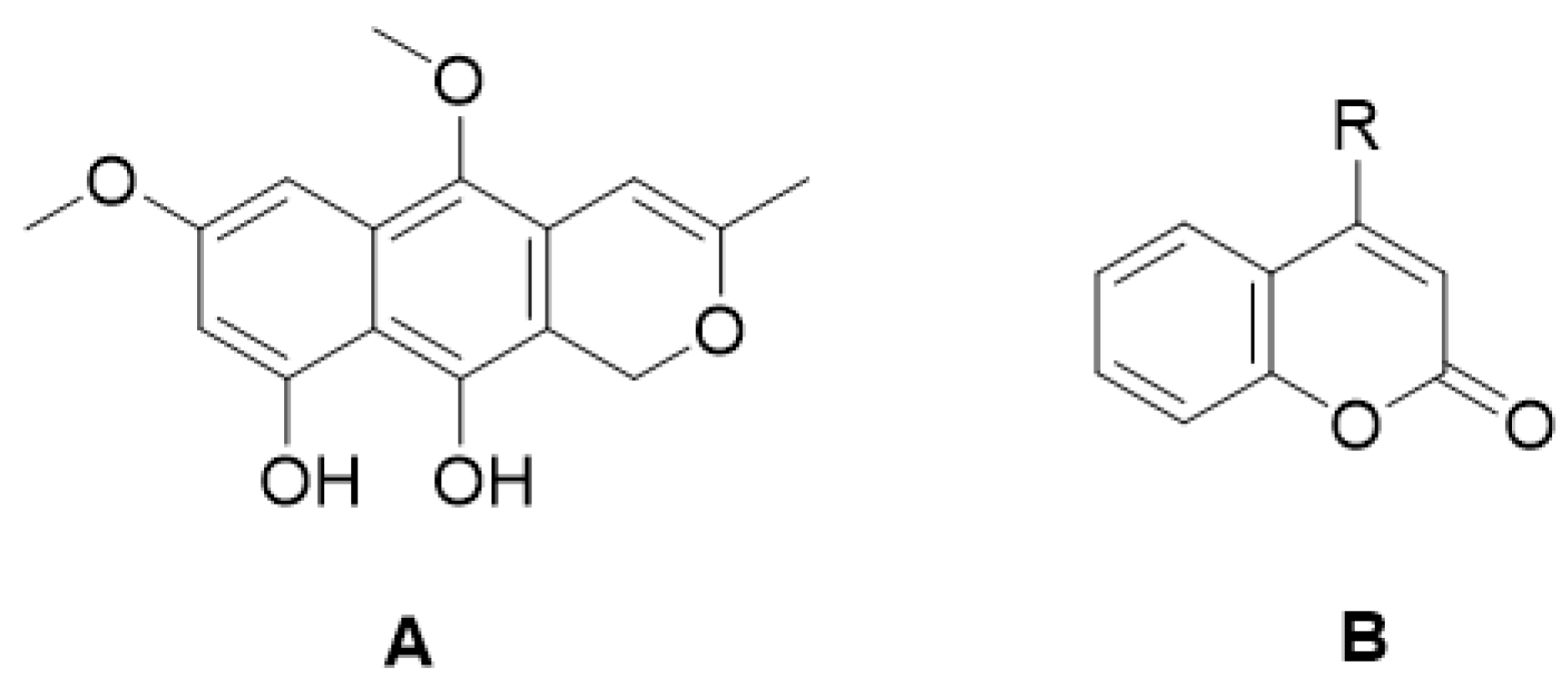


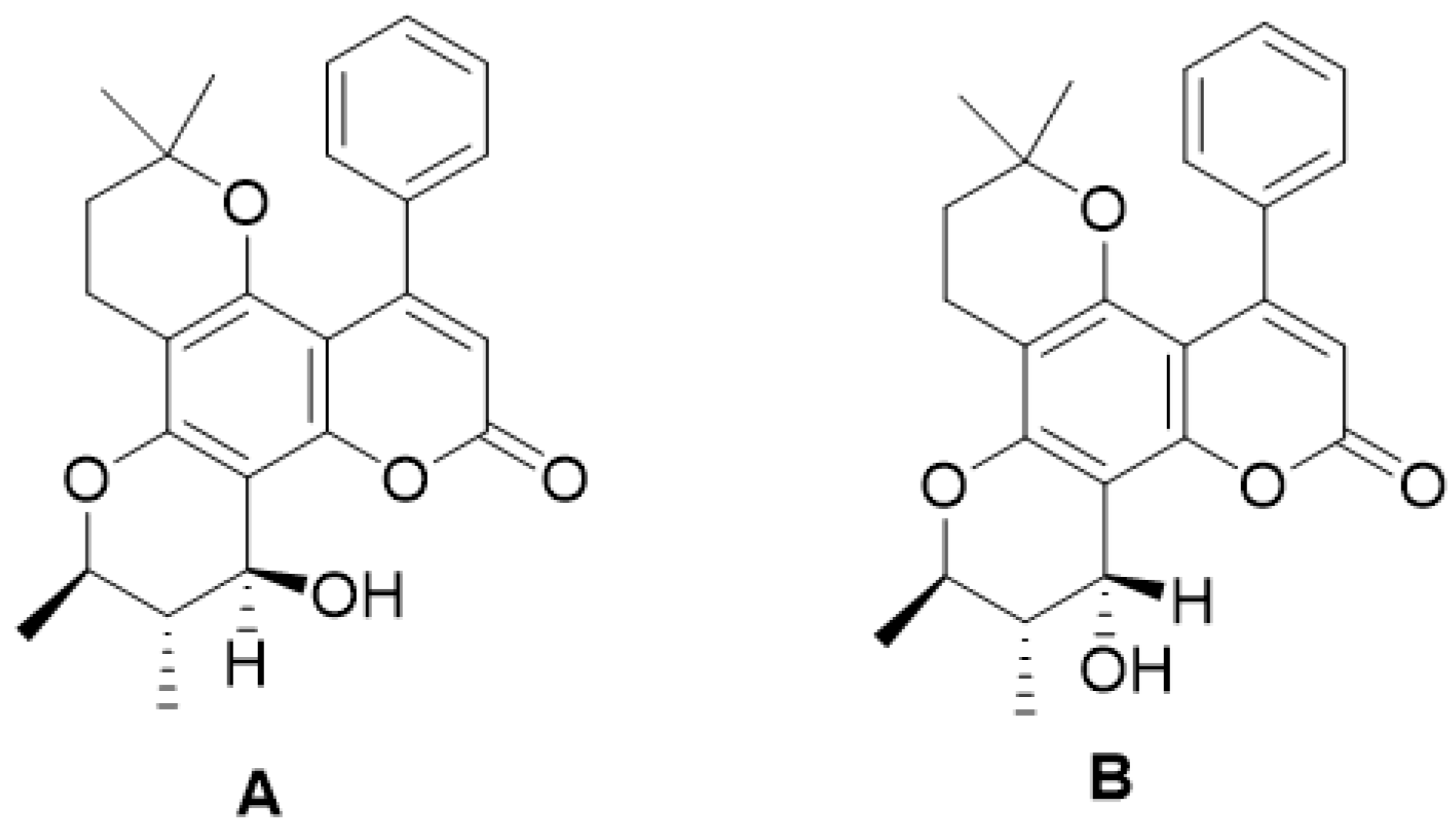
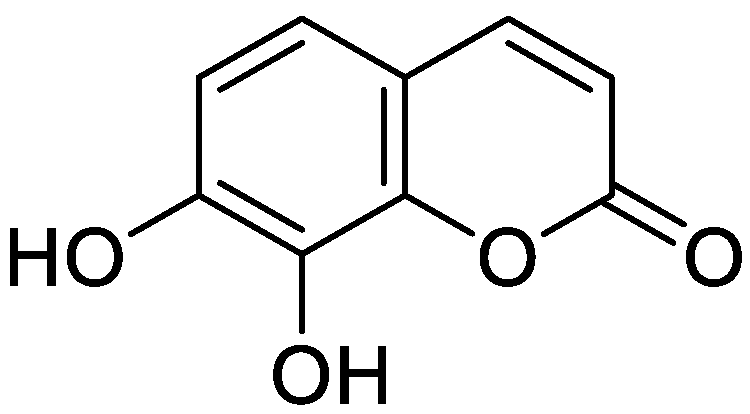
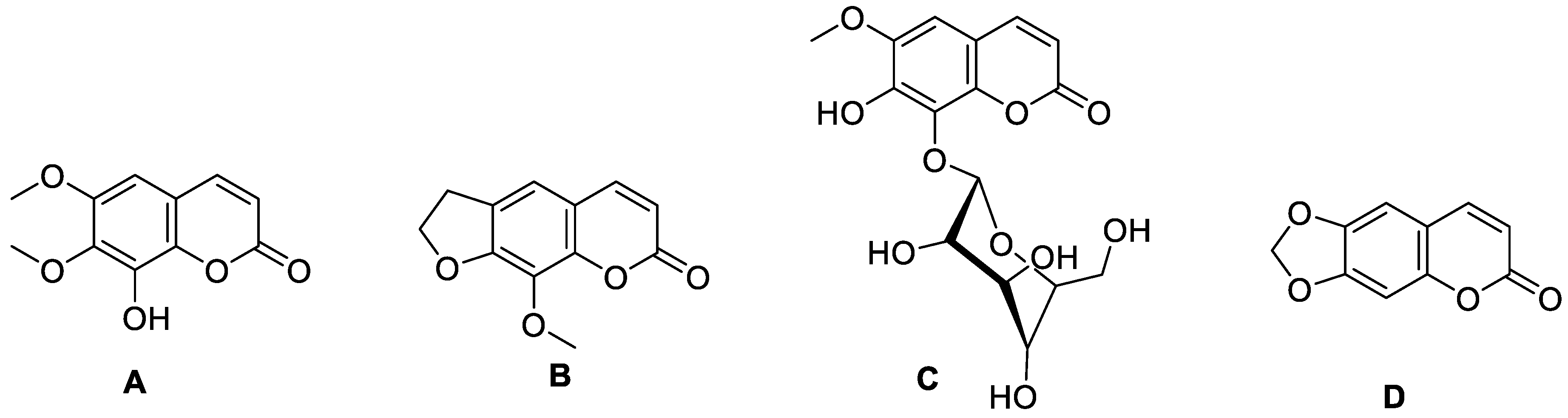


| Type of Coumarin | Structure | Examples of Coumarins | Reference |
|---|---|---|---|
| Single coumarin |  Umbelliferone (7-hydroxycoumarin) | Esculetin | [15] |
| Ostrutin | [16] | ||
| Osthole | [17] | ||
| Novobiocin | [18] | ||
| Coumermycin | [19] | ||
| Umbelliferone | [20] | ||
| Fraxidine | [21] | ||
| Ferudenol | [22] | ||
| Furanocoumarin |  Imperatorin | Isopimpinellin | [23] |
| Psoralen | [24] | ||
| Bergaptene | [25] | ||
| Methoxsalene | [26] | ||
| Marmelosin | [27] | ||
| Pyranocoumarin | 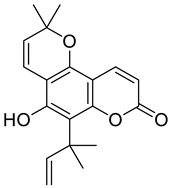 Nordentatin | 1. Linear type: | [28] |
| Grandivitin | [29] | ||
| Agasyllin | [30] | ||
| Aegelinol de Benzonatate | |||
| Xanthylethine | [31] | ||
| 2. Angular type: | |||
| Inophyllum A, B, C, E, P, G1 and G2 | [32] | ||
| Calanolide A, B and F | [33] | ||
| (+)-Dihydrocalanolide A and B | |||
| Pseudocordatolide | [34] | ||
| Biscoumarin |  Dicoumarol | Biscoumarin | [35] |
| Dihydrofuranocoumarin | 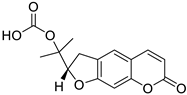 Felamidine | Anthogenol | [36] |
| Marmesin | [37] | ||
| Rutaretin | [38] | ||
| Phenycoumarins | 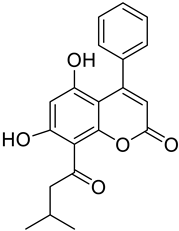 Isodispare B | Dispardiol B | [39] |
| Mammea A/AB | [40] | ||
| Disparinol D | [41] | ||
| Disparpropylinol B | [42] |
| Type of Cancer | Pharmacological Function | Structure | Substitute | Reference |
|---|---|---|---|---|
