Recyclable Magnetic Iron Immobilized onto Chitosan with Bridging Cu Ion for the Enhanced Adsorption of Methyl Orange
Abstract
1. Introduction
2. Results and Discussion
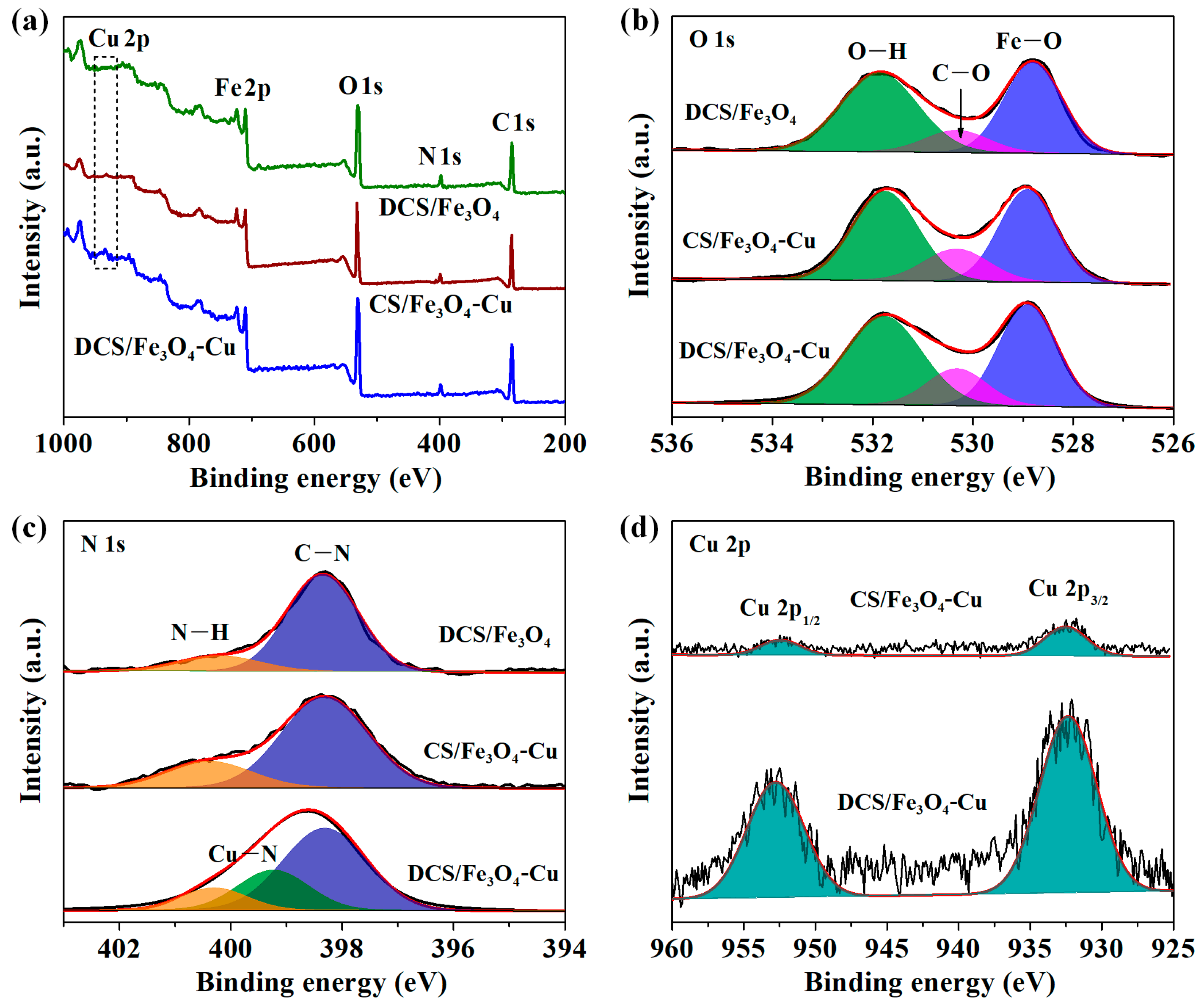
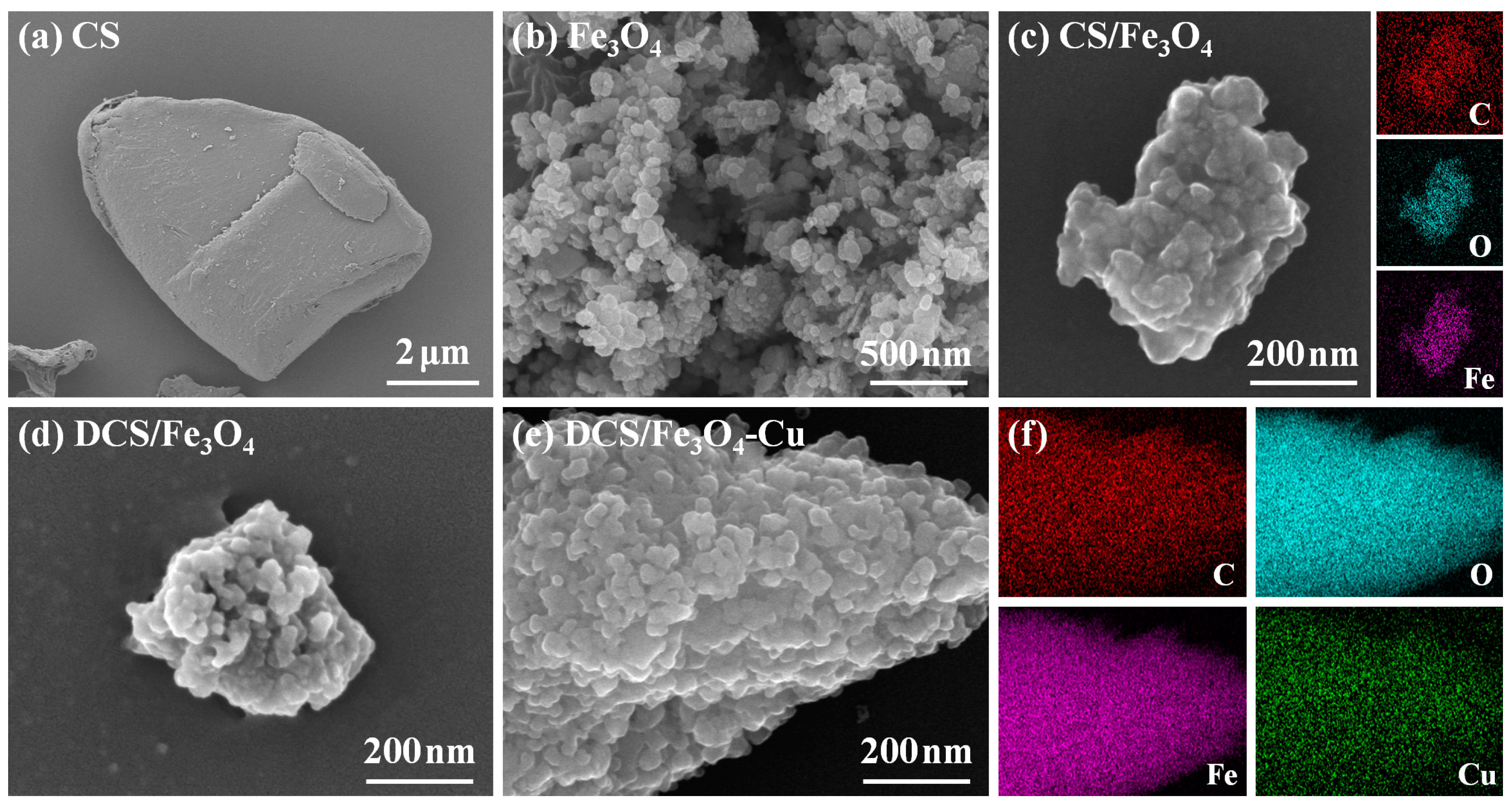
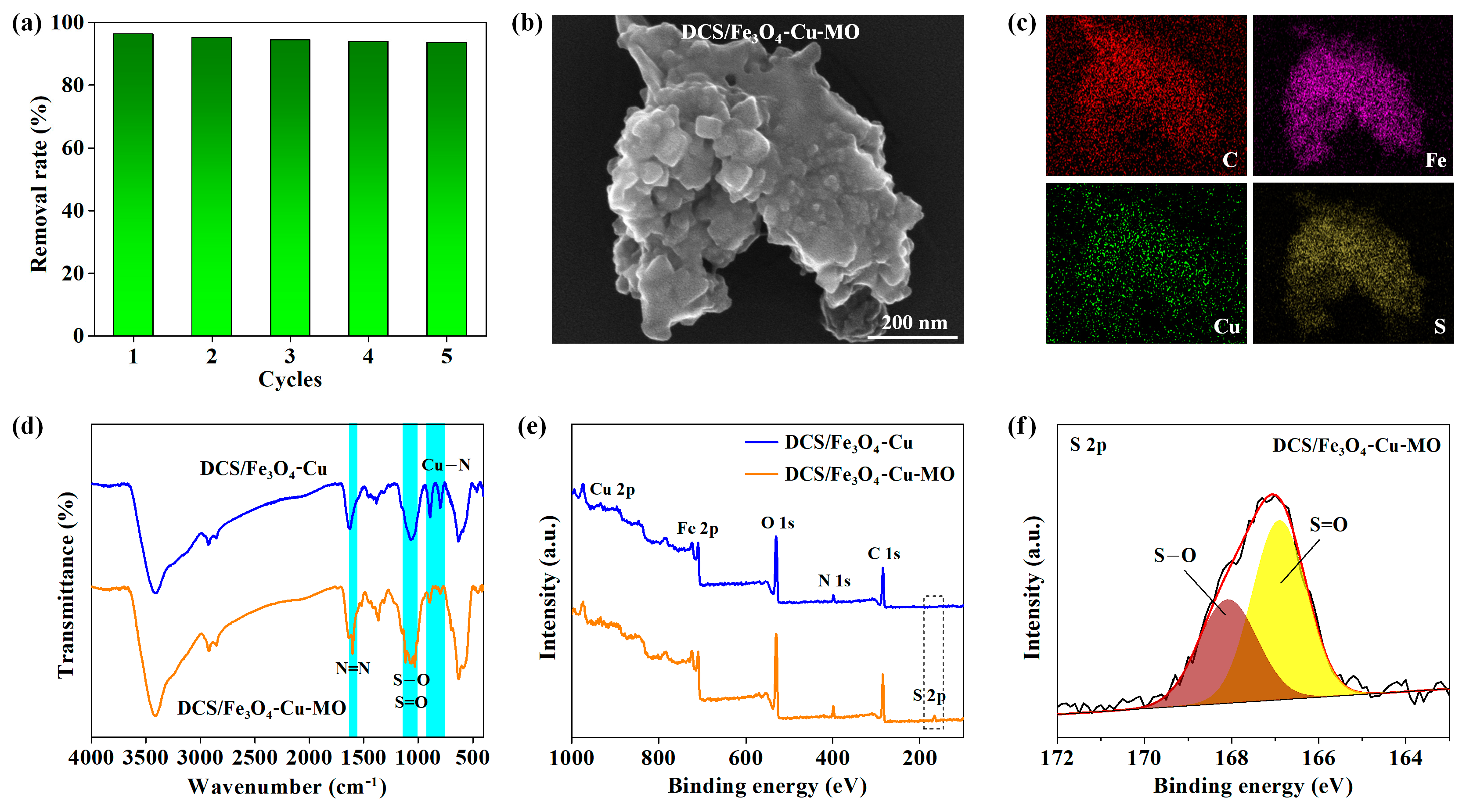
2.1. Physical Properties of DCS/Fe3O4-Cu
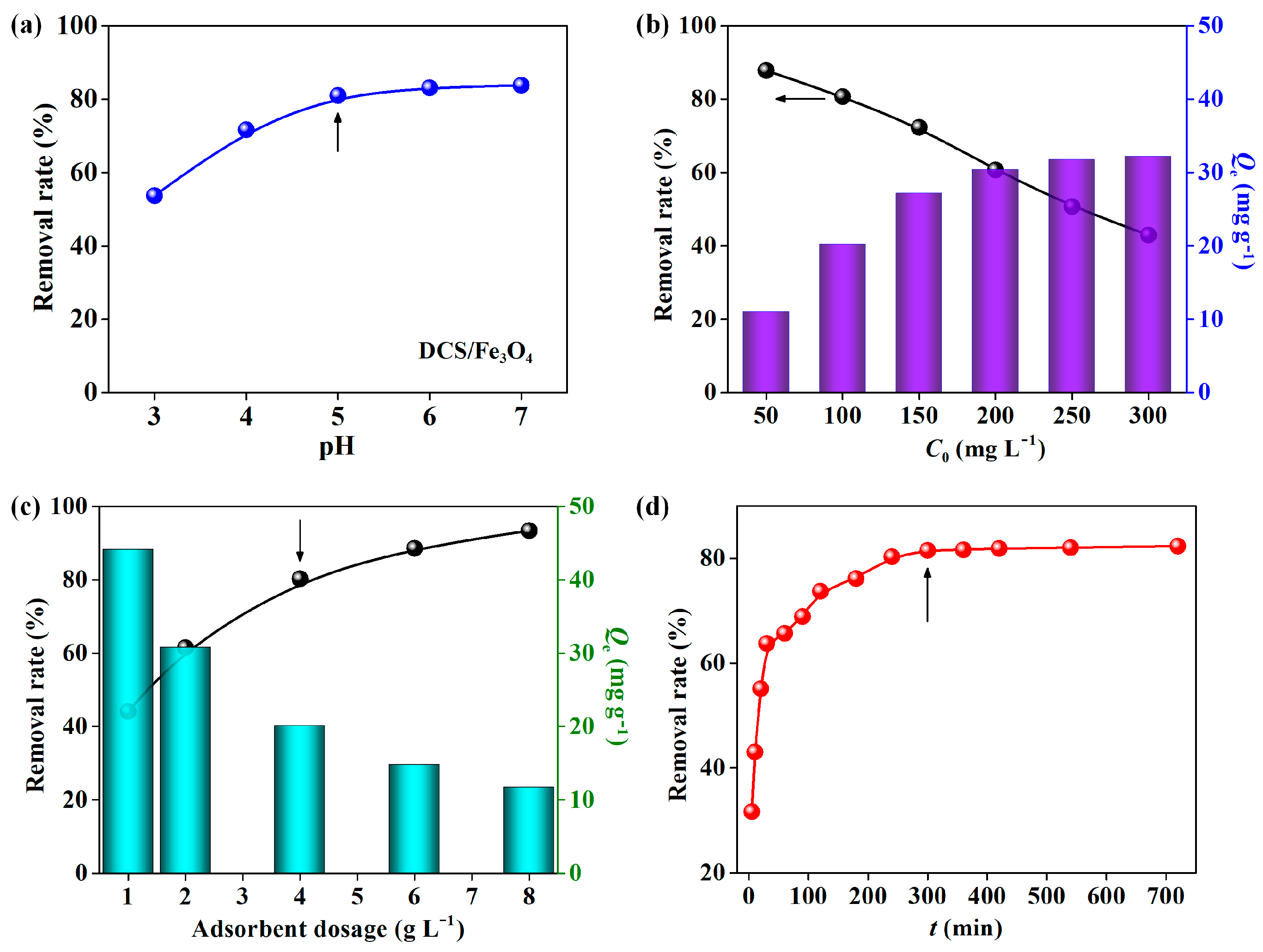
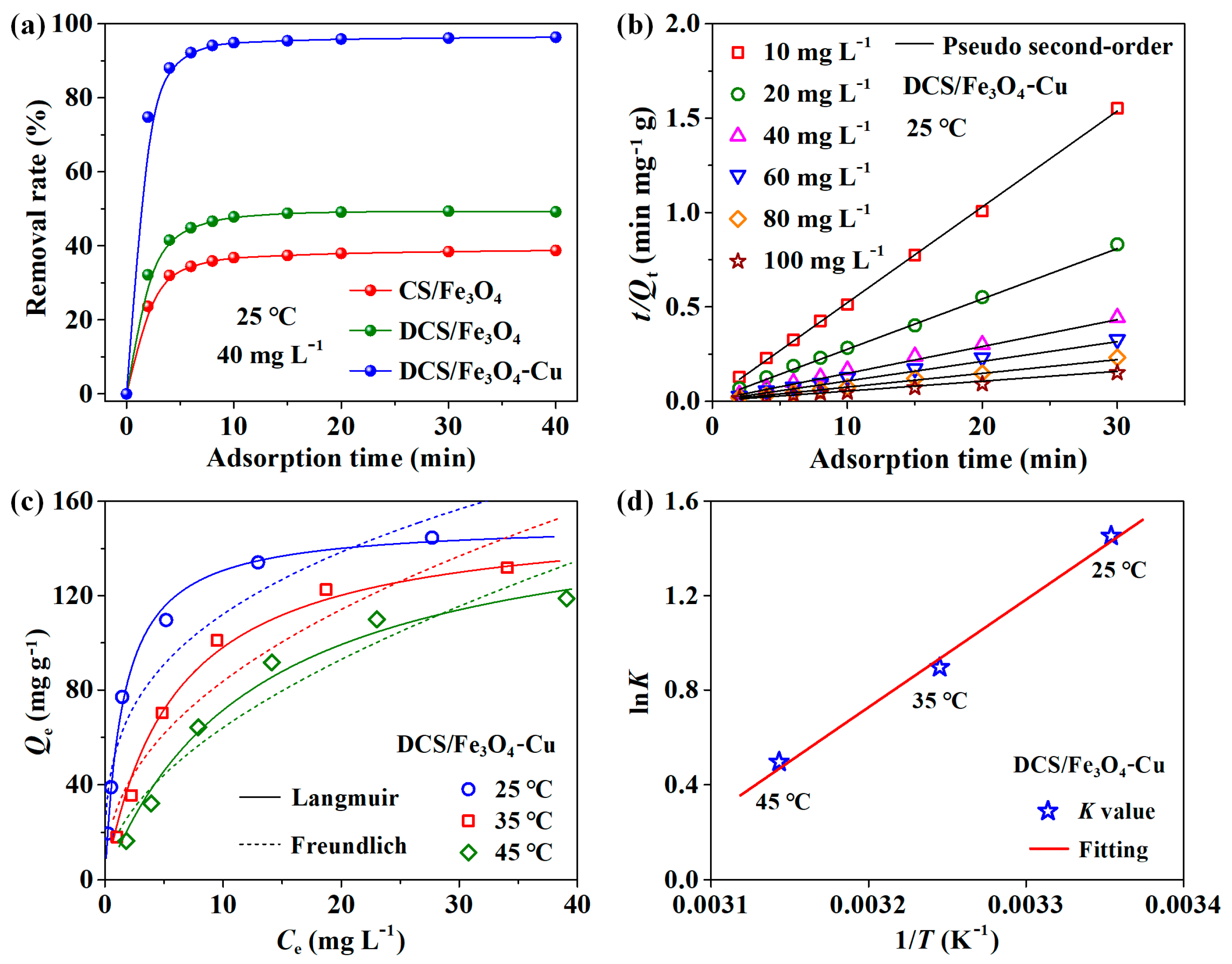
2.2. Adsorption of MO on DCS/Fe3O4-Cu
2.3. Regeneration Study of DCS/Fe3O4-Cu
3. Materials and Methods
3.1. Materials and Reagents
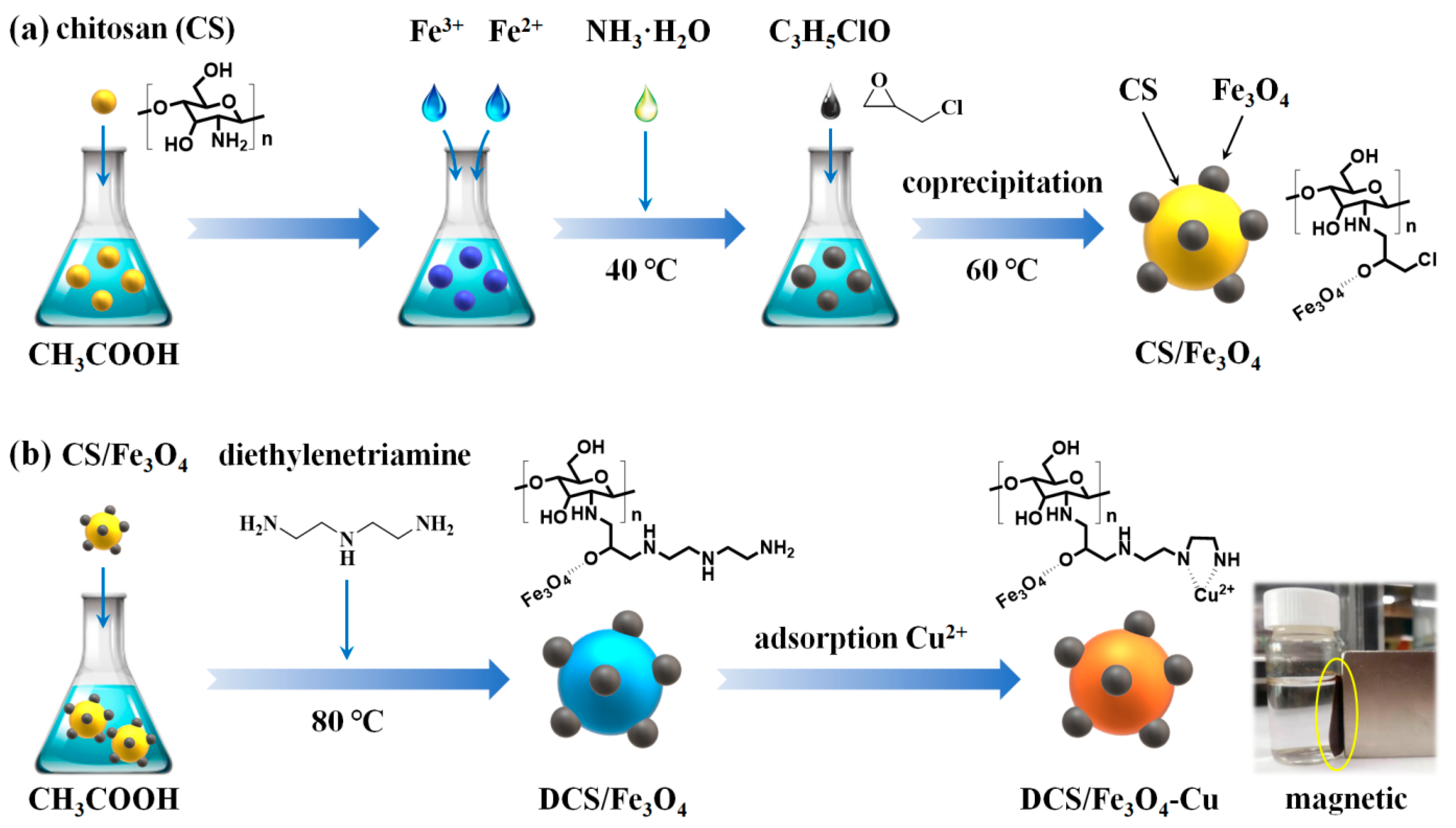
3.2. Synthesis of CS/Fe3O4
3.3. Synthesis of DCS/Fe3O4-Cu
3.4. Characterization
3.5. Adsorption Experiments
3.6. Desorption and Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gallego-Schmid, A.; Tarpani, R.R.Z. Life cycle assessment of wastewater treatment in developing countries: A review. Water Res. 2019, 153, 63–79. [Google Scholar] [CrossRef]
- Hong, H.J.; Ban, G.; Kim, H.S.; Jeong, H.S.; Park, M.S. Fabrication of cylindrical 3D cellulose nanofibril (CNF) aerogel for continuous removal of copper (Cu2+) from wastewater. Chemosphere 2021, 278, 130288. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bagotia, N.; Yadav, S.; Sharma, A.K.; Kumar, S. In-situ fabrication of surfactant modified CNT-based novel bio-composite and its performance evaluation for simultaneous removal of anionic dyes: Optimization by Box-Behnken design. Sep. Purif. Technol. 2022, 284, 120262. [Google Scholar] [CrossRef]
