Abstract
Macrophages are crucial components of the immune system and play a critical role in the initial defense against pathogens. They are highly heterogeneous and plastic and can be polarized into classically activated macrophages (M1) or selectively activated macrophages (M2) in response to local microenvironments. Macrophage polarization involves the regulation of multiple signaling pathways and transcription factors. Here, we focused on the origin of macrophages, the phenotype and polarization of macrophages, as well as the signaling pathways associated with macrophage polarization. We also highlighted the role of macrophage polarization in lung diseases. We intend to enhance the understanding of the functions and immunomodulatory features of macrophages. Based on our review, we believe that targeting macrophage phenotypes is a viable and promising strategy for treating lung diseases.
1. Introduction
Macrophages are a crucial cell type in the innate immune system, possessing complex physiological functions and a wide distribution throughout the body. They not only serve as the first line of defense against pathogens in the host’s innate immune response, but also play a vital role in maintaining organismal homeostasis by participating in waste removal and tissue repair [1]. Macrophages are an extremely heterogeneous population with a high degree of plasticity [2,3]. Depending on the stimulus or local microenvironment, macrophages can polarize into multiple phenotypes, mainly consisting of classically activated macrophages (M1) and selectively activated macrophages (M2) [4,5,6,7].
In the lungs, macrophages constitute the most abundant immune cell population, comprising approximately 70% [8,9]. They are associated with the pathophysiology of a diverse array of lung diseases, including acute lung injury (ALI), chronic obstructive pulmonary disease (COPD), asthma, tuberculosis, and lung cancer [10,11,12,13,14]. During the pathology of various diseases, macrophages are critical in regulating immune homeostasis by regulating the M1/M2 polarization. Macrophage polarization has garnered significant attention as a research hotspot in recent years, and its regulation holds promise as a novel therapeutic strategy for disease treatment [15]. In the current review, we discuss the origin of macrophages, the phenotype and polarization of macrophages, the signaling pathways related to macrophage polarization, and the role of macrophage polarization in lung diseases. We aim to enhance the understanding of the functions and immunomodulatory features of macrophages.
2. Origin of Macrophages
Initially, it was widely believed that tissue macrophages in health and disease were derived from circulating monocytes [16,17]. However, the discovery of resident tissue macrophages in multiple organ systems that originate from the yolk sac during embryonic development has led to a dramatic shift in researchers’ understanding of macrophage origin [9,18]. At present, a large body of evidence demonstrates that tissue macrophages have a dual origin [19,20]. Macrophages can either differentiate from circulating monocytes derived from bone marrow stem cells or derive from primitive macrophages originating from the embryonic yolk sac and fetal liver. These primitive macrophages take up residence during the prenatal embryonic development, where they proliferate to form a resident tissue macrophage population [21] (Figure 1).
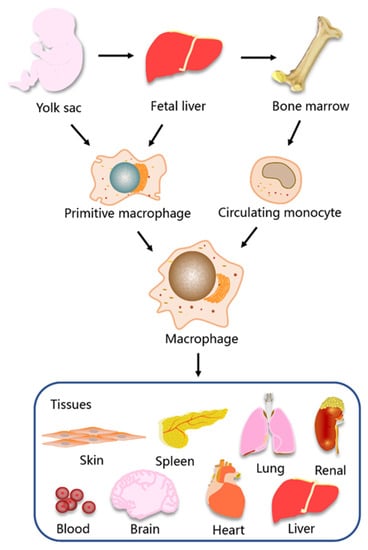
Figure 1.
The origin and tissue distribution of macrophages. Primitive macrophages are derived from the yolk sac and fetal liver and circulating monocytes are derived from bone marrow. Macrophages are widely distributed in tissues and organs of the body, including blood, skin, brain, heart, lung, liver, spleen, and kidney.
Macrophages are distributed in almost all organs and tissues throughout the body, where they play a diverse range of physiological roles during growth and development [22,23,24]. In some organs and tissues, macrophages are given unique names, such as microglia in the brain, alveolar macrophages in the lungs and Kupffer cells in the liver [23,25,26]. Alveolar macrophages and Kupffer cells derive from fetal liver mononuclear cells, while microglia derive from yolk sac erythromyeloid progenitors in early embryonic development. The origin and diversity of macrophages in different tissues are significant for both homeostatic functions and pathology. In general, embryonic-derived macrophages play a crucial role in maintaining tissue homeostasis, and macrophages derived from bone marrow monocytes are associated with host defense responses and pathological signaling [1,16,20]. However, each organ and its surrounding environment influence the function and properties of macrophages [27,28].
3. Phenotype and Polarization of Macrophages
Mature macrophages exhibit high plasticity and heterogeneity in phenotype and function, enabling them to respond and adapt to various environmental signals. Based on the concept of type-1/type-2 helper T (h-) cell polarization, phenotypically polarized macrophages are defined according to two main activation states, namely M1 and M2 macrophages (Figure 2). The nomenclature of M1 and M2 macrophages was first proposed by Mills et al. and has been widely used to define the major subsets of macrophages for a long time [5]. Upon sensing changes in the local tissue microenvironment, macrophages can activate different signal cascades, leading to the polarization into different phenotypes, or even switching from one phenotype to another. Many physiological and pathological states of the body are closely related to the proportion of the two macrophage phenotypes [7,15,29].
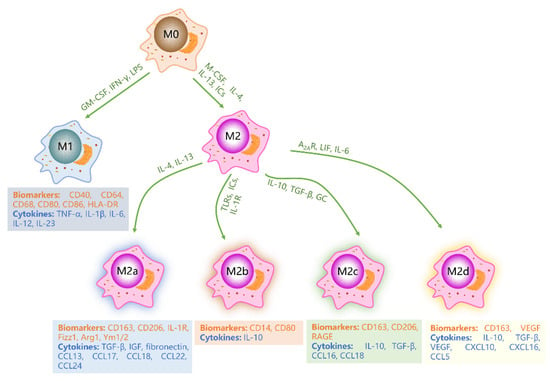
Figure 2.
Schematic diagram of macrophage subtypes. Under stimulation by GM-CSF, IFN-γ, and LPS, M0 macrophages polarize into M1 macrophages. Alternatively, M-CSF, IL-4, IL-13, and IC stimulation lead to polarization of M0 macrophages into M2 macrophages. Various cytokines further induce M2 macrophages to differentiate into M2a, M2b, M2c, and M2d phenotypes. M1, M2a, M2b, M2c and M2d macrophages express distinct biomarkers and secrete diverse cytokines. Green letters represent cytokines or factors that promote macrophage polarization. Orange letters represent biomarkers of macrophages. Blue letters indicate cytokines secreted by macrophages.
3.1. M1 Macrophages
M1 macrophages, also known as “classically activated (proinflammatory) macrophages”, are activated by granulocyte-macrophage colony-stimulating factor (GM-CSF), Toll-like receptor (TLR) ligands such as LPS, or Th1-type cytokines such as IFN-γ [15,30,31,32]. These macrophages are characterized by a high expression of proinflammatory cytokines and high antigen presentation, which play a critical role in promoting inflammation, killing microorganisms, and fighting against tumors [6,33,34]. M1 macrophages release a large number of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-1β, IL-6, IL-12 and IL-23 [35,36]. Moreover, M1 macrophages highly express CD40, CD64, CD68, CD80, CD86, and human leukocyte DR (HLA-DR) antigen [1,3,26]. In normal tissues, the proportion of M1 macrophages is precisely regulated, and increases during inflammation [29,37]. At the early stage of the inflammatory response, M1 macrophages engulf foreign pathogens and remove bacteria and cell debris. However, excessive secretion of pro-inflammatory cytokines can worsen the inflammatory reaction, resulting in tissue damage and blocked wound healing [3,37].
