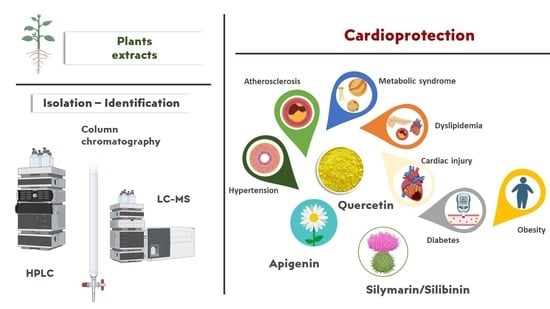
Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin
Abstract
1. Introduction
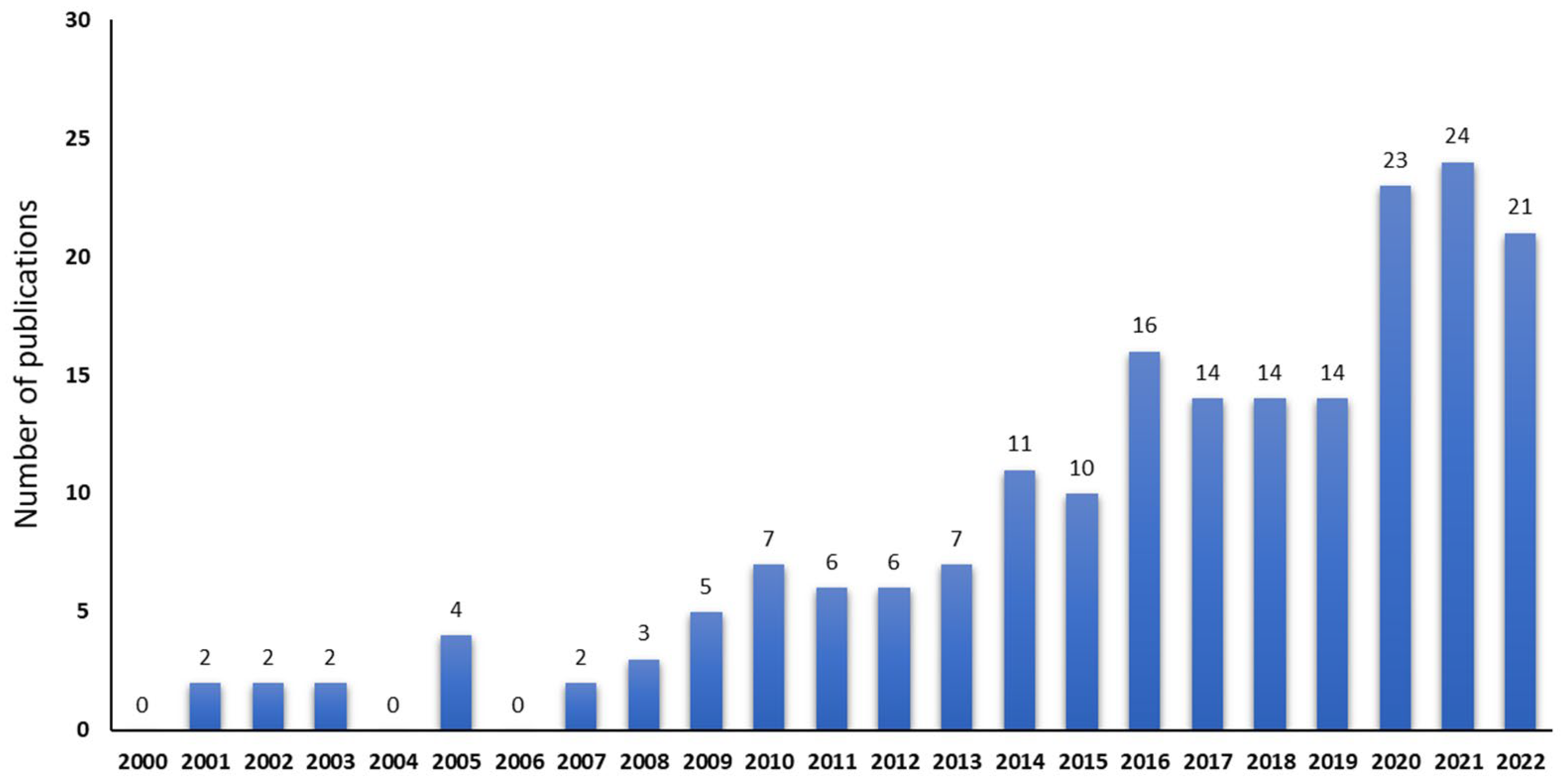
2. Literature Search Strategy
3. Results and Discussion
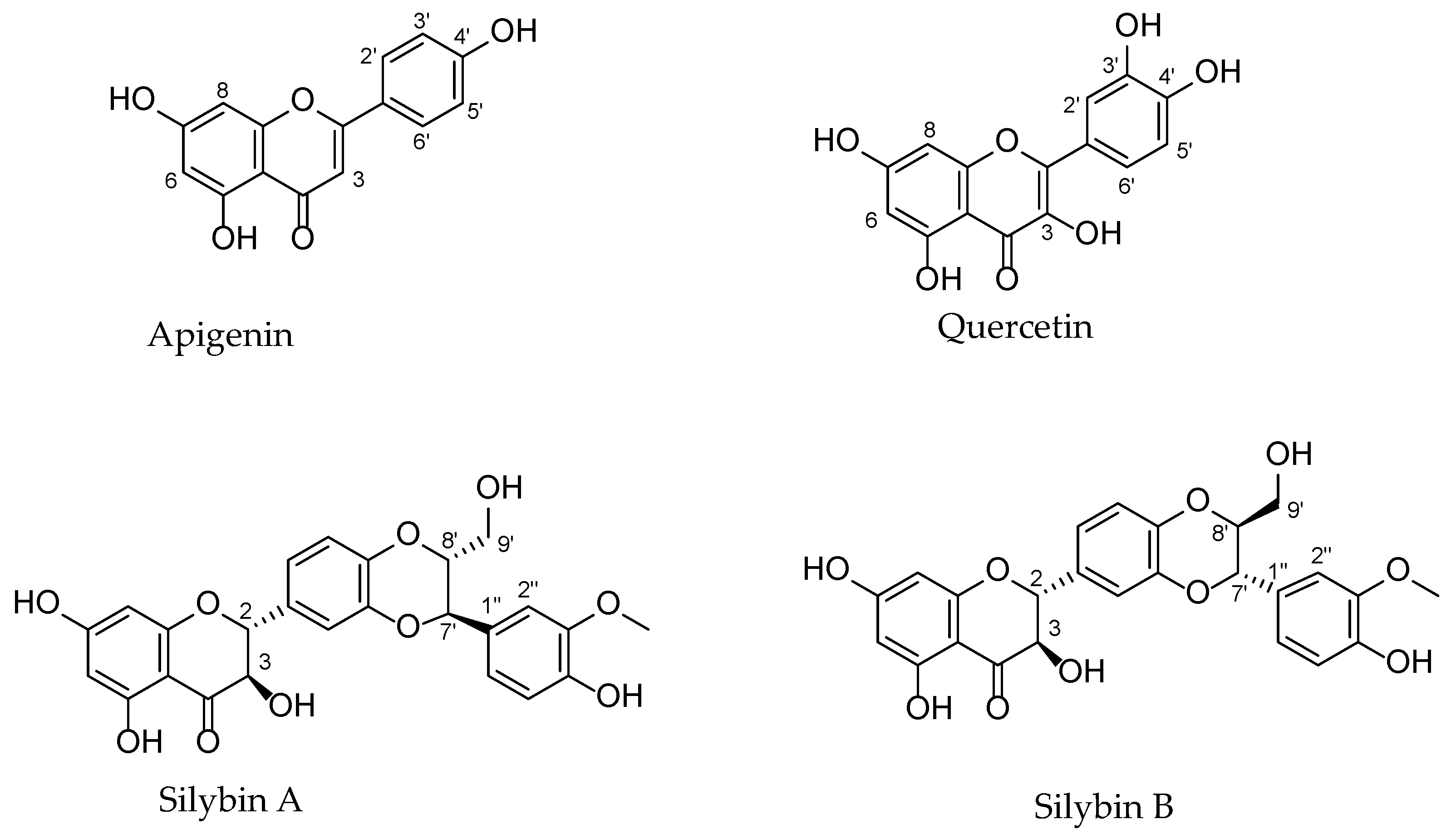
3.1. Chemical Structure, Plant Origin (Family), Methods of Isolation, and Identification
3.1.1. Apigenin
Methods for Isolation: Column Chromatography and Preparative HPLC
Methods for Identification: HPLC and LC-MS Analysis
3.1.2. Quercetin
Methods for Isolation: Column Chromatography and Preparative HPLC
Methods for Identification: HPLC and LC-MS Analysis
3.1.3. Silymarin Extract and Constituents
Methods for Isolation and Identification
| Botanical Name (Family) | Extract/Residue-Fraction | Plant Parts | Method/Solvents | References |
|---|---|---|---|---|
| Apigenin | ||||
| Ailanthus excelsa Roxb. [A. excelsus Roxb.] (Simaroubaceae) | 70% Methanol/Ethyl acetate (isolation) | L. | CC (Sephadex LH-20) | [22] |
| Chrysanthemum morifolium Ramat. (Asteraceae) | Aqueous, Ethanol (identification) | Fl. | LC-MS | [36] |
| Cynara cardunculus L. (Asteraceae) | Aqueous (identification) | L. | HPLC analysis | [35] |
| Gentiana veitchiorum Hemsl. (Gentianaceae) | 70% Methanol (identification) | Fl. | HPLC-MS/MS 0.1% formic acid/water and methanol | [31] |
| 70% Methanol (isolation) | CC (silica gel)/CHCl3-MeOH (100:1 to 1:1), Semi-prep HPLC/MeCN-H2O | |||
| Matricaria recutita L. (Asteraceae) | 70% Methanol (isolation) | L. | CC (Sephadex LH-20)/acetone | [30] |
| Merremia tridentata (L.) Hallier f. (Convolvulaceae) | Aqueous, 50% Ethanol (isolation; identification) | Stem; R. | CC (silica gel)/MeOH, CHCl3 | [34] |
| HPLC-DAD | ||||
| Petroselinum crispum (Mill.) Nym. ex A.W. Hill (Apiaceae) | Aqueous/Ethyl acetate (isolation) | L. | CC (Sephadex LH-20)/EtOH | [26] |
| Premna foetida Renw. ex Blume (Lamiaceae) | Methanol (identification) | L. | RP-HPLC/0.1% H3PO4: ACN (gradient system) | [27] |
| Chloroform (isolation) | CC | |||
| Platycodon grandiflorum (Jacq.) A. DC. [P. grandiflorum A. DC.] (Campanulaceae) | Ethanol/Ethyl acetate (isolation) | Fl. | CC (silica gel)/CH2Cl2: MeOH (19:1 to 9:1) | [25] |
| Morus indica L. (Moraceae) | 80% Methanol (isolation) | L. | prep-HPLC | [32] |
| Sophora alopecuroides L. (Leguminosae) | 75% Ethanol/Ethyl acetate (isolation) | A.p.; R.; S. | CC (Sephadex LH-20)/MeOH | [33] |
| Teucrium polium L. (Lamiaceae) | Methanol (isolation) | A.p. | CC (silica gel)/different solvent systems | [23,24] |
| CC (Sephadex LH-20)/MeOH | ||||
| Ziziphora clinopodioides Lam. (Lamiaceae) | Hydroalcoholic (80% Ethanol:20% Water)/Dichloromethane (isolation) | Whole plant | CC (Sephadex LH-20) Flash CC (silica gel) | [28] |
| Quercetin | ||||
| Acacia arabica (Lam.) Willd. (Leguminosae) | Hot water (isolation) | B. | RP-HPLC | [59] |
| Allium victorialis L. (Alliaceae; Liliaceae p) | 50% Ethanol/Ethyl acetate (isolation) | L. | CC | [44] |
| Anacardium humile A.St.-Hil. (Anacardiaceae) | 98% Ethanol (identification) | L. | HPLC-ESI-MS/MS)/water acidified with formic acid (0.1% v/v) and MeOH | [66] |
| Artemisia capillaris Thunb. (Asteraceae) | Methanol (isolation) | Whole plant | CC | [43] |
| Bauhinia megalandra Griseb. (Leguminosae) | Methanol/Ethyl acetate-acetone (8:2) (isolation) | L. | CC (Sephadex LH-20) | [74] |
| Bauhinia strychnifolia Craib. (Leguminosae) | Ethanol/Ethyl acetate (isolation; identification) | Stem | CC (Sephadex LH-20) | [61] |
| LC-QTOF/MS | ||||
| Bryophyllum pinnatum (Lam.) Oken (Crassulaceae) | Methanol/Ethyl acetate (isolation) | L. | CC (silica gel)/ MeOH:EtOAc:H2O (5:3:2) | [53] |
| Carya illinoinensis (Wangenh.) K. Koch (Juglandaceae) | 70% Ethanol (isolation) | B. | CC | [40] |
| Cordia boissieri A.DC. (Boraginaceae) | Hydroalcoholic/Ethyl acetate (isolation) | L. | CC (polyamide, Sephadex LH-20) | [51] |
| Coreopsis lanceolata L. (Asteraceae) | Methanol/Ethyl acetate (isolation) | Fl. | RP-CC/MeOH:H2O; CH3CN:H2O, CC (Sephadex LH-20)/MeOH | [57] |
| Coreopsis tinctoria Nutt. (Asteraceae) | Ethanol (isolation) | Flower buds | ODS-RP-18 column/MeOH: H2O, CC (Sephadex LH-20)/MeOH | [46] |
| Crataegus pinnatifida Bge. var. major N.E.Br. [C. pinnatifida f. major (N.E.Br.) W.Lee] (Rosaceae) | 70% Ethanol | L. | CC | [60] |
| Cuscuta pedicellata Ledeb. (Convolvulaceae) | Ethanol (isolation) | Whole plant | CC | [48] |
| Cyclocarya paliurus (Batal.) Iljinsk. (Juglandaceae; Cyclocaryaceae p) | 75% Ethanol/Chloroform (isolation) | B. | CC (silica gel, Sephadex LH-20) | [41] |
| Cynanchum acutum L. (Asclepiadaceae; Apocynaceae p) | Methanol/Ethyl acetate (isolation) | Whole plant | CC (Sephadex LH-20) | [58] |
