Density Functional Study on Adsorption of NH3 and NOx on the γ-Fe2O3 (111) Surface
Abstract
1. Introduction
2. Results
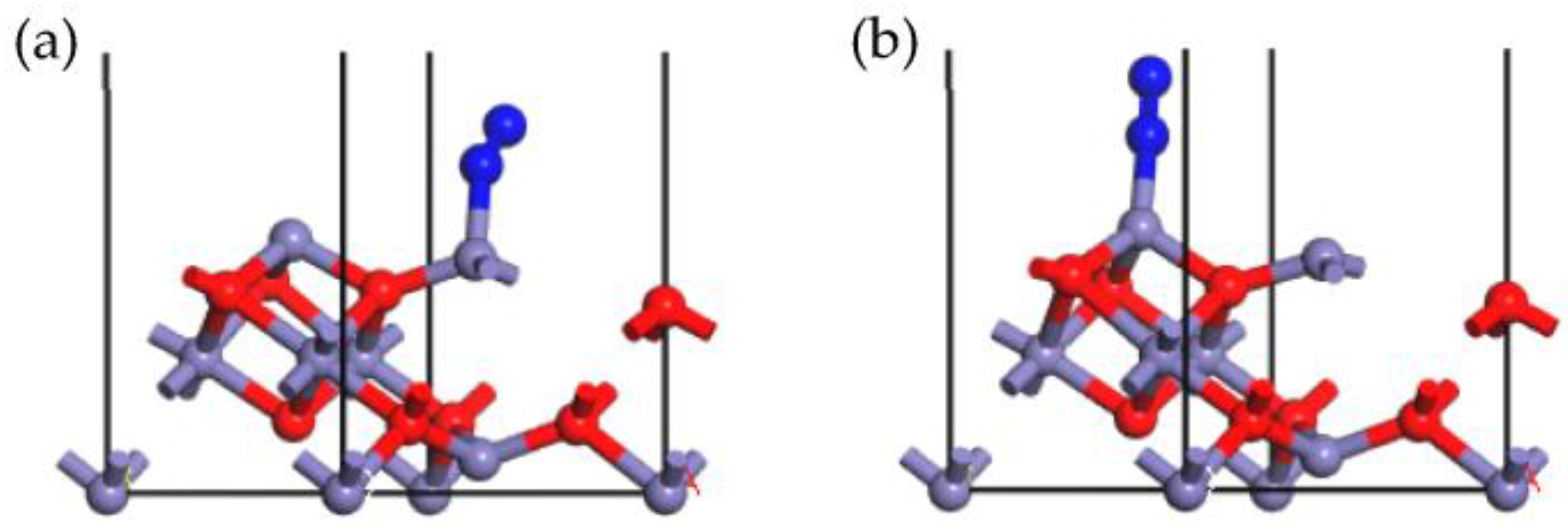
2.1. Adsorption of NH3 onto γ-Fe2O3 (111) Surface
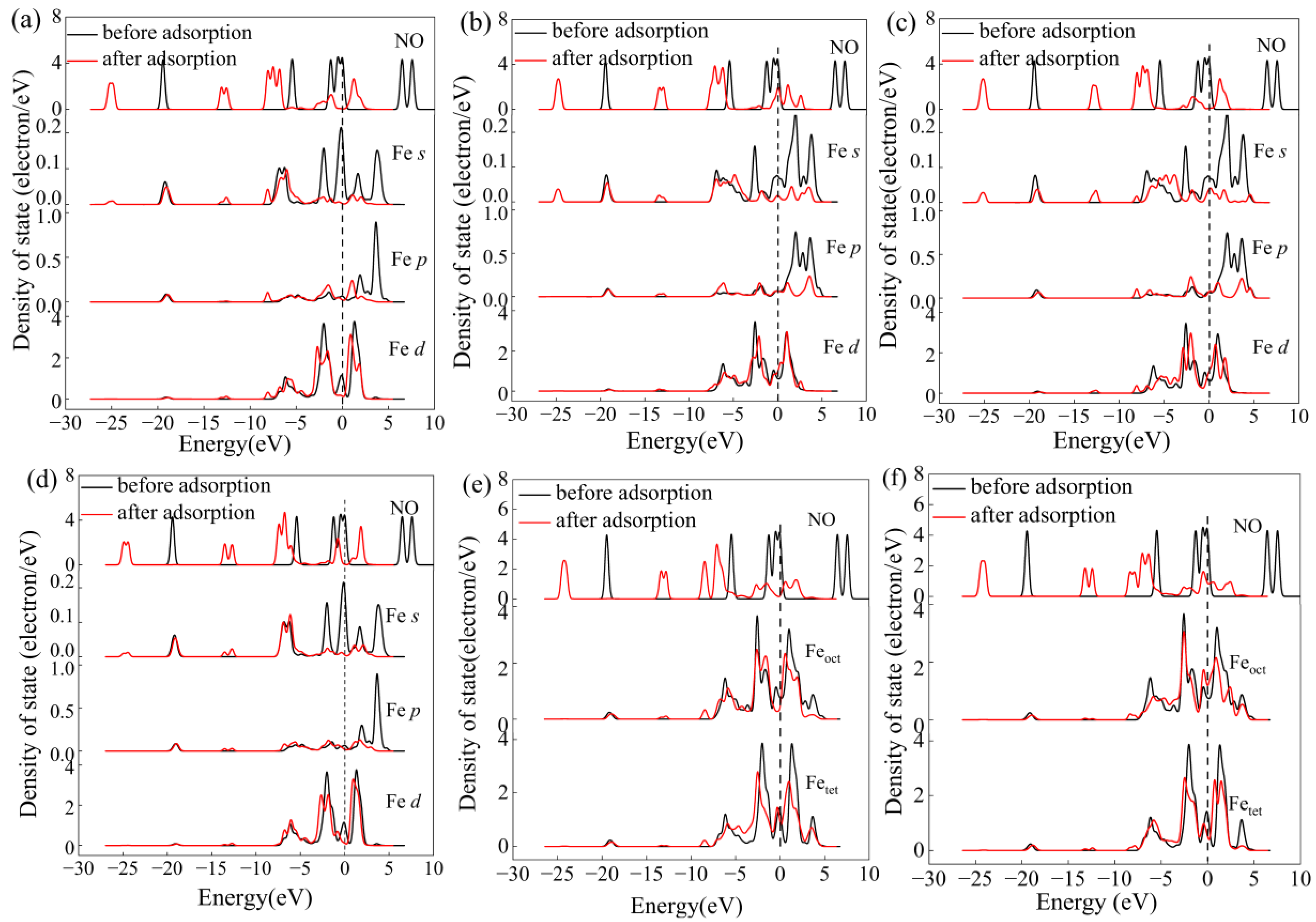
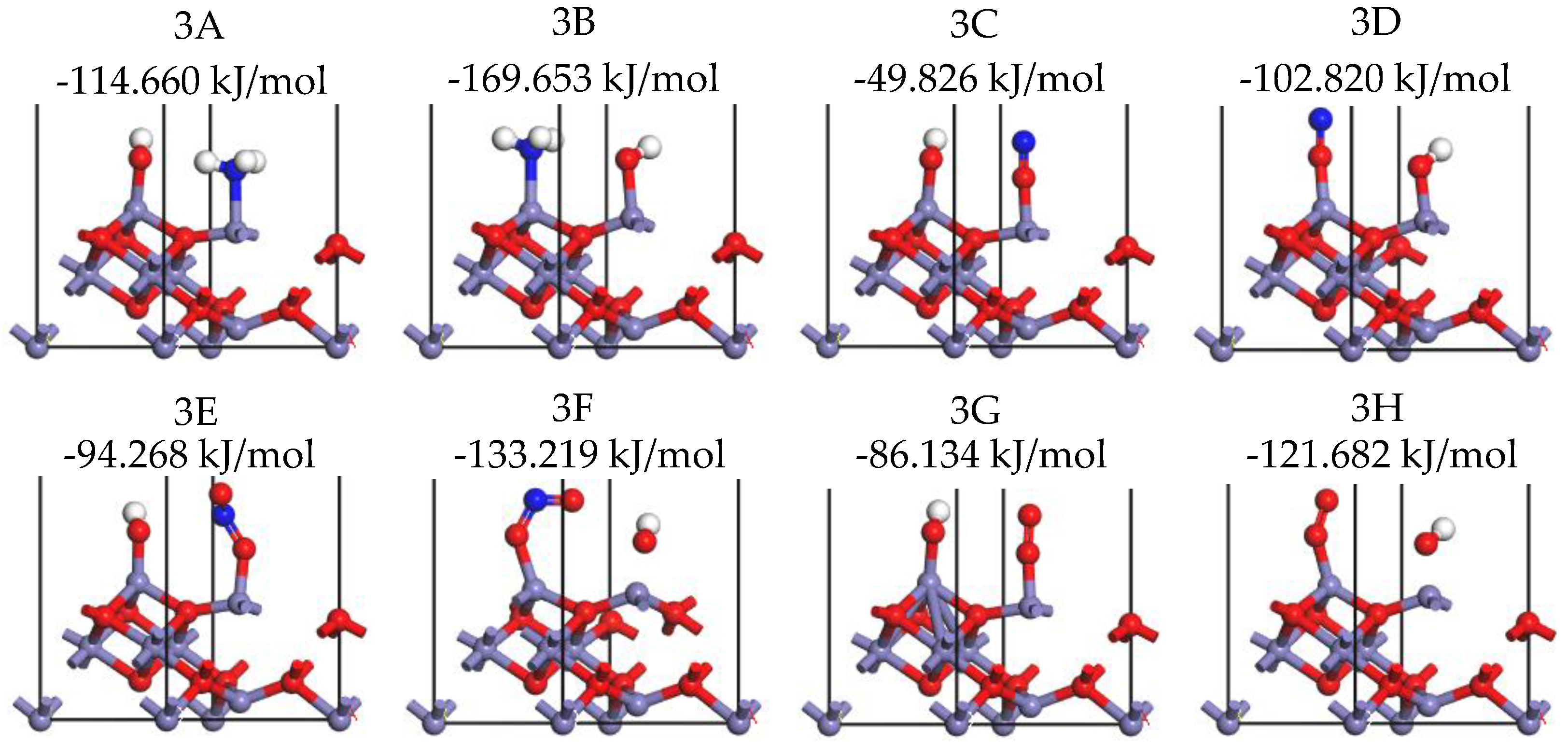
2.2. Adsorption of NO onto γ-Fe2O3 (111) Surface
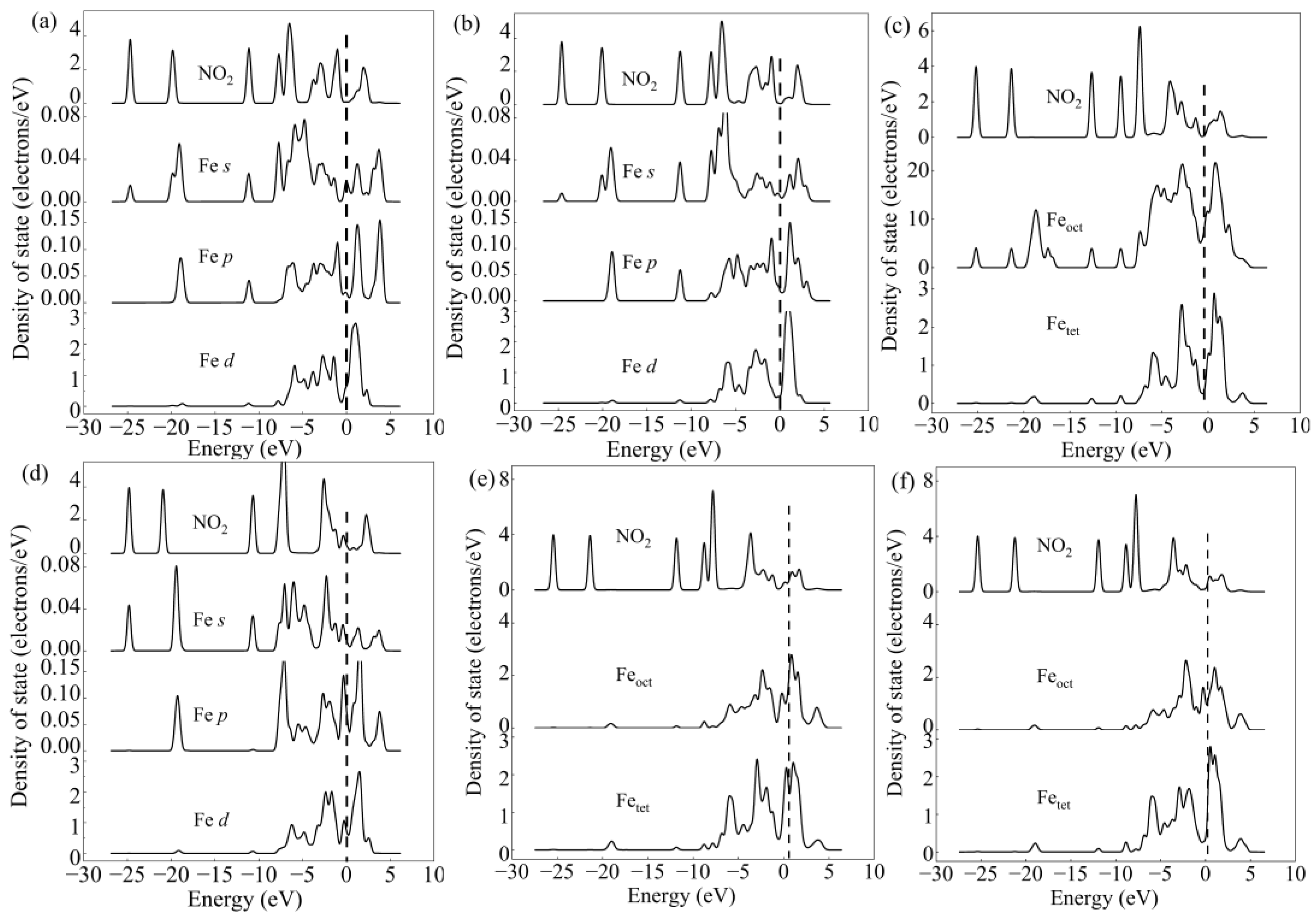
2.3. Adsorption of NO2 onto γ-Fe2O3 (111) Surface
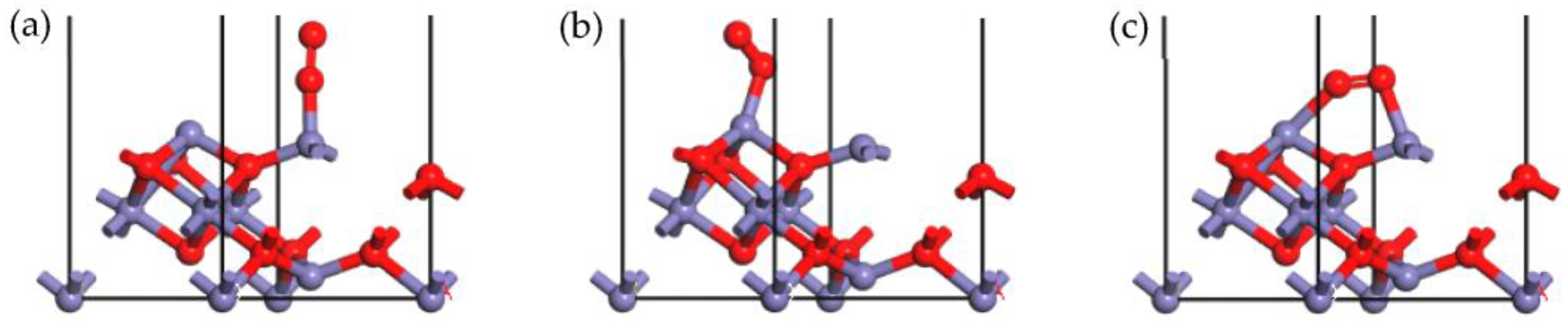
2.4. Adsorption of Other Molecules onto the γ-Fe2O3 (111) Surface
3. Discussion
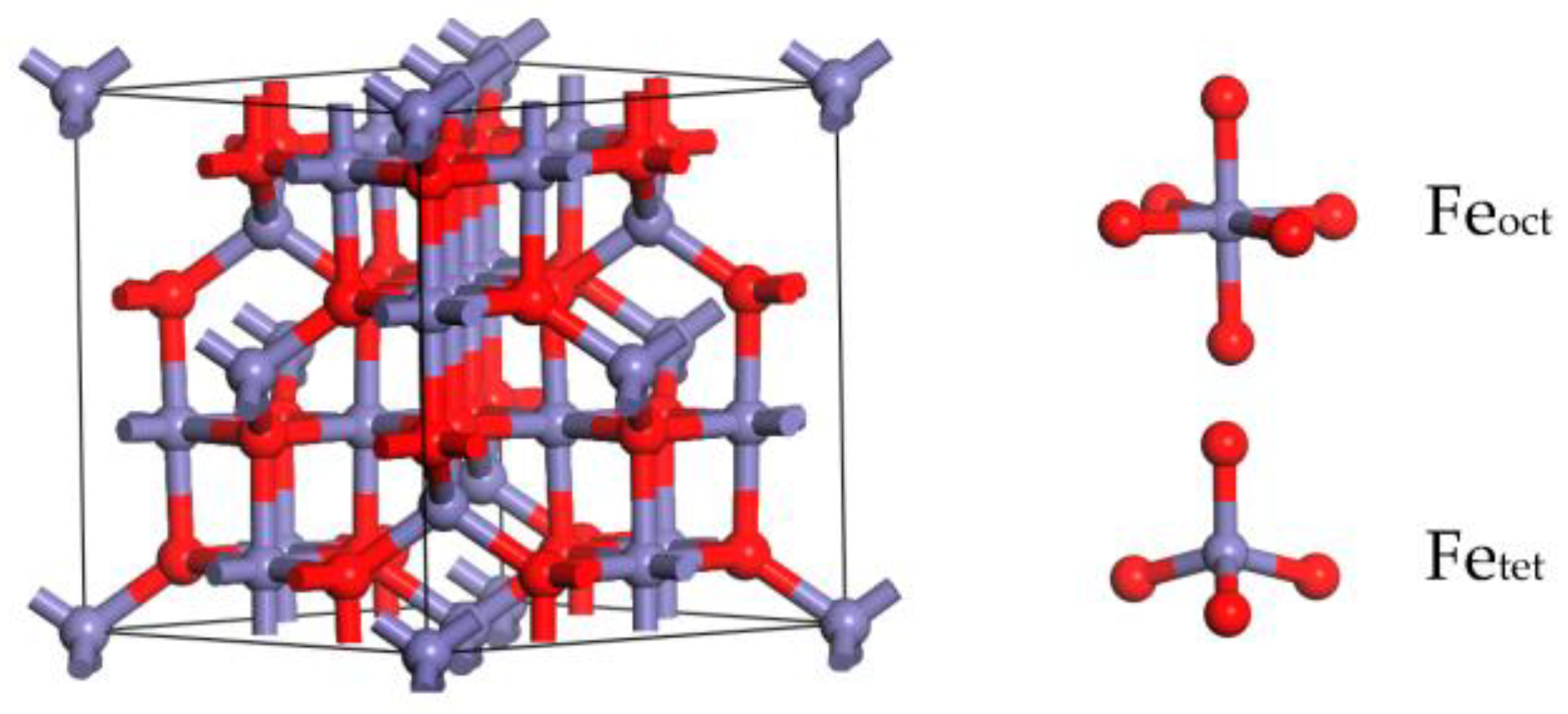
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakatsuji, T.; Miyamoto, A. Removal technology for nitrogen oxides and sulfur oxides from exhaust gases. Catal. Today 1991, 10, 21–31. [Google Scholar] [CrossRef]
- Bauerle, G.; Wu, S.; Nobe, K. Parametric and durability studies of NOx reduction with NH3 on V2O5 catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1978, 17, 117–122. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Liu, Z.; Ihl Woo, S. Recent advances in catalytic DeNOx science and technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156, 371–377. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Yao, G.; Wang, F.; Wang, X.; Gui, K. Magnetic field effects on selective catalytic reduction of NO by NH3 over Fe2O3 catalyst in a magnetically fluidized bed. Energy 2010, 35, 2295–2300. [Google Scholar] [CrossRef]
- Yao, G.; Gui, K.; Wang, F. Low-Temperature De-NOx by Selective Catalytic Reduction Based on Iron-Based Catalysts. Chem. Eng. Technol. 2010, 33, 1093–1098. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G.; Turco, M.; Kotur, E.; Willey, R. Adsorption, activation, and oxidation of ammonia over SCR catalysts. J. Catal. 1995, 157, 523–535. [Google Scholar] [CrossRef]
- Apostolescu, N.; Geiger, B.; Hizbullah, K.; Jan, M.; Kureti, S.; Reichert, D.; Schotta, F.; Weisweiler, W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts. Appl. Catal. B Environ. 2006, 62, 104–114. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Ma, L.; Peng, Y.; Hamidreza, A.; Chang, H.; Li, J. Comparison on the performance of α-Fe2O3 and γ-Fe2O3 for selective catalytic reduction of nitrogen oxides with ammonia. Catal. Lett. 2013, 143, 697–704. [Google Scholar] [CrossRef]
- Kato, A.; Matsuda, S.; Nakajima, F.; Imanari, M.; Watanabe, Y. Reduction of nitric oxide with ammonia on iron oxide-titanium oxide catalyst. J. Phys. Chem. 1981, 85, 1710–1713. [Google Scholar] [CrossRef]