3.2. M2 Macrophages
M2 macrophages, also known as “selectively activated macrophages”, can be activated by macrophage colony-stimulating factor (M-CSF), Th2-type cytokines such as IL-4 and IL-13, immune complexes (ICs), and complement components [7,26]. M2 macrophages play a critical role in anti-inflammatory and immunomodulatory processes and participate in tissue repair and remodeling. M2 macrophages secrete anti-inflammatory cytokines, including transforming growth factor-β (TGF-β), IL-10, and chemokines CCL18 and CCL22 [31]. Generally, M2 macrophages highly express CD163, CD206, CD209, scavenger receptor A (SR-A), SR-B1, CCR2, CXCR1, and CXCR2 [29,38]. According to different microenvironments and stimuli, M2 macrophages can be further divided into four different subtypes: M2a, M2b, M2c, and M2d [31,39]. M2a macrophages are produced by non-polarized macrophages in response to IL-4 and IL-13 stimulation and are associated with allergic reactions. M2a macrophages express high levels of CD163, CD206, IL-1R, Fizz1, Arg1, and Ym1/2, and secrete profibrotic factors required for tissue repair such as TGF-β, insulin-like growth factor (IGF), fibronectin, CCL13, CCL17, CCL18, CCL22, and CCL24, and collect eosinophils, basophils, and Th2 cells [3,7,40,41,42]. M2b macrophages are induced by TLRs, ICs, and IL-1R, and participate in anti-inflammatory responses and immune regulation. M2b macrophages are characterized by a high expression of CD14 and CD80 and secrete anti-inflammatory cytokines such as IL-10. They also recruit regulatory T cells, which help fight inflammation [43,44,45]. M2c macrophages, induced by IL-10, TGF-β, and glucocorticoid (GC), play a key role in inhibiting immune responses and promoting tissue repair. These macrophages express high levels of CD163, CD206, and advanced glycation end product receptors (RAGE) on their surfaces, and secrete high levels of IL-10, TGF-β, CCL16, and CCL18, and recruit eosinophils and resting T cells [41,42,46,47]. M2d macrophages, also known as tumor-associated macrophages (TAMs), are induced by adenosine A2 receptor (A2AR), leukemia inhibitory factor (LIF) and IL-6, and mainly inhibit inflammatory reaction and promote angiogenesis and tumor growth [48,49]. These macrophages express high levels of CD163 and vascular endothelial growth factor (VEGF), and secrete significant amounts of IL-10, TGF-β, CXCL10, CXCL16, and CCL5 [3,50].
3.3. Signaling Pathways Regulating Macrophage Polarization
Macrophage polarization is a complex process involving multi-factor interactions and is regulated by multiple intracellular signaling molecules and pathways. In recent years, researchers have been studying these pathways in-depth. There are five well-defined signaling pathways involved in macrophage polarization: the PI3K/AKT signaling pathway, TLRs/NF-κB signaling pathway, JAK-STAT signaling pathway, Notch signaling pathway, and JNK signaling pathway [51,52] (Figure 3). In different microenvironments, these pathways individually or collectively regulate macrophage polarization to achieve a dynamic balance between M1 and M2 macrophages.
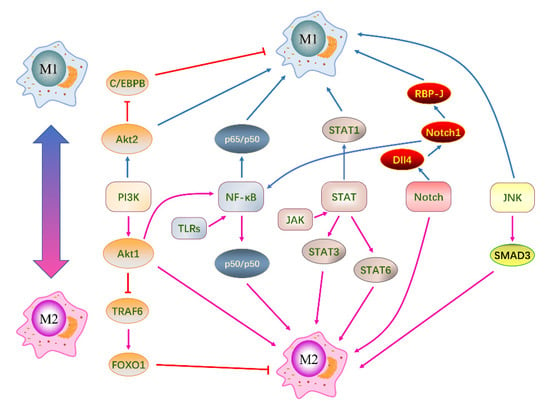
Figure 3.
Signaling pathways associated with macrophage polarization. There are five primary signaling pathways: PI3K/AKT signaling pathway, TLRs/NF-κB signaling pathway, JAK-STAT signaling pathway, Notch signaling pathway, and JNK signaling pathway. These pathways modulate macrophage polarization individually or in conjunction with others. Negative regulation between adjacent molecules is indicated by red lines, while positive regulation is represented by arrows. Molecules that promote macrophage polarization toward the M1 phenotype are depicted by blue arrows, and those that promote M2 polarization are shown by pink arrows.
3.3.1. PI3K/AKT Signaling Pathway
The PI3K/AKT signaling pathway is crucial for cell growth, development, migration, and macrophage polarization [53]. PI3K is a phosphatidylinositol kinase that phosphorylates phosphatidylinositol bisphosphate (PIP2) to produce phosphatidylinositol trisphosphate (PIP3). Cell signaling factors activate class I PI3K, which converts the second messenger PIP2 to PIP3. PIP3 binds to and activates AKT. The activated AKT then regulates cellular functions by regulating the transcription of downstream target genes. The PI3K/Akt1 pathway inhibits TRAF6 and upregulates IRAK-M, the TLR4 repressor, to inactive transcription factor FOXO1, which promotes M2 macrophage polarization by inhibiting TLR4 target genes [54]. Conversely, inhibiting PI3K enhances NF-κB activation and iNOS synthesis, promoting M1 macrophage polarization. Inhibition of the negative regulator of PI3K leads to a decrease in macrophage pro-inflammatory cytokine secretion and induces the synthesis of M2 macrophage surface markers, predisposing macrophage phenotype to M2 [54,55]. Studies have shown that Akt1 and Akt2 have different effects on macrophage polarization. Akt1-deficient macrophages expressed high levels of iNOS, TNF-α, and IL-6. Akt2-deficient macrophages expressed high levels of Fizz1 and IL-10 [53]. Akt1 promotes M2 macrophage polarization and inhibits M1 macrophage polarization. In contrast, Akt2 promotes M1 macrophages and inhibits M2 macrophages.
3.3.2. TLRs/NF-κB Signaling Pathway
In the resting state, NF-κB binds to IκB in an inactive form in the cytoplasm. Upon phosphorylation of IκB, it dissociates from NF-κB, allowing free NF-κB to enter the nucleus and bind to the gene regulatory regions, thereby regulating gene expression [56]. The TLRs/NF-κB signaling pathway plays an important role in macrophage polarization, with all TLRs except TLR3 being able to activate and dissociate NF-κB. TLRs binding to specific ligands, such as PAMP and cytokines, initiate NF-κB-mediated signaling processes that increase the expression of pro-inflammatory-related genes, predisposing the macrophage phenotype towards M1 [57]. The first TLR identified was TLR4, and NF-κB is a key transcriptional regulator of TLR4-induced polarization of macrophages toward the M1. TLR4 activates kinases through both MyD88-dependent and MyD88-independent pathways to phosphorylate IκB, promoting the activation of NF-κB. This leads to the expression of M1 macrophage-related genes by binding to specific DNA sequences in the nucleus [58]. Inhibition of NF-κB reduces TNF-α, IL-1β, and IL-6 in the LPS-induced lung injury model, thereby inhibiting M1 macrophage polarization [59].
3.3.3. JAK-STAT Signaling Pathway
The JAK/STAT signaling pathway is widely involved in important physiological processes, including cell development and immune regulation [60,61]. This pathway involves four kinds of JAK (JAK1, JAK2, JAK3, and TYK2) and seven transcription factors STAT (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6). STAT protein family members are key transcription factors regulating M1/M2 polarization of macrophages [60]. When stimulated by IFN-γ, STAT1 in the JAK/STAT signaling pathway is activated, leading to the secretion of pro-inflammatory factors and promoting the polarization of M1 macrophages [62]. IFN-α and IFN-β inhibit STAT1 phosphorylation and M1 macrophage polarization through negative feedback regulation [63]. IL-4 is a key cytokine promoting the polarization of macrophages towards M2 phenotype by activating the STAT3 signaling pathway [64]. In addition, IL-4 binds to its receptor to activate JAK, which on the one hand directly mediates STAT6 phosphorylation to induce M2 macrophage polarization. Meanwhile, phosphorylated STAT6 binds to KLF4 and PPAR-γ to promote M2 macrophage polarization [65]. Suppressors of cytokine signaling (SOCS) are inhibitors of the JAK/STAT signaling pathway. SOCS1-7 directly inhibit JAK activity by binding to JAK or regulate macrophage polarization by inhibiting STAT phosphorylation with competing receptor binding sites [66]. SOCS1 is an endogenous inhibitor of the STAT1 pathway, inhibiting the JAK/STAT1 signaling pathway through its KIR and SH-2 domains and promoting M2 macrophage polarization. As a negative regulator of STAT3, SOCS3 up-regulation can inhibit M2 macrophage polarization [67]. At present, there are few studies on the regulation of macrophage polarization by the JAK/STAT2/4/5 pathway, and the mechanism requires further exploration.
3.3.4. Notch Signaling Pathway
The Notch signaling pathway has complex and diverse functions that are involved in important physiological processes such as immune cell development, angiogenesis, and embryonic development, and are closely related to tumor formation and some neurological diseases. There are four kinds of Notch receptors: Notch1, Notch2, Notch3, and Notch4, which are expressed in various tissues and organs. Notch ligands are classified into two classes: Delta-like and Jagged, with the former consisting of Dll1, Dll3, and Dll4, and the latter consisting of Jagged1 and Jagged2 [68]. The interaction of the Notch receptor and Notch ligand results in the cascade activation of Notch signaling. After the Notch receptor is activated, its intracellular domain (NICD) translocates from the medial cell membrane into the nucleus, activating the transcription of promoter containing the RBP-J recognition site to activate the Notch signaling pathway under the action of the depolymerase and metalloproteinase domain (ADAM)-type protein and γ-secretase complex [69,70]. Studies have shown that the Dll4/Notch1 signaling axis plays an essential role in promoting M1 polarization of macrophages, while blocking the M2 polarization of macrophages and the expression of related cytokines [71]. The specific mechanism is that ligand Dll4 binds Notch1 receptor to form a complex to activate downstream ADAM protease and γ-secretase, leading to NICD entering the nucleus and interacting with RBP-J to promote the polarization of M1 macrophages. In the high-fat diet-induced obesity model, the activation of the Notch1 signaling pathway promotes the M1 polarization of adipose tissue macrophages [72]. Recent studies have shown that NICD can also directly bind to NF-κB protein, acting independently of RBP-J, namely the non-classical Notch signaling pathway [73].