| Dillenia indica Blanco (Dilleniaceae) | Methanol/Ethyl acetate (isolation) | L. | CC | [45] |
| Geigeria alata (DC), Oliv. and Hiern. [G. alata Benth. and Hook.f. ex Oliv.] (Asteraceae) | 80% Ethanol/Chloroform, Ethyl acetate (isolation) | n.d. | CC (silica gel)/DCM:MeOH | [56] |
| Lactuca serriola L. (Asteraceae) | Methanol (isolation) | A.p. | n.d. | [55] |
| Leonurus cardiaca L. (Lamiaceae) | 70% Ethanol (identification) | A.p. | HPLC | [63] |
| Mandevilla moricandiana Woodson (Apocynaceae) | Hydroalcoholic (70% Ethanol: 30% Water)/Ethyl acetate (identification) | L. | UHPLC-DAD-ESI-MSn | [65] |
| Phyllanthus emblica L. (Euphorbiaceae) | Methanol (isolation) | Fr. | CC (silica gel)/CHCl3: MeOH | [54] |
| Polygonum hyrcanicum Rech.f. (Polygonaceae) | Methanol/Ethyl acetate (isolation) | A.p. | CC (silica gel, Sephadex LH-20) | [42] |
| Pueraria thomsonii Benth (Fabaceae) | 75% Ethanol/Ethyl acetate (isolation; identification) | L. | CC (silica gel, SHP-20P) | [62] |
| HPLC-DAD | ||||
| Sarcopyramis nepalensis Wall. (Melastomataceae) | 70% Ethanol/Ethyl acetate (isolation) | Whole plant | CC (Sephadex LH-20/MeOH) | [47] |
| Sophora alopecuroides L. (Leguminosae) | 75% Ethanol/Ethyl acetate (isolation) | A.p.; R.; S. | CC (Sephadex LH-20)/MeOH | [33] |
| Tetracera indica Merr. [T. indica (Christm. and Panz.) Merr.] (Dilleniaceae) | Ethanol/Ethyl acetate (isolation) | Stems | CC (Silica gel, Sephadex LH-20) | [50] |
| Toona sinensis (A.Juss.) M.Roem. (Meliaceae) | 80% Ethanol/Chloroform, Ethyl acetate (isolation) | L. | CC (silica gel)/n-hexane:EtOAc:MeOH, capillary electrophoresis using silica gel CC | [49] |
| Ugni molinae Turcz. (Myrtaceae) | Aqueous (identification) | Fr. | HPLC/1% HCOOH: ACN | [64] |
| Xenophyllum poposum (Phil.) V.A.Funk (Asteraceae) | Hydroalcoholic (Ethanol:Water, 1:1)/Ethyl acetate (isolation; identification) | A.p. | CC | [52] |
| HPLC-DAD-MS/MS | ||||
| Flavonolignans and extracts of Silybum marianum (L.) Gaertn. (Asteraceae) | ||||
| Silymarin constituents | n.d. (identification) | n.d. | HPLC-DAD/H2O + 0.1% HCOOH; MeOH + 0.1% HCOOH | [69] |
| Silychristin | n.d. (isolation) | prep-HPLC | [69] | |
| S. marianum | Ethyl acetate (identification) | S. | HPLC/H3PO4: MeOH: H2O (0.5:35:65–0.5:50:50 v/v/v) | [72] |
| S. marianum | Ethanol:Water (1:1) (identification) | S. | HPLC-DAD/ water with 0.1% formic acid; MeOH (1:1) | [73] |
3.2. Physicochemical and Biopharmaceutical Properties
3.2.1. Apigenin
3.2.2. Quercetin
3.2.3. Silymarin Extract and Constituents
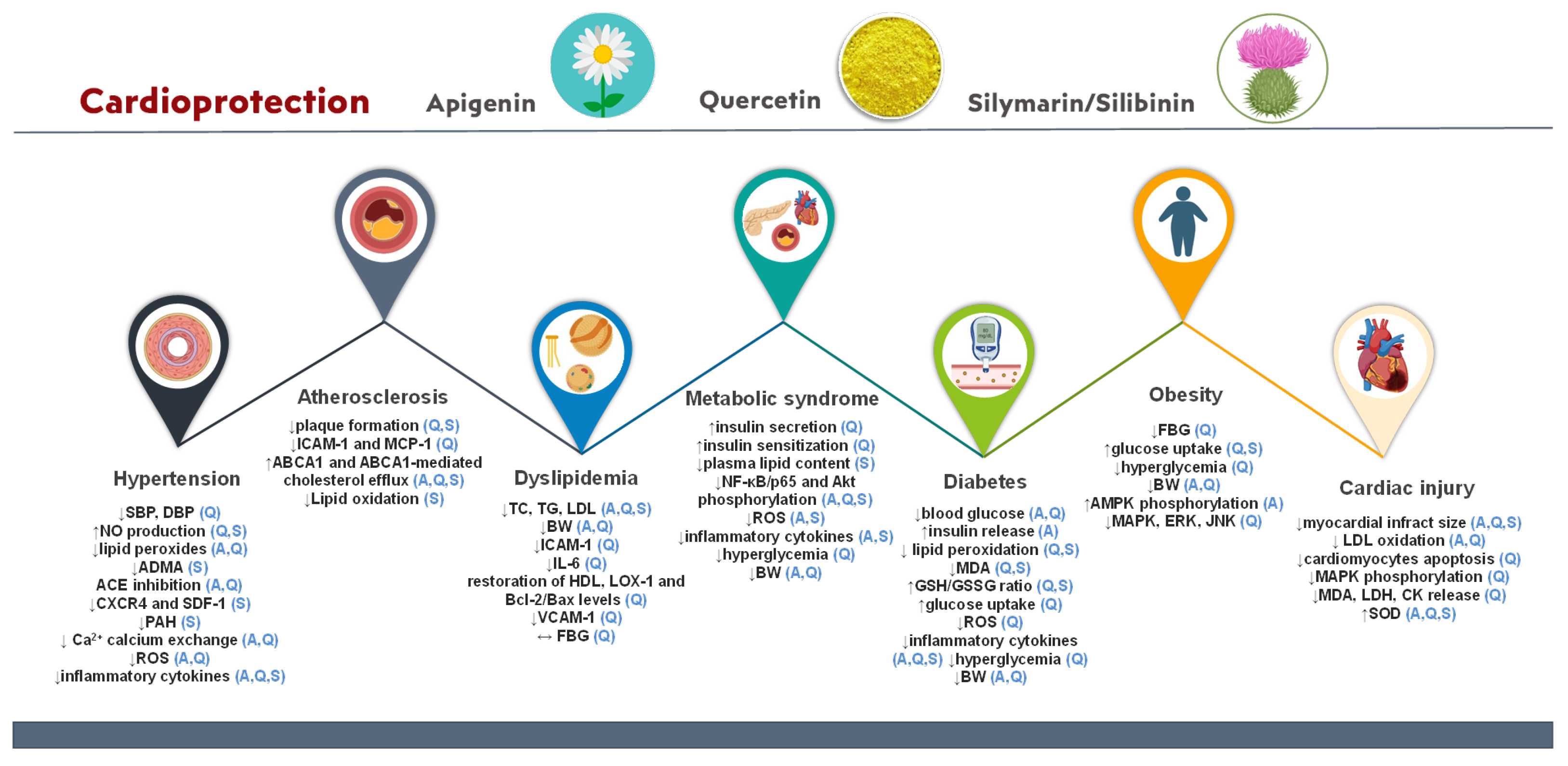
3.3. Bio-Actives’ Cardiovascular Prevention Activity Based on Preclinical and Clinical Studies
3.3.1. Hypertension
3.3.2. Diabetes
3.3.3. Dyslipidemia
3.3.4. Atherosclerosis
3.3.5. Obesity
3.3.6. Cardiac Injury
3.3.7. Metabolic Syndrome
| Cardiovascular Disease | Mechanism | Bio-Active | References |
|---|---|---|---|
| Hypertension | ↓SBP and DBP | quercetin | [96,109,110] |
| ↓ADMA | silibinin | [18] | |
| ↓CXCR4 and SDF-1 | |||
| ↓PAH | |||
| ↓ROS, ↓oxidative stress, and ↓MCP-1 | apigenin, quercetin | [11,93,94,95,96] | |
| ↓overproduction of eNOS and cNOS | [21,103,104,105] | ||
| ↓lipid peroxides | [97] | ||
| Activation of AMPK/SIRT1 | [98,99] | ||
| ACE inhibition | [22,102] | ||
| Inhibition of calcium exchange | [46,64] | ||
| ↑NO production/bioavailability and vasorelaxation | quercetin, silibinin | [29,35,41,94,95,96] | |
| ↓inflammatory cytokines (IL-1β, IL-6, IL-10, TNF-α, and MCP-1) | apigenin, quercetin, silibinin | [11,18,95] | |
| Diabetes | Restoration of Bcl-2/Bax levels | apigenin | [126] |
| ↓ TNF-α and IL-6 | [125] | ||
| ↓ CK-MB and LDH | [124] | ||
| ↑insulin release and sensitivity | [24] | ||
| Inhibition of PKCβII activation | [104] | ||
| ↓ICAM-1 and E-selectin | [36] | ||
| ↓ROS oxidative stress | quercetin | [118] | |
| ↑glucose uptake via GLUT4 stimulation | [115,116,117,119] | ||
| DPP-IV inhibition | [57] | ||
| ↓ TNF-α, IL-1β, and IFNγ | silibinin/silymarin | [122,123,133] | |
| ↓pancreatic protein damage and creatinine levels | [135] | ||
| ↓blood glucose levels | apigenin, quercetin | [32,54] | |
| Inhibition of myocardial fibrosis and cardiac remodeling | apigenin, silymarin | [122,126,128,129,130] | |
| Inhibition of lipid peroxidation, ↓MDA, and ↑GSH/GSSG ratio | quercetin, silymarin | [72,118,131,132] | |
| ↓NF-κB/p65 and Akt phosphorylation | apigenin, quercetin, silibinin | [36,49,58,95,123,126] | |
| Dyslipidemia | Restoration of HDL, LOX-1, and Bcl-2/Bax levels | quercetin | [139,142,143] |
| ↓ICAM-1, ↓IL-6, and ↓VCAM-1 | [144,145,146] | ||
| ↓lipid accumulation | apigenin, quercetin | [139,146] | |
| ↓BW | [140,141] | ||
| ↓levels of TC, TG, and LDL | apigenin, quercetin, silymarin | [140,141] | |