- Kato, A.; Matsuda, S.; Kamo, T.; Nakajima, F.; Kuroda, H.; Narita, T. Reaction between nitrogen oxide (NOx) and ammonia on iron oxide-titanium oxide catalyst. J. Phys. Chem. 1981, 85, 4099–4102. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Liu, Y.; Shan, W.; Shi, X.; Zhang, C. Influence of calcination temperature on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Catal. Today 2011, 164, 520–527. [Google Scholar] [CrossRef]
- Liu, F.; He, H. Structure− Activity Relationship of Iron Titanate Catalysts in the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C 2010, 114, 16929–16936. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C. Novel iron titanate catalyst for the selective catalytic reduction of NO with NH3 in the medium temperature range. Chem. Commun. 2008, 17, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D.; He, H.; Qu, Z.; Yang, C.; Zhou, Q.; Jia, J. Nanosized cation-deficient Fe−Ti spinel: A novel magnetic sorbent for elemental mercury capture from flue gas. ACS Appl. Mater. Interfaces 2011, 3, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, J.; Wang, C.; Chen, J.; Ma, L.; Chang, H.; Chen, L.; Peng, Y.; Yan, N. Fe–Ti spinel for the selective catalytic reduction of NO with NH3: Mechanism and structure–activity relationship. Appl. Catal. B: Environ. 