3.3.5. JNK Signaling Pathway
The MAPK pathway is a conserved tertiary kinase cascade, consisting of MKKK, MKK, and MAPK [74]. The JNK signaling pathway, an important branch of the MAPK pathway, is mainly involved in the functional transformation of adipose tissue macrophages. In obesity, growth factors, cytokines, and saturated fatty acids can activate JNK, leading to the transformation of macrophages into M1 phenotype. At the same time, macrophages also activate the M2 macrophage transcription factor SMAD3, which limits the formation of M1 macrophages [75]. LPS upregulates CCL-2 expression through the TLR4/MyD88 signaling pathway, thereby activating JNK and transcription factors of NF-κB/AP-1, which promote M1 macrophages polarization [76,77]. Furthermore, in IL-4 activated macrophages, K63 ubiquitination of MSR1 leads to JNK activation, promoting the transition from an anti-inflammatory to a pro-inflammatory state and promoting M1 polarization of macrophages [78].
4. The Role of Macrophage Polarization in Lung Disease
4.1. Macrophages in Lung Tissues
Lung tissue macrophages constitute the most abundant immune cell population in the lungs and play a pivotal role in regulating the local microenvironment and immune response [79,80]. They are widely distributed throughout the lung and alveolar tissues and participate in almost all physiological and pathological processes of the lungs [26,81]. There are three main types of lung macrophages: alveolar macrophages (AMs), interstitial macrophages (IMs), and bronchial macrophages (BMs), of which AMs account for over 90% [82]. BMs, isolated from induced sputum, have been less studied [83]. IMs, located in the mesenchyme, interact with mesenchymal lymphocytes to enhance specific immune responses, typically via antigen-presenting macrophages [80,84]. AMs reside in the alveolar lumen and play a crucial role in maintaining lung homeostasis and immune regulation [85,86,87]. Based on their functional status and origin, AMs can be further divided into two subgroups: long-term resident AMs and recruited AMs. Long-lived resident AMs are a uniform, stationary, and immunosuppressed population, whereas recruited AMs are formed when peripheral blood mononuclear cells are recruited into the alveolar lumen in response to certain stimuli [14]. AMs are extremely plastic and heterogeneous. On the one hand, this property drives their rapid response to stimuli and targeted specific immune functions. On the other hand, this property promotes AMs polarization into functionally distinct phenotypes, with different cytokine secretion profiles that mediate pro-inflammatory or anti-inflammatory responses [26,88]. Most studies have focused on AMs in humans and mice.
4.2. Macrophage Polarization and Lung Disease
Recently, studies have highlighted the involvement of macrophages in the pathophysiology of various lung diseases, including ALI, COPD, asthma, tuberculosis, and lung cancer (Figure 4). Modulating macrophage phenotypes can regulate the immune homeostasis of these diseases, providing a novel strategy for their treatment. In vitro and in vivo studies have confirmed that many drugs capable of regulating macrophage polarization exhibit potential in treating lung diseases (Table 1).
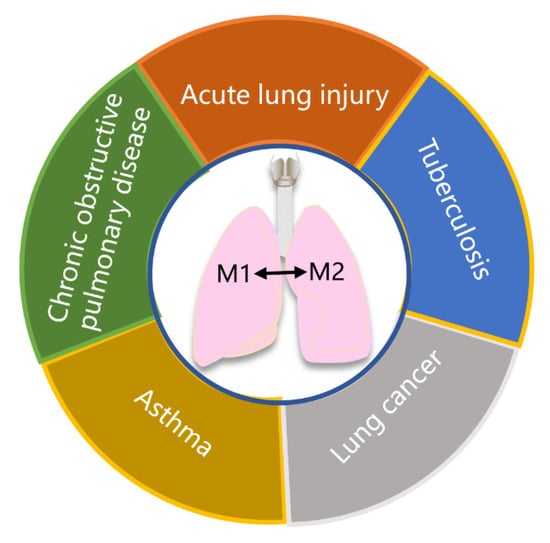
Figure 4.
Schematic diagram of important lung diseases involving macrophage polarization. Macrophage polarization plays a crucial role in the pathophysiology of various lung diseases, including acute lung injury, chronic obstructive pulmonary disease, asthma, tuberculosis, and lung cancer.

Table 1.
Major phenotypes of macrophages in lung diseases and potential therapeutic agents.
4.2.1. Macrophage Polarization and ALI
ALI is a manifestation of the systemic inflammatory response syndrome that affects the lungs, and is characterized by an acute inflammatory response in the alveoli and lung parenchyma [14]. Various infectious and non-infectious factors can trigger ALI, such as bacteria, viruses, pneumonia, aspiration, and trauma [107]. Under the influence of these inducers, macrophages and inflammatory cells infiltrate the lung tissues and release numerous cytokines and inflammatory mediators. This leads to the impairment of the integrity of alveolar epithelial cells and pulmonary microvascular endothelial cells, resulting in pulmonary edema. Then, pulmonary edema causes impaired gas exchange and hypoxemia. Acute respiratory distress syndrome (ARDS) may occur if the condition worsens [108]. Macrophages in lung tissue play an important role in all stages of ALI, with AMs being especially prominent as the largest proportion of macrophages [109].
During ALI exudation, a large number of inflammatory mediators and inflammatory cells accumulate in the lungs, causing diffuse alveolar injury and increased permeability of pulmonary capillaries. This results in protein exudation, pulmonary tissue edema, and damage to epithelial barrier and capillary endothelium [14]. M1 macrophages play a pivotal role in the initiation of ALI exudation. During this process, TLRs or other recognition receptors are induced and activated, leading to the polarization of alveolar macrophages towards M1 macrophages and the release of various inflammatory factors, such as IL-1β, IL-6, IL-12, MCP-1, MIP-2, TNF-α, and ROS [110]. Ultimately, this leads to diffuse alveolar injury. Macrophages polarize to the M1 phenotype by activating the classical JAK/STAT1 signaling pathway, where IFN-γ binding to IFN-γR on the surface of macrophages activates signal molecules such as JAK1, JAK2, and STAT1. This promotes the release of inflammatory cytokines, causing macrophages to polarize to M1 [66,111]. SOCS, mainly SOCS1 and SOCS3, negatively regulate the JAK/STAT1 pathway [112].
Following the exudation phase, the convalescence phase is the second stage of ALI. During this phase, a new extracellular matrix is produced in the alveoli, which is accompanied by neovascularization, thereby promoting repair of the injured lung tissue. M2 macrophages play a key role in the regulation of ALI recovery and tissue repair. These macrophages are capable of enhancing the expression of IL-10, fibronectin 1, and TGF-β, while inhibiting the expression of pro-inflammatory cytokines and slowing down the injury of epithelial cells. This enables the promotion of lung tissue repair [113,114]. In addition, a large number of M2 macrophages can further release anti-inflammatory factors, such as IL-4 and IL-10 through phagocytosis of apoptotic neutrophils, thus inhibiting inflammation and facilitating the recovery from a lung injury [115,116]. During ALI recovery, the transformation of macrophages from M1 phenotype to M2 is also regulated by several pathways, including the JAK/STAT and IRF signaling pathways [117].
Pulmonary fibrosis is the advanced stage of the ALI pathologic processes, characterized by the proliferation of fibroblasts and excessive extracellular matrix (ECM) deposition [117]. At this stage, M1 and M2 macrophages are recruited to the site of the lung tissue injury to regulate the fibrosis process, following the destruction of the basement membrane. M1 macrophages play a significant role in matrix degradation through direct and indirect production of MMP and a variety of anti-fibrotic cytokines [118,119]. MMP production is particularly important for ECM remodeling, and it helps to reduce the pathological fibroplasia observed in the late stage of ALI. Interestingly, M2 macrophages promote fibroplasia in lung tissue. They express significant levels of anti-inflammatory cytokines and tissue inhibitors of metalloproteinases, promoting ECM deposition in the lung tissue [14,120]. Therefore, the degree of pulmonary fibrosis depends on the balance between M1 and M2 macrophages in the local microenvironment of the lung tissue injury.
Macrophages are critical immune cells involved in all stages of ALI. Currently, drugs such as dexamethasone, prednisone, and ulinastatin are commonly used in clinical treatments for ALI [121]. In recent years, researchers have focused on developing novel drugs that can regulate macrophage polarization for ALI treatment. Several studies have shown that drugs such as 5-methoxyflavone, zanubrutinib, canagliflozin, and κ-Opioid receptor agonist U50448H can inhibit macrophages with the M1 phenotype and promote macrophages with the M2 phenotype, which provides a potential candidate drug for the treatment of ALI [89,90,91,92].
