Diosmin and Bromelain Stimulate Glutathione and Total Thiols Production in Red Blood Cells
Abstract
1. Introduction
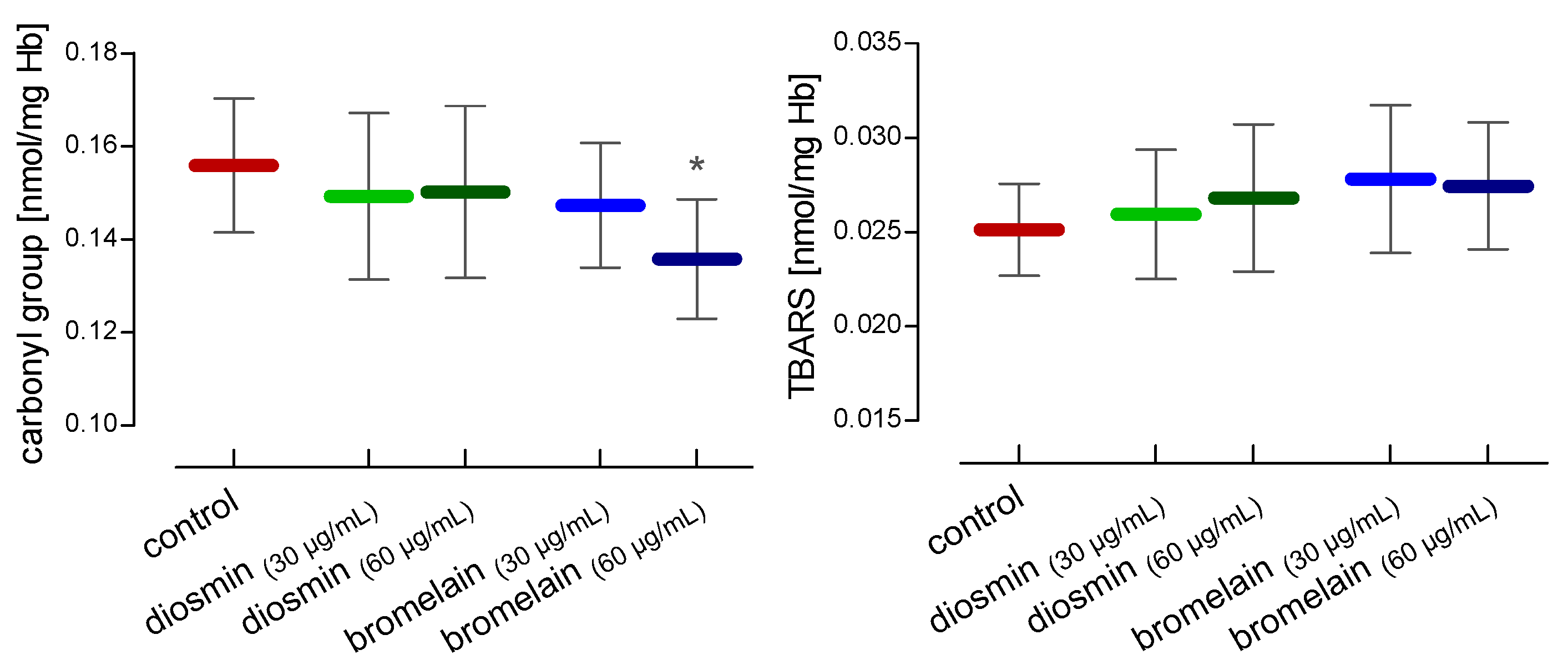
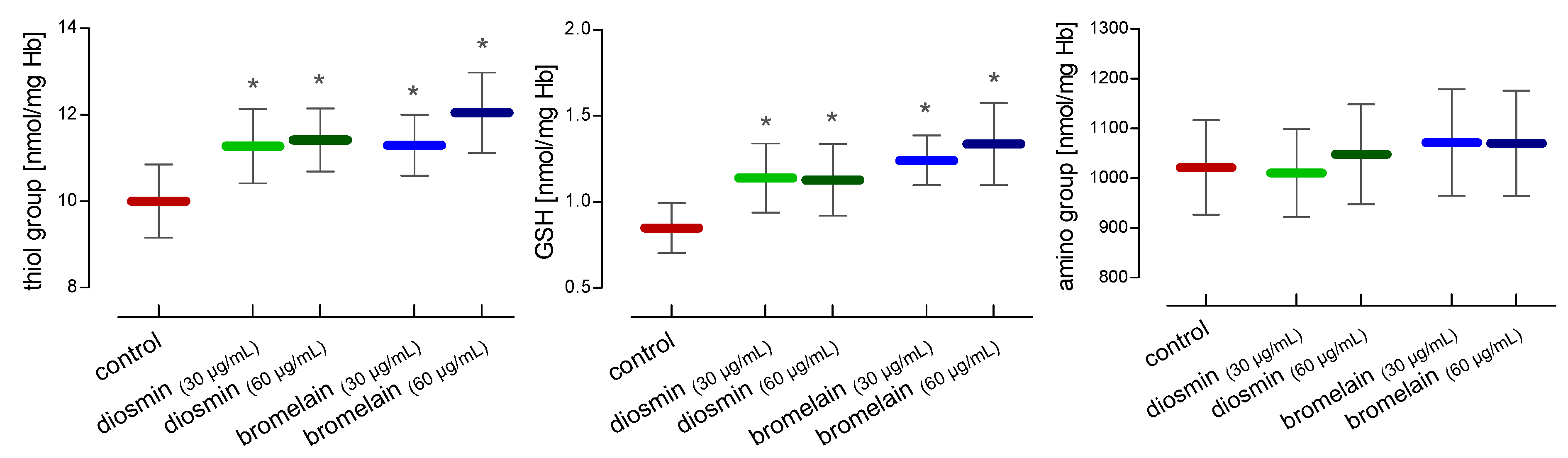
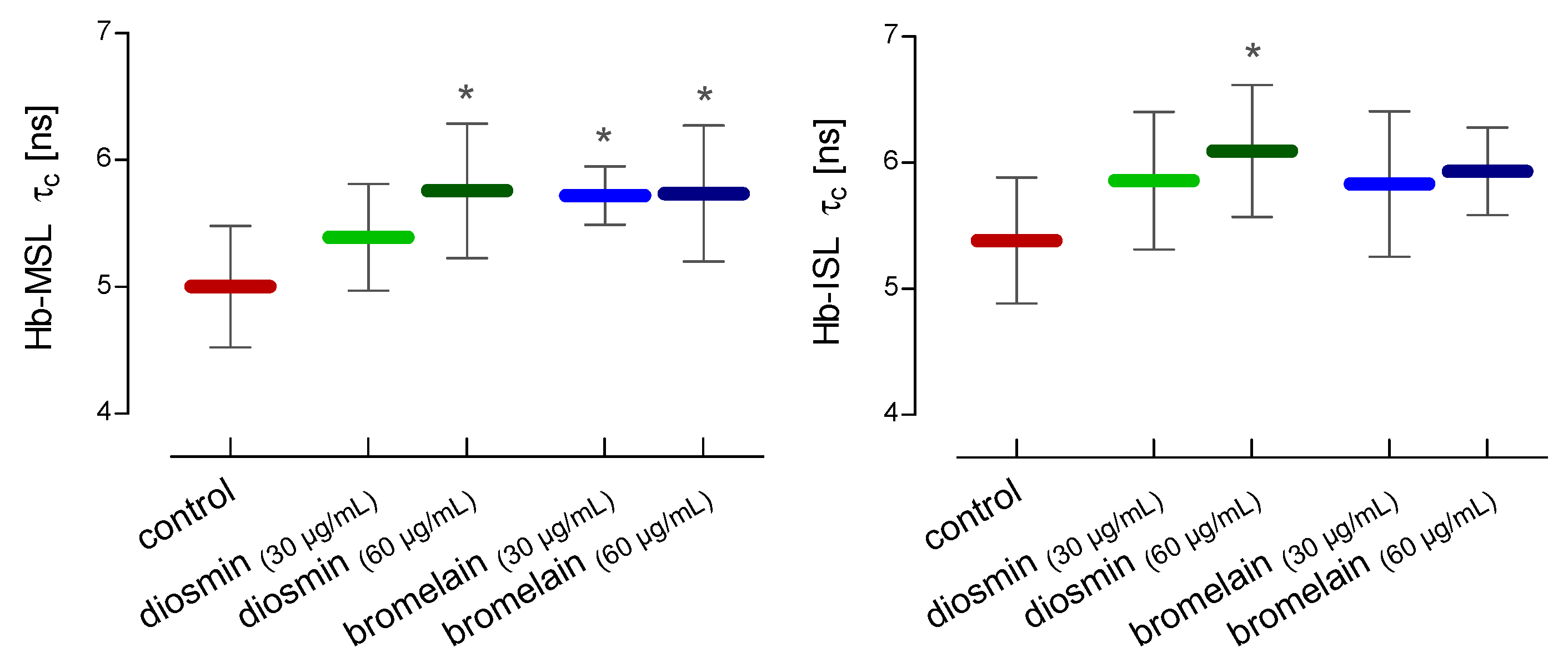
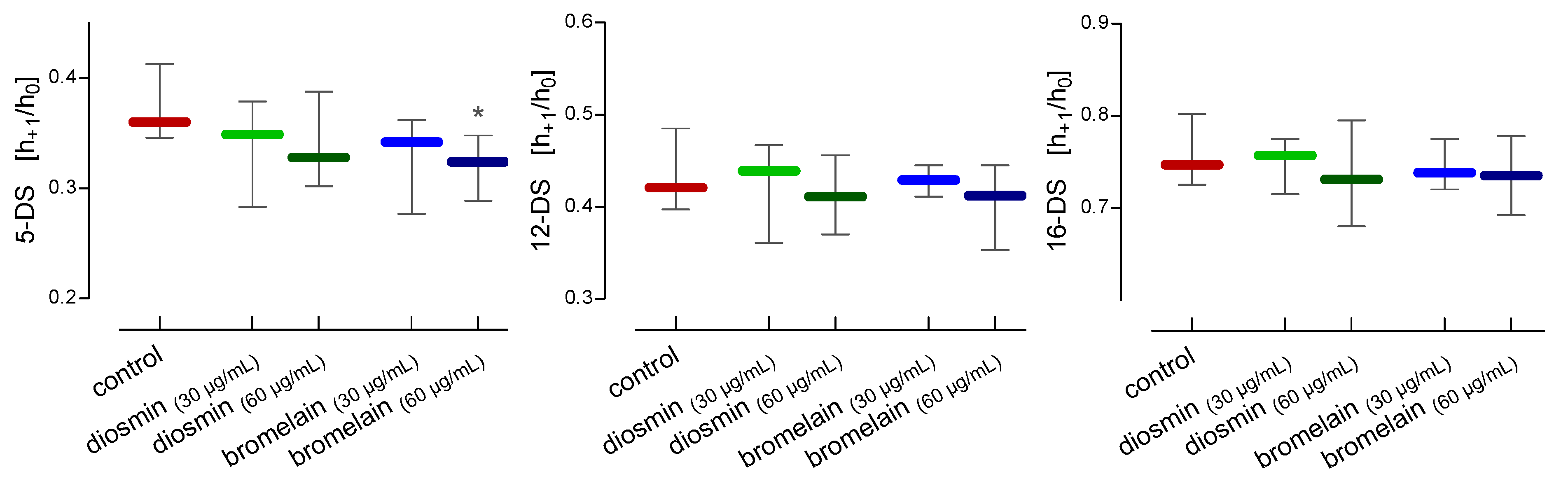
2. Results
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Red Blood Cells Isolation
4.3. Hemolysate Preparation
4.4. Determination of Carbonyl Groups
4.5. Determination of Thiobarbituric Reactive Substances
4.6. Determination of Thiol Groups
4.7. Determination of Glutathione Content
4.8. Determination of Amino Groups
4.9. Total Non-Enzymatic Antioxidant Capacity
4.10. Electron Paramagnetic Resonance (EPR)
4.11. Internal Viscosity of RBCs
4.12. The RBCs Internal Peptides and Proteins Changes
4.13. The Conformational State of Hemolysate Proteins
4.14. RBCs Membrane Fluidity
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RBCs | erythrocytes |
| EPR | electron paramagnetic resonance |
| TBARS | thiobarbituric acid reactive substances |
| GSH | glutathione |
| NEAC | non-enzymatic antioxidant capacity |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
| ROS | reactive oxygen species |
| MSL | 4-Maleimido-TEMPO |
| ISL | 4-(2-Iodoacetamido)-TEMPO |
| 5-DS | 5-doxy-stearic acid |
| 12-DS | 12-doxy-stearic acid |
| 16-DS | 16-doxy-stearic acid |
References
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef] [PubMed]
- Kilit, A.C.; Köse, O.; Imi, G.; Aydemir, E. Research Article Anticancer and antimicrobial activities of diosmin. Genet. Mol. Res. 2021, 20, gmr18752. [Google Scholar] [CrossRef]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative Stress in Ischemic Heart Disease. Oxid. Med. Cell. Longev. 2020, 2020, 6627144. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pari, L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem. Biol. Interact. 2012, 195, 43–51. [Google Scholar] [CrossRef]
- Mustafa, S.; Akbar, M.; Khan, M.A.; Sunita, K.; Parveen, S.; Pawar, J.S.; Massey, S.; Agarwal, N.R.; Husain, S.A. Plant metabolite diosmin as the therapeutic agent in human diseases. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100122. [Google Scholar] [CrossRef]