- Chandrakant, R.H.; Ananda, J.J.; Dipak, V.P.; Naresh, M.M.; Aniruddha, B.P. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar]
- Laurel, A.S.; Kathryn, M.R.; Ruthann, A.R. Review of organic wastewater compound concentrations and removal in onsite wastewater treatment systems. Environ. Sci. Technol. 2017, 51, 7304–7317. [Google Scholar]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Laing, G.D. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, Q.F.; Liu, C.S.; Yu, D.J.; Pu, X.Y.; Wang, Q.Q.; Gao, H.; Zhang, B.N.; Cha, D.S. Selective adsorption toward Hg(II) and inhibitory effect on bacterial growth occurring on thiosemicarbazide-functionalized chitosan microsphere surface. ACS Appl. Mater. Interfaces 2018, 10, 40302–40316. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Li, Z.; Qu, L.; Han, R. Fabrication of zirconium (IV)-loaded chitosan/Fe3O4/graphene oxide for efficient removal of alizarin red from aqueous solution. Carbohydr. Polym. 2020, 248, 116792. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Adsorption of Cu(II) and Co(II) from aqueous solution using lignosulfonate/chitosan adsorbent. Int. J. Biol. Macromol. 2020, 163, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hamzaa, M.F.; Wei, Y.; Mira, H.I.; Adel Abdel-Rahman, A.-H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef]
