Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status
Abstract
:1. Introduction
2. Results
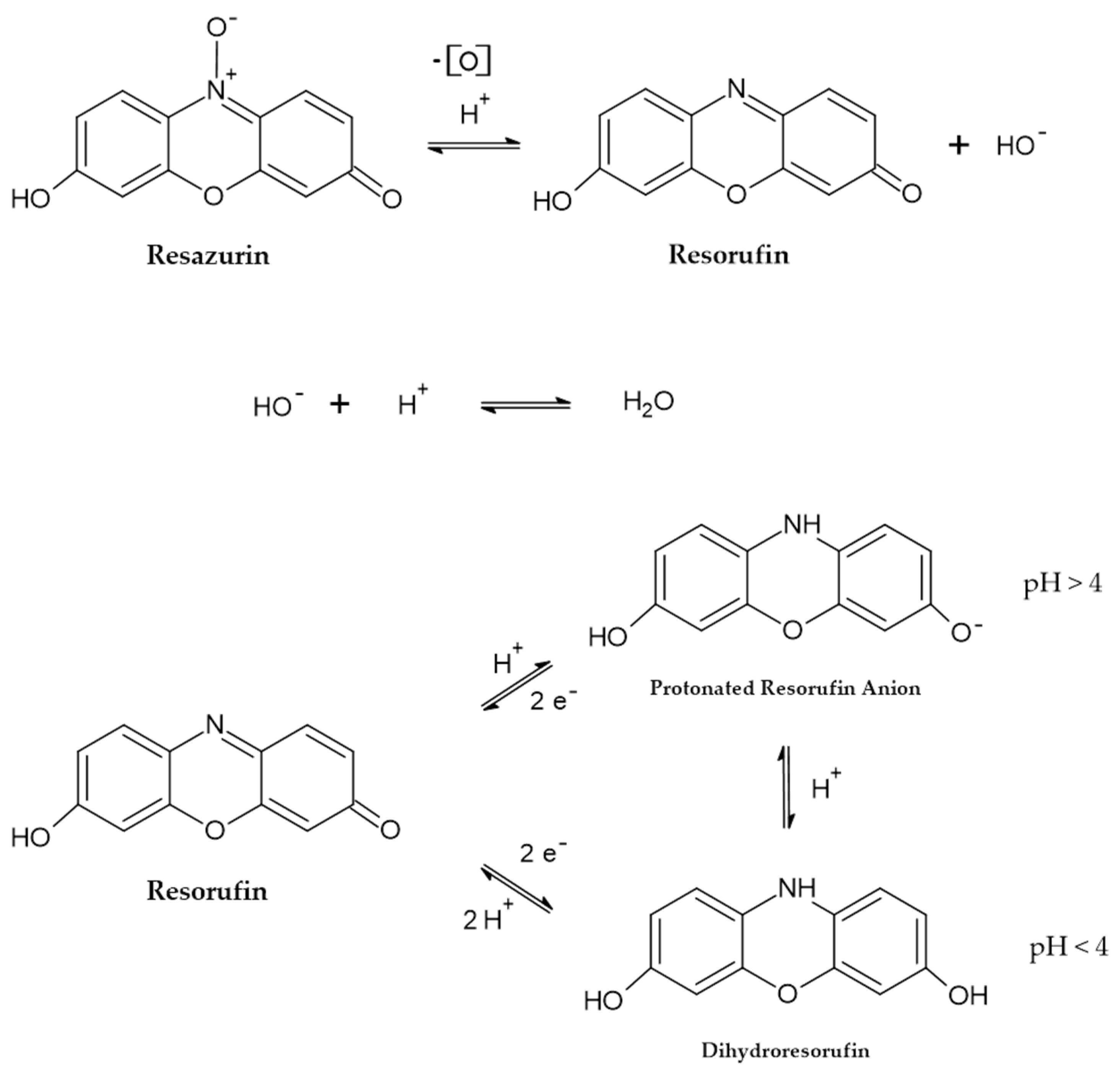
2.1. The Consecutive Reactions of Resazurin and Their Kinetics
2.2. Assumptions and Limitations
2.2.1. All Living Cells Have Equal Reducing Power and Non-Cellular Conversion of A to B Is Non-Significant
2.2.2. Instrumental Factors in Fluorimetry
2.2.3. Only Varies While Other Factors Are Constant When Comparing Conditions (a) and (b)
2.2.4. Viability vs. Metabolic Activity
2.3. Practical Examples from Literature
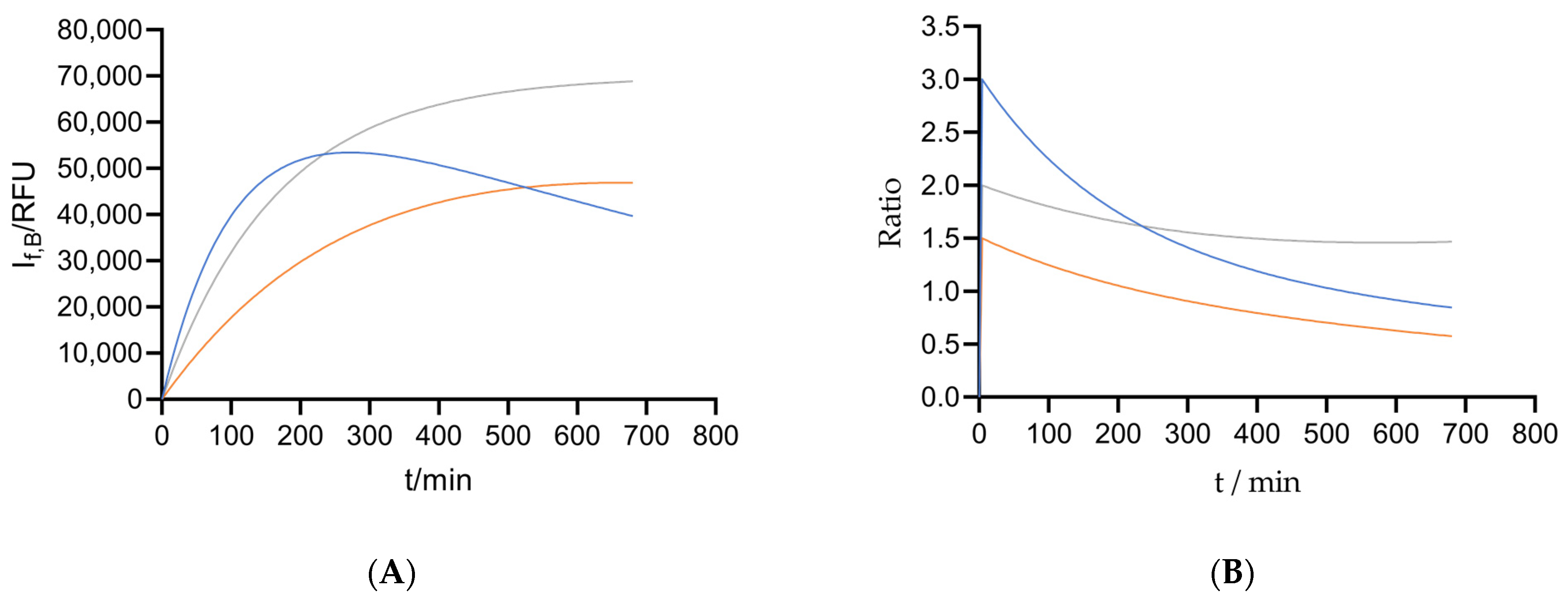
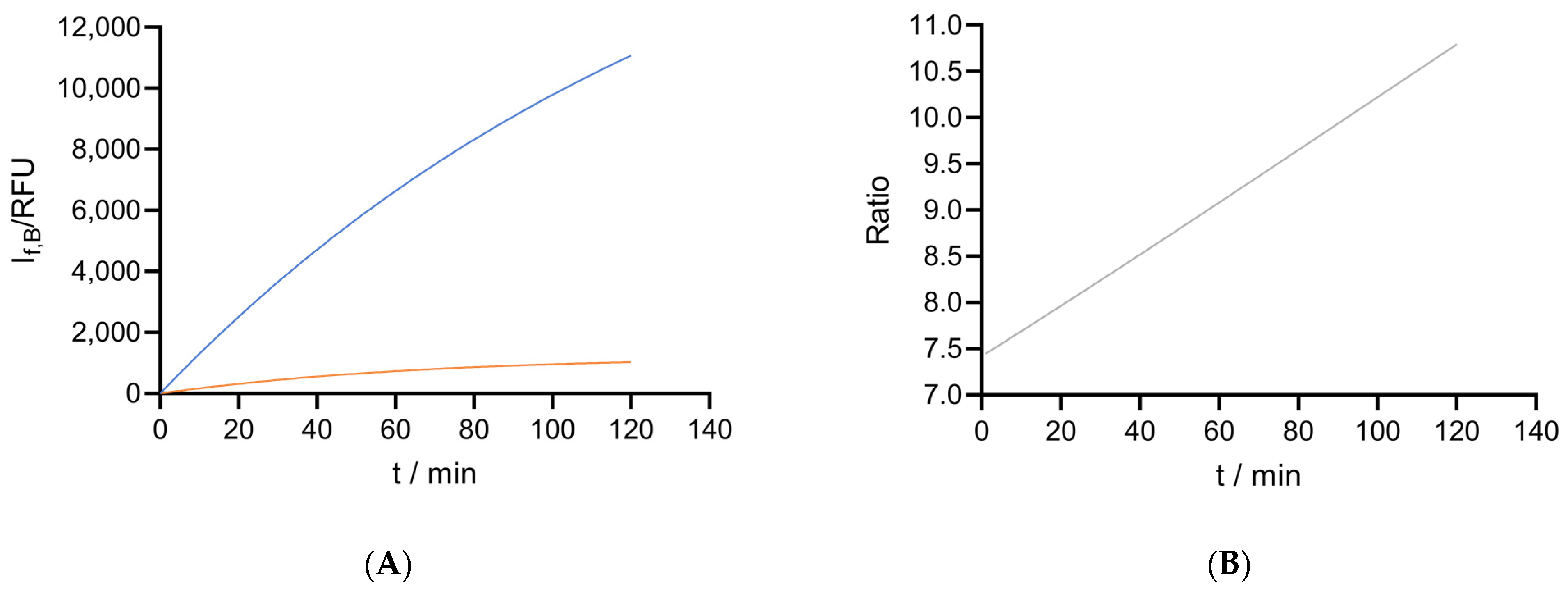
2.3.1. Comparing the Reducing Power of Different Types of Cells
2.3.2. Cytotoxicity: The Effect of Solutes on Cell Metabolic Activity
3. Conclusions
Good Practice in MTT and Resazurin Assay
- (1)
- Use as low a resazurin concentration as possible because toxicity issues and technical spectroscopic biases may arise. The minimal concentration you can use depends on the signal-to-noise ratio of your detection system.
- (2)
- Test for the reducing power of the medium in the absence of cells. Make sure it is nonsignificant when compared to cell reducing power in the absence of any toxic agents.
- (3)
- Use data obtained at the shortest time interval possible, i.e., resazurin reduction data (formation of resorufin) should be registered as soon as possible after resazurin addition to cells, provided that the read-out is above the detection limit of the experimental setup you are using.