| Atherosclerosis | ↓proinflammatory cytokines | apigenin | [11,155,159,190] |
| ↓ICAM-1 and MCP-1 | quercetin | [156,158] | |
| ↓elastin degradation, ↓macrophage infiltration, and ↓MMP-9 and VCAM-1 expression | [168,169,170] | ||
| ↓LDL oxidation | silymarin | [165] | |
| Induction of autophagy and foam cell formation | apigenin, quercetin | [154,155,156,157] | |
| ↓atherosclerotic plaque formation | quercetin, silymarin | [17,166] | |
| ↑ABCA1 and ABCA1-mediated cholesterol efflux | apigenin, quercetin, silymarin | [159,160,161,162,163,164] | |
| ↓inflammation via TLR-4/NF-κB signaling pathway | |||
| Obesity | ↓BW | apigenin, quercetin | [140,176,177] |
| ↑AMPK phosphorylation | apigenin | [173] | |
| ↓fatty acid-binding protein 4 and stearoyl-CoA desaturase | |||
| Downregulation of MAPK, ERK, and JNK | quercetin | [177] | |
| ↑glucose uptake | quercetin, silymarin | [59,69,160,178] | |
| ↓fasting blood glucose levels | quercetin | [42,43,45,61,62] | |
| ↓activity of pancreatic lipase and fatty acid synthase | apigenin | [174] | |
| Cardiac injury | Inhibition of cardiomyocyte apoptosis via the PI3K/Akt and SIRT1/TMBIM6 pathways | quercetin | [12,182,183] |
| Stimulation of mitophagy events | [184] | ||
| Impedes Ca2+ influx via L-type Ca2+ channels | [186] | ||
| Inhibition of MAPK phosphorylation and MDA, LDH, and CK release | [187] | ||
| ↓MAPK | [194] | ||
| Anti-platelet activity | apigenin, quercetin | [26] | |
| ↓LDL oxidation | [27] | ||
| ↓myocardial infract size | apigenin, quercetin, silibinin | [189,191,192] | |
| ↑SOD activity | |||
| ↓ER and oxidative stress, reverse of inflammation via the NF-kB pathway | silibinin | [193] | |
| Metabolic syndrome | ↑insulin secretion and sensitization | quercetin | [120,121] |
| ↓plasma lipid content | silymarin | [200] | |
| ↑NAD+ levels in liver | apigenin, silymarin | [75,195] | |
| ↓inflammatory cytokines | [75,196,197] | ||
| ↓ROS production and oxidative stress in β pancreatic cells |
| Cardiovascular Disease | Study Design | Main Outcomes | Bio-Active | Ref. |
|---|---|---|---|---|
| Hypertension | Meta-analysis: Seven RCTs, 587 pts, HTN, healthy individuals | ↓SBP | quercetin | [112] |
| Meta-analysis: Ten RCTs, 841 pts, HTN, healthy individuals | ↓SBP and DBP | [113] | ||
| Cohort study, 15,662 pts, healthy individuals | No effect on hypertension incidence | [114] | ||
| Diabetes | Non-controlled pilot study, 15 pts, T2DM | ↓glycosylated hemoglobin, ↓basal insulin, ↓TSH, ↓usCRP, ↓both SBP, ↓DBP | quercetin | [137] |
| Meta-analysis: Ten clinical trials, 700 pts, healthy, T2DM, NAFLD | ↓FBG, ↓HbA1c, ↓insulin, ↓TC, ↓TG, ↓LDL, ↑HDL | silymarin | [15] | |
| Meta-analysis: Five RCTs, 270 pts, healthy, T2DM | ↓FBG, ↓HbA1c | [138] | ||
| Dyslipidemia | Meta-analysis: Five RCTs, 442 pts, healthy, T2DM, HTN, hyperlipidemia | ↓TG | quercetin | [147] |
| Meta-analysis: Sixteen RCTs, 1575 pts, healthy, HTN, T2DM, hypercholesterolemic | ↓TC, ↔TG, ↓LDL | [148] | ||
| Double-blinded, placebo-controlled cross-over study, 175 pts, overweight with high-CVD risk | ↓LDL | [149] | ||
| Randomized, double-blinded, placebo-controlled cross-over trial, 70 pts, overweight-to-obese patients with pre-hypertension | ↔FBG, ↔LDL | [150] | ||
| Meta-analysis: Five RCTs, 270 pts, healthy, T2DM | ↔lipid levels | silymarin | [138] | |
| Meta-analysis: Eight RCTs, 195 pts, T2DM | ↓FBG, ↓HbA1c, ↓LDL, ↓MDA, ↑HDL | [139] | ||
| Meta-analysis: Ten RCTs, 620 pts, hyperlipidemic | ↓TC, ↓TG, ↓LDL, ↑HDL | [152] | ||
| Obesity | Randomized, placebo-controlled, double-blind trial, 110 pts, MS | ↓BW, ↓SBP, ↓DBP, ↓TC, ↓LDL, ↓fasting plasma insulin | quercetin | [179] |
| Double-blind crossover study, 49 pts, healthy with different APOE isoforms | ↓waist circumference, ↓TG, ↑HDL | [180] | ||
| Meta-analysis: Seven RCTs, 896 pts, healthy, obese, HTN | ↓SBP, ↓DBP, ↔BW, ↔BMI, ↔waist circumference, ↔waist-to-hip ratio | [181] | ||
| Double-blinded, placebo-controlled cross-over study, 172 pts, overweight, high-CVD risk phenotype | ↓SBP, ↓ox-LDL, ↔TNF-a, ↔C-reactive protein | [149] | ||
| Metabolic syndrome | Meta-analysis: Eighteen RCTs, 987 pts, HTN, overweight, MS, T2DM, NAFLD | ↓SBP, ↓DBP, ↓TC, ↓TG, ↓LDL, ↑HDL, ↓glucose levels | quercetin | [202] |
| Meta-analysis: Nine RCTs, 781 pts, HTN, T2DM, obesity, PCOS | ↔FBG, ↔HbA1c, ↓insulin, | [203] |
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thiriet, M. Cardiovascular Disease: An Introduction. In Vasculopathies; Biomathematical and Biomechanical Modeling of the Circulatory and Ventilatory Systems; Springer: Cham, Switzerland, 2018; Volume 8, pp. 1–90. ISBN 978-3-319-89314-3. [Google Scholar]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 December 2022).