- Senthamizhselvan, O.; Manivannan, J.; Silambarasan, T.; Raja, B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014, 736, 131–137. [Google Scholar] [CrossRef]
- Tong, N.; Zhang, Z.; Zhang, W.; Qiu, Y.; Gong, Y.; Yin, L.; Qiu, Q.; Wu, X. Diosmin alleviates retinal edema by protecting the blood-retinal barrier and reducing retinal vascular permeability during ischemia/reperfusion injury. PLoS ONE 2013, 8, e61794. [Google Scholar] [CrossRef]
- Lewinska, A.; Siwak, J.; Rzeszutek, I.; Wnuk, M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol. In Vitro 2015, 29, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Arroo, R.; Sari, S.; Huang, S. Flavonoids as Inducers of Apoptosis and Autophagy in Breast Cancer. In Discovery and Development of Anti-Breast Cancer Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 147–196. ISBN 9780128212776. [Google Scholar]
- Dutta, S.; Bhattacharyya, D. Enzymatic, antimicrobial and toxicity studies of the aqueous extract of Ananas comosus (pineapple) crown leaf. J. Ethnopharmacol. 2013, 150, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef]
- Ley, C.M.; Tsiami, A.; Ni, Q.; Robinson, N. A review of the use of bromelain in cardiovascular diseases. Zhong Xi Yi Jie He Xue Bao 2011, 9, 702–710. [Google Scholar] [CrossRef]
- Tochi, B.N.; Wang, Z.; Xu, S.Y.; Zhang, W. Therapeutic Application of Pineapple Protease (Bromelain): A Review. Pakistan J. Nutr. 2008, 7, 513–520. [Google Scholar] [CrossRef]
- Metzig, C.; Grabowska, E.; Eckert, K.; Rehse, K.; Maurer, H.R. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo 1999, 13, 7–12. [Google Scholar]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef]
- Chisci, G.; Fredianelli, L. Therapeutic Efficacy of Bromelain in Alveolar Ridge Preservation. Antibiotics 2022, 11, 1542. [Google Scholar] [CrossRef]