| Melanoma B16-F10 | In an in vitro study, 4-hydroxycoumarin demonstrated its therapeutic effect as an antineoplastic. By inhibiting the stability of the cytoskeleton, adhesion, and motility of the melanoma cell line B16-F10. | 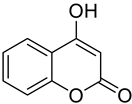 4-Hydroxycoumarin | An OH group at position 4 of the basic coumarin core | [153] |
| Lung | It was shown that 7-hydroxycoumarin exhibits an antiproliferative effect towards lung cancer cells (SK-LU-1, 1.3.15 and 3A5A). | 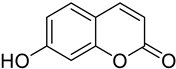 7-Hydroxycumarin | OH at position 7 of the coumarin (7-hydroxycoumarin Umbelliferone Basic nucleus) | [154] |
| Breast/ Ovarian/ Prostate/ Kidney | The synthesis of new 4,7-disubstituted coumarin derivatives as apoptosis-inducing agents targeting galectin-1 was performed by evaluating the cytotoxic effect on cancer cell lines: MCF7, SKOV3, PC-3, DU145, and HEK293T. Compound 7q* conjugate showed a potential antiproliferative effect against PC-3 prostate cancer cell lines by inhibiting the G1 phase of the cell cycle, reducing Gal-1 protein levels in a dose-dependent manner. | 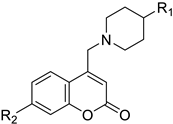 R1=COOC2H5 R2=4-NO2-C6H4-CH2 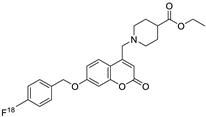 | Compound: 7q* substituent R2 (4-NO2-C6H4-CH2). | [155] |
| Prostate | Overexpression of 1-lipoxygenase-1 (15-LOX-1) is considered a malignancy factor for prostate cancer. Synthetic derivatives of farnesyloxycoumarin (3f, 4f, 7f)** are potential inhibitors of 15-LOX-1, especially 7-farnesyloxycoumarin exerting cytotoxic and anticancer effects against prostate cancer PC-3 cells. | 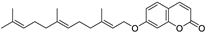 7-Farnesyloxycoumarin | Prenylated coumarins derived from farnesyloxycoumarins | [156] |