- Motaghi, H.; Arabkhani, P.; Parvinnia, M.; Asfaram, A. Simultaneous adsorption of cobalt ions, azo dye, and imidacloprid pesticide on the magnetic chitosan/activated carbon@UiO-66 bio-nanocomposite: Optimization, mechanisms, regeneration, and application. Sep. Purif. Technol. 2022, 284, 120258. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Zeng, G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Dang, Q.F.; Liu, C.S.; Cha, D.S.; Yu, Z.Z.; Zhu, W.J.; Fan, B. Uptake of Pb(II) and Cd(II) on chitosan microsphere surface successively grafted by methyl acrylate and diethylenetriamine. ACS Appl. Mater. Interfaces 2017, 9, 11144–11155. [Google Scholar] [CrossRef]
- Qu, Y.; Qin, L.; Liu, X.; Yang, Y. Magnetic Fe3O4/ZIF-8 composite as an effective and recyclable adsorbent for phenol adsorption from wastewater. Sep. Purif. Technol. 2022, 294, 121169. [Google Scholar] [CrossRef]
- Parandhaman, T.; Pentela, N.; Ramalingam, B.; Samanta, D.; Das, S.K. Metal nanoparticle loaded magnetic-chitosan microsphere: Water dispersible and easily separable hybrid metal nano-biomaterial for catalytic applications. ACS Sustain. Chem. Eng. 2017, 5, 489–501. [Google Scholar] [CrossRef]
