Updated Progress of the Copper-Catalyzed Borylative Functionalization of Unsaturated Molecules
Abstract
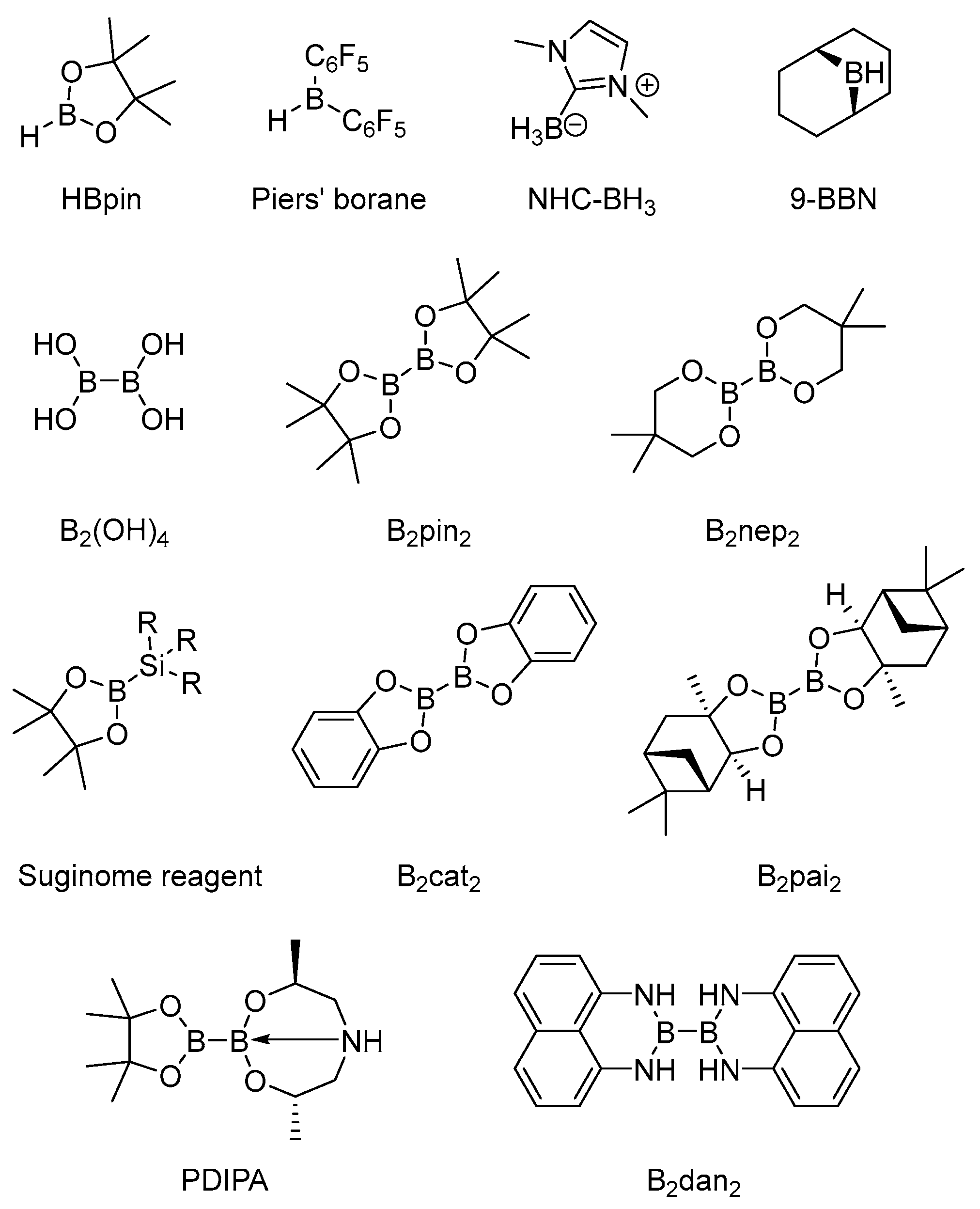
1. Introduction
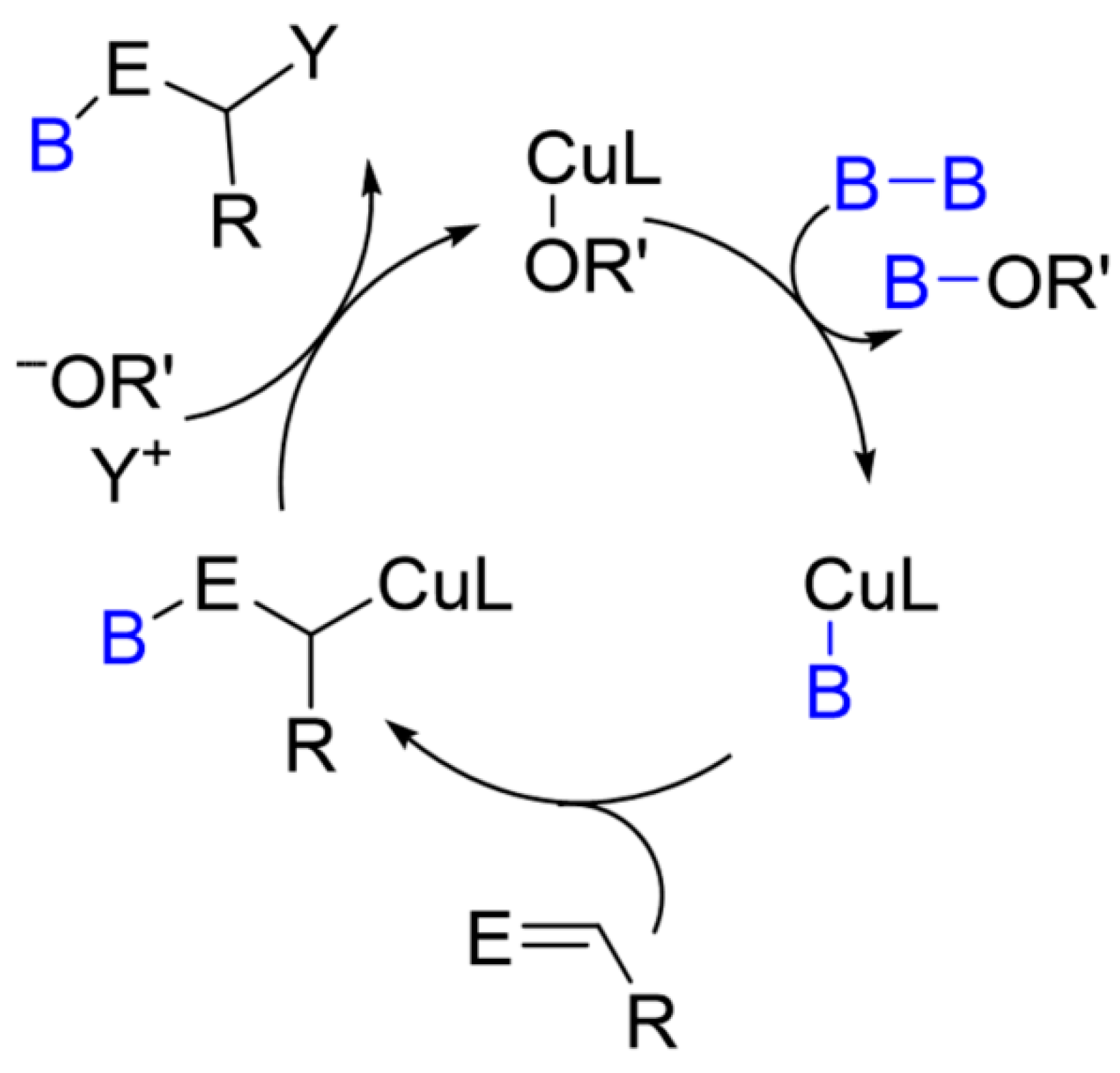
2. Reaction of Copper-Boryl Species with C-C Multiple Bonds
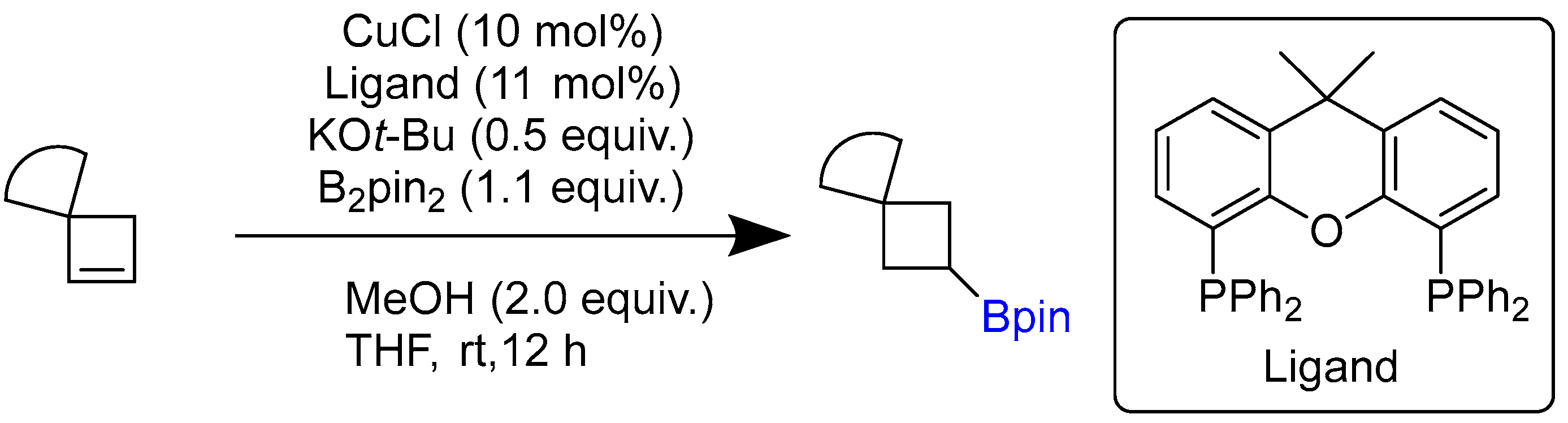
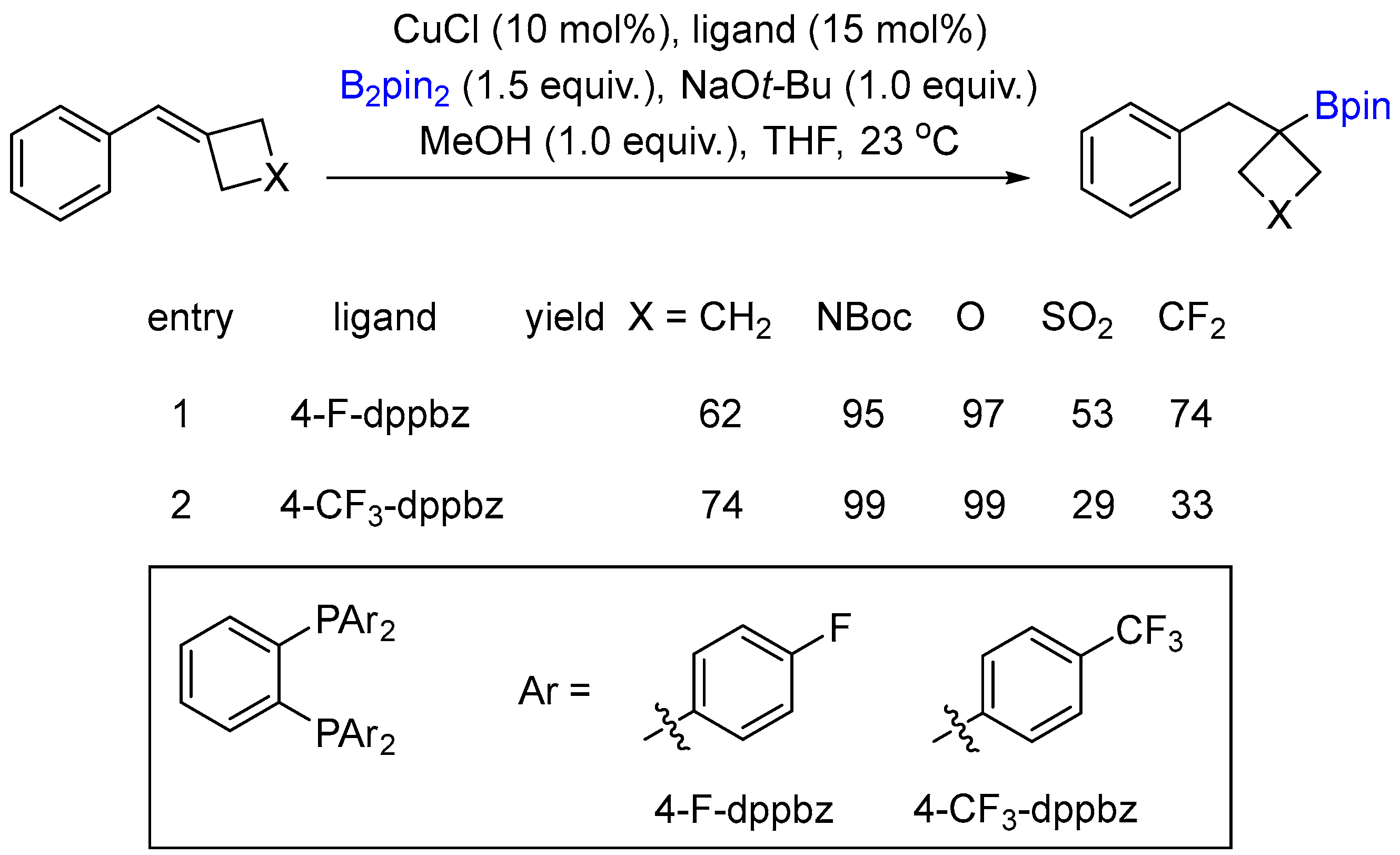
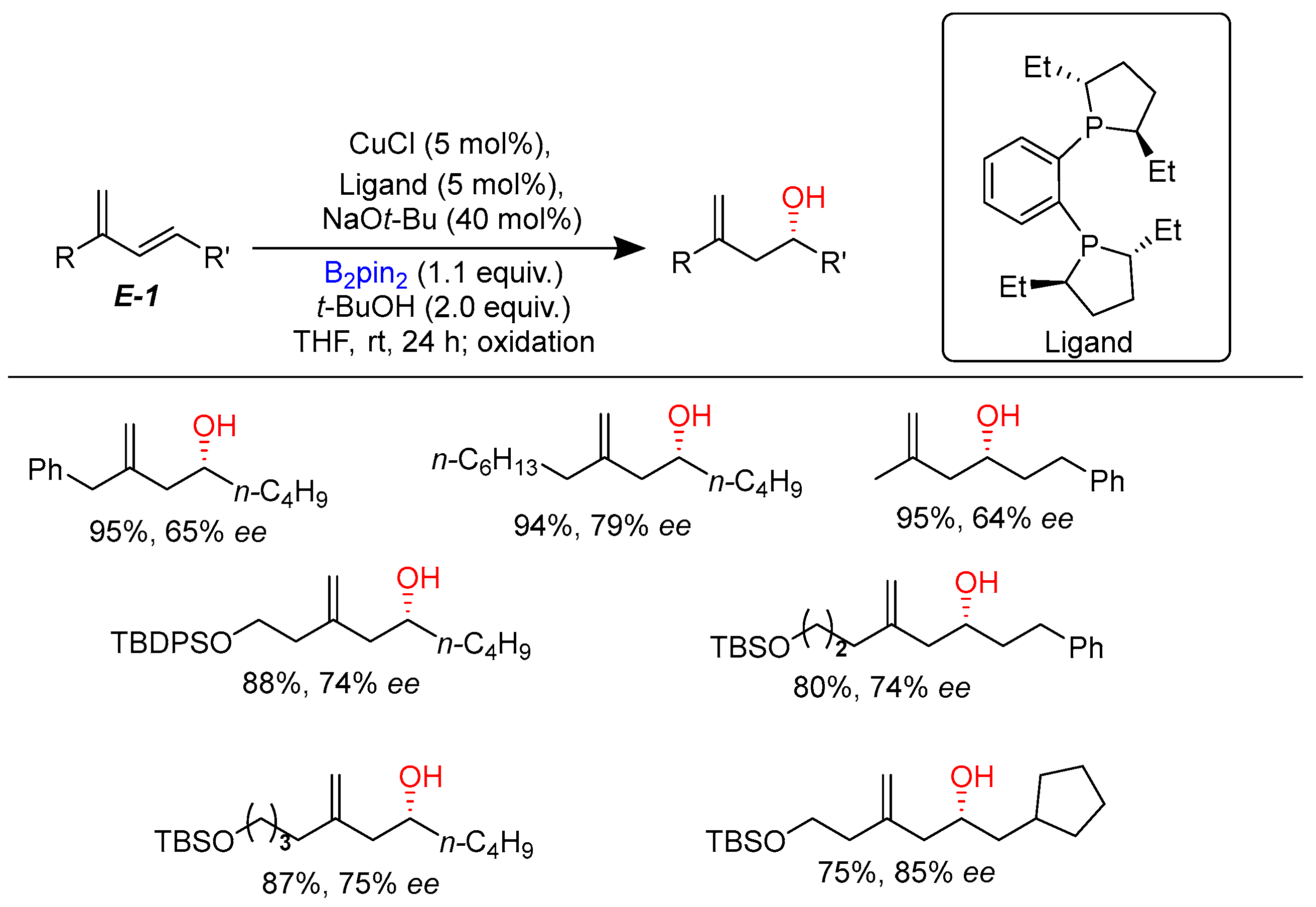
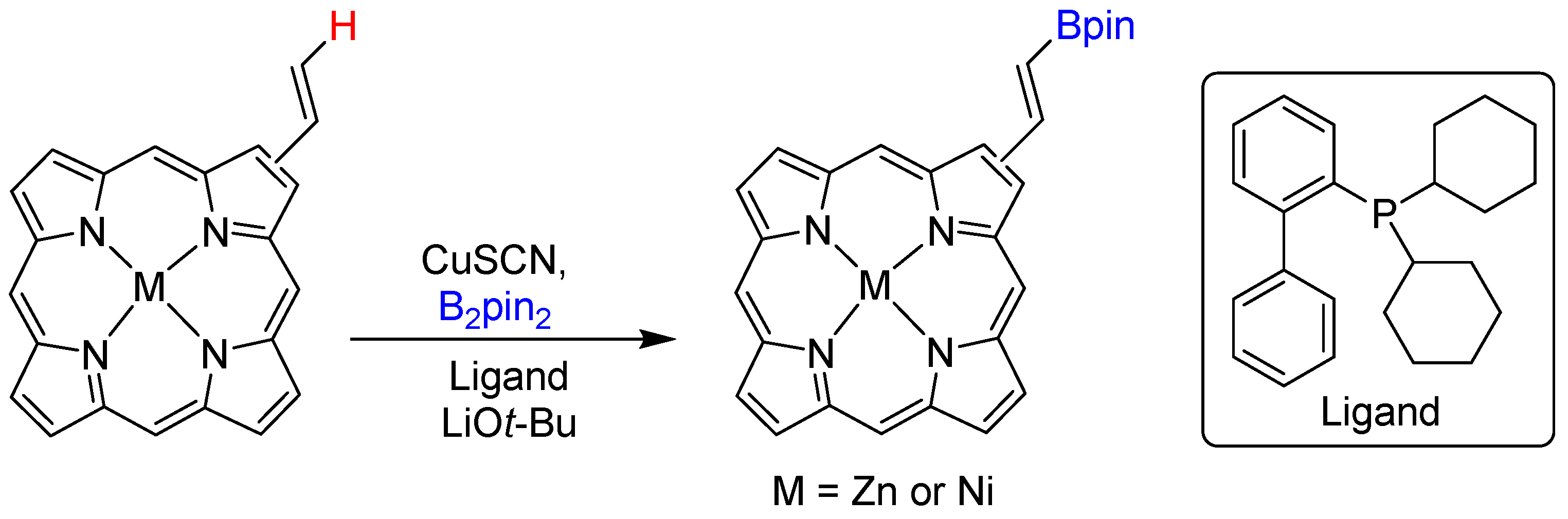
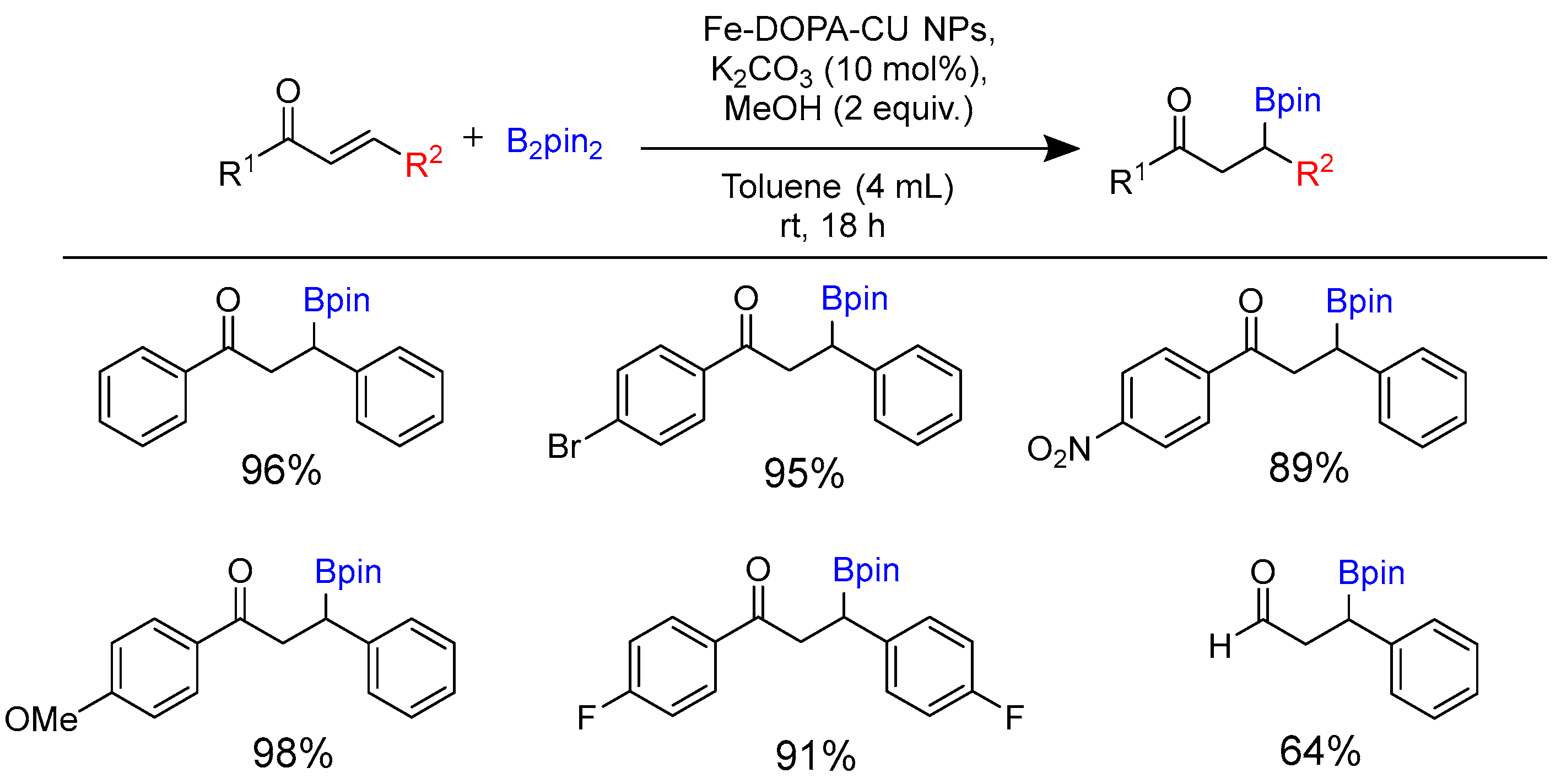
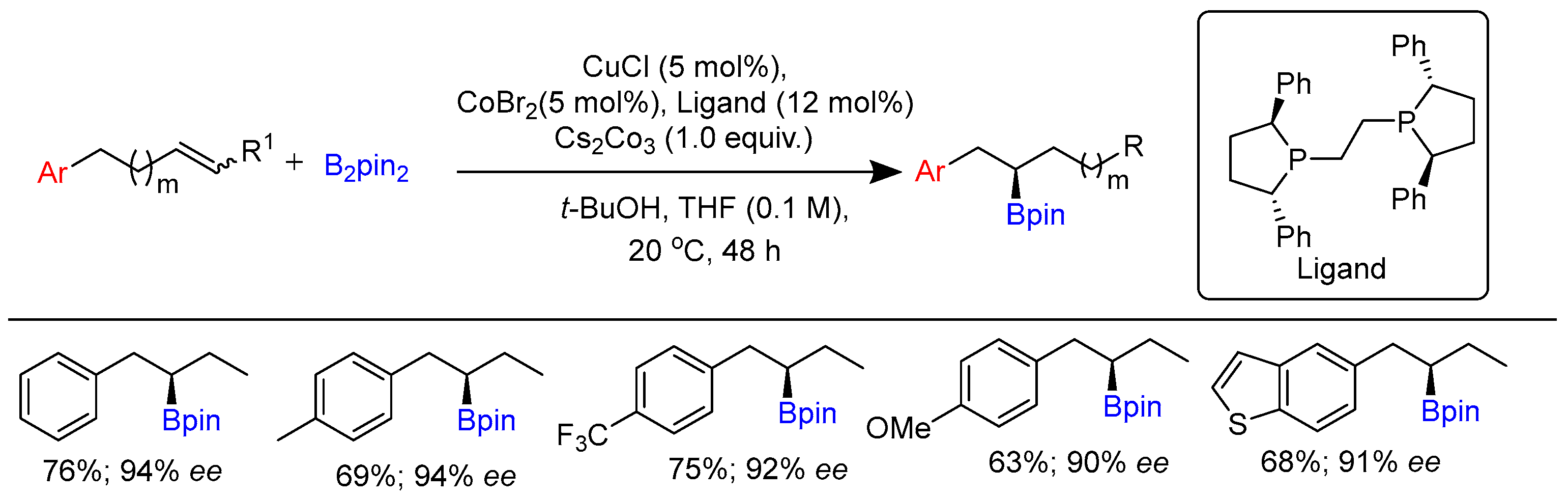
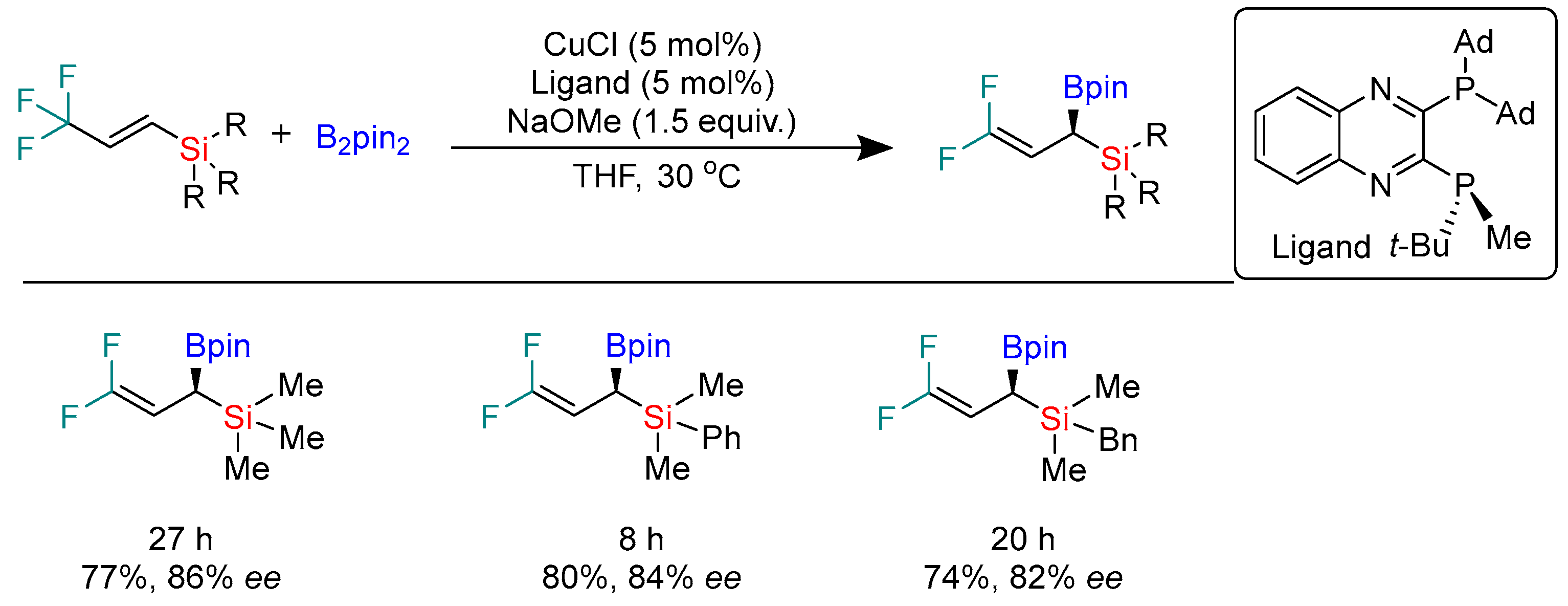
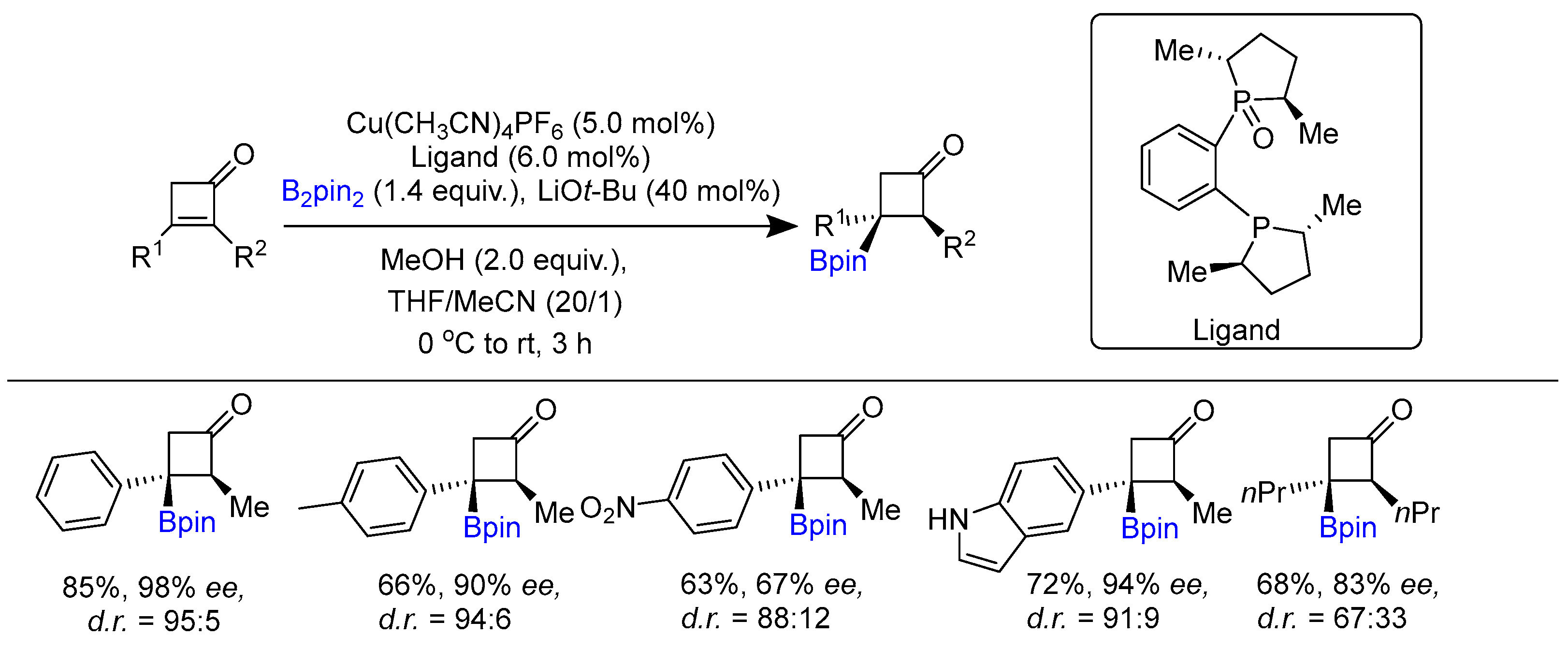
2.1. Hydroboration of Alkenes
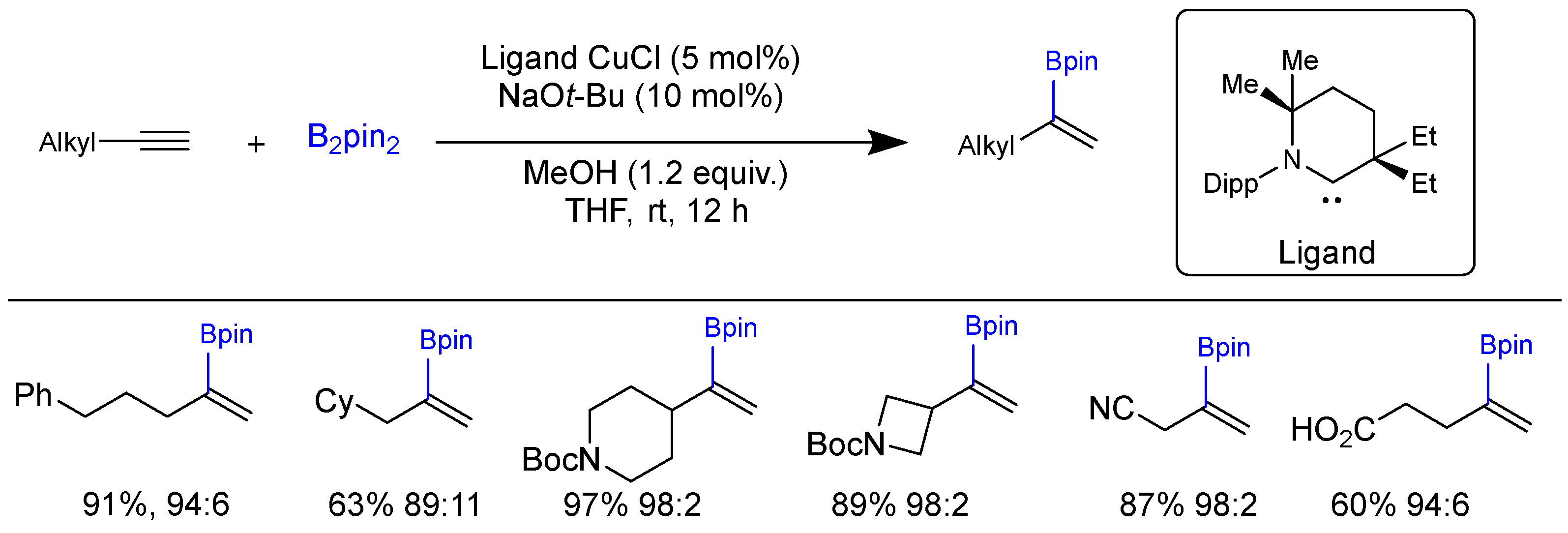
2.2. Hydroboration of Alkynes
3. Carboborylation of C-C Multiple Bonds
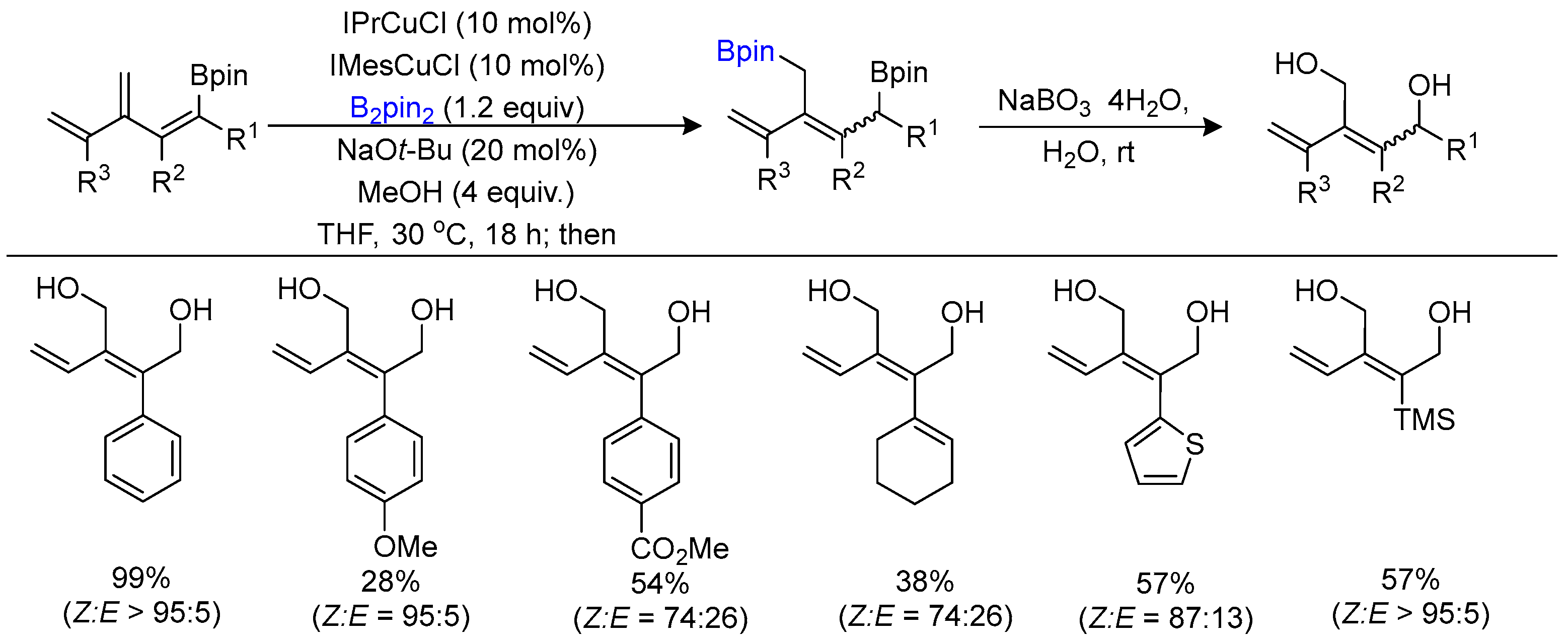
3.1. Carboborylation of Alkenes
3.2. Carboborylation of Allenes
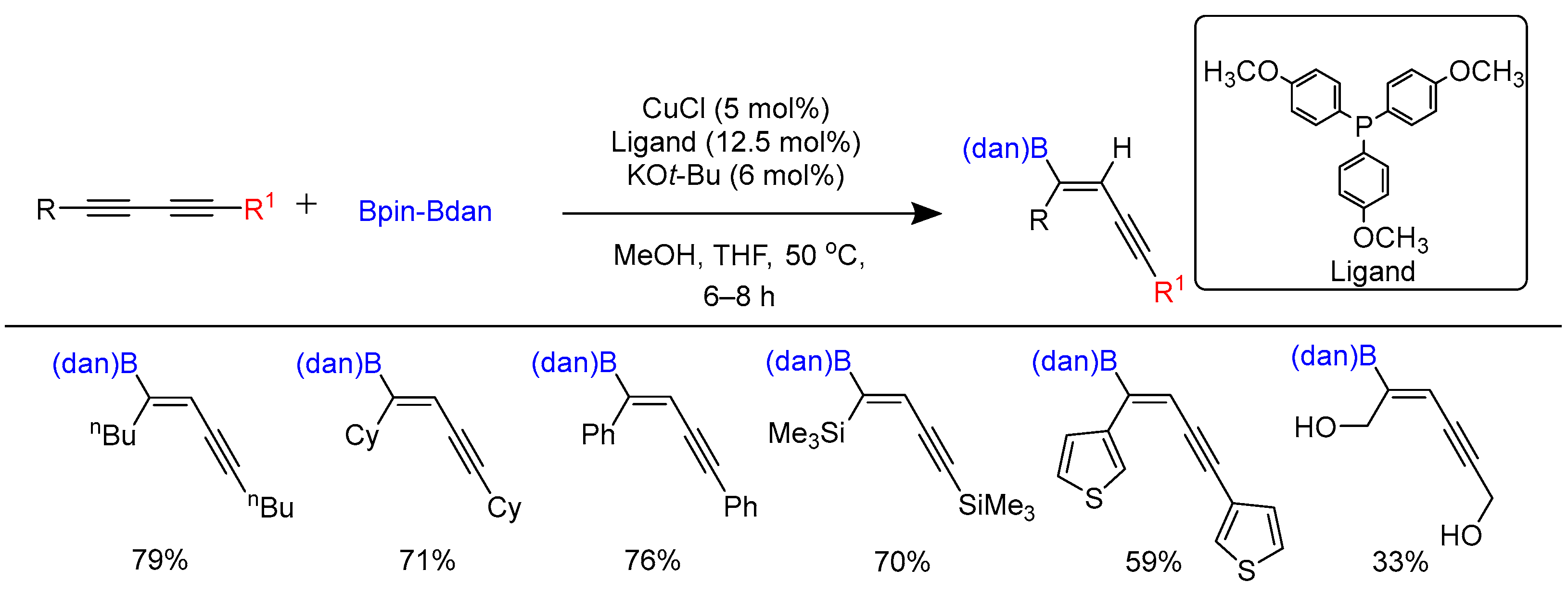
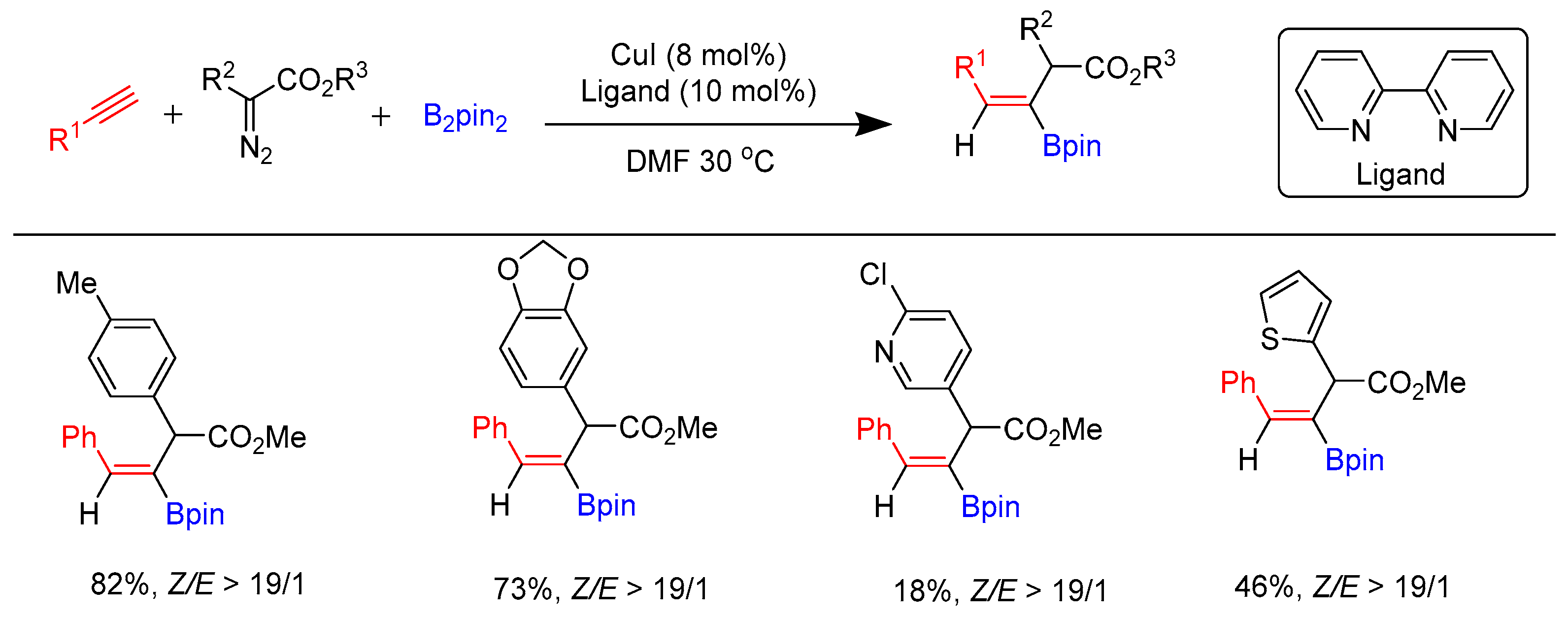
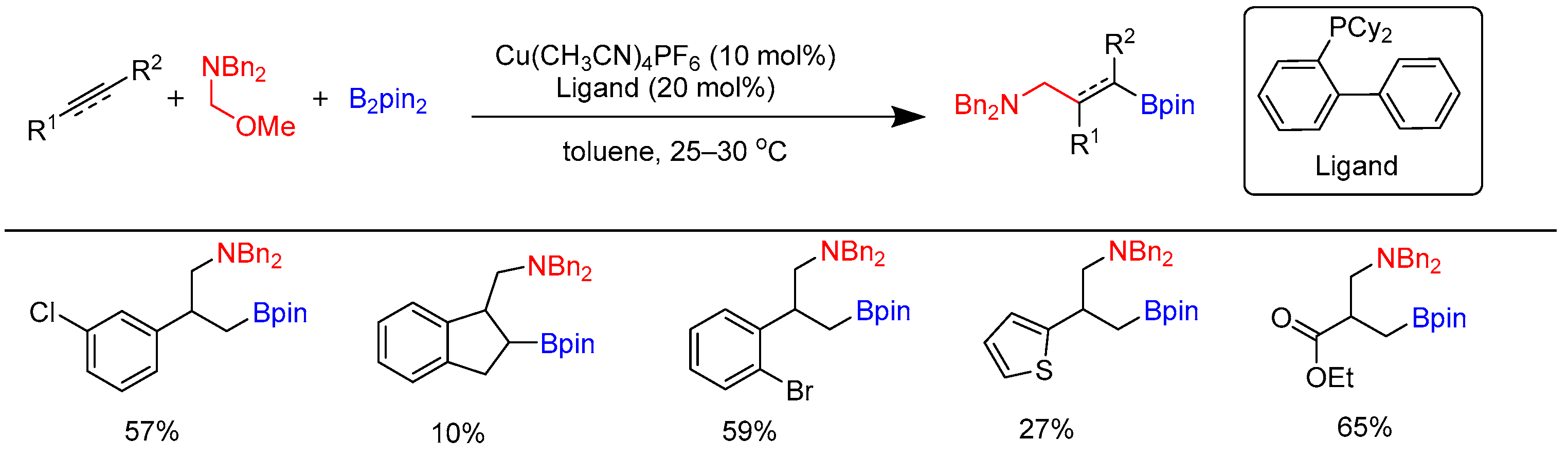
3.3. Carboborylation of Alkynes
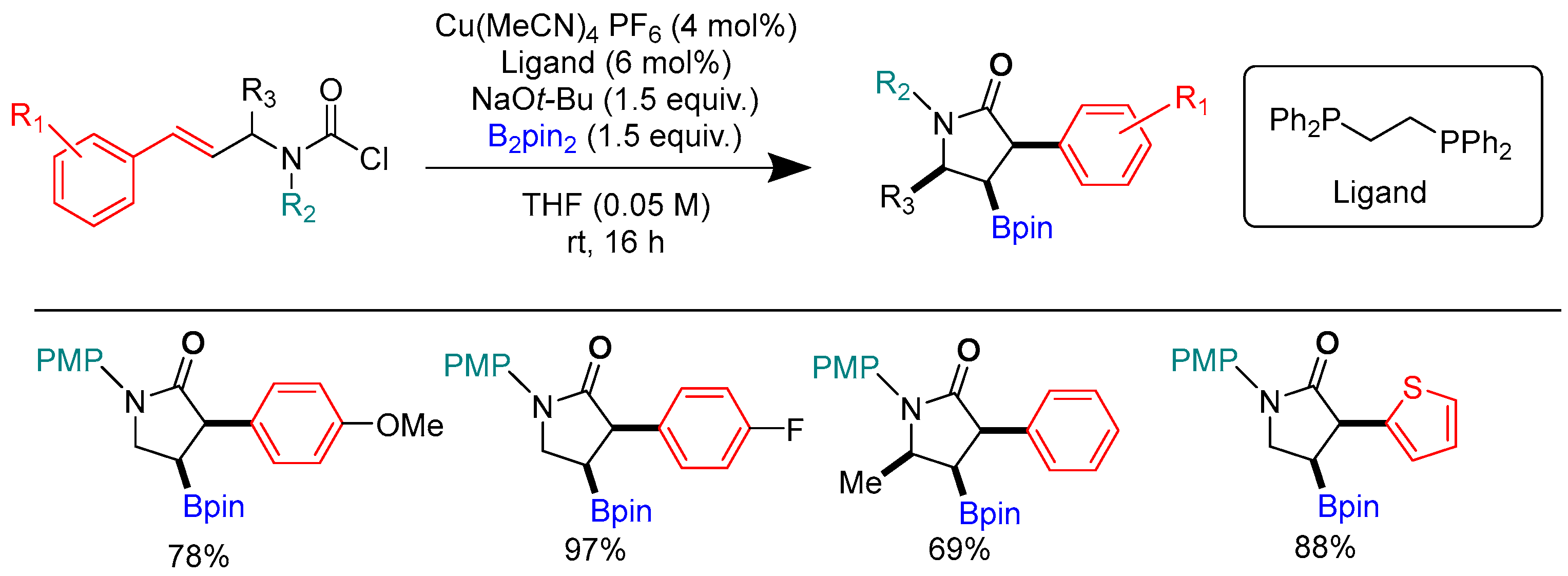
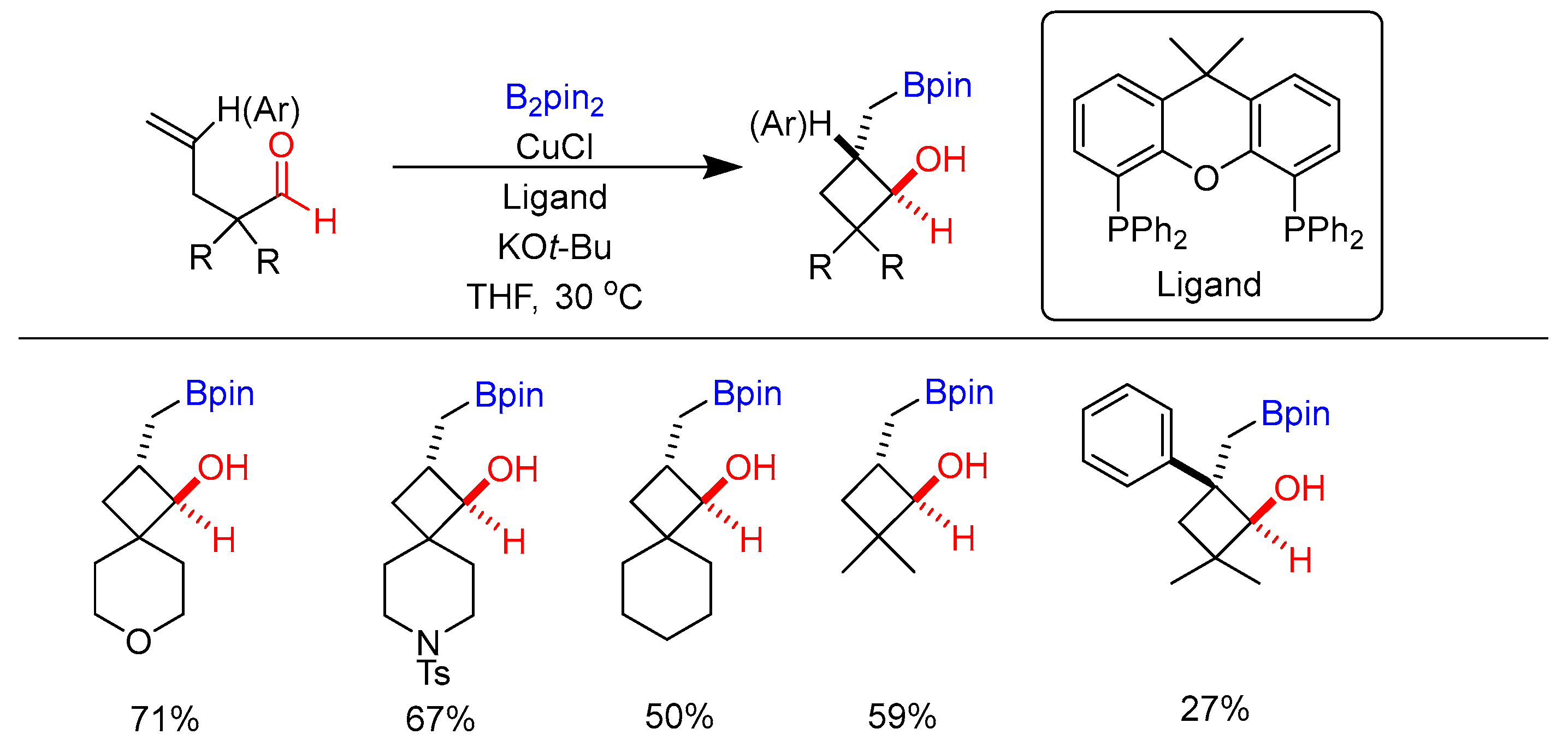
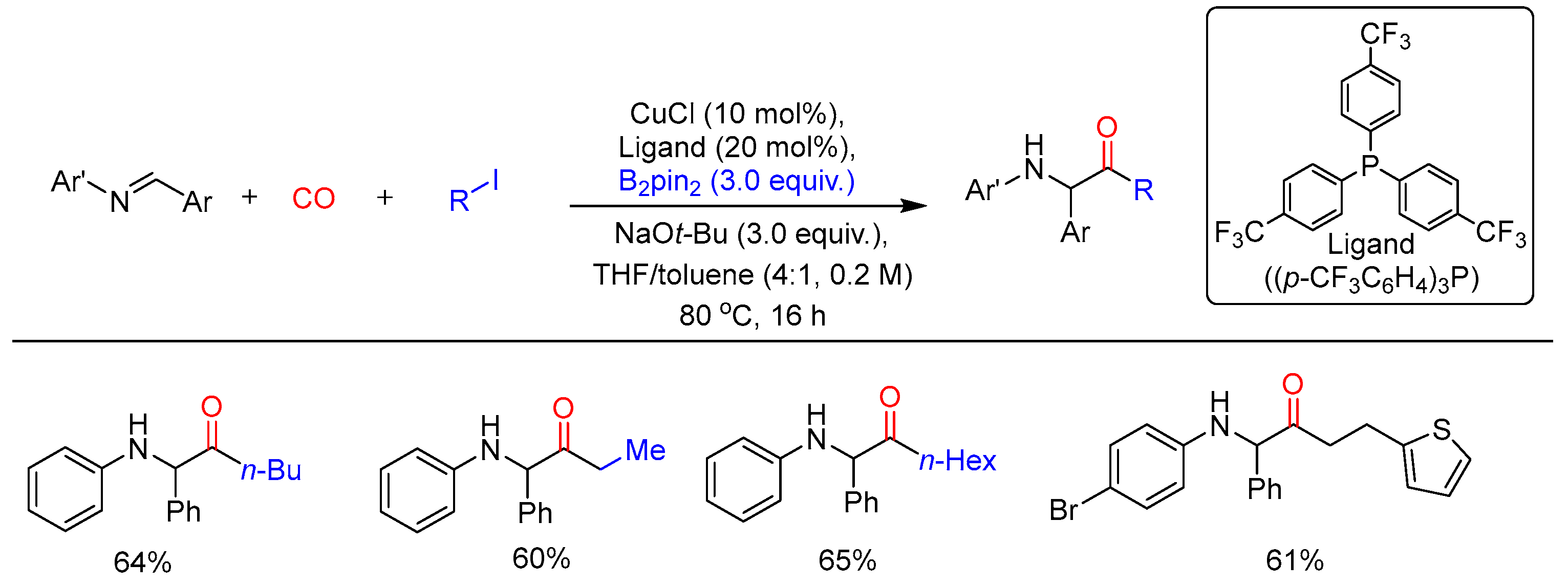
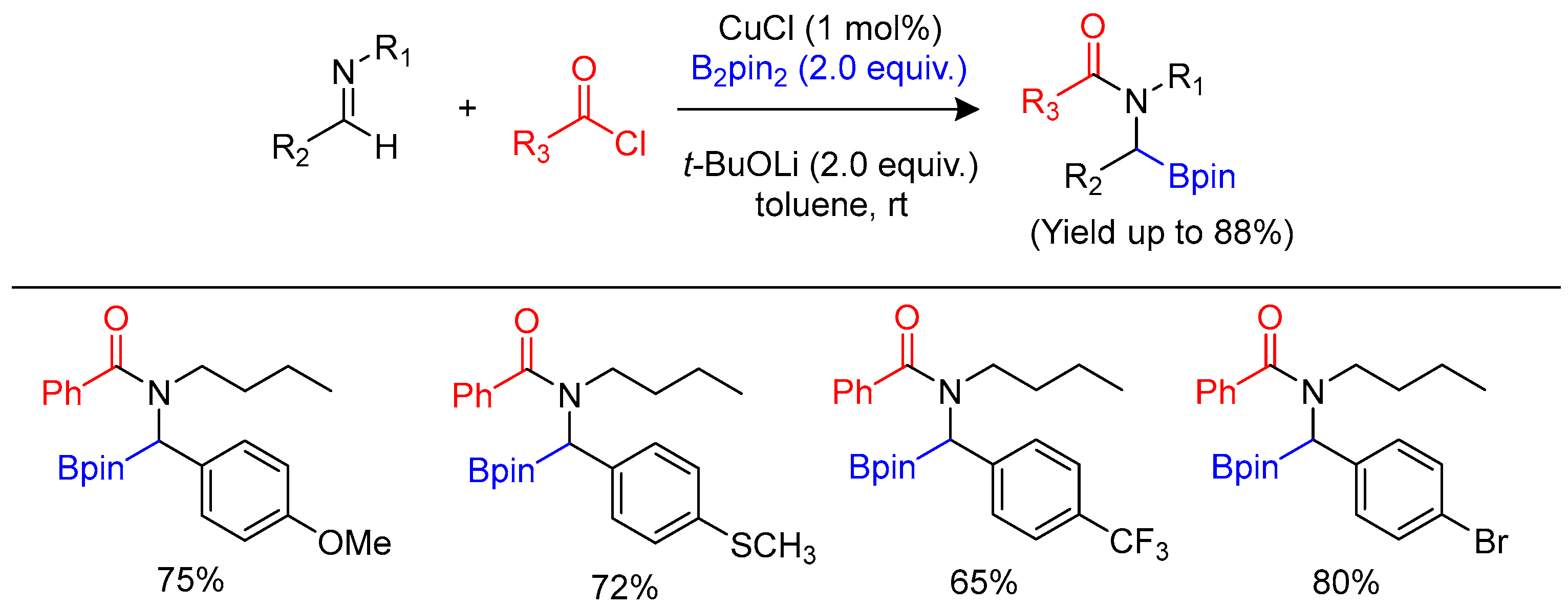
3.4. Carboborylation of Imine and Carbonyl Derivatives
4. Heteroboration of C-C Multiple Bonds
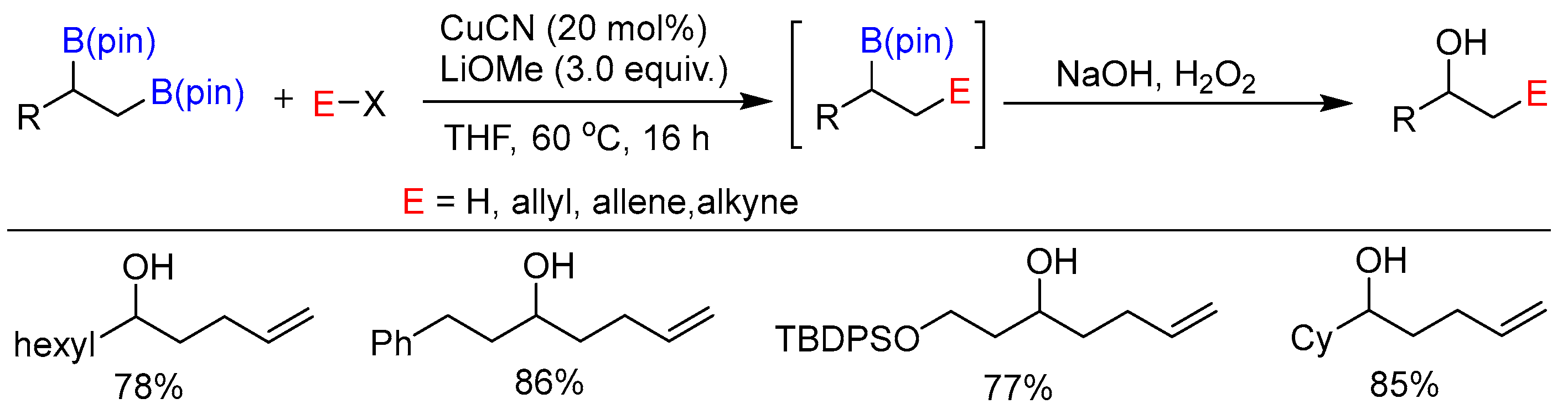
5. Multiboration
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, W.; Oestreich, M. Beyond Carbon: Enantioselective and enantiospecific reactions with catalytically generated boryl-and silylcopper intermediates. ACS Cent. Sci. 2020, 6, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G. Structure, properties, and preparation of boronic acid derivatives. overview of their reactions and applications. In Boronic Acids; Hall, D.G., Ed.; WILEY-VCH: Hoboken, NY, USA, 2006; pp. 1–99. [Google Scholar]
- Mkhalid, I.A.; Barnard, H.J.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C-H Activation for the construction of C-B bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Manna, S.; Dass, K.K.; Nandy, S.; Aich, D.; Paul, S.; Panda, S. A new avenue for the preparation of organoboron compounds via nickel catalysis. Coord. Chem. Rev. 2021, 448, 214165. [Google Scholar] [CrossRef]