2012, 117, 73–80. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, J.; Du, G.; Zheng, J.; Zhu, Z. In situ modifying of carbon tube-in-tube nanostructures with highly active Fe2O3 nanoparticles. Nanotechnology 2008, 19, 205605. [Google Scholar] [CrossRef]
- Yang, S.; Liu, C.; Chang, H.; Ma, L.; Qu, Z.; Yan, N.; Wang, C.; Li, J. Improvement of the activity of γ-Fe2O3 for the selective catalytic reduction of NO with NH3 at high temperatures: NO reduction versus NH3 oxidization. Ind. Eng. Chem. Res. 2013, 52, 5601–5610. [Google Scholar] [CrossRef]
- Liang, H.; Gui, K.; Zha, X. DRIFTS study of γFe2O3 nano-catalyst for low-temperature selective catalytic reduction of NOx with NH3. Can. J. Chem. Eng. 2016, 94, 1668–1675. [Google Scholar] [CrossRef]
- Ramis, G.; Larrubia, M. An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3/Al2O3 SCR catalysts. J. Mol. Catal. A Chem. 2004, 215, 161–167. [Google Scholar] [CrossRef]
- Larrubia, M.; Ramis, G.; Busca, G. An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3-TiO2 SCR catalysts. Appl. Catal. B Environ. 2001, 30, 101–110. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Gilardoni, F.; Weber, J.; Baiker, A. Mechanism of the vanadium oxide-catalyzed selective reduction of NO by NH3. A quantum chemical modeling. J. Phys. Chem. A 1997, 101, 6069–6076. [Google Scholar] [CrossRef]
- Gilardoni, F.; Weber, J.; Baiker, A. Density functional investigation of the mechanism of the selective catalytic reduction of NO by NH3 over vanadium oxide model clusters. Int. J. Quantum Chem. 1997, 61, 683–688. [Google Scholar] [CrossRef]
- Rethwisch, D.; Dumesic, J. Adsorptive and catalytic properties of supported metal oxides. 2. Infrared spectroscopy of nitric oxide adsorbed on supported iron oxides. J. Phys. Chem. 1986, 90, 1625–1630. [Google Scholar] [CrossRef]
- Guo, P.; Guo, X.; Zheng, C. Roles of γ-Fe2O3 in fly ash for mercury removal: Results of density functional theory study. Appl. Surf. Sci. 2010, 256, 6991–6996. [Google Scholar] [CrossRef]
- Rohrbach, A.; Hafner, J. Molecular adsorption of NO on NiO (100): DFT and DFT+ U calculations. Phys. Rev. B 2005, 71, 045405. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Junaid, A. NO and NO2 adsorption on Al2O3 and Ga modified Al2O3 surfaces: A density functional theory study. J. Phys. Chem. C 2010, 114, 4445–4450. [Google Scholar] [CrossRef]