- Martín-Caballero, J.; Wéry, A.S.J.; Reinoso, S.; Artetxe, B.; Felices, L.S.; Bakkali, B.E.; Trautwein, G.; Alcañiz-Monge, J.; Vilas, J.L.; Gutiérrez-Zorrilla, J.M. A robust open framework formed by decavanadate clusters and copper(II) complexes of macrocyclic polyamines: Permanent microporosity and catalytic oxidation of cycloalkanes. Inorg. Chem. 2016, 55, 4970–4979. [Google Scholar] [CrossRef]
- Li, J.; Jiang, B.; Liu, Y.; Qiu, C.; Hu, J.; Qian, G.; Guo, W.; Ngo, H.H. Preparation and adsorption properties of magnetic chitosan composite adsorbent for Cu2+ removal. J. Clean. Prod. 2017, 158, 51–58. [Google Scholar] [CrossRef]
- Qiu, H.; Ling, C.; Yuan, R.; Liu, F.; Li, A. Bridging effects behind the coadsorption of copper and sulfamethoxazole by a polyamine-modified resin. Chem. Eng. J. 2019, 362, 422–429. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Yuan, Y.; Liu, F.; Zhu, C.; Ling, C.; Li, A. Insight into Cu(II) adsorption on polyamine resin in the presence of HEDP by tracking the evolution of amino groups and Cu(II)-HEDP complexes. ACS Sustain. Chem. Eng. 2019, 7, 5256–5263. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, Q.; Liu, J.; Tu, T. Metallo-aerogels derived from chitosan with encapsulated metal nanoparticles as robust, efficient and selective nanocatalysts towards reduction of nitroarenes. Nano Res. 2021, 14, 59–65. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Luo, S.Y.; Zong, M.H.; Lou, W.Y. Multi-functional magnetic hydrogels based on millettia speciosa champ residue cellulose and chitosan: Highly efficient and reusable adsorbent for congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Dang, Q.F.; Liu, C.S.; Cha, D.S.; Zhang, H.F.; Zhu, W.J.; Zhang, Q.Q.; Fan, B. Preparation and characterization of poly(maleic acid)-grafted cross-linked chitosan microspheres for Cd(II) adsorption. Carbohydr. Polym. 2017, 172, 28–39. [Google Scholar] [CrossRef]
- Min, L.L.; Yang, L.M.; Wu, R.X.; Zhong, L.B.; Yuan, Z.H.; Zheng, Y.M. Enhanced adsorption of arsenite from aqueous solution by an iron-doped electrospun chitosan nanofiber mat: Preparation, characterization and performance. J. Colloid Interface Sci. 2019, 535, 255–264. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Z.; Chen, N.; Feng, C.; Lei, Z.; Zhang, Z. Insight into efficient phosphorus removal/recovery from enhanced methane production of waste activated sludge with chitosan-Fe supplementation. Water Res. 2020, 187, 116427. [Google Scholar] [CrossRef]
- Liu, T.; Gou, S.; He, Y.; Fang, S.; Zhou, L.; Gou, G.; Liu, L. N-Methylene Phosphonic Chitosan Aerogels for Efficient Capture of Cu2+ and Pb2+ from Aqueous Environment. Carbohydr. Polym. 2021, 269, 118355. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Chuvilin, A.L.; Sobolev, V.I.; Stolyarova, S.G.; Shubin, Y.V.; Asanov, I.P.; Ishchenko, A.V.; Magnani, G.; Riccò, M.; Okotrub, A.V.; et al. Copper on carbon materials: Stabilization by nitrogen doping. J. Mater. Chem. A 2017, 5, 10574–10583. [Google Scholar] [CrossRef]
- Shen, C.; Li, H.; Wen, Y.; Zhao, F.; Zhang, Y.; Wu, D.; Li, F. Spherical Cu2O-Fe3O4@chitosan bifunctional catalyst for coupled Cr-organic complex oxidation and Cr (VI) capture-reduction. Chem. Eng. J. 2020, 383, 123105. [Google Scholar] [CrossRef]
- Raza, H.; Yildiz, I.; Yasmeen, F.; Munawar, K.S.; Ashfaq, M.; Abbas, M.; Ahmed, M.; Younus, H.A.; Zhang, S.; Ahmad, N. Synthesis of a 2D copper(II)-carboxylate framework having ultrafast adsorption of organic dyes. J. Colloid Interface Sci. 2021, 602, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, X.; Wu, G.; Wang, J.; Zhang, T. Quaternized salicylaldehyde Schiff base modified mesoporous silica for efficiently sensing Cu(II) ions and their removal from aqueous solution. Appl. Surf. Sci. 2020, 527, 146803. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Li, X. Adsorption performance and mechanism of iron-loaded biochar to methyl orange in the presence of Cr6+ from dye wastewater. J. Hazard. Mater. 2021, 415, 125749. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Dewapriya, P.; Prasad, P.; Chang, Y.; Huang, X.; Wang, Y.; Gong, X.; Hopkins, T.E.; Fu, C.; Thomas, K.V.; et al. Efficient removal of perfluorinated chemicals from contaminated water sources using magnetic fluorinated polymer sorbents. Angew. Chem. Int. Ed. 2022, 134, e202213071. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Xu, Y.; Shen, H.; Kong, X.; Xu, H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Lu, P.; He, S.; Zhou, Y.; Zhang, Y. Adsorption, micellization and antimicrobial activity of formyl-containing cationic surfactant in diluted aqueous solutions. J. Mol. Liq. 2021, 325, 115168. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, L.; Chen, H.; Liu, X.; Hong, C.; Chen, D.; Pan, B. Mechanistically understanding adsorption of methyl orange, indigo carmine, and methylene blue onto ionic/nonionic polystyrene adsorbents. J. Hazard. Mater. 2021, 418, 126300. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.W.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: A facile method to designer biochar fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Zhong, X.; Wan, H.; Lin, Y.; Chen, G.; Wu, D.; Zheng, Z.; Liu, X.; Zhang, Y. N-doped bimetallic sulfides hollow spheres derived from metal-organic frameworks toward cost-efficient and high performance oxygen evolution reaction. Appl. Surf. Sci. 2022, 591, 153173. [Google Scholar] [CrossRef]