| Colon | Coumarin derivatives were tested in HCT116 colorectal cancer cell line, reducing its viability in a time- and concentration-dependent manner. Coumarin polysulfides accumulate in the G2-M phase of the cell cycle inducing apoptosis, decreasing balc-2, increased bax, cytochrome release, and inhibition of caspases 3-7 and cdc25C. They are considered potent antineoplastic agents. | 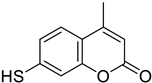 7-Mercapto-4-methyl-2H-chromen-2-one | Coumarin derivatives diallyl polysulfides (di-coumarin polysulfides) (di-coumarin polysulfides) | [157] |
| Lung/ Skin/ Kidney/ | Several of these compounds showed antiproliferative activity. Compounds 14 and 17*** showed higher apoptotic activity. They became promising candidates for cancer treatment. | 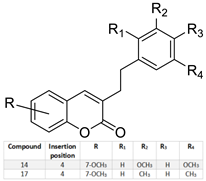 | Several of these compounds showed antiproliferative activity. Compounds 14 and 17*** showed higher apoptotic activity. They became promising candidates for cancer treatment. | [158] |
| Breast/ Human oral epidermoid carcinoma | The cytotoxic effect of compound 9c was tested against breast cancer (MDA-MB-231) and human oral epidermoid carcinoma cell line (KB) cell lines. This compound showed antiproliferative, apoptotic activity, regulating ROS and caspase-3 levels. | 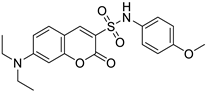 (7-(Diethylamino)-N-(4-methoxyphenyl)-2-oxo-2H-chromene-3-sulfonamide) | Coumarin derivatives with sulfonamide and amide substituents | [159] |
| Breast and Lung | This triad was shown to have a cytotoxic effect and a selective inhibition towards CDK4. These compounds were tested in breast cancer cell lines (MCF-7) and human lung carcinoma (A-549) and the IC50 was evaluated with good results. | 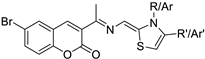 thiazole-hydrazono-coumarin | 6-bromo-3-(substituted thiazolidine-2-ylidene) hydrazineylidene)-coumarin derivatives with different substituents at the 3-position of the coumarin nucleus | [160] |