- Ren, D.; Gui, K. Study of the adsorption of NH3 and NOx on the nano-γFe2O3 (001) surface with density functional theory. Appl. Surf. Sci. 2019, 487, 171–179. [Google Scholar] [CrossRef]
- Bowker, M.; Hutchings, G.; Davies, P.; Edwards, D.; Davies, R.; Shaikhutdinov, S.; Freund, H. Surface structure of γ-Fe2O3 (111). Surf. Sci. 2012, 606, 1594–1599. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Du, X.; Ding, J.; Liu, F. Metal–Metal Interactions of Ternary Spinel for Efficient NH3 Selective Catalytic Reduction of NOx at a Low Temperature. Energy Fuels 2020, 34, 15424–15432. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Miao, S.; Liu, J.; Yu, Y.; Ding, J. Mechanistic landscape of HCl-mediated Hg0 capture by magnetite. J. Phys. Chem. C 2019, 123, 30434–30442. [Google Scholar] [CrossRef]
- Ren, D.; Gui, K.; Gu, S. Quantum chemistry study of SCR-NH3 nitric oxide reduction on Ce-doped γFe2O3 catalyst surface. Mol. Catal. 2021, 502, 111373. [Google Scholar] [CrossRef]
- Baetzold, R.; Yang, H. Computational study on surface structure and crystal morphology of γ-Fe2O3: Toward deterministic synthesis of nanocrystals. J. Phys. Chem. B 2003, 107, 14357–14364. [Google Scholar] [CrossRef]