- Hemming, D.; Fritzemeier, R.; Westcott, S.A.; Santos, W.L.; Steel, P.G. Copper-boryl mediated organic synthesis. Chem. Soc. Rev. 2018, 47, 7477–7494. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-catalyzed borylative difunctionalization of π-systems. ACS Catal. 2020, 10, 11578–11622. [Google Scholar] [CrossRef]
- Yoshida, H.; Kawashima, S.; Takemoto, Y.; Okada, K.; Ohshita, J.; Takaki, K. Copper-catalyzed borylation reactions of alkynes and arynes. Angew. Chem. Int. Ed. Engl. 2012, 51, 235–238. [Google Scholar] [CrossRef]
- Alam, S.; Karim, R.; Khan, A.; Pal, A.K.; Maruani, A. Copper-catalyzed preparation of alkenylboronates and arylboronates. Eur. J. Org. Chem. 2021, 2021, 6115–6160. [Google Scholar] [CrossRef]
- Shin, M.; Kim, M.; Hwang, C.; Lee, H.; Kwon, H.; Park, J.; Lee, E.; Cho, S.H. Facile synthesis of alpha-boryl-substituted allylboronate esters using stable bis[(pinacolato)boryl]methylzinc reagents. Org. Lett. 2020, 22, 2476–2480. [Google Scholar] [CrossRef]
- Laitar, S.D.; Müller, P.; Sadighi, P.J. Efficient homogeneous catalysis in the reduction of CO2 to CO. J. Am. Chem. Soc. 2005, 127, 17196–17197. [Google Scholar] [CrossRef]
- Ito, H.; Yamanaka, H.; Tateiwab, J.; Hosomi, A. Boration of an a,b-enone using a diboron promoted by a copper(I)–phosphine mixture catalyst. Tetrahedron Lett. 2000, 41, 6821–6825. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishiyama, T.; Miyaura, N. A borylcopper species generated from bis(pinacolato)diboron and its additions to a,b-unsaturated carbonyl compounds and terminal alkynes. J. Organomet. Chem. 2001, 625, 47–53. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Lewis acidity of boron compounds. Coord. Chem. Rev. 2014, 270, 75–88. [Google Scholar] [CrossRef]
- Wang, D.-K.; Li, L.; Xu, Q.; Zhang, J.; Zheng, H.; Wei, W.-T. 1,3-Difunctionalization of alkenes: State-of-the-art and future challenges. Org. Chem. Front. 2021, 8, 7037–7049. [Google Scholar] [CrossRef]
- Hu, J.; Ferger, M.; Shi, Z.; Marder, T.B. Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 2021, 50, 13129–13188. [Google Scholar] [CrossRef]