4.2.2. Macrophage Polarization and COPD
COPD is an inflammatory disease of the lungs that affects both the lung parenchyma and airways, resulting in the obstruction of small airways and emphysema [13]. This disease is characterized by progressive airflow restriction and recurrent inflammation, massive mucus secretion in the central airway, fibrosis of the small airway, and destruction of lung parenchyma. Smoking is the primary cause of COPD, which induces the activation and aggregation of macrophages [122]. These induced and activated macrophages play a crucial role in the pathogenesis of COPD by participating in inflammation, tissue repair, phagocytosis, and regulating the pulmonary microenvironment.
In COPD, inflammatory responses affect bronchi, bronchioles, and lung parenchyma, resulting in progressive airflow restriction and intrapulmonary hypoxia. At the early stage, M1 macrophages release pro-inflammatory mediators, such as IL-1, TNF-α, NO, ROS, CCL2, and CXCL1, to stimulate the adaptive immune response and remove foreign irritants [123]. In the middle and late stages, M2 macrophages become dominate, inhibiting excessive inflammatory responses and maintaining homeostasis [124]. In addition, M2 macrophages have the function of tissue repair and can secrete a variety of anti-inflammatory factors in the lung tissue of COPD patients [125]. Chronic lung inflammation due to long-term cigarette smoke exposure can cause macrophages to overexpress CD206, IL-10, and TGF-β, driving them to polarize into M2 phenotype and promote tissue repair, as studies have shown [126].
Phagocytosis is a critical function of macrophages in maintaining immune function. M2 macrophages are the primary cells responsible for pulmonary phagocytosis and clearance in COPD. Studies have confirmed that reduced phagocytosis capacity of pulmonary macrophages in COPD patients is associated with a decrease in key markers of M2 macrophage, such as CD206 and CD163, indicating the importance of M2 macrophages in phagocytosis [127]. Additionally, as COPD progresses, the number of M2 macrophages decreases, reducing their ability to phagocytose the accumulated debris from earlier stages of the disease, resulting in the weaker phagocytic activity of macrophages in the middle and late stages of COPD.
Macrophages exhibit tissue-specific characteristics, and their polarization phenotypes vary across different tissues. The most obvious example is the difference in macrophage phenotype between the airway and alveolar lumen in patients with COPD. In the airway, pro-inflammatory M1 macrophages dominate, while anti-inflammatory M2 macrophages are predominant in the alveolar lumen [128]. Moreover, M2 macrophages accounted for the majority of sputum and bronchial alveolar lavage fluid in patients with COPD [13,123]. COPD is a chronic, progressive disease and different pulmonary microenvironments in its progressive stage can alter the polarization of macrophages. Clinical studies have reported a reduction in the proportion of anti-inflammatory phenotype M2a macrophages in the lung tissues of COPD patients, but this proportion returns to a normal amount after a year of treatment [129].
Currently, the treatment of COPD relies mainly on the use of bronchodilators, anti-inflammatory drugs, and anti-infective drugs [13]. While these medications have significant therapeutic effects, they can also cause side effects. Changes in the lung microenvironment can induce different phenotypes of macrophages, resulting in different functional properties. Therefore, modulating the macrophage phenotype in COPD is a novel approach to treat COPD. Ulinastatin, a drug that inhibits M1 macrophages, has been shown to protect the lungs of mice with COPD and may be a potential candidate drug for the treatment of COPD [93].
4.2.3. Macrophage Polarization and Asthma
Asthma is a heterogeneous chronic lung disease characterized by airway inflammation, reversible airflow obstruction, airway remodeling, and bronchial hyperresponsiveness [130]. Allergic asthma, which is caused by allergic stimulation, is the most common type, while non-allergic asthma, caused by non-allergic stimulation, is another common form. Allergens trigger bronchoconstriction and increase the number of eosinophils in the lungs in allergic asthma, while non-allergic asthma usually occurs in adults with a neutrophilic lung infiltration [131]. Although eosinophils and neutrophils are considered as key factors in asthma pathogenesis, recent studies have found that the macrophage phenotypes change in asthma, suggesting an important role for macrophages in asthma.
Macrophages are abundant in lung tissue, but their role in asthma pathology appears to be more related to functional changes than quantitative differences. There is no significant difference in the percentage of macrophages in lung tissue and bronchoalveolar lavage fluid between asthmatic patients and control groups [132]. The polarization and phenotype of macrophages are usually related to the type of asthma. Robbe et al. replicated an allergic asthma mouse model and a non-allergic asthma mouse model to identify M1 or M2 macrophages in different types of asthma [133]. The results showed that macrophages had M1 polarization in the non-allergic asthma mouse model, while macrophages had M2 polarization in the allergic asthma mouse model. This indicates that M1 macrophages are the primary effector cells in non-allergic asthma, while M2 macrophages are the dominant effector cells in allergic asthma. M1 macrophages cause asthma airway inflammation by producing pro-inflammatory cytokines such as TNF-α and IL-6 [134]. In addition, M1 macrophages have been implicated in the pathophysiology of severe asthma, especially in patients who do not respond well to corticosteroids [21,135]. In the pathogenesis of asthma, M2 macrophages promote allergic inflammation and airway hyperreactivity, and through collagen deposition, they induce airway remodeling, leading to asthma exacerbation [12].
Reducing inflammation is a crucial strategy for treating asthma. Corticosteroids are commonly used in clinical practice to inhibit the production of proinflammatory cytokines. Targeting the NLRP3 inflammasome is a potential strategy for treating asthma. Inhibition of NLRP3 inflammasome can prevent airway hyperreactivity and inflammation in mice with severe asthma by inhibiting the expression of IL-1β and Th-2 [136]. Recently, some studies have proposed a novel strategy for suppressing the inflammatory response in asthma by regulating macrophage polarization. Azithromycin has been successfully used for the treatment of asthma by promoting a shift in macrophages from the M1 phenotype to the M2 [94]. Furthermore, protostemonine, emodin, and tiotropium have been found to inhibit the polarization of M2 macrophages and reduce inflammation in asthma patients [95,96,97]. Obviously, regulating macrophage polarization holds great promise as a treatment for asthma. In allergic asthma, M1 macrophages should be inhibited, while in non-allergic asthma, M2 macrophages should be restricted.
4.2.4. Macrophage Polarization and Tuberculosis
Tuberculosis (TB) is a chronic infectious disease caused by the bacterium mycobacterium tuberculosis (MTB), which can infect organs throughout the body, most commonly the lungs. A key histological feature of TB is the tuberculous granuloma [137].
MTB primarily infects macrophages, and macrophage polarization plays a crucial role in MTB infection and the formation and development of tuberculous granulomas [138]. M1 and M2 macrophages coexist in tuberculous granuloma, but M1 macrophages are dominant in the early stages of infection and gradually converted to M2 macrophages in the later stages. Therefore, M1 macrophages are considered the primary cells promoting the formation of granuloma, while M2 macrophages inhibit granuloma formation [11]. In rat models, after injection with Bacillus Calmette–Guérin, a large number of M1 macrophages were detected at the peak of liquefaction necrosis in tuberculous lesions, while M2 macrophages were rare [139].
MTB secretes proteins that can affect macrophage polarization. For example, tubercule-specific peptides, such as E6, E7, and C14 in ESAT-6 and CFP-10 secreted by MTB have varying effects on macrophage polarization [140,141]. E6 and C14 promote M2 polarization of macrophages, while E7 has a bidirectional regulatory effect on macrophage polarization. In the early stage, E7 induces macrophages to M1 polarization through the JNK pathway, but in the later stage, it promotes M2 polarization via the JAK/STAT pathway. In addition, the heat shock protein HSP16.3 of MTB has been found to promote the polarization of macrophages from M1 to M2 and inhibit the development of inflammation [142].
The frontline treatment of MTB infection is a combination of four drugs: isoniazid, rifampicin, pyrazinamide, and ethambutol. These drugs aid in the polarization of M1 macrophages to the M2 phenotype [98]. However, the challenge of treating TB is further complicated by the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB. Thus, there is an urgent need to develop new anti-TB drugs and combination treatment regimens [143]. At present, most of the studies on macrophage polarization in TB have focused on the structure and function of MTB. To screen new therapeutic drugs for TB that target macrophage polarization, further research is needed.
4.2.5. Macrophage Polarization and Lung Cancer
Lung cancer has the highest mortality rate among all types of cancer worldwide, with an average five-year survival rate of only about 20% [144]. It is mainly divided into two types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with NSCLC accounting for about 80% of all lung cancer cases [10]. In the past, the treatment of lung cancer mainly focused on tumor cells. However, with the development of medical research, the tumor microenvironment (TME) in lung cancer has gradually gained attention. Tumor-associated macrophages (TAMs) are macrophages that infiltrate or aggregate in TME and can affect the entire process of lung cancer development.