- Salgado, M.T.; Cao, Z.; Nagababu, E.; Mohanty, J.G.; Rifkind, J.M. Red blood cell membrane-facilitated release of nitrite-derived nitric oxide bioactivity. Biochemistry 2015, 54, 6712–6723. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Devika, M.S.; Vysakh, A.; Vijeesh, V.; Jisha, N.; Ruby, P.M. Flavonoid Glycoside Diosmin Induces Apoptosis and Cell Cycle Arrest in DLD-1 Human Colon Cancer Cell Line. J. Biol. Act. Prod. Nat. 2022, 12, 232–242. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wei, P.-L.; Makondi, P.T.; Chen, W.-T.; Huang, C.-Y.; Chang, Y.-J. Bromelain inhibits the ability of colorectal cancer cells to proliferate via activation of ROS production and autophagy. PLoS ONE 2019, 14, e0210274. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.; Kim, M.; Jin, E.-J. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim. Cells Syst. 2018, 22, 334–340. [Google Scholar] [CrossRef]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef]
- Pauzi, A.Z.M.; Yeap, S.K.; Abu, N.; Lim, K.L.; Omar, A.R.; Aziz, S.A.; Chow, A.L.T.; Subramani, T.; Tan, S.G.; Alitheen, N.B. Combination of cisplatin and bromelain exerts synergistic cytotoxic effects against breast cancer cell line MDA-MB-231 in vitro. Chin. Med. 2016, 11, 46. [Google Scholar] [CrossRef]
- Gwozdzinski, L.; Pieniazek, A.; Bernasinska-Slomczewska, J.; Hikisz, P.; Gwozdzinski, K. Alterations in the Plasma and Red Blood Cell Properties in Patients with Varicose Vein: A Pilot Study. Cardiol. Res. Pract. 2021, 2021, 5569961. [Google Scholar] [CrossRef]
- Faivre, B.; Menu, P.; Labrude, P.; Vigneron, C. Hemoglobin autooxidation/oxidation mechanisms and methemoglobin prevention or reduction processes in the bloodstream. Literature review and outline of autooxidation reaction. Artif. Cells Blood Substit. Immobil. Biotechnol. 1998, 26, 17–26. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 2014, 5, 500. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef]
- van ‘t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Raj Rai, S.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol. Dis. 2008, 40, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Minnich, V.; Smith, M.B.; Brauner, M.J.; Majerus, P.W. Glutathione biosynthesis in human erythrocytes. I. Identification of the enzymes of glutathione synthesis in hemolysates. J. Clin. Invest. 1971, 50, 507–513. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef]