- Dherbassy, Q.; Manna, S.; Talbot, F.J.T.; Prasitwatcharakorn, W.; Perry, G.J.P.; Procter, D.J. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020, 11, 11380–11393. [Google Scholar] [CrossRef]
- Ito, H.; Miya, T.; Sawamura, M. Practical procedure for copper(I)-catalyzed allylic boryl substitution with stoichiometric alkoxide base. Tetrahedron 2012, 68, 3423–3427. [Google Scholar] [CrossRef]
- Han, J.T.; Yun, J. Asymmetric synthesis of alpha-chiral beta-hydroxy allenes: Copper-catalyzed gamma-selective borylative coupling of vinyl arenes and propargyl phosphates. Chem. Commun. 2019, 55, 9813–9816. [Google Scholar] [CrossRef]
- Manna, S. Copper-Catalyzed Diastereo-and enantioselective borylative cyclization. Catalysts 2022, 12, 734. [Google Scholar] [CrossRef]
- Novoa, L.; Trulli, L.; Fernandez, I.; Parra, A.; Tortosa, M. Regioselective monoborylation of spirocyclobutenes. Org. Lett. 2021, 23, 7434–7438. [Google Scholar] [CrossRef]
- Kang, T.; Erbay, T.G.; Xu, K.L.; Gallego, G.M.; Burtea, A.; Nair, S.K.; Patman, R.L.; Zhou, R.; Sutton, S.C.; McAlpine, I.J.; et al. Multifaceted substrate-ligand interactions promote the copper-catalyzed hydroboration of benzylidenecyclobutanes and related compounds. ACS Catal. 2020, 10, 13075–13083. [Google Scholar] [CrossRef]
- Xu, R.; Rohde, L.N.; Diver, S.T. Regioselective Cu-catalyzed hydroboration of 1,3-disubstituted-1,3-dienes: Functionalization of conjugated dienes readily accessible through ene–yne metathesis. ACS Catal. 2022, 12, 6434–6443. [Google Scholar] [CrossRef]
- Chaves-Pouso, A.; Rivera-Chao, E.; Fananas-Mastral, M. Copper-catalyzed protoboration of borylated dendralenes: A regio-and stereoselective access to functionalized 1,3-dienes. Chem. Commun. 2020, 56, 12230–12233. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, E.S.; Kozhemyakin, G.L.; Tyurin, V.S.; Frolova, V.V.; Lonin, I.S.; Ponomarev, G.V.; Buryak, A.K.; Zamilatskov, I.A. Direct C-H borylation of vinylporphyrins via copper catalysis. Org. Biomol. Chem. 2022, 20, 1926–1932. [Google Scholar] [CrossRef]