- Popiolek-Kalisz, J.; Fornal, E. The Impact of Flavonols on Cardiovascular Risk. Nutrients 2022, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective Effects of Different Flavonoids against Myocardial Ischaemia/Reperfusion Injury in Langendorff-Perfused Rat Hearts. JPP 2013, 65, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between Flavonoids and Cardiovascular Disease Incidence or Mortality in European and US Populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Wang, H. Protective Roles of Apigenin Against Cardiometabolic Diseases: A Systematic Review. Front. Nutr. 2022, 9, 875826. [Google Scholar] [CrossRef]
- Gao, H.-L.; Yu, X.-J.; Hu, H.-B.; Yang, Q.-W.; Liu, K.-L.; Chen, Y.-M.; Zhang, Y.; Zhang, D.-D.; Tian, H.; Zhu, G.-Q.; et al. Apigenin Improves Hypertension and Cardiac Hypertrophy Through Modulating NADPH Oxidase-Dependent ROS Generation and Cytokines in Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 721–736. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of Silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The Food Plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and Clinical Evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- Radjabian, T.; Huseini, H.F. Anti-Hyperlipidemic and Anti-Atherosclerotic Activities of Silymarins from Cultivated and Wild Plants of Silybum marianum L. With Different Content of Flavonolignans. Iran. J. Pharmacol. Ther. 2010, 9, 6367. [Google Scholar]
- Zhang, T.; Kawaguchi, N.; Yoshihara, K.; Hayama, E.; Furutani, Y.; Kawaguchi, K.; Tanaka, T.; Nakanishi, T. Silibinin Efficacy in a Rat Model of Pulmonary Arterial Hypertension Using Monocrotaline and Chronic Hypoxia. Respir. Res. 2019, 20, 79. [Google Scholar] [CrossRef]
- International Plant Names Index (IPNI). Available online: https://www.ipni.org/ (accessed on 11 December 2022).
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef]
- Paredes, M.; Romecín, P.; Atucha, N.; O’Valle, F.; Castillo, J.; Ortiz, M.; García-Estañ, J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients 2018, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of Angiotensin Converting Enzyme (ACE) by Flavonoids Isolated From Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef]
- Esmaeili, M.; Zohari, F.; Sadeghi, H. Antioxidant and Protective Effects of Major Flavonoids from Teucrium polium on β -Cell Destruction in a Model of Streptozotocin-Induced Diabetes. Planta Med. 2009, 75, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Sadeghi, H. Pancreatic Β-Cell Protective Effect of Rutin and Apigenin Isolated from Teucrium polium. Pharmacologyonline 2009, 2, 341–353. [Google Scholar]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the Flowers of Platycodon grandiflorum with Inhibitory Activity on Advanced Glycation End Products and Rat Lens Aldose Reductase in Vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef]
- Chaves, D.S.A.; Frattani, F.S.; Assafim, M.; de Almeida, A.P.; Zingali, R.B.; Costa, S.S. Phenolic Chemical Composition of Petroselinum crispum Extract and Its Effect on Haemostasis. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef]
- Dianita, R.; Jantan, I. Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds. Molecules 2019, 24, 1469. [Google Scholar] [CrossRef] [PubMed]
- Senejoux, F.; Demougeot, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Girard-Thernier, C. Bioassay-Guided Isolation of Vasorelaxant Compounds from Ziziphora clinopodioides Lam. (Lamiaceae). Fitoterapia 2012, 83, 377–382. [Google Scholar] [CrossRef]
- Senejoux, F.; Girard, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Demougeot, C. Mechanisms of Vasorelaxation Induced by Ziziphora clinopodioides Lam. (Lamiaceae) Extract in Rat Thoracic Aorta. J. Ethnopharmacol. 2010, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Wang, Z.; Guillen Quispe, Y.N.; Lim, S.S.; Yu, J.M. Evaluation of Aldose Reductase, Protein Glycation, and Antioxidant Inhibitory Activities of Bioactive Flavonoids in Matricaria recutita L. and Their Structure-Activity Relationship. J. Diabetes Res. 2018, 2018, 3276162. [Google Scholar] [CrossRef]
- Dou, X.; Zhou, Z.; Ren, R.; Xu, M. Apigenin, Flavonoid Component Isolated from Gentiana veitchiorum Flower Suppresses the Oxidative Stress through LDLR-LCAT Signaling Pathway. Biomed. Pharmacother. 2020, 128, 110298. [Google Scholar] [CrossRef]
- Anandan, S.; Urooj, A. Hypoglycemic Effects of Apigenin from Morus indica in Streptozotocin Induced Diabetic Rats. IJCRR. 2021, 13, 100–105. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Huang, Q.; Duan, H.; Zhao, G.; Liu, L.; Li, Y. Flavonoids from Sophora alopecuroides L. Improve Palmitate-Induced Insulin Resistance by Inhibiting PTP1B Activity in Vitro. Bioorg. Med. Chem. Lett. 2021, 35, 127775. [Google Scholar] [CrossRef] [PubMed]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In Vitro and in Vivo Antidiabetic Activity, Isolation of Flavonoids, and in Silico Molecular Docking of Stem Extract of Merremia tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef]
- Rossoni, G.; Grande, S.; Galli, C.; Visioli, F. Wild Artichoke Prevents the Age-Associated Loss of Vasomotor Function. J. Agric. Food Chem. 2005, 53, 10291–10296. [Google Scholar] [CrossRef] [PubMed]
- Lii, C.-K.; Lei, Y.-P.; Yao, H.-T.; Hsieh, Y.-S.; Tsai, C.-W.; Liu, K.-L.; Chen, H.-W. Chrysanthemum morifolium Ramat. Reduces the Oxidized LDL-Induced Expression of Intercellular Adhesion Molecule-1 and E-Selectin in Human Umbilical Vein Endothelial Cells. J. Ethnopharmacol. 2010, 128, 213–220. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive Effects of Quercetin in the Central Nervous System: Focusing on the Mechanisms of Actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Quercetin And Its Derivatives: Chemical Structure And Bioactivity—A Review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.; Nabavi, S.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Salama, M.M.; Abd-elrahman, E.H.; El-Maraghy, S.A. Antidiabetic Activity of Phenolic Compounds from Pecan Bark in Streptozotocin-Induced Diabetic Rats. Phytochem. Lett. 2011, 4, 337–341. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Guan, X.-L.; Li, J.; Deng, S.-P.; Li, L.-Q.; Tang, M.-T.; Huang, J.-G.; Chen, Z.-Z.; Yang, R.-Y. Hypoglycemic Effects and Constituents of the Barks of Cyclocarya paliurus and Their Inhibiting Activities to Glucosidase and Glycogen Phosphorylase. Fitoterapia 2011, 82, 1081–1085. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Asghari, B.; Saeidnia, S.; Ajani, Y.; Mirjani, M.; Malmir, M.; Dolatabadi Bazaz, R.; Hadjiakhoondi, A.; Salehi, P.; Hamburger, M.; et al. In Vitro α-Glucosidase Inhibitory Activity of Phenolic Constituents from Aerial Parts of Polygonum hyrcanicum. DARU J. Pharm. Sci. 2012, 20, 37. [Google Scholar] [CrossRef]
- Nurul Islam, M.; Jung, H.A.; Sohn, H.S.; Kim, H.M.; Choi, J.S. Potent α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors from Artemisia capillaris. Arch. Pharm. Res. 2013, 36, 542–552. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.-J.; Yu, S.Y.; Ku, S.-K.; Kim, M.-H.; Kim, J.S. Effects of Allium victorialis Leaf Extracts and Its Single Compounds on Aldose Reductase, Advanced Glycation End Products and TGF-Β1 Expression in Mesangial Cells. BMC Complement. Altern. Med. 2013, 13, 251. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents from Dillenia indica. Biomed. Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-H.; Zhao, J.; Jin, H.-T.; Cao, Y.; Ming, T.; Zhang, L.-L.; Hu, M.-Y.; Hamlati, H.; Pang, S.-B.; Ma, X.-P. Vasorelaxant Effects of the Extracts and Some Flavonoids from the Buds of Coreopsis tinctoria. Pharm. Biol. 2013, 51, 1158–1164. [Google Scholar] [CrossRef]
- Tan, C.; Zuo, J.; Yi, X.; Wang, P.; Luo, C.; Hu, Y.; Yi, H.; Qiao, W. Phenolic Constituents from Sarcopyramis nepalensis and Their α-Glucosidase Inhibitory Activity. Afr. J. Trad. Compl. Alt. Med. 2015, 12, 156. [Google Scholar] [CrossRef]
- Zekry, S.H.; Abo-elmatty, D.M.; Zayed, R.A.; Radwan, M.M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Effect of Metabolites Isolated from Cuscuta pedicellata on High Fat Diet-Fed Rats. Med. Chem. Res. 2015, 24, 1964–1973. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, M.; Zhang, J. Quercetin Isolated from Toona sinensis Leaves Attenuates Hyperglycemia and Protects Hepatocytes in High-Carbohydrate/High-Fat Diet and Alloxan Induced Experimental Diabetic Mice. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Ahmed, Q.U.; Soad, S.Z.M.; Latip, J.; Taher, M.; Syafiq, T.M.F.; Sarian, M.N.; Alhassan, A.M.; Zakaria, Z.A. Flavonoids from Tetracera indica Merr. Induce Adipogenesis and Exert Glucose Uptake Activities in 3T3-L1 Adipocyte Cells. BMC Complement. Altern. Med. 2017, 17, 431. [Google Scholar] [CrossRef]