| Bone marrow (myeloma) | This compound showed an inhibitory effect against two enzymes, carbonic anhydrase, and lipoxygenase, which are involved in the development of cancer, so this compound is considered a promising target for inhibiting these enzymes. | 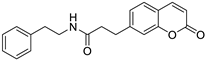 2-(2-(2-Oxo-2H-chromen-7-yl) oxy)-N-phenethylacetamide | 6,7-substituted coumarins derived from 7-hydroxycoumarins | [161] |
| Prostate/ Melanoma/ Kidney | A coumarinic derivative 3,3’-methylenebis(4-hydroxycoumarin) “Dicoumarol” was shown to have an antineoplastic effect when tested in vitro in prostate, kidney, and melanoma cancer cell lines, as it inhibits cell proliferation by affecting mitotic spindle microtubules in the cell cycle phases. | 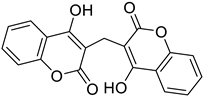 3,3’-methylenebis(4-hydroxycoumarin) | In position 3 there is a methylene bond | [162] |
| Bladder | 7-isopentenyloxycoumarin was shown to have anticancer effects by demonstrating a cytotoxic effect against bladder cancer cell lines (5673 cells) by inducing apoptosis and inhibition of the G2/M phases of the cell cycle. | 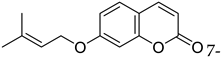 7-isopentenyloxycoumarin 7-isopentenyloxycoumarin | Isopentenyl group | [163] |
| Small cell lung | 7,8-Dihydroxy-4-methylcoumarin was shown to induce apoptosis tested in A549 lung cancer cell lines by activating the mitochondria-mediated caspase-dependent pathway. |  7,8-Dihydroxy-4-methylcoumarin | Methyl group at position 4 and 7,8 Hydroxy. | [164] |