TAMs are highly malleable, able to polarize to different phenotypes and perform different functions depending on the microenvironmental stimuli. Studies have shown that M1 TAMs are dominant in the early stage of lung cancer, while M2 TAMs become dominant in the middle and late stages [145]. M2 TAMs play crucial roles in the growth, angiogenesis, metastasis, and invasion of lung cancer. Severe infiltration of M2 TAMs is associated with a worse prognosis and lower survival rates in patients. Oct4 expression in lung cancer cells has been shown to promote the polarization of macrophages towards M2 TAMs by upregulating M-CSF, thus leading to tumor growth and metastasis [146]. In mouse models of lung cancer, hypoxia has been found to increase M2 TAMs and promote the expression of IL-10, VEGF, and HIF-1α, further promoting macrophage invasion and cancer cell metastasis [147]. Knockout of IFN-γ in a mouse model of lung cancer resulted in TAMs polarizing into the M2 phenotype and produced larger lung tumors than control mice [148]. In addition, M2 TAMs may promote the expression of EMT and CRYAB, which in turn promote the invasion and metastasis of lung cancer cells [149].
M2 TAMs play a critical role in promoting tumor angiogenesis and local tumor invasion. Studies have demonstrated that M2 TAMs can increase the expression of VEGF-A and VEGF-C in NSCLC cells, leading to the formation of blood vessels and lymphatic vessels at the tumor site [150]. G-Rh2 has been found to induce M2 TAMs to differentiate into M1 phenotype, thereby inhibiting the expression of angiogenic factors and the migration of lung cancer cells [151]. In addition, M2 TAMs also inhibited tumor immunity. M2 TAMs stimulated by Th2 cells release a variety of cytokines, enzymes, and chemokines in the lung TME, which recruit regulatory T cells or inhibit cytotoxic T cell activity, leading to immune tolerance [144,152]. ILT4 is induced by activation of the EGFR-AKT and ERK1/2 signaling pathways in NSCLC cells. Overexpression of ILT4 has been found to recruit M2 TAMs that impair T cell response and inhibit tumor immunity, while inhibition of ILT4 can prevent immunosuppression and inhibit tumor growth [153].
Given the critical role of TAMs in the occurrence and development of lung cancer, targeting TAMs with therapeutic strategies may enhance the efficacy of systemic chemotherapy and immunotherapy for lung cancer. Currently, three strategies are being considered to inhibit the development of lung cancer: changing the phenotype of TAMs, preventing the recruitment of TAMs, and depleting TAMs. Studies have shown that CD40 is involved in the differentiation of monocytes into M1 TAMs and can reverse M2 TAMs into M1. CD40-targeted agonists are being investigated in combination with chemotherapy or immune-checkpoint inhibitors in preliminary clinical trials for NSCLC [154]. TAMs receptor inhibitors are another therapeutic approach to reversing the transformation of M2 TAMs into M1. TAMs receptors, including Tyro3, Axl, and MERTK, polarize TAMs toward the M2 phenotype [155]. In mouse NSCLC models, studies have demonstrated that MERTK’s small molecule inhibitor UNC2025 can inhibit tumor metastasis [156].
Chemokines, cytokines, and complement mediators contribute to the recruitment of TAMs in the tumor microenvironment. Therefore, regulating these factors to inhibit TAM recruitment is an interesting research direction. At present, the CCL2-CCR2 and CSF-1-CSF-1R signaling pathways have been studied extensively [157,158]. The CCL2-CCR2 signaling pathway is known to recruit TAMs and promote tumor neovascularization and the rapid growth of cancer cells. In a mouse model of lung cancer, it was found that knockout of the CCR2 gene or use of the CCR2 inhibitors inhibited the recruitment of TAMs and promoted their polarization into M1 phenotype, ultimately inhibiting the progression of lung cancer [159].
The number of TAMs in the tumor tissue is closely related to the prognosis of the disease, and higher numbers are associated with faster tumor growth. Therefore, consumption of TAMs in TME is an effective strategy for inhibiting tumor growth. CSF-1R is expressed in TAMs, and CSF-1R inhibitors can deplete M2 TAMs in TME. Currently, inhibitors of CSF-1R monoclonal antibodies combined with immunotherapy drugs are being tested in clinical trials for NSCLC treatment [160]. Targeting cell surface molecules, such as CD52, SR-A, folicacid receptor-β (FR-β), and CD206 can also lead to the depletion of M2 TAMs, thereby inhibiting angiogenesis and delaying tumor progression [161].
Taken together, targeting TAMs is an innovative therapeutic for lung cancer treatment. While significant progress has been made in developing novel anti-tumoral drugs that target TAMs, the clinical benefits of some of these drugs remain to be determined [162]. Several studies have demonstrated the potential of drugs such as imatinib, β-elemene, resveratrol, paeoniflorin, paeoniflorin, hydroxychloroquine, astragaloside IV, puerarin, and gefitinib for lung cancer treatment by inhibiting the polarization of M2 TAMs or transforming M2 TAMs into M1 [99,100,101,102,103,104,105,106].
5. Conclusions
Macrophages are the major population of immune cells and play a crucial role in pathogen recognition, clearance, and innate and acquired immune responses. They can be polarized into M1 and M2 phenotypes depending on the local microenvironment. In this review, we focused on the origin of macrophages, the phenotype and polarization of macrophages, as well as the signaling pathways involved in macrophage polarization. We also highlighted the role of macrophage polarization in lung diseases. Many drugs that can regulate the polarization of macrophages have the potential to treat lung diseases. Thus, we believe targeting and modulating macrophage phenotypes is an effective and promising strategy for the treatment of lung diseases.
Author Contributions
L.Z., Z.X. and L.D. provided ideas. L.D. wrote the original manuscript and designed the figures. L.Z., Z.X., L.D., Z.J., T.X., F.L., H.D., Y.Z. and S.L. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Sichuan province’s “14th five-year plan” Sichuan pig major science and technology project (No. 2021ZDZX0010), the Chongqing municipal technology innovation and application development project (No. cstc2021jscx-dxwt BX0007), the research and integration demonstration of key technologies for improving quality and efficiency of Sichuan pig industry chain (No. 2020YFN0147) and the agricultural industry technology system of Sichuan provincial department of agriculture (No. CARS-SVDIP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Li, H.; Ciric, B.; Yang, J.; Xu, H.; Fitzgerald, D.; Elbehi, M.; Fonseca-Kelly, Z.; Yu, S.; Zhang, G.; Rostami, A. Intravenous tolerance modulates macrophage classical activation and antigen presentation in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2009, 208, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Rong, L.; Cong, Y.; Shen, L.; Zhang, N.; Wang, B. Macrophage polarization: An effective approach to targeted therapy of inflammatory bowel disease. Expert Opin. Ther. Targets 2021, 25, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.; Kincaid, K.; Alt, J.; Heilman, M.; Hill, A. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. (Baltim. Md. 1950) 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Z.; Fu, L.; Xu, T. Macrophage Polarization in the Development and Progression of Ovarian Cancers: An Overview. Front. Oncol. 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sugimoto, C.; Arainga, M.; Alvarez, X.; Didier, E.S.; Kuroda, M.J. In Vivo Characterization of Alveolar and Interstitial Lung Macrophages in Rhesus Macaques: Implications for Understanding Lung Disease in Humans. J. Immunol. 2014, 192, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Jessop, F.; Trout, K.L.; Holian, A.; Migliaccio, C. Inflammatory Cells of the Lung: Macrophages-ScienceDirect. Compr. Toxicol. (Third Ed.) 2018, 15, 94–114. [Google Scholar]