- Payne, M.; Teter, M.; Allan, D.; Arias, T.; Joannopoulos, J. CASTEP 4.2 Academic version, licensed under the UKCP-MSI Agreement. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- White, J.; Bird, D. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954. [Google Scholar] [CrossRef]
- Nayak, S.; Nooijen, M.; Bernasek, S.; Blaha, P. Electronic structure study of CO adsorption on the Fe (001) surface. J. Phys. Chem. B 2001, 105, 164–172. [Google Scholar] [CrossRef]


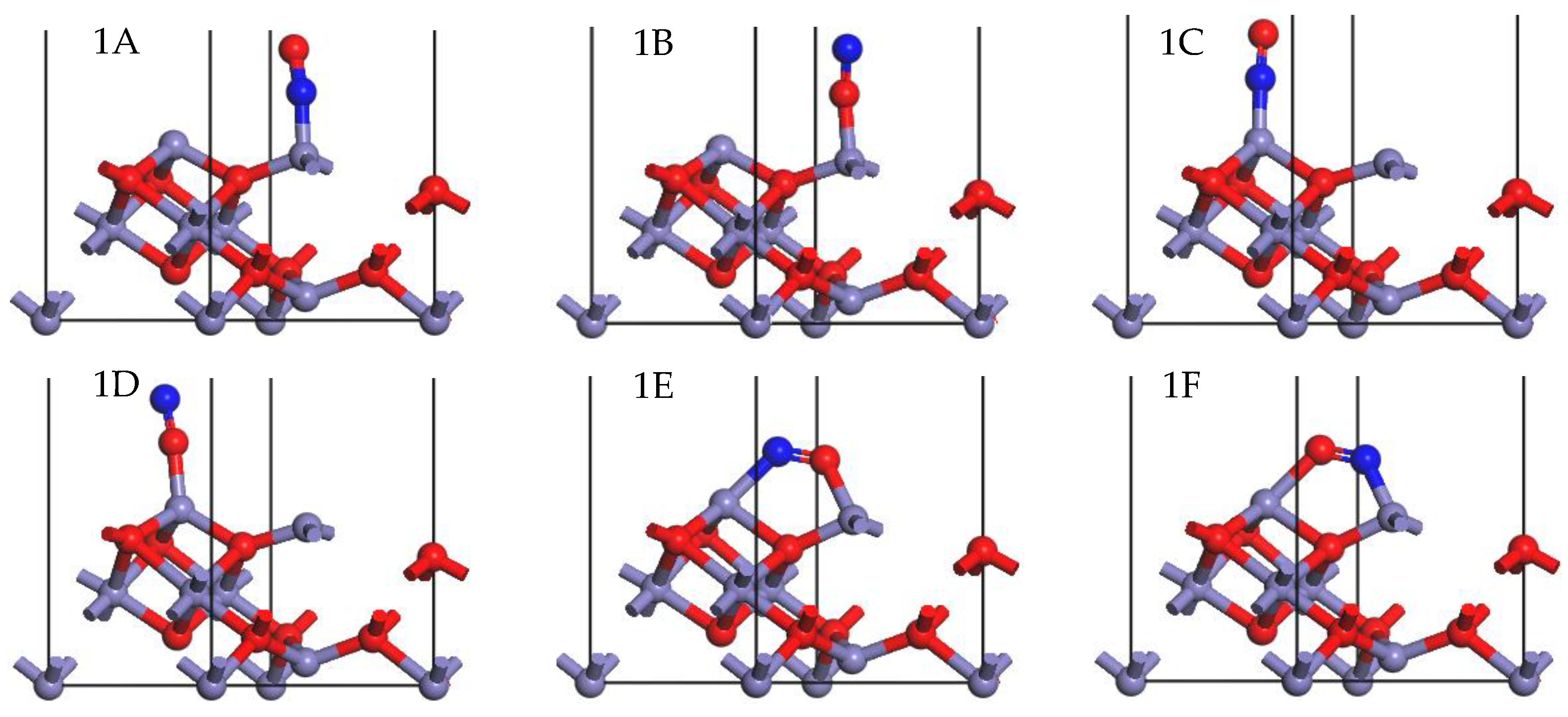




| Adsorption Site | Adsorption Energy (kJ/mol) | Hirshfeld Charge (e) | Bond Length (Å) | |
|---|---|---|---|---|
| a | Feoct | −8.935 | 0.10 | 2.101 |
| b | Fetet | −90.729 | 0.15 | 2.130 |
| c | Lattice O | −34.620 | −0.09 | 1.851 |
| Adsorption Site | Adsorption Energy (kJ/mol) | Hirshfeld Charge (e) | Fe-N Bond Length (Å) | Fe-O Bond Length (Å) | |
|---|---|---|---|---|---|
| 1A | Feoct | −287.159 | −0.14 | 1.697 | |
| 1B | Feoct | −177.547 | −0.18 | 1.811 | |
| 1C | Fetet | −195.665 | −0.10 | 1.701 | |
| 1D | Fetet | −70.593 | −0.14 | 1.802 | |
| 1E | Fetet-Feoct | −237.602 | −0.18 | 1.941 | 1.851 |
| 1D | Fetet-Feoct | −316.855 | −0.16 | 1.718 | 2.002 |