- Owis, A.I.; Abo-youssef, A.M.; Osman, A.H. Leaves of Cordia Boissieri, A. DC. as a Potential Source of Bioactive Secondary Metabolites for Protection against Metabolic Syndrome-Induced in Rats. Z. Naturforsch. C 2017, 72, 107–118. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Kuzmicic, J.; Carvajal, L.; Muñoz, F.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Norambuena-Soto, I.; Chiong, M.; et al. Vasodilator and Hypotensive Effects of Pure Compounds and Hydroalcoholic Extract of Xenophyllum poposum (Phil) V.A Funk (Compositae) on Rats. Phytomedicine 2018, 50, 99–108. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Olofinsan, K.A.; Teralı, K.; Ghali, U.M.; Ajiboye, T.O. Bioactivity-Guided Isolation of Antidiabetic Principles from the Methanolic Leaf Extract of Bryophyllum pinnatum. J. Food Biochem. 2018, 42, e12627. [Google Scholar] [CrossRef]
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-Diabetic Activity of Quercetin Extracted from Phyllanthus emblica L. Fruit: In Silico and in Vivo Approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar] [CrossRef]
- Hussein, N.; Amen, Y.; Abdel Bar, F.; Halim, A.F.; Saad, H.-E.A. Antioxidants and α-Glucosidase Inhibitors from Lactuca serriola L. Rec. Nat. Prod. 2020, 14, 410–415. [Google Scholar] [CrossRef]
- Fadul, E.; Nizamani, A.; Rasheed, S.; Adhikari, A.; Yousuf, S.; Parveen, S.; Gören, N.; Alhazmi, H.A.; Choudhary, M.I.; Khalid, A. Anti-Glycating and Anti-Oxidant Compounds from Traditionally Used Anti-Diabetic Plant Geigeria alata (DC) Oliv. & Hiern. Nat. Prod. Res. 2020, 34, 2456–2464. [Google Scholar] [CrossRef]
- Kim, B.-R.; Paudel, S.; Nam, J.-W.; Jin, C.; Lee, I.-S.; Han, A.-R. Constituents of Coreopsis lanceolata Flower and Their Dipeptidyl Peptidase IV Inhibitory Effects. Molecules 2020, 25, 4370. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Ibrahim, A.K.; Elfaky, M.A.; Habib, E.S.; Mahamed, M.I.; Mehanna, E.T.; Darwish, K.M.; Khodeer, D.M.; Ahmed, S.A.; Elhady, S.S. Antioxidant and Anti-Inflammatory Activity of Cynanchum acutum L. Isolated Flavonoids Using Experimentally Induced Type 2 Diabetes Mellitus: Biological and In Silico Investigation for NF-ΚB Pathway/MiR-146a Expression Modulation. Antioxidants 2021, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Identification of Multiple Pancreatic and Extra-Pancreatic Pathways Underlying the Glucose-Lowering Actions of Acacia arabica Bark in Type-2 Diabetes and Isolation of Active Phytoconstituents. Plants 2021, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, M.; Wang, S.; Zhang, J.; Du, Y.; Zhao, Y.; Zheng, X.; Ma, B. Phenolic Compounds from the Leaves of Crataegus pinnatifida Bge. var. major N.E.Br. And Their Lipid-Lowering Effects. Bioorg. Med. Chem. Lett. 2021, 47, 128211. [Google Scholar] [CrossRef]
- Praparatana, R.; Maliyam, P.; Barrows, L.R.; Puttarak, P. Flavonoids and Phenols, the Potential Anti-Diabetic Compounds from Bauhinia strychnifolia Craib. Stem. Molecules 2022, 27, 2393. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Zhang, N.-N.; Guo, S.; Liu, S.-J.; Hou, Y.-F.; Li, S.; Ho, C.-T.; Bai, N.-S. Glycosides and Flavonoids from the Extract of Pueraria thomsonii Benth Leaf Alleviate Type 2 Diabetes in High-Fat Diet plus Streptozotocin-Induced Mice by Modulating the Gut Microbiota. Food Funct. 2022, 13, 3931–3945. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.; Jakstas, V.; Majiene, D.; Baniene, R.; Kuršvietiene, L.; Masteikova, R.; Savickas, A.; Toleikis, A.; Trumbeckaite, S. The Effect of Leonurus cardiaca Herb Extract and Some of Its Flavonoids on Mitochondrial Oxidative Phosphorylation in the Heart. Planta Med. 2014, 80, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Jofré, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and Vasodilator Activity of Ugni molinae Turcz. (Murtilla) and Its Modulatory Mechanism in Hypotensive Response. Oxid. Med. Cell. Longev. 2016, 2016, 6513416. [Google Scholar] [CrossRef]
- Ferreira, L.L.D.M.; Leão, V.d.F.; de Melo, C.M.; de Machado, T.B.; Amaral, A.C.F.; da Silva, L.L.; Simas, N.K.; Muzitano, M.F.; Leal, I.C.R.; Raimundo, J.M. Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction. Pharmaceutics 2021, 13, 1173. [Google Scholar] [CrossRef]
- De Lima Júnior, J.P.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a Novel Source of Antioxidant, Antiglycation and α-Amylase Inhibitors Molecules with Potential for Management of Oxidative Stress and Diabetes. J. Ethnopharmacol. 2021, 268, 113667. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of Milk Thistle (Silybum marianum L. Gaertn.), a Medicinal Weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Lee, D.Y.-W.; Liu, Y. Molecular Structure and Stereochemistry of Silybin A, Silybin B, Isosilybin A, and Isosilybin B, Isolated from Silybum marianum (Milk Thistle). J. Nat. Prod. 2003, 66, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Pferschy-Wenzig, E.-M.; Atanasov, A.G.; Malainer, C.; Noha, S.M.; Kunert, O.; Schuster, D.; Heiss, E.H.; Oberlies, N.H.; Wagner, H.; Bauer, R.; et al. Identification of Isosilybin A from Milk Thistle Seeds as an Agonist of Peroxisome Proliferator-Activated Receptor Gamma. J. Nat. Prod. 2014, 77, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P. Medicinal Natural Products A Biosynthtic Approach, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Křen, V.; Valentová, K. Silybin and Its Congeners: From Traditional Medicine to Molecular Effects. Nat. Prod. Rep. 2022, 39, 1264–1281. [Google Scholar] [CrossRef]
- Palomino, O.; Gouveia, N.; Ramos, S.; Martín, M.; Goya, L. Protective Effect of Silybum marianum and Silibinin on Endothelial Cells Submitted to High Glucose Concentration. Planta Med. 2016, 83, 97–103. [Google Scholar] [CrossRef] [PubMed]
- İnceören, N.; Emen, S.; Çeken Toptancı, B.; Kızıl, G.; Kızıl, M. In Vitro Inhibition of Advanced Glycation End Product Formation by Ethanol Extract of Milk Thistle (Silybum marianum L.) Seed. S. Afr. J. Bot. 2022, 149, 682–692. [Google Scholar] [CrossRef]
- Estrada, O.; Hasegawa, M.; Gonzalez-Mujíca, F.; Motta, N.; Perdomo, E.; Solorzano, A.; Méndez, J.; Méndez, B.; Zea, E.G. Evaluation of Flavonoids From Bauhinia megalandra Leaves as Inhibitors of Glucose-6-Phosphatase System. Phytother. Res. 2005, 19, 859–863. [Google Scholar] [CrossRef]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic Properties and Drug Interactions of Apigenin, a Natural Flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. AOs 2021, 10, 1643. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- El Daibani, A.A.; Xi, Y.; Luo, L.; Mei, X.; Zhou, C.; Yasuda, S.; Liu, M.-C. Sulfation of Hesperetin, Naringenin and Apigenin by the Human Cytosolic Sulfotransferases: A Comprehensive Analysis. Nat. Prod. Res. 2020, 34, 797–803. [Google Scholar] [CrossRef]
- Bak, M.J.; Das Gupta, S.; Wahler, J.; Suh, N. Role of Dietary Bioactive Natural Products in Estrogen Receptor-Positive Breast Cancer. Semin. Cancer Biol. 2016, 40–41, 170–191. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Bafandeh, F. The Cardiovascular Protective Effects of Chrysin: A Narrative Review on Experimental Researches. CHAMC 2019, 17, 17–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Suo, W.; Zhang, X.; Lv, J.; Liu, Z.; Liu, R. Roles and Mechanisms of Quercetin on Cardiac Arrhythmia: A Review. Biomed. Pharmacother. 2022, 153, 113447. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Pattanayak, P.; Awasthi, A.; Alruwaili, N.K.; Zafar, A.; Almawash, S.; Gulati, M.; Singh, S.K. Quality by Design-Based Optimization of Formulation Parameters to Develop Quercetin Nanosuspension for Improving Its Biopharmaceutical Properties. S. Afr. J. Bot. 2022, 149, 798–806. [Google Scholar] [CrossRef]
- Rao, L. A Review on Quercetin: Assessment of the Pharmacological Potentials and Various Formulations Strategies. Int. J. Pharm. Sci. Rev. Res. 2020, 64, 139–144. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Muñoz-Reyes, D.; Morales, A.I.; Prieto, M. Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney. Antioxidants 2021, 10, 909. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef]