- Sedighzadeh, S.S.; Khoshbin, A.P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. A narrative review of tumor-associated macrophages in lung cancer: Regulation of macrophage polarization and therapeutic implications. Transl. Lung Cancer Res. 2021, 10, 1889. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, Q.; Guo, Y.; Chen, J.; Xiong, G.; Peng, Y.; Ye, J.; Li, J. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas in vitro. PLoS ONE 2015, 10, e0129744. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Akata, K.; van Eeden, S.F. Lung macrophage functional properties in chronic obstructive pulmonary disease. Int. J. Mol. Sci. 2020, 21, 853. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, J.; Shuai, W.; Meng, J.; Feng, J.; Han, Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 2020, 69, 883–895. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, mechanisms and significance of macrophage plasticity. Annu. Rev. Pathol. 2020, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- van Furth, R. Mononuclear Phagocytes: Biology of Monocytes and Macrophages; Springer Science & Business Media: Berlin, Germany, 2013; Chapter 1; pp. 3–9. [Google Scholar]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.-L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Wei, J.; Besner, G.E. M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J. Surg. Res. 2015, 197, 126–138. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018, 18, 689–702. [Google Scholar] [CrossRef]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Yamasaki, K.; van Eeden, S.F. Lung macrophage phenotypes and functional responses: Role in the pathogenesis of COPD. Int. J. Mol. Sci. 2018, 19, 582. [Google Scholar] [CrossRef] [PubMed]
- Jordão, M.J.C.; Sankowski, R.; Brendecke, S.M.; Sagar; Locatelli, G.; Tai, Y.-H.; Tay, T.L.; Schramm, E.; Armbruster, S.; Hagemeyer, N. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 2019, 363, eaat7554. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Soulat, D. Latest perspectives on macrophages in bone homeostasis. Pflug. Arch. Eur. J. Physiol. 2017, 469, 517–525. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage polarization in chronic inflammatory diseases: Killers or builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef]
- Abumaree, M.; Al Jumah, M.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.; Fatani, A.; Chamley, L.; Knawy, B. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem. Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I. Diverse macrophages polarization in tumor microenvironment. Arch. Pharmacal Res. 2016, 39, 1588–1596. [Google Scholar] [CrossRef]
- Houghton, A.M.; Hartzell, W.O.; Robbins, C.S.; Gomis-Rüth, F.X.; Shapiro, S.D. Macrophage elastase kills bacteria within murine macrophages. Nature 2009, 460, 637–641. [Google Scholar] [CrossRef]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco) bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Sakamoto, A.; Yamamoto, T.; Narahara, S.; Sugiuchi, H.; Yamaguchi, Y. Differential regulation of IL-23 production in M1 macrophages by TIR8/SIGIRR through TLR4-or TLR7/8-mediated signaling. Cytokine 2017, 99, 310–315. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.P.; Christmann, B.S.; Dunaway, C.W.; Morris, A.; Steele, C. Experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 303, L469–L475. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Q.; Zheng, D.; Sun, Y.; Wang, C.; Yu, X.; Wang, Y.; Lee, V.W.; Zheng, G.; Tan, T.K. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013, 84, 745–755. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Bao, Y.; Zhang, T.; Ainiwaer, D.; Xiong, X.; Wang, G.; Sun, Z. The serum soluble Klotho alleviates cardiac aging and regulates M2a/M2c macrophage polarization via inhibiting TLR4/Myd88/NF-κB pathway. Tissue Cell 2022, 76, 101812. [Google Scholar] [CrossRef] [PubMed]
- Ohama, H.; Asai, A.; Ito, I.; Suzuki, S.; Kobayashi, M.; Higuchi, K.; Suzuki, F. M2b macrophage elimination and improved resistance of mice with chronic alcohol consumption to opportunistic infections. Am. J. Pathol. 2015, 185, 420–431. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Santegoets, K.C.; Van Bon, L.; Wenink, M.H.; Tak, P.P.; Radstake, T.R.; Baeten, D.L. Soluble immune complexes shift the TLR-induced cytokine production of distinct polarized human macrophage subsets towards IL-10. PLoS ONE 2012, 7, e35994. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Lurier, E.B.; Dalton, D.; Dampier, W.; Raman, P.; Nassiri, S.; Ferraro, N.M.; Rajagopalan, R.; Sarmady, M.; Spiller, K.L. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 2017, 222, 847–856. [Google Scholar] [CrossRef]
- Tian, L.; Yu, Q.; Liu, D.; Chen, Z.; Zhang, Y.; Lu, J.; Ma, X.; Huang, F.; Han, J.; Wei, L. Epithelial–mesenchymal Transition of Peritoneal Mesothelial Cells Is Enhanced by M2c Macrophage Polarization. Immunol. Investig. 2022, 51, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, S.; Hasko, G.; Wu, D.; Leibovich, S.J. Suppression of PLCβ2 by endotoxin plays a role in the adenosine A2A receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am. J. Pathol. 2009, 175, 2439–2453. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010, 20, 701–712. [Google Scholar] [CrossRef]
- Wu, H.; Xu, J.B.; He, Y.L.; Peng, J.J.; Zhang, X.H.; CHEN, C.Q.; Li, W.; Cai, S.R. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J. Surg. Oncol. 2012, 106, 462–468. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. Chem.Texts 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Pottle, J.; Ni, M.; He, Y.; Zhang, Z.Y.; Buckner, J.H. Negative regulation of TLR signaling in myeloid cells—Implications for autoimmune diseases. Immunol. Rev. 2016, 269, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, R.; Wang, X.; Liu, F.; Zhao, J.; Yao, Q.; Zhi, W.; He, Z.; Niu, X. Nobiletin-ameliorated lipopolysaccharide-induced inflammation in acute lung injury by suppression of NF-κB pathway in vivo and vitro. Inflammation 2018, 41, 996–1007. [Google Scholar] [CrossRef]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef]
- Gao, Q.; Liang, X.; Shaikh, A.S.; Zang, J.; Xu, W.; Zhang, Y. JAK/STAT signal transduction: Promising attractive targets for immune, inflammatory and hematopoietic diseases. Curr. Drug Targets 2018, 19, 487–500. [Google Scholar] [CrossRef]
- Xu, D.; Li, Q.; Zhou, Y.; Shen, Y.; Lai, W.; Hao, T.; Ding, Y.; Mai, K.; Ai, Q. Functional analysis and regulation mechanism of interferon gamma in macrophages of large yellow croaker (Larimichthys crocea). Int. J. Biol. Macromol. 2022, 194, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hou, J.; Zhou, Y.; Yang, Y.; Cao, X. Type I IFN–inducible downregulation of microRNA-27a feedback inhibits antiviral innate response by upregulating Siglec1/TRIM27. J. Immunol. 2016, 196, 1317–1326. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520. [Google Scholar] [CrossRef]
- Daniel, B.; Nagy, G.; Horvath, A.; Czimmerer, Z.; Cuaranta-Monroy, I.; Poliska, S.; Hays, T.T.; Sauer, S.; Francois-Deleuze, J.; Nagy, L. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 2018, 46, 4425–4439. [Google Scholar] [CrossRef] [PubMed]
- Letellier, E.; Haan, S. SOCS2: Physiological and pathological functions. Front. Bioscience-Elite 2016, 8, 189–204. [Google Scholar]
- Liang, Y.B.; Tang, H.; Chen, Z.B.; Zeng, L.J.; Wu, J.G.; Yang, W.; Li, Z.Y.; Ma, Z.F. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type. Mol. Med. Rep. 2017, 16, 6405–6411. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch signaling in development, tissue homeostasis, and disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Van Baarlen, J.; Storm, G.; Prakash, J. The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Andersson, E.R.; Sandberg, R.; Lendahl, U. Notch signaling: Simplicity in design, versatility in function. Development 2011, 138, 3593–3612. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Smith, S.; Foldi, J.; Zhao, B.; Chung, A.Y.; Outtz, H.; Kitajewski, J.; Shi, C.; Weber, S. Notch–RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 2012, 13, 642–650. [Google Scholar] [CrossRef]
- Miranda, K.; Yang, X.; Bam, M.; Murphy, E.A.; Nagarkatti, P.S.; Nagarkatti, M. MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. Int. J. Obes. 2018, 42, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch signaling regulates immune responses in atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef]
- Oh, J.; Riek, A.E.; Zhang, R.M.; Williams, S.A.; Darwech, I.; Bernal-Mizrachi, C. Deletion of JNK2 prevents vitamin-D-deficiency-induced hypertension and atherosclerosis in mice. J. Steroid Biochem. Mol. Biol. 2018, 177, 179–186. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Hasan, A.; Shenouda, S.; Wilson, A.; Kochumon, S.; Ali, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. TLR4/MyD88-mediated CCL2 production by lipopolysaccharide (endotoxin): Implications for metabolic inflammation. J. Diabetes Metab. Disord. 2018, 17, 77–84. [Google Scholar] [CrossRef]