- Gwozdziński, K. A spin label study of the action of cupric and mercuric ions on human red blood cells. Toxicology 1991, 65, 315–323. [Google Scholar] [CrossRef]
- McConnell, H.M.; Hamilton, C.L. Spin-labeled hemoglobin derivatives in solution and in single crystals. Proc. Natl. Acad. Sci. USA 1968, 60, 776–781. [Google Scholar] [CrossRef]
- Ohnishi, S.; Maeda, T.; Ito, T.; Hwang, K.J.; Tyuma, I. Spin-labeled hemoglobin subunits. Biochemistry 1968, 7, 2662–2666. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Wisniewska, A. Physical properties of lipid bilayer membranes: Relevance to membrane biological functions. Acta Biochim. Pol. 2000, 47, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L.C. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Lande, M.B.; Donovan, J.M.; Zeidel, M.L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 1995, 106, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Guţu, M.; Rusu, V.; Ştefănescu, C. Fluiditatea membranară—Parametru biofizic in relaţie cu procesele de transport membranare. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 153–162. [Google Scholar] [PubMed]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- Garnier, M.; Perret, G.; Pilardeau, P.; Vaysse, J.; Rolland, Y.; Uzzan, B.; Vassy, R. Effect of diosmin upon red blood cell deformability and osmotic fragility. Relationship with lipid content. Methods Find. Exp. Clin. Pharmacol. 1988, 10, 259–262. [Google Scholar] [PubMed]
- Drabkin, D.L. Spectrophotometric studies; the crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. J. Biol. Chem. 1946, 164, 703–723. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Stocks, J.; Dormandy, T.L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Haematol. 1971, 20, 95–111. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Diplock, A.T.; Symons, M.C.R. Techniques in Free Radical Research; Elsevier Science: Amsterdam, The Netherlands; New York, NY, USA, 1991; ISBN 9780080858913. [Google Scholar]