- Sornsuvit, C.; Hongwiset, D.; Yotsawimonwat, S.; Toonkum, M.; Thongsawat, S.; Taesotikul, W. The Bioavailability and Pharmacokinetics of Silymarin SMEDDS Formulation Study in Healthy Thai Volunteers. Evid. Based Complement. Altern. Med. 2018, 2018, 1507834. [Google Scholar] [CrossRef]
- Kellici, T.F.; Ntountaniotis, D.; Leonis, G.; Chatziathanasiadou, M.; Chatzikonstantinou, A.V.; Becker-Baldus, J.; Glaubitz, C.; Tzakos, A.G.; Viras, K.; Chatzigeorgiou, P.; et al. Investigation of the Interactions of Silibinin with 2-Hydroxypropyl-β-Cyclodextrin through Biophysical Techniques and Computational Methods. Mol. Pharm. 2015, 12, 954–965. [Google Scholar] [CrossRef]
- Tvrdý, V.; Pourová, J.; Jirkovský, E.; Křen, V.; Valentová, K.; Mladěnka, P. Systematic Review of Pharmacokinetics and Potential Pharmacokinetic Interactions of Flavonolignans from Silymarin. Med. Res. Rev. 2021, 41, 2195–2246. [Google Scholar] [CrossRef]
- Ma, J.; Chen, X. Advances in Pathogenesis and Treatment of Essential Hypertension. Front. Cardiovasc. Med. 2022, 9, 1003852. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M. Quercetin Decreases the Activity of Matrix Metalloproteinase-2 and Ameliorates Vascular Remodeling in Renovascular Hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, G.B.; Vinayagam, R.; Do, G.S.; Oh, S.Y.; Yang, S.J.; Kwon, J.B.; Singh, M. Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages. Molecules 2022, 27, 7432. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin Improves Vascular Endothelial Function through Promotion of Autophagy in Hypertensive Rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Chakravarthi, S.; Bangra Kulur, A.; Yee, T.M. Plant Flavone Apigenin Protects against Cyclosporine-Induced Histological and Biochemical Changes in the Kidney in Rats. Biomed. Prev. Nutr. 2014, 4, 589–593. [Google Scholar] [CrossRef]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.-L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and Its Metabolites Improve Vessel Function by Inducing ENOS Activity via Phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, L.; Zhang, B.; Deng, Z.; Li, H. Synergistic Protection of Quercetin and Lycopene against Oxidative Stress via SIRT1-Nox4-ROS Axis in HUVEC Cells. Curr. Res. Nutr. Food Sci. 2022, 5, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hsu, C.-N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Häckl, L.P.N.; Cuttle, G.; Sanches Dovichi, S.; Lima-Landman, M.T.; Nicolau, M. Inhibition of Angiotensin-Converting Enzyme by Quercetin Alters the Vascular Response to Bradykinin and Angiotensin I. Pharmacology 2002, 65, 182–186. [Google Scholar] [CrossRef]
- Palmieri, D.; Perego, P.; Palombo, D. Apigenin Inhibits the TNFα-Induced Expression of ENOS and MMP-9 via Modulating Akt Signalling through Oestrogen Receptor Engagement. Mol. Cell Biochem. 2012, 371, 129–136. [Google Scholar] [CrossRef]
- Qin, W.; Ren, B.; Wang, S.; Liang, S.; He, B.; Shi, X.; Wang, L.; Liang, J.; Wu, F. Apigenin and Naringenin Ameliorate PKCβII-Associated Endothelial Dysfunction via Regulating ROS/Caspase-3 and NO Pathway in Endothelial Cells Exposed to High Glucose. Vasc. Pharmacol. 2016, 85, 39–49. [Google Scholar] [CrossRef]
- Jin, B.; Qian, L.; Chen, S.; Li, J.; Wang, H.; Bruce, I.C.; Lin, J.; Xia, Q. Apigenin Protects Endothelium-Dependent Relaxation of Rat Aorta against Oxidative Stress. Eur. J. Pharmacol. 2009, 616, 200–205. [Google Scholar] [CrossRef]
- Wei, X.; Gao, P.; Pu, Y.; Li, Q.; Yang, T.; Zhang, H.; Xiong, S.; Cui, Y.; Li, L.; Ma, X.; et al. Activation of TRPV4 by Dietary Apigenin Antagonizes Renal Fibrosis in Deoxycorticosterone Acetate (DOCA)–Salt-Induced Hypertension. Clin. Sci. 2017, 131, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Demirci, B.; Dost, T.; Gokalp, F.; Birincioglu, M. Silymarin Improves Vascular Function of Aged Ovariectomized Rats: Silymarin And Postmenopausal Endothelium. Phytother. Res. 2014, 28, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Hong, Y.; Huang, Z.-Q. Protective Effects of Silybin on Human Umbilical Vein Endothelial Cell Injury Induced by H2O2 in Vitro. Vasc. Pharmacol. 2005, 43, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive Effects of the Flavonoid Quercetin in Spontaneously Hypertensive Rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Elbarbry, F.; Abdelkawy, K.; Moshirian, N.; Abdel-Megied, A.M. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int. J. Mol. Sci. 2020, 21, 6554. [Google Scholar] [CrossRef]
- Li Volti, G.; Salomone, S.; Sorrenti, V.; Mangiameli, A.; Urso, V.; Siarkos, I.; Galvano, F.; Salamone, F. Effect of Silibinin on Endothelial Dysfunction and ADMA Levels in Obese Diabetic Mice. Cardiovasc. Diabetol. 2011, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef]
- Yao, Z.; Dai, K.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Sun, S.; Wang, X.; Jia, Q.; et al. Low Dietary Quercetin Intake by Food Frequency Questionnaire Analysis Is Not Associated with Hypertension Occurrence. Clin. Nutr. 2021, 40, 3748–3753. [Google Scholar] [CrossRef]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-Activated Protein Kinase and Enhancement of Basal Glucose Uptake in Muscle Cells by Quercetin and Quercetin Glycosides, Active Principles of the Antidiabetic Medicinal Plant Vaccinium Vitis-Idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Pharmacogn. Mag. 2015, 11, 74. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective Effect of Quercetin on Hyperglycemia, Oxidative Stress and DNA Damage in Alloxan Induced Type 2 Diabetic Mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef]
- Mokashi, P.; Khanna, A.; Pandita, N. Flavonoids from Enicostema Littorale Blume Enhances Glucose Uptake of Cells in Insulin Resistant Human Liver Cancer (HepG2) Cell Line via IRS-1/PI3K/Akt Pathway. Biomed. Pharmacother. 2017, 90, 268–277. [Google Scholar] [CrossRef]
- Boydens, C.; Pauwels, B.; Vanden Daele, L.; Van de Voorde, J. Protective Effect of Resveratrol and Quercetin on in Vitro-Induced Diabetic Mouse Corpus Cavernosum. Cardiovasc. Diabetol. 2016, 15, 46. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective Effect of Quercetin on Streptozotocin-Induced Diabetic Peripheral Neuropathy Rats through Modulating Gut Microbiota and Reactive Oxygen Species Level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Chu, C.; Gao, X.; Li, X.; Zhang, X.; Ma, R.; Jia, Y.; Li, D.; Wang, D.; Xu, F. Involvement of Estrogen Receptor-α in the Activation of Nrf2-Antioxidative Signaling Pathways by Silibinin in Pancreatic β-Cells. Biomol. Ther. 2020, 28, 163–171. [Google Scholar] [CrossRef]
- Mohammadi, H.; Manouchehri, H.; Changizi, R.; Bootorabi, F.; Khorramizadeh, M.R. Concurrent Metformin and Silibinin Therapy in Diabetes: Assessments in Zebrafish (Danio Rerio) Animal Model. J. Diabetes Metab. Disord. 2020, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, U.; Chandrayan, G.; Patil, C.; Arya, D.; Suchal, K.; Agrawal, Y.; Ojha, S.; Goyal, S. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats Mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin Ameliorates Streptozotocin-Induced Diabetic Nephropathy in Rats via MAPK-NF-ΚB-TNF-α and TGF-Β1-MAPK-Fibronectin Pathways. Am. J. Physiol. Renal Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef]
- Liu, H.-J.; Fan, Y.-L.; Liao, H.-H.; Liu, Y.; Chen, S.; Ma, Z.-G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.-Z. Apigenin Alleviates STZ-Induced Diabetic Cardiomyopathy. Mol. Cell Biochem. 2017, 428, 9–21. [Google Scholar] [CrossRef]
- Meng, S.; Yang, F.; Wang, Y.; Qin, Y.; Xian, H.; Che, H.; Wang, L. Silymarin Ameliorates Diabetic Cardiomyopathy via Inhibiting TGF-Β1/Smad Signaling: Silymarin Ameliorates DCM. Cell Biol. Int. 2019, 43, 65–72. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Ingles, J.; Goldstein, J.; Thaxton, C.; Caleshu, C.; Corty, E.W.; Crowley, S.B.; Dougherty, K.; Harrison, S.M.; McGlaughon, J.; Milko, L.V.; et al. Evaluating the Clinical Validity of Hypertrophic Cardiomyopathy Genes. Circ. Genom. Precis. Med. 2019, 12, e002460. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; de Marvao, A.; Dawes, T.J.W.; et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Moreland, N.; La Grange, L.; Montoya, R. Impact of in Utero Exposure to EtOH on Corpus Callosum Development and Paw Preference in Rats: Protective Effects of Silymarin. BMC Complement. Altern. Med. 2002, 2, 10. [Google Scholar] [CrossRef]