- Shegavi, M.L.; Saini, S.; Bhawar, R.; Vishwantha, M.D.; Bose, S.K. Recyclable copper nanoparticles-catalyzed hydroboration of alkenes and β-borylation of α,β-unsaturated carbonyl compounds with bis(pinacolato)diboron. Adv. Synth. Catal. 2021, 363, 2408–2416. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Yin, J.; Xiong, T.; Zhang, Q. Remote site-selective asymmetric protoboration of unactivated alkenes anabled by bimetallic relay catalysis. Angew. Chem. Int. Ed. 2022, 61, e202202713. [Google Scholar] [CrossRef]
- Oyama, N.; Akiyama, S.; Kubota, K.; Imamoto, T.; Ito, H. Cu(I)-catalyzed enantioselective γ-boryl substitution of trifluoromethyl- and silyl-substituted alkenes. Eur. J. Org. Chem. 2022, 2022, e202200664. [Google Scholar] [CrossRef]
- Cui, M.; Zhao, Z.Y.; Oestreich, M. Boosting the enantioselectivity of conjugate borylation of alpha,beta-disubstituted cyclobutenones with monooxides of chiral C(2)-symmetric bis(phosphine) ligands. Chem. Eur. J. 2022, 28, e202202163. [Google Scholar] [CrossRef]
- Nguyen, K.; Clement, H.A.; Bernier, L.; Coe, J.W.; Farrell, W.; Helal, C.J.; Reese, M.R.; Sach, N.W.; Lee, J.C.; Hall, D.G. Catalytic enantioselective synthesis of a cis-β-boronyl cyclobutylcarboxyester scaffold and its highly diastereoselective nickel/photoredox dual-catalyzed Csp3–Csp2 cross-coupling to access elusive trans-β-aryl/heteroaryl cyclobutylcarboxyesters. ACS Catal. 2021, 11, 404–413. [Google Scholar] [CrossRef]
- Gao, Y.; Yazdani, S.; Kendrick, A.t.; Junor, G.P.; Kang, T.; Grotjahn, D.B.; Bertrand, G.; Jazzar, R.; Engle, K.M. Cyclic (alkyl)(amino)carbene ligands enable Cu-catalyzed Markovnikov protoboration and protosilylation of terminal alkynes: A versatile portal to functionalized alkenes. Angew. Chem. Int. Ed. 2021, 60, 19871–19878. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakrabortty, R.; Kumar, S.; Das, A.; Ganesh, V. Copper-Catalyzed Protoboration of 1,3-Diynes as a Platform for Iterative Functionalization. ACS Catal. 2022, 12, 11660–11666. [Google Scholar] [CrossRef]
- Kehr, G.; Erker, G. 1,1-Carboboration. Chem. Commun. 2012, 48, 1839–1850. [Google Scholar] [CrossRef]