- (4)
- Observance of the three previous rules allows quantitative ratiometric analysis, i.e., the fold variation of the measured response (fluorescence intensity or UV-Vis. absorption) is the fold variation of metabolic activity or intrinsic reducing power of cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; InTechOpen: London, UK, 2018; Chapter 1; ISBN 978-1-78923-419-0. [Google Scholar]
- Neufeld, B.H.; Tapia, J.B.; Lutzke, A.; Reynolds, M.M. Small Molecule Interferences in Resazurin and MTT-Based Metabolic Assays in the Absence of Cells. Anal. Chem. 2018, 90, 6867–6876. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Çakir, S.; Arslan, E. Voltammetry of Resazurin at a Mercury Electrode. Chem. Pap. 2010, 64, 386–394. [Google Scholar] [CrossRef]
- Coutinho, A.; Prieto, M. Ribonuclease T1 and Alcohol Dehydrogenase Fluorescence Quenching by Acrylamide: A Laboratory Experiment for Undergraduate Students. J. Chem. Educ. 1993, 70, 425–428. [Google Scholar] [CrossRef]
- da Poian, A.T.; Castanho, M.A.R.B. Integrative Human Biochemistry, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2021; Chapter 4; ISBN 978-3-030-48739-3. [Google Scholar]
- Uzarski, J.S.; DiVito, M.D.; Wertheim, J.A.; Miller, W.M. Essential Design Considerations for the Resazurin Reduction Assay to Noninvasively Quantify Cell Expansion within Perfused Extracellular Matrix Scaffolds. Biomaterials 2017, 129, 163–175. [Google Scholar] [CrossRef]
- Lavogina, D.; Lust, H.; Tahk, M.-J.; Laasfeld, T.; Vellama, H.; Nasirova, N.; Vardja, M.; Eskla, K.-L.; Salumets, A.; Rinken, A.; et al. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors 2022, 12, 196. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. Available online: https://pubmed.ncbi.nlm.nih.gov/23805433/ (accessed on 15 December 2022).
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic Reduction of Resazurin; Location within the Cell for Cytotoxicity Assays. Biotechnol. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira-da-Silva, B.; Castanho, M.A.R.B. Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status. Molecules 2023, 28, 2283. https://doi.org/10.3390/molecules28052283
Vieira-da-Silva B, Castanho MARB. Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status. Molecules. 2023; 28(5):2283. https://doi.org/10.3390/molecules28052283
Chicago/Turabian StyleVieira-da-Silva, Beatriz, and Miguel A. R. B. Castanho. 2023. "Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status" Molecules 28, no. 5: 2283. https://doi.org/10.3390/molecules28052283
APA StyleVieira-da-Silva, B., & Castanho, M. A. R. B. (2023). Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status. Molecules, 28(5), 2283. https://doi.org/10.3390/molecules28052283