- Malekinejad, H.; Rezabakhsh, A.; Rahmani, F.; Hobbenaghi, R. Silymarin Regulates the Cytochrome P450 3A2 and Glutathione Peroxides in the Liver of Streptozotocin-Induced Diabetic Rats. Phytomedicine 2012, 19, 583–590. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Xu, F.; Liu, W.; Hayashi, T.; Onodera, S.; Tashiro, S.; Ikejima, T. Involvement of Estrogen Receptors in Silibinin Protection of Pancreatic β-Cells from TNFα- or IL-1β-Induced Cytotoxicity. Biomed. Pharmacother. 2018, 102, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Recoba, R.; Barrón, H.; Alvarez, C.; Favari, L. Silymarin Increases Antioxidant Enzymes in Alloxan-Induced Diabetes in Rat Pancreas. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 136, 205–212. [Google Scholar] [CrossRef]
- Miranda, L.M.O.; da Cunha Agostini, L.; de Lima, W.G.; Camini, F.C.; Costa, D.C. Silymarin Attenuates Hepatic and Pancreatic Redox Imbalance Independent of Glycemic Regulation in the Alloxan-Induced Diabetic Rat Model. Biomed. Environ. Sci. 2020, 33, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of Dietary Flavonoids with Risk of Type 2 Diabetes, and Markers of Insulin Resistance and Systemic Inflammation in Women: A Prospective Study and Cross-Sectional Analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef]
- Sales, D.S.; Carmona, F.; de Azevedo, B.C.; Taleb-Contini, S.H.; Bartolomeu, A.C.D.; Honorato, F.B.; Martinez, E.Z.; Pereira, A.M.S. Eugenia punicifolia (Kunth) DC. as an Adjuvant Treatment for Type-2 Diabetes Mellitus: A Non-Controlled, Pilot Study. Phytother. Res. 2014, 28, 1816–1821. [Google Scholar] [CrossRef]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin Ameliorates Insulin Resistance and Lipid Accumulation by Endoplasmic Reticulum Stress and SREBP-1c/SREBP-2 Pathway in Palmitate-Induced HepG2 Cells and High-Fat Diet–Fed Mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.-I.; Ha, T.-Y. Quercetin Reduces High-Fat Diet-Induced Fat Accumulation in the Liver by Regulating Lipid Metabolism Genes: Anti-Obesity Effect Of Quercetin. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.A.; Elshikh, M.S.; Mohamed, M.O.; Darweesh, M.F.; Hussein, D.S.; Almutairi, S.M.; Embaby, A.S. Quercetin Mitigates the Adverse Effects of High Fat Diet on Pancreatic and Renal Tissues in Adult Male Albino Rats. J. King Saud Univ. Sci. 2022, 34, 101946. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.-C.; Du, C.; Wang, L.-N.; Xiao, Y.-H. Effects of Apigenin on the Expression of LOX-1, Bcl-2, and Bax in Hyperlipidemia Rats. Chem. Biodivers. 2021, 18, e2100049. [Google Scholar] [CrossRef]
- Gobalakrishnan, S. Effect of Silybin on Lipid Profile in Hypercholesterolaemic Rats. J. Clin. Diagn. Res. 2016, 10, FF01. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.-M.; Tong, Q.; et al. Silymarin Ameliorates Metabolic Dysfunction Associated with Diet-Induced Obesity via Activation of Farnesyl X Receptor. Front. Pharmacol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Jiang, L.-Y.; Wang, Y.-C.; Ma, D.-F.; Li, X. Quercetin Attenuates Atherosclerosis via Modulating Oxidized LDL-Induced Endothelial Cellular Senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effects of Quercetin Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The Effects of Quercetin Supplementation on Lipid Profiles and Inflammatory Markers among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin Reduces Systolic Blood Pressure and Plasma Oxidised Low-Density Lipoprotein Concentrations in Overweight Subjects with a High-Cardiovascular Disease Risk Phenotype: A Double-Blinded, Placebo-Controlled Cross-over Study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a Quercetin-Rich Onion Skin Extract on 24 h Ambulatory Blood Pressure and Endothelial Function in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomised Double-Blinded Placebo-Controlled Cross-over Trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Pourmasoumi, M.; Mohammadi, H.; Symonds, M.; Miraghajani, M. The Effects of Silymarin Supplementation on Metabolic Status and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Med. 2018, 41, 311–319. [Google Scholar] [CrossRef]
- Mohammadi, H.; Hadi, A.; Arab, A.; Moradi, S.; Rouhani, M.H. Effects of Silymarin Supplementation on Blood Lipids: A Systematic Review and Meta-analysis of Clinical Trials. Phytother. Res. 2019, 33, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ravari, S.S.; Talaei, B.; Gharib, Z. The Effects of Silymarin on Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Obes. Med. 2021, 26, 100368. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, P.; Liu, Y.; Wen, G.; Fu, X.; Sun, X. Inhibition of Autophagy Ameliorates Atherogenic Inflammation by Augmenting Apigenin-Induced Macrophage Apoptosis. Int. Immunopharmacol. 2015, 27, 24–31. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Hutton, D.A.; Brunt, V.E.; VanDongen, N.S.; Ziemba, B.P.; Casso, A.G.; Greenberg, N.T.; Mercer, A.N.; Rossman, M.J.; Campisi, J.; et al. Apigenin Restores Endothelial Function by Ameliorating Oxidative Stress, Reverses Aortic Stiffening, and Mitigates Vascular Inflammation with Aging. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H185–H196. [Google Scholar] [CrossRef]
- Huwait, E.A.; Saddeek, S.Y.; Al-Massabi, R.F.; Almowallad, S.J.; Pushparaj, P.N.; Kalamegam, G. Antiatherogenic Effects of Quercetin in the THP-1 Macrophage Model In Vitro, With Insights Into Its Signaling Mechanisms Using In Silico Analysis. Front. Pharmacol. 2021, 12, 698138. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, P.; Li, F.; Liu, Q.; Qin, S.; Zhou, G.; Xu, X.; Si, Y.; Guo, S. Quercetin Improves Macrophage Reverse Cholesterol Transport in Apolipoprotein E-Deficient Mice Fed a High-Fat Diet. Lipids Health Dis. 2017, 16, 9. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin Attenuates Atherosclerotic Inflammation and Adhesion Molecule Expression by Modulating TLR-NF-ΚB Signaling Pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhou, H.-F.; Liang, Y.; Zhao, G.-J. Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation. Cell. Physiol. Biochem. 2018, 47, 2170–2184. [Google Scholar] [CrossRef]
- Wang, L.; Rotter, S.; Ladurner, A.; Heiss, E.; Oberlies, N.; Dirsch, V.; Atanasov, A. Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules 2015, 21, 55. [Google Scholar] [CrossRef]
- Puteri, M.U.; Azmi, N.U.; Kato, M.; Saputri, F.C. PCSK9 Promotes Cardiovascular Diseases: Recent Evidence about Its Association with Platelet Activation-Induced Myocardial Infarction. Life 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Zhao, C.-H.; Yao, X.-L.; Zhang, H. Quercetin Attenuates High Fructose Feeding-Induced Atherosclerosis by Suppressing Inflammation and Apoptosis via ROS-Regulated PI3K/AKT Signaling Pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin Protects against Atherosclerosis by Regulating the Expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, H.; Shen, D.; Chen, C.; Xing, S.; Dou, F.; Jia, Q. Effect of Quercetin on Atherosclerosis Based on Expressions of ABCA1, LXR-α and PCSK9 in ApoE-/- Mice. Chin. J. Integr. Med. 2020, 26, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Garelnabi, M.; Mahini, H.; Wilson, T. Quercetin Intake with Exercise Modulates Lipoprotein Metabolism and Reduces Atherosclerosis Plaque Formation. J. Int. Soc. Sports Nutr. 2014, 11, 22. [Google Scholar] [CrossRef]
- Saragusti, A.C.; Ortega, M.G.; Cabrera, J.L.; Estrin, D.A.; Marti, M.A.; Chiabrando, G.A. Inhibitory Effect of Quercetin on Matrix Metalloproteinase 9 Activity Molecular Mechanism and Structure–Activity Relationship of the Flavonoid–Enzyme Interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Kondo, M.; Izawa-Ishizawa, Y.; Goda, M.; Hosooka, M.; Kagimoto, Y.; Saito, N.; Matsuoka, R.; Zamami, Y.; Chuma, M.; Yagi, K.; et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. Int. J. Mol. Sci. 2020, 21, 7226. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, M.; Lopes-Virella, M.F.; Huang, Y. Quercetin Inhibits Matrix Metalloproteinase-1 Expression in Human Vascular Endothelial Cells through Extracellular Signal-Regulated Kinase. Arch. Biochem. 2001, 391, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Myoung, H.-J.; Kim, G.; Nam, K.-W. Apigenin Isolated from the Seeds of Perilla frutescens Britton var crispa (Benth.) Inhibits Food Intake in C57BL/6J Mice. Arch. Pharm. Res. 2010, 33, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Fujimori, K. Antiadipogenic Effect of Dietary Apigenin through Activation of AMPK in 3T3-L1 Cells. J. Agric. Food Chem. 2011, 59, 13346–13352. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Lasa, A.; Abendaño, N.; Fernández-Quintela, A.; Mosqueda-Solís, A.; Garcia-Sobreviela, M.P.; Arbonés-Mainar, J.M.; Portillo, M.P. Phenolic Compounds Apigenin, Hesperidin and Kaempferol Reduce in Vitro Lipid Accumulation in Human Adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Che, D.N.; Shin, J.Y.; Kang, H.J.; Kim, J.H.; Jang, S.I. Anti-obesity Effects of Enzyme-treated Celery Extract in Mice Fed with High-fat Diet. J. Food Biochem. 2020, 44, e13105. [Google Scholar] [CrossRef]