- Xu, N.; Kong, Z.; Wang, J.Z.; Lovinger, G.J.; Morken, J.P. Copper-Catalyzed Coupling of Alkyl Vicinal Bis(boronic Esters) to an Array of Electrophiles. J. Am. Chem. Soc. 2022, 144, 17815–17823. [Google Scholar] [CrossRef] [PubMed]
- Torelli, A.; Whyte, A.; Polishchuk, I.; Bajohr, J.; Lautens, M. Stereoselective Construction of γ-Lactams via Copper-Catalyzed Borylacylation. Org. Lett. 2020, 22, 7915–7919. [Google Scholar] [CrossRef] [PubMed]
- Maza, R.J.; Royes, J.; Carbo, J.J.; Fernandez, E. Consecutive borylcupration/C-C coupling of gamma-alkenyl aldehydes towards diastereoselective 2-(borylmethyl)cycloalkanols. Chem. Commun. 2020, 56, 5973–5976. [Google Scholar] [CrossRef]
- Larin, E.M.; Loup, J.; Polishchuk, I.; Ross, R.J.; Whyte, A.; Lautens, M. Enantio-and diastereoselective conjugate borylation/Mannich cyclization. Chem. Sci. 2020, 11, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Dherbassy, Q.; Manna, S.; Shi, C.; Prasitwatcharakorn, W.; Crisenza, G.E.M.; Perry, G.J.P.; Procter, D.J. Enantioselective Copper-Catalyzed Borylative Cyclization for the Synthesis of Quinazolinones. Angew. Chem. Int. Ed. 2021, 60, 14355–14359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, L.; Lin, H.Z.; Deng, J.T.; Zhang, X.Z.; Peng, J.B. Copper-catalyzed 1,2-Borylacylation of 1,3-Enynes: Synthesis of β-Alkynyl ketones. Chem. Commun. 2022, 58, 5968–5971. [Google Scholar] [CrossRef]
- Suliman, A.M.Y.; Ahmed, E.M.A.; Gong, T.; Fu, Y. Three-component reaction of gem-difluorinated cyclopropanes with alkenes and B2pin2 for the synthesis of monofluoroalkenes. Chem. Commun. 2021, 57, 6400–6403. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.P.; Wu, X.F. Copper-catalyzed borofunctionalization of styrenes with B2pin2 and CO. Chem. Sci. 2021, 12, 13777–13781. [Google Scholar] [CrossRef]
- Akiyama, S.; Oyama, N.; Endo, T.; Kubota, K.; Ito, H. A Copper(I)-Catalyzed Radical-Relay Reaction Enabling the Intermolecular 1,2-Alkylborylation of Unactivated Olefins. J. Am. Chem. Soc. 2021, 143, 5260–5268. [Google Scholar] [CrossRef]