- Liu, S.; Lu, C.; Liu, Y.; Zhou, X.; Sun, L.; Gu, Q.; Shen, G.; Guo, A. Hyperbaric oxygen alleviates the inflammatory response induced by LPS through inhibition of NF-κB/MAPKs-CCL2/CXCL1 signaling pathway in cultured astrocytes. Inflammation 2018, 41, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Härtlova, A.; Gierliński, M.; Prescott, A.; Castellvi, J.; Losa, J.H.; Petersen, S.K.; Wenzel, U.A.; Dill, B.D.; Emmerich, C.H. Triggering MSR1 promotes JNK-mediated inflammation in IL-4-activated macrophages. EMBO J. 2019, 38, e100299. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Liegeois, M.; Legrand, C.; Desmet, C.J.; Marichal, T.; Bureau, F. The interstitial macrophage: A long-neglected piece in the puzzle of lung immunity. Cell. Immunol. 2018, 330, 91–96. [Google Scholar] [CrossRef]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef]
- Balhara, J.; Gounni, A. The alveolar macrophages in asthma: A double-edged sword. Mucosal Immunol. 2012, 5, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, M.; Bodzenta-Lukaszyk, A.; Kowal, K.; Dabrowska, M. Bronchial macrophages in asthmatics reveal decreased CD16 expression and substantial levels of receptors for IL-10, but not IL-4 and IL-7. Folia Histochem. Cytobiol. 2007, 45, 181–189. [Google Scholar]
- Fathi, M.; Johansson, A.; Lundborg, M.; Orre, L.; Sköld, C.M.; Camner, P. Functional and morphological differences between human alveolar and interstitial macrophages. Exp. Mol. Pathol. 2001, 70, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Peão, M.; Aguas, A.; De Sa, C.; Grande, N. Morphological evidence for migration of particle-laden macrophages through the interalveolar pores of Kohn in the murine lung. Cells Tissues Organs 1993, 147, 227–232. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Brieland, J.K.; Jones, M.L.; Clarke, S.J.; Baker, J.B.; Warren, J.S.; Fantone, J.C. Effect of acute inflammatory lung injury on the expression of monocyte chemoattractant protein-i (MCP-i) in rat pulmonary alveolar macrophages. Am. Respir. Cell Mol. Biol. 1992, 7, 134–139. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Liang, P.; Wang, L.; Yang, S.; Pan, X.; Li, J.; Zhang, Y.; Liang, Y.; Li, J.; Zhou, B. 5-Methoxyflavone alleviates LPS-mediated lung injury by promoting Nrf2-mediated the suppression of NOX4/TLR4 axis in bronchial epithelial cells and M1 polarization in macrophages. J. Inflamm. 2022, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Li, S.; Liang, J.; Liu, Z.; Cui, Y.; Gao, J.; Yang, Z.; Li, L.; Zhou, H. Zanubrutinib ameliorates lipopolysaccharide-induced acute lung injury via regulating macrophage polarization. Int. Immunopharmacol. 2022, 111, 109138. [Google Scholar] [CrossRef]
- Lin, F.; Song, C.; Zeng, Y.; Li, Y.; Li, H.; Liu, B.; Dai, M.; Pan, P. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. Int. Immunopharmacol. 2020, 88, 106969. [Google Scholar] [CrossRef]
- Gao, G.-J.; Song, D.-D.; Li, L.; Zhao, F.; Sun, Y.-J. κ-Opioid Receptor Agonist U50448H Protects against Acute Lung Injury in Rats with Cardiopulmonary Bypass via the CAP-NLRP3 Signaling Pathway. Evidence-Based Complement. Altern. Med. 2022, 2022, 2868135. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, Z.; Yu, H. Effect of different treatments on macrophage differentiation in chronic obstructive pulmonary disease and repeated pulmonary infection. Saudi J. Biol. Sci. 2020, 27, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Ebrahimi Samangani, A.; Kargari, A.; Kiani Nejad, A.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Y.; Li, X.; Shen, Y.; Ding, Y.; Zhu, H.; Liu, F.; Yu, K.; Sun, L.; Qian, F. Protostemonine attenuates alternatively activated macrophage and DRA-induced asthmatic inflammation. Biochem. Pharmacol. 2018, 155, 198–206. [Google Scholar] [CrossRef]
- Song, Y.-d.; Li, X.-z.; Wu, Y.-x.; Shen, Y.; Liu, F.-f.; Gao, P.-p.; Sun, L.; Qian, F. Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model. Acta Pharmacol. Sin. 2018, 39, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Jinno, M.; Tanaka, A.; Uno, T.; Uchida, Y.; Manabe, R.; Ida, H.; Kuwahara, N.; Fukuda, Y.; Kimura, T. The effect of muscarinic M3 receptor blockage in development of M2 macrophages in allergic inflammation. J. Allergy Clin. Immunol. 2019, 143, AB293. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Sui, L.; Xu, H.; Piao, Q.; Liu, Y.; Qu, X.; Sun, Y.; Song, L.; Li, D. Antibiotics induce polarization of pleural macrophages to M2-like phenotype in patients with tuberculous pleuritis. Sci. Rep. 2017, 7, 14982. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, J.; Zhang, B.; Liang, G.; Chen, X.; Yao, F.; Xu, X.; Wu, H.; He, Q.; Ding, L. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 2018, 133, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, M.; Li, N.; Li, Z.; Li, H.; Shao, S.; Zou, K.; Zou, L. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem. Biophys. Res. Commun. 2017, 490, 514–520. [Google Scholar] [CrossRef]
- Sun, L.; Chen, B.; Jiang, R.; Li, J.; Wang, B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell. Immunol. 2017, 311, 86–93. [Google Scholar] [CrossRef]
- Qi, W.; Gang-Ling, C.; Ya-Juan, L.; Yang, C.; Fang-Zhen, L. Paeoniflorin inhibits macrophage-mediated lung cancer metastasis. Chin. J. Nat. Med. 2015, 13, 925–932. [Google Scholar]
- Li, Y.; Cao, F.; Li, M.; Li, P.; Yu, Y.; Xiang, L.; Xu, T.; Lei, J.; Tai, Y.Y.; Zhu, J. Hydroxychloroquine induced lung cancer suppression by enhancing chemo-sensitization and promoting the transition of M2-TAMs to M1-like macrophages. J. Exp. Clin. Cancer Res. 2018, 37, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cui, W.-Q.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.-L.; Gong, W.-Y.; Dong, J.-C.; Liu, B.-J. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 1–16. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, J.; Wang, B.; Liu, M.; Zhao, J.; Yang, M.; Li, Y. Puerarin inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK 1/2 pathway. Int. J. Oncol. 2017, 50, 545–554. [Google Scholar] [CrossRef]
- Tariq, M.; Zhang, J.-q.; Liang, G.-k.; He, Q.-j.; Ding, L.; Yang, B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 1501–1511. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Sun, Y.; Yi, X.; Wei, X.; Liu, W.; Zhang, Q.; Yi, H.; Chen, G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann. Transl. Med. 2020, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mallampalli, R.K. Correction: The Acute Respiratory Distress Syndrome: From Mechanism to Translation. J. Immunol. 2015, 194, 5569. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiu, H.; Zhang, S.; Zhang, G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018, 2018, 1264913. [Google Scholar] [CrossRef]
- Laskin, D.L.; Malaviya, R.; Laskin, J.D. Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol. Sci. 2019, 168, 287–301. [Google Scholar] [CrossRef]
- Minutti, C.M.; García-Fojeda, B.; Sáenz, A.; de Las Casas-Engel, M.; Guillamat-Prats, R.; de Lorenzo, A.; Serrano-Mollar, A.; Corbí, Á.L.; Casals, C. Surfactant protein A prevents IFN-γ/IFN-γ receptor interaction and attenuates classical activation of human alveolar macrophages. J. Immunol. 2016, 197, 590–598. [Google Scholar] [CrossRef]
- Linossi, E.M.; Babon, J.J.; Hilton, D.J.; Nicholson, S.E. Suppression of cytokine signaling: The SOCS perspective. Cytokine Growth Factor Rev. 2013, 24, 241–248. [Google Scholar] [CrossRef]
- Herold, S.; Mayer, K.; Lohmeyer, J. Acute lung injury: How macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2011, 2, 65. [Google Scholar] [CrossRef]
- Cheng, P.; Li, S.; Chen, H. Macrophages in lung injury, repair, and fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef]
- Shirey, K.A.; Pletneva, L.M.; Puche, A.C.; Keegan, A.D.; Prince, G.A.; Blanco, J.C.; Vogel, S.N. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4-, and IFN-β-dependent. Mucosal Immunol. 2010, 3, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.-w.; Shi, Y.; Zheng, Y.-j.; Ju, M.-j.; He, H.-y.; Ma, G.-g.; Hao, G.-w.; Luo, Z. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J. Transl. Med. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, N.; Pandey, R.; Tripathi, Y.B. Role of Macrophage Polarization in Acute Respiratory Distress Syndrome. J. Respir. 2021, 1, 260–272. [Google Scholar] [CrossRef]
- Strieter, R.M. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc. Am. Thorac. Soc. 2008, 5, 305–310. [Google Scholar] [CrossRef]
- Lupher, M.L., Jr.; Gallatin, W.M. Regulation of fibrosis by the immune system. Adv. Immunol. 2006, 89, 245–288. [Google Scholar] [PubMed]