| Breast and Colon | A new coumarinic derivative containing [3,4-d] imidazole-4(1H)-one was synthesized and its biological effect, named “compound 35”, was evaluated. It showed anticancer activity against colon cancer cell lines HCT116 and breast cancer MCF-7, affecting apoptosis and inhibiting the G0/G1 phases of the cell cycle. | 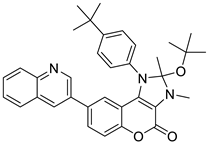 2-(4-(2-(tert-butoxy)-2,3-dimethyl-4-oxo-8-(quinolin-3-yl)-2,3-dihydrochromeno [3,4-d] imidazol-1 490 (4H)-yl) phenyl)-2-methylpropanenitrile (Compound 35) | [3,4-d] imidazole-4(1H)-one derivatives | [165] |
| Lung | Isofraxidin was shown to possess apoptosis-inducing activity against A549 lung cancer cell lines demonstrating its effectiveness as a potential anticancer agent. | 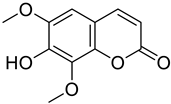 7-hydroxy-6, 8-dimethoxy coumarin (Isofraxidin) | OH group at position 7 and methoxy at positions 6 and 8 | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. https://doi.org/10.3390/molecules28052413
Flores-Morales V, Villasana-Ruíz AP, Garza-Veloz I, González-Delgado S, Martinez-Fierro ML. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules. 2023; 28(5):2413. https://doi.org/10.3390/molecules28052413
Chicago/Turabian StyleFlores-Morales, Virginia, Ana P. Villasana-Ruíz, Idalia Garza-Veloz, Samantha González-Delgado, and Margarita L. Martinez-Fierro. 2023. "Therapeutic Effects of Coumarins with Different Substitution Patterns" Molecules 28, no. 5: 2413. https://doi.org/10.3390/molecules28052413
APA StyleFlores-Morales, V., Villasana-Ruíz, A. P., Garza-Veloz, I., González-Delgado, S., & Martinez-Fierro, M. L. (2023). Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules, 28(5), 2413. https://doi.org/10.3390/molecules28052413