- Yang, L.M.; Zeng, H.H.; Liu, X.L.; Ma, A.J.; Peng, J.B. Copper catalyzed borocarbonylation of benzylidenecyclopropanes through selective proximal C-C bond cleavage: Synthesis of γ-boryl-γ,δ-unsaturated carbonyl compounds. Chem. Sci. 2022, 13, 7304–7309. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.P.; Geng, H.Q.; Wu, X.F. Copper-Catalyzed Boroaminomethylation of Olefins to γ-Boryl Amines with CO as C1 Source. Angew. Chem. Int. Ed. 2022, 61, e202211455. [Google Scholar] [CrossRef]
- Wu, F.P.; Wu, X.F. Pd/Cu-Catalyzed amide-enabled selectivity-reversed borocarbonylation of unactivated alkenes. Chem. Sci. 2021, 12, 10341–10346. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Kim, S.T.; Baik, M.H.; Yun, J. Direct Stereoconvergent Allylation of Chiral Alkylcopper Nucleophiles with Racemic Allylic Phosphates. Chem. Eur. J. 2020, 26, 2592–2596. [Google Scholar] [CrossRef]
- Yang, Z.; Li, P.; Lu, H.; Li, G. Copper-Catalyzed Asymmetric Borylacylation of Styrene and Indene Derivatives. J. Org. Chem. 2021, 86, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Endo, K.; Ito, H. Regio-and Stereoselective Synthesis of Multi-Alkylated Allylic Boronates through Three-Component Coupling Reactions between Allenes, Alkyl Halides, and a Diboron Reagent. J. Am. Chem. Soc. 2021, 143, 13865–13877. [Google Scholar] [CrossRef]
- Hu, H.; Ji, G.C.; Jiang, L.; Bi, S.; Jiang, Y.Y.; Liu, Y. Ligand-Controlled Regiodivergent Cyanoboration of Internal Allenes by Copper Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202210338. [Google Scholar] [CrossRef]
- Perez-Saavedra, B.; Velasco-Rubio, A.; Rivera-Chao, E.; Varela, J.A.; Saa, C.; Fananas-Mastral, M. Catalytic Lewis Base Additive Enables Selective Copper-Catalyzed Borylative alpha-C-H Allylation of Alicyclic Amines. J. Am. Chem. Soc. 2022, 144, 16206–16216. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Tambe, S.D.; Cho, E.J. Diastereoselective Reductive Cyclization of Allene-Tethered Ketoamines via Copper-Catalyzed Cascade Carboboronation and Protodeborylation. Bull. Korean Chem. Soc. 2021, 42, 683–690. [Google Scholar] [CrossRef]
- Deng, H.; Dong, Y.; Shangguan, Y.; Yang, F.; Han, S.; Wu, J.; Liang, B.; Guo, H.; Zhang, C. Copper-Catalyzed Three-Component Carboboronation of Allenes Using Highly Strained Cyclic Ketimines as Electrophiles. Org. Lett. 2021, 23, 4431–4435. [Google Scholar] [CrossRef]