- Wu, T.; Jing, M.; Tian, Y.; Yang, L.; Hu, J.; Cao, X.; Zou, G.; Hou, H.; Ji, X. Surface-driven energy storage behavior of dual-heteroatoms functionalized carbon material. Adv. Funct. Mater. 2019, 29, 1900941. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, D.; Jin, P.; Guo, W.; Liu, J.; Wang, W.; Li, P.; Cao, Y.; Zhang, L.; Zhang, Y. Recyclable Magnetic Iron Immobilized onto Chitosan with Bridging Cu Ion for the Enhanced Adsorption of Methyl Orange. Molecules 2023, 28, 2307. https://doi.org/10.3390/molecules28052307
Teng D, Jin P, Guo W, Liu J, Wang W, Li P, Cao Y, Zhang L, Zhang Y. Recyclable Magnetic Iron Immobilized onto Chitosan with Bridging Cu Ion for the Enhanced Adsorption of Methyl Orange. Molecules. 2023; 28(5):2307. https://doi.org/10.3390/molecules28052307
Chicago/Turabian StyleTeng, Daoguang, Peng Jin, Wenhuan Guo, Jiang Liu, Wei Wang, Peng Li, Yijun Cao, Ling Zhang, and Ying Zhang. 2023. "Recyclable Magnetic Iron Immobilized onto Chitosan with Bridging Cu Ion for the Enhanced Adsorption of Methyl Orange" Molecules 28, no. 5: 2307. https://doi.org/10.3390/molecules28052307
APA StyleTeng, D., Jin, P., Guo, W., Liu, J., Wang, W., Li, P., Cao, Y., Zhang, L., & Zhang, Y. (2023). Recyclable Magnetic Iron Immobilized onto Chitosan with Bridging Cu Ion for the Enhanced Adsorption of Methyl Orange. Molecules, 28(5), 2307. https://doi.org/10.3390/molecules28052307