- Tsoutsou, P.G.; Gourgoulianis, K.I.; Petinaki, E.; Germenis, A.; Tsoutsou, A.G.; Mpaka, M.; Efremidou, S.; Molyvdas, P.-A. Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir. Med. 2006, 100, 938–945. [Google Scholar] [CrossRef]
- Hao, D.-L.; Wang, Y.-J.; Yang, J.-Y.; Xie, R.; Jia, L.-Y.; Cheng, J.-T.; Ma, H.; Tian, J.-X.; Guo, S.-S.; Liu, T. The Alleviation of LPS-Induced Murine Acute Lung Injury by GSH-Mediated PEGylated Artesunate Prodrugs. Front. Pharmacol. 2022, 13, 860492. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Digilio, F.A.; Galderisi, U.; Peluso, G. The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Hu, X.; Wang, H.; Han, C.; Cao, X. Src promotes anti-inflammatory (M2) macrophage generation via the IL-4/STAT6 pathway. Cytokine 2018, 111, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S. Altered macrophage function in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2013, 10, S180–S185. [Google Scholar] [CrossRef] [PubMed]
- Oliveira da Silva, C.; Monte-Alto-Costa, A.; Renovato-Martins, M.; Viana Nascimento, F.J.; dos Santos Valença, S.; Lagente, V.; Pôrto, L.C.; Victoni, T. Time course of the phenotype of blood and bone marrow monocytes and macrophages in the lung after cigarette smoke exposure in vivo. Int. J. Mol. Sci. 2017, 18, 1940. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: Implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2007, 37, 748–755. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; McAlinden, K.; Kim, R.Y.; Ward, C.; Hackett, T.-L.; Walters, E.H.; Sohal, S.S. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lin, M.-C.; Lee, C.-H.; Liu, S.-F.; Wang, C.-C.; Fang, W.-F.; Chao, T.-Y.; Wu, C.-C.; Wei, Y.-F.; Chang, H.-C. Defective formyl peptide receptor 2/3 and annexin A1 expressions associated with M2a polarization of blood immune cells in patients with chronic obstructive pulmonary disease. J. Transl. Med. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- van der Veen, T.A.; de Groot, L.E.; Melgert, B.N. The different faces of the macrophage in asthma. Curr. Opin. Pulm. Med. 2020, 26, 62. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Girodet, P.-O.; Nguyen, D.; Mancini, J.D.; Hundal, M.; Zhou, X.; Israel, E.; Cernadas, M. Alternative macrophage activation is increased in asthma. Am. J. Respir. Cell Mol. Biol. 2016, 55, 467–475. [Google Scholar] [CrossRef]
- Robbe, P.; Draijer, C.; Borg, T.R.; Luinge, M.; Timens, W.; Wouters, I.M.; Melgert, B.N.; Hylkema, M.N. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am. J. Physiology-Lung Cell. Mol. Physiol. 2015, 308, L358–L367. [Google Scholar] [CrossRef] [PubMed]
- Gosset, P.; Tsicopoulos, A.; Wallaert, B.; Vannimenus, C.; Joseph, M.; Tonnel, A.-B.; Capron, A. Increased secretion of tumor necrosis factor α and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J. Allergy Clin. Immunol. 1991, 88, 561–571. [Google Scholar] [CrossRef]
- Oriss, T.B.; Raundhal, M.; Morse, C.; Huff, R.E.; Das, S.; Hannum, R.; Gauthier, M.C.; Scholl, K.L.; Chakraborty, K.; Nouraie, S.M. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight 2017, 2, e91019. [Google Scholar] [CrossRef]
- Rossios, C.; Pavlidis, S.; Hoda, U.; Kuo, C.-H.; Wiegman, C.; Russell, K.; Sun, K.; Loza, M.J.; Baribaud, F.; Durham, A.L. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J. Allergy Clin. Immunol. 2018, 141, 560–570. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Long, R.Y.; Liu, X.H.; Liu, Q.Y.; Li, M.; Luo, M.J. Research progress on the effect of pathogenic bacteria on macrophage polarization (in Chinese). Chin. J. Immunol. 2018, 34, 1748. [Google Scholar]
- Teng, O.; Ang, C.K.E.; Guan, X.L. Macrophage–bacteria interactions—A lipid-centric relationship. Front. Immunol. 2017, 8, 1836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Liu, Z.; Zhang, Y.J.; Zhang, T.W.; Rong, M.Y.; Jia, C.Y. Changes in macrophage polarization in a rat model of tuberculous wound (in Chinese). Chin. J. Inj. Repair 2018, 13, 30. [Google Scholar]
- Shen, D.H.; Fang, Y.M.; Shen, Y.M.; Liu, G.B.; Yao, Y.N.; Lai, X.M. Effects of tuberculous specific peptides E6, E7 and C14 on the polarization of mononuclear macrophage subtypes (in Chinese). Chin. Pharm. Biotechnol. 2016, 11, 216–223. [Google Scholar]
- Seghatoleslam, A.; Hemmati, M.; Ebadat, S.; Movahedi, B.; Mostafavi-Pour, Z. Macrophage immune response suppression by recombinant mycobacterium tuberculosis antigens, the ESAT-6, CFP-10, and ESAT-6/CFP-10 fusion proteins. Iran. J. Med. Sci. 2016, 41, 296. [Google Scholar]
- Liu, Q.Y.; Li, S.S.; Qin, H.; Luo, J.M. Effect of heat shock proteins on macrophages in tuberculous tuberculosis infection (in Chinese). Mod. Immunol. 2017, 37, 69–73. [Google Scholar]
- Tanner, L.; Mashabela, G.T.; Omollo, C.C.; de Wet, T.J.; Parkinson, C.J.; Warner, D.F.; Haynes, R.K.; Wiesner, L. Intracellular Accumulation of Novel and Clinically Used TB Drugs Potentiates Intracellular Synergy. Microbiol. Spectr. 2021, 9, e0043421. [Google Scholar] [CrossRef]
- Xu, F.; Wei, Y.; Tang, Z.; Liu, B.; Dong, J. Tumor-associated macrophages in lung cancer: Friend or foe? Mol. Med. Rep. 2020, 22, 4107–4115. [Google Scholar]
- Qian, B.-Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-S.; Shiau, A.-L.; Su, B.-H.; Hsu, T.-S.; Wang, C.-T.; Su, Y.-C.; Tsai, M.-S.; Feng, Y.-H.; Tseng, Y.-L.; Yen, Y.-T. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J. Hematol. Oncol. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Ma, S.; Dong, R.; Meng, W.; Ying, M.; Weng, Q.; Chen, Z.; Ma, J.; Fang, Q. Tumor hypoxia enhances non-small cell lung cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget 2014, 5, 9664. [Google Scholar] [CrossRef]
- Redente, E.F.; Dwyer-Nield, L.D.; Barrett, B.S.; Riches, D.W.; Malkinson, A.M. Lung tumor growth is stimulated in IFN-γ-/- mice and inhibited in IL-4Rα-/- mice. Anticancer. Res. 2009, 29, 5095–5101. [Google Scholar]
- Guo, Z.; Song, J.; Hao, J.; Zhao, H.; Du, X.; Li, E.; Kuang, Y.; Yang, F.; Wang, W.; Deng, J. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.-Y.; Hewitt, S.M. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.-l. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer 2019, 136, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, A.; Zhang, F.; Yang, Z.; Wang, S.; Fang, Y.; Li, J.; Wang, J.; Shi, W.; Wang, L. ILT4 inhibition prevents TAM-and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics 2021, 11, 3392. [Google Scholar] [CrossRef]
- Richards, D.M.; Sefrin, J.P.; Gieffers, C.; Hill, O.; Merz, C. Concepts for agonistic targeting of CD40 in immuno-oncology. Human Vaccines Immunother. 2020, 16, 377–387. [Google Scholar] [CrossRef]
- Myers, K.V.; Amend, S.R.; Pienta, K.J. Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for macrophages in the tumor microenvironment. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Cummings, C.T.; Zhang, W.; Davies, K.D.; Kirkpatrick, G.D.; Zhang, D.; DeRyckere, D.; Wang, X.; Frye, S.V.; Earp, H.S.; Graham, D.K. Small Molecule Inhibition of MERTK Is Efficacious in Non–Small Cell Lung Cancer Models Independent of Driver Oncogene StatusA Novel MERTK Small Molecule Inhibitor in NSCLC. Mol. Cancer Ther. 2015, 14, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Low-Marchelli, J.M.; Ardi, V.C.; Vizcarra, E.A.; Van Rooijen, N.; Quigley, J.P.; Yang, J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013, 73, 662–671. [Google Scholar] [CrossRef]
- Gomez-Roca, C.A.; Cassier, P.A.; Italiano, A.; Cannarile, M.; Ries, C.; Brillouet, A.; Mueller, C.; Jegg, A.-M.; Meneses-Lorente, G.; Baehner, M. Phase I study of RG7155, a novel anti-CSF1R antibody, in patients with advanced/metastatic solid tumors. Am. Soc. Clin. Oncol. 2015, 33, 3005. [Google Scholar] [CrossRef]
- Schmall, A.; Al-Tamari, H.M.; Herold, S.; Kampschulte, M.; Weigert, A.; Wietelmann, A.; Vipotnik, N.; Grimminger, F.; Seeger, W.; Pullamsetti, S.S. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am. J. Respir. Crit. Care Med. 2015, 191, 437–447. [Google Scholar] [CrossRef]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.-M.; Ries, C.H.; Rüttinger, D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Kandefer-Szerszeń, M. Tumor-associated macrophages as target for antitumor therapy. Arch. Immunol. Ther. Exp. 2018, 66, 97–111. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Gorain, B.; Alhakamy, N.A.; Md, S. Role of therapeutic agents on repolarisation of tumour-associated macrophage to halt lung cancer progression. J. Drug Target. 2020, 28, 166–175. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).