- Yu, S.-H.; Gong, T.-J.; Fu, Y. Three-Component Borylallenylation of Alkynes: Access to Densely Boryl-Substituted Ene-allenes. Org. Lett. 2020, 22, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kim, N.; Mendoza, S.D.; Yazdani, S.; Faria Vieira, A.; Liu, M.; Kendrick, A.; Grotjahn, D.B.; Bertrand, G.; Jazzar, R.; et al. (CAAC)Copper Catalysis Enables Regioselective Three-Component Carboboration of Terminal Alkynes. ACS Catal. 2022, 12, 7243–7247. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, B.; Wu, R.; Zhu, S. Cu-catalyzed carboboration of acetylene with Michael acceptors. Chem. Sci. 2022, 13, 7604–7609. [Google Scholar] [CrossRef]
- Chaves-Pouso, A.; Álvarez-Constantino, A.M.; Fañanás-Mastral, M. Enantio-and Diastereoselective Copper-Catalyzed Allylboration of Alkynes with Allylic gem-Dichlorides. Angew. Chem. Int. Ed. 2021, 61, e202117696. [Google Scholar] [CrossRef]
- Li, Z.; Sun, J. Copper-Catalyzed 1,1-Boroalkylation of Terminal Alkynes: Access to Alkenylboronates via a Three-Component Reaction. Org. Lett. 2021, 23, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yin, P.; Wu, X.F. Regioselective catalytic carbonylation and borylation of alkynes with aryldiazonium salts toward α-unsubstituted β-boryl ketones. Chem. Sci. 2022, 13, 12122–12126. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, L.; Wang, S.; Dai, Y.; Feng, Y.; Gong, C.; Tao, C. Copper-catalyzed borylative aminomethylation of C-C double and triple bonds with N,O-acetal. Chem. Commun. 2021, 57, 3279–3282. [Google Scholar] [CrossRef]
- Wu, F.P.; Wu, X.F. Ligand-Controlled Copper-Catalyzed Regiodivergent Carbonylative Synthesis of α-Amino Ketones and α-Boryl Amides from Imines and Alkyl Iodides. Angew. Chem. Int. Ed. 2021, 60, 695–700. [Google Scholar] [CrossRef]