- Egwim, I.O.; Gruber, H.J. Spectrophotometric measurement of mercaptans with 4,4’-dithiodipyridine. Anal. Biochem. 2001, 288, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Senft, A.P.; Dalton, T.P.; Shertzer, H.G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Crowell, E.A.; Ough, C.S.; Bakalinsky, A. Determination of Alpha Amino Nitrogen in Musts and Wines by TNBS Method. Am. J. Enol. Vitic. 1985, 36, 175–177. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.D. Determining intracellular viscosity from the rotational motion of spin labels. Methods Enzymol. 1986, 127, 239–249. [Google Scholar] [CrossRef]
- Keith, A.; Bulfield, G.; Snipes, W. Spin-labeled Neurospora mitochondria. Biophys. J. 1970, 10, 618–629. [Google Scholar] [CrossRef]
- Berliner, L.J. The spin-label approach to labeling membrane protein sulfhydryl groups. Ann. N. Y. Acad. Sci. 1983, 414, 153–161. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwozdzinski, L.; Bernasinska-Slomczewska, J.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pieniazek, A. Diosmin and Bromelain Stimulate Glutathione and Total Thiols Production in Red Blood Cells. Molecules 2023, 28, 2291. https://doi.org/10.3390/molecules28052291
Gwozdzinski L, Bernasinska-Slomczewska J, Wiktorowska-Owczarek A, Kowalczyk E, Pieniazek A. Diosmin and Bromelain Stimulate Glutathione and Total Thiols Production in Red Blood Cells. Molecules. 2023; 28(5):2291. https://doi.org/10.3390/molecules28052291
Chicago/Turabian StyleGwozdzinski, Lukasz, Joanna Bernasinska-Slomczewska, Anna Wiktorowska-Owczarek, Edward Kowalczyk, and Anna Pieniazek. 2023. "Diosmin and Bromelain Stimulate Glutathione and Total Thiols Production in Red Blood Cells" Molecules 28, no. 5: 2291. https://doi.org/10.3390/molecules28052291
APA StyleGwozdzinski, L., Bernasinska-Slomczewska, J., Wiktorowska-Owczarek, A., Kowalczyk, E., & Pieniazek, A. (2023). Diosmin and Bromelain Stimulate Glutathione and Total Thiols Production in Red Blood Cells. Molecules, 28(5), 2291. https://doi.org/10.3390/molecules28052291