| Adsorption Site | Adsorption Energy (kJ/mol) | Hirshfeld Charge (e) | |
|---|---|---|---|
| a | Feoct | −86.538 | −0.10 |
| b | Fetet | −123.374 | −0.08 |
| Adsorption Site | Adsorption Energy (kJ/mol) | Hirshfeld Charge (e) | |
|---|---|---|---|
| a | Feoct | −75.266 | 0.09 |
| b | Fetet | −112.224 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Wang, L.; Dong, L.; Hu, H.; Ren, D. Density Functional Study on Adsorption of NH3 and NOx on the γ-Fe2O3 (111) Surface. Molecules 2023, 28, 2371. https://doi.org/10.3390/molecules28052371
Huang W, Wang L, Dong L, Hu H, Ren D. Density Functional Study on Adsorption of NH3 and NOx on the γ-Fe2O3 (111) Surface. Molecules. 2023; 28(5):2371. https://doi.org/10.3390/molecules28052371
Chicago/Turabian StyleHuang, Wei, Liang Wang, Lu Dong, Hongyun Hu, and Dongdong Ren. 2023. "Density Functional Study on Adsorption of NH3 and NOx on the γ-Fe2O3 (111) Surface" Molecules 28, no. 5: 2371. https://doi.org/10.3390/molecules28052371
APA StyleHuang, W., Wang, L., Dong, L., Hu, H., & Ren, D. (2023). Density Functional Study on Adsorption of NH3 and NOx on the γ-Fe2O3 (111) Surface. Molecules, 28(5), 2371. https://doi.org/10.3390/molecules28052371