- Xia, Q.; Chang, H.R.; Li, J.; Wang, J.Y.; Peng, Y.Q.; Song, G.H. Tunable Synthesis of α-Amino Boronic Esters from Available Aldehydes and Amines through Sequential One-Pot Dehydration and Copper-Catalyzed Borylacylation. J. Org. Chem. 2020, 85, 2716–2724. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, P.; Zhou, J.; Hu, Z.; Xu, T. Modular and Fast Synthesis of Versatile Secondary α, α-Dialkyl Boronates via Deoxygenative Alkylboration of Aldehydes. Angew. Chem. Int. Ed. 2022, 61, e202214213. [Google Scholar] [CrossRef]
- Larin, E.M.; Torelli, A.; Loup, J.; Lautens, M. One-Pot, Three-Step Synthesis of Benzoxazinones via Use of the Bpin Group as a Masked Nucleophile. Org. Lett. 2021, 23, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Nishino, S.; Nishii, Y.; Hirano, K. Anti-Selective synthesis of β-boryl-α-amino acid derivatives by Cu-catalysed borylamination of α, β-unsaturated esters. Chem. Sci. 2022, 13, 14387–14394. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, H.; Wang, M.; Liao, J. Copper-Catalyzed Enantioselective Aminoboration of Styrenes with 1,2-Benzisoxazole as Nitrogen Source. Acta Chim. Sin. 2020, 78, 1229. [Google Scholar] [CrossRef]
- Zhao, S.; Ge, S. Synergistic Hydrocobaltation and Borylcobaltation Enable Regioselective Migratory Triborylation of Unactivated Alkenes. Angew. Chem. Int. Ed. 2022, 61, e202116133. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.-P.; Spannenberg, A.; Wu, X.-F. Cu/Pd-catalyzed borocarbonylative trifunctionalization of alkynes and allenes: Synthesis of β-geminal-diboryl ketones. Sci. China. Chem. 2021, 64, 2142–2153. [Google Scholar] [CrossRef]
- Wu, F.P.; Luo, X.; Radius, U.; Marder, T.B.; Wu, X.F. Copper-Catalyzed Synthesis of Stereodefined Cyclopropyl Bis(boronates) from Alkenes with CO as the C1 Source. J. Am. Chem. Soc. 2020, 142, 14074–14079. [Google Scholar] [CrossRef]
- Geng, H.-Q.; Li, W.; Zhao, Y.; Wu, X.-F. Copper-catalyzed synthesis of cyclopropyl bis(boronates) from aryl olefins and carbon monoxide. Org. Chem. Front. 2022, 9, 4943–4948. [Google Scholar] [CrossRef]
- Li, J.; Ge, S. Copper-Catalyzed Quadruple Borylation of Terminal Alkynes to Access sp(3)-Tetra-Organometallic Reagents. Angew. Chem. Int. Ed. 2022, 61, e202213057. [Google Scholar] [CrossRef]



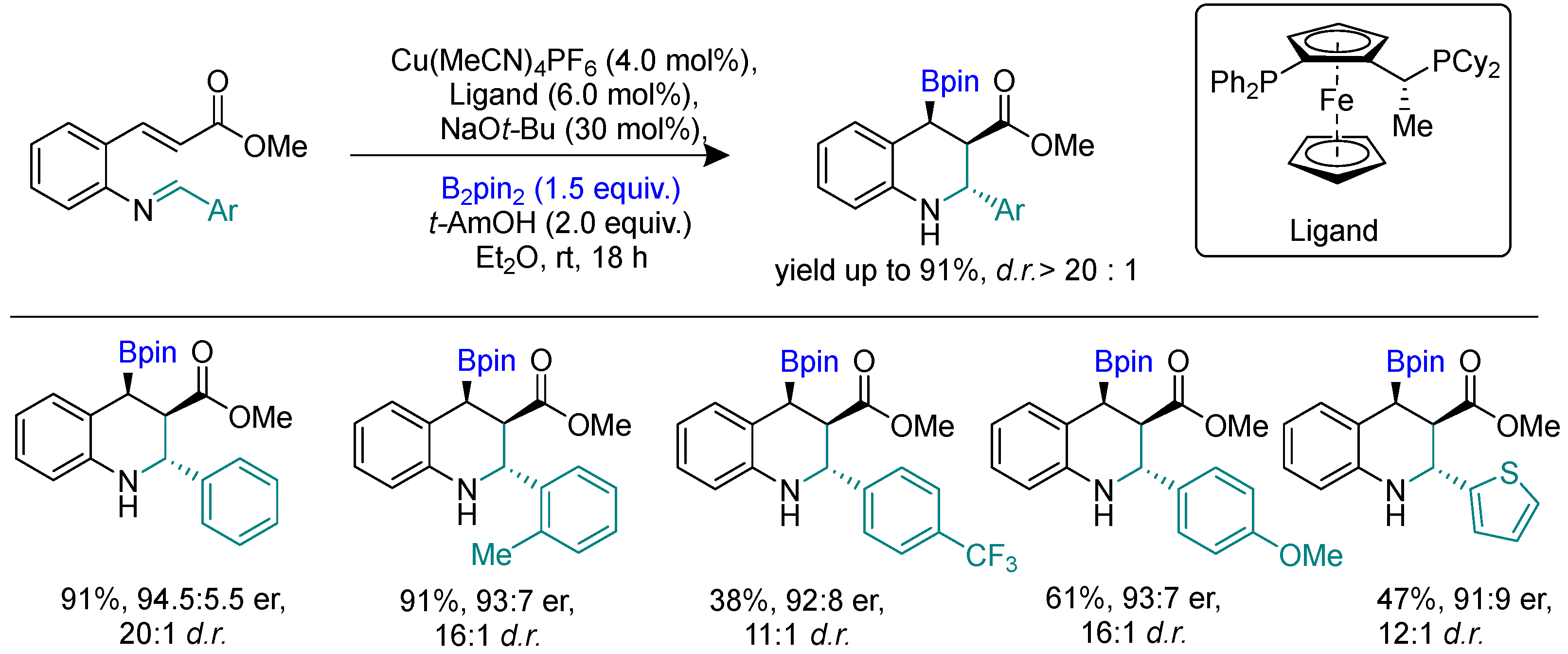
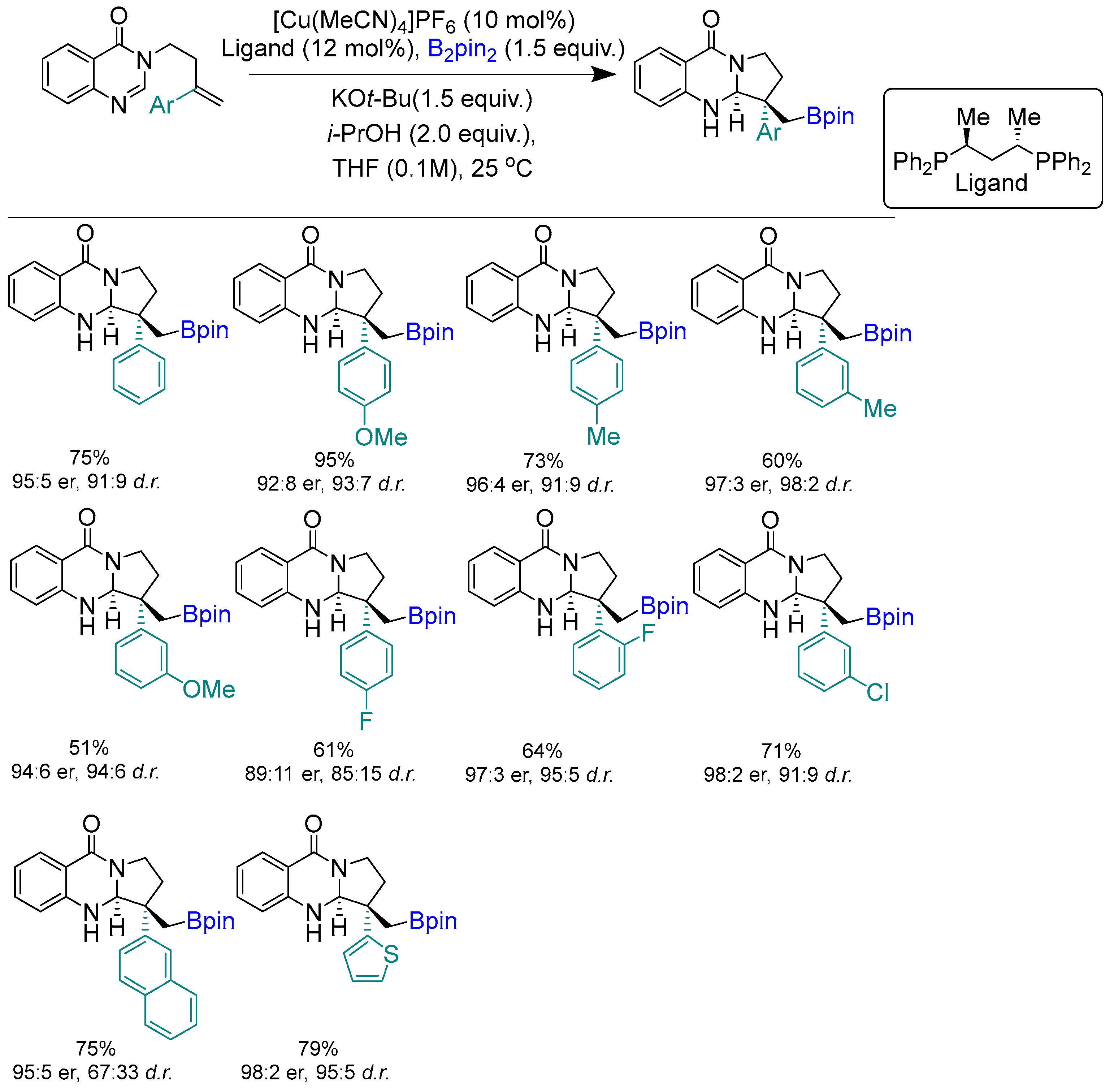