- Sun, T.; Ding, W.; Xu, T.; Ao, X.; Yu, T.; Li, M.; Liu, Y.; Zhang, X.; Hou, L.; Wang, J. Parkin Regulates Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury by Targeting Cyclophilin-D. Antioxid. Redox. Signal. 2019, 31, 1177–1193. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, M.; Cai, H.; Chen, J.; Lin, Y.; Wang, F.; Wang, L.; Zhang, X.; Liu, J. The Activity Comparison of Six Dietary Flavonoids Identifies That Luteolin Inhibits 3T3-L1 Adipocyte Differentiation through Reducing ROS Generation. J. Nutr. Biochem. 2022, 112, 109208. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin Alleviates Obesity-Associated Metabolic Syndrome by Regulating the Composition of the Gut Microbiome. Front. Microbiol. 2021, 12, 805827. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The Anti-Obesity Effect of Quercetin Is Mediated by the AMPK and MAPK Signaling Pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef]
- Fang, X.-K.; Gao, J.; Zhu, D.-N. Kaempferol and Quercetin Isolated from Euonymus alatus Improve Glucose Uptake of 3T3-L1 Cells without Adipogenesis Activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Shatylo, V.; Antoniuk-Shcheglova, I.; Naskalova, S.; Bondarenko, O.; Havalko, A.; Krasnienkov, D.; Zabuga, O.; Kukharskyy, V.; Guryanov, V.; Vaiserman, A. Cardio-Metabolic Benefits of Quercetin in Elderly Patients with Metabolic Syndrome. PharmaNutrition 2021, 15, 100250. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of Quercetin on Traits of the Metabolic Syndrome, Endothelial Function and Inflammation in Men with Different APOE Isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of Quercetin Supplementation on Plasma Lipid Profiles, Blood Pressure, and Glucose Levels: A Systematic Review and Meta-Analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.Z.; Wu, Y.; Ke, J.J.; He, X.H.; Wang, Y.L. Quercetin Postconditioning Attenuates Myocardial Ischemia/Reperfusion Injury in Rats through the PI3K/Akt Pathway. Braz. J. Med. Biol. Res. 2013, 46, 861–867. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, Z.; Li, X.; Li, X.; Cao, T.; Bi, Y.; Zhou, J.; Chen, X.; Yu, D.; Zhu, L.; et al. Protective Effect of Quercetin on Posttraumatic Cardiac Injury. Sci. Rep. 2016, 6, 30812. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Meng, Q.; ShiyuanWang; Yan, P.; Wang, X.; Luo, D.; Zhou, X.; Ji, R. Quercetin Improves Cardiomyocyte Vulnerability to Hypoxia by Regulating SIRT1/TMBIM6-Related Mitophagy and Endoplasmic Reticulum Stress. Oxid. Med. Cell Long. 2021, 2021, 5529913. [Google Scholar] [CrossRef]
- Bali, E.; Ergin, V.; Rackova, L.; Bayraktar, O.; Küçükboyacı, N.; Karasu, Ç. Olive Leaf Extracts Protect Cardiomyocytes against 4-Hydroxynonenal-Induced Toxicity In Vitro: Comparison with Oleuropein, Hydroxytyrosol, and Quercetin. Planta Med. 2014, 80, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, M.; Zeng, H.; Liu, P.; Zhu, X.; Zhou, F.; Liu, J.; Zhang, J.; Dong, Z.; Tang, Y.; et al. Quercetin Attenuates Ethanol-Induced Iron Uptake and Myocardial Injury by Regulating the Angiotensin II-L-Type Calcium Channel. Mol. Nutr. Food Res. 2018, 62, 1700772. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Retamal, C.; Schupper, D.; Vergara-Hernández, D.; Saha, S.; Profumo, E.; Buttari, B.; Saso, L. Antioxidant Cardioprotection against Reperfusion Injury: Potential Therapeutic Roles of Resveratrol and Quercetin. Molecules 2022, 27, 2564. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Z.; Xu, L.; Sun, A.; Fu, X.; Zhang, L.; Jing, L.; Lu, A.; Dong, Y.; Jia, Z. Protective Effect of Apigenin on Ischemia/Reperfusion Injury of the Isolated Rat Heart. Cardiovasc. Toxicol. 2015, 15, 241–249. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Hu, J.; Li, X.; Zhang, X.; Li, Z. Apigenin Attenuates Myocardial Ischemia/Reperfusion Injury via the Inactivation of P38 Mitogen-activated Protein Kinase. Mol. Med. Rep. 2015, 12, 6873–6878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Liu, Z.; Ma, Z.; An, D.; Xu, D. Apigenin Attenuates Myocardial Infarction-Induced Cardiomyocyte Injury by Modulating Parkin-Mediated Mitochondrial Autophagy. J. Biosci. 2020, 45, 75. [Google Scholar] [CrossRef]
- Rao, P. Cardioprotective Activity of Silymarin in Ischemia-Reperfusion-Induced Myocardial Infarction in Albino Rats. Exp. Clin. Cardiol. 2007, 12, 179. [Google Scholar]
- Albadrani, G.M.; BinMowyna, M.N.; Bin-Jumah, M.N.; El–Akabawy, G.; Aldera, H.; AL-Farga, A.M. Quercetin Prevents Myocardial Infarction Adverse Remodeling in Rats by Attenuating TGF-Β1/Smad3 Signaling: Different Mechanisms of Action. Saudi J. Biol. Sci. 2021, 28, 2772–2782. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, H.; Wang, Q.; Hou, J.-W.; Mao, Z.-J.; Li, Y.-G. Protective Role of Silibinin against Myocardial Ischemia/Reperfusion Injury-Induced Cardiac Dysfunction. Int. J. Biol. Sci. 2020, 16, 1972–1988. [Google Scholar] [CrossRef]
- Tan, X.; Xian, W.; Li, X.; Chen, Y.; Geng, J.; Wang, Q.; Gao, Q.; Tang, B.; Wang, H.; Kang, P. Mechanisms of Quercetin against Atrial Fibrillation Explored by Network Pharmacology Combined with Molecular Docking and Experimental Validation. Sci. Rep. 2022, 12, 9777. [Google Scholar] [CrossRef]
- Bouderba, S.; Sanchez-Martin, C.; Villanueva, G.R.; Detaille, D.; Koceïr, E.A. Beneficial Effects of Silibinin against the Progression of Metabolic Syndrome, Increased Oxidative Stress, and Liver Steatosis in Psammomys Obesus, a Relevant Animal Model of Human Obesity and Diabetes. J. Diabetes 2014, 6, 184–192. [Google Scholar] [CrossRef]
- Prakash, P.; Singh, V.; Jain, M.; Rana, M.; Khanna, V.; Barthwal, M.K.; Dikshit, M. Silymarin Ameliorates Fructose Induced Insulin Resistance Syndrome by Reducing de Novo Hepatic Lipogenesis in the Rat. Eur. J. Pharmacol. 2014, 727, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Alex, R.; Bellner, L.; Raffaele, M.; Licari, M.; Vanella, L.; Stec, D.E.; Abraham, N.G. Milk Thistle Seed Cold Press Oil Attenuates Markers of the Metabolic Syndrome in a Mouse Model of Dietary-induced Obesity. J. Food Biochem. 2020, 44, e13522. [Google Scholar] [CrossRef] [PubMed]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective Effect of Dietary Flavonoid Quercetin against Lipemic-Oxidative Hepatic Injury in Hypercholesterolemic Rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-R.; Chen, Z.; Fang, K.; Xu, J.-X.; Ge, J.-F. Protective Effect of Quercetin against the Metabolic Dysfunction of Glucose and Lipids and Its Associated Learning and Memory Impairments in NAFLD Rats. Lipids Health Dis. 2021, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Wat, E.; Wang, Y.; Chan, K.; Law, H.W.; Koon, C.M.; Lau, K.M.; Leung, P.C.; Yan, C.; Lau, C.B.S. An in Vitro and in Vivo Study of a 4-Herb Formula on the Management of Diet-Induced Metabolic Syndrome. Phytomedicine 2018, 42, 112–125. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.; Dumont, J.; et al. Impact of Flavonols on Cardiometabolic Biomarkers: A Meta-Analysis of Randomized Controlled Human Trials to Explore the Role of Inter-Individual Variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of Quercetin Supplementation on Glycemic Control among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomou, E.-M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules 2023, 28, 2387. https://doi.org/10.3390/molecules28052387
Tomou E-M, Papakyriakopoulou P, Skaltsa H, Valsami G, Kadoglou NPE. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules. 2023; 28(5):2387. https://doi.org/10.3390/molecules28052387
Chicago/Turabian StyleTomou, Ekaterina-Michaela, Paraskevi Papakyriakopoulou, Helen Skaltsa, Georgia Valsami, and Nikolaos P. E. Kadoglou. 2023. "Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin" Molecules 28, no. 5: 2387. https://doi.org/10.3390/molecules28052387
APA StyleTomou, E.-M., Papakyriakopoulou, P., Skaltsa, H., Valsami, G., & Kadoglou, N. P. E. (2023). Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules, 28(5), 2387. https://doi.org/10.3390/molecules28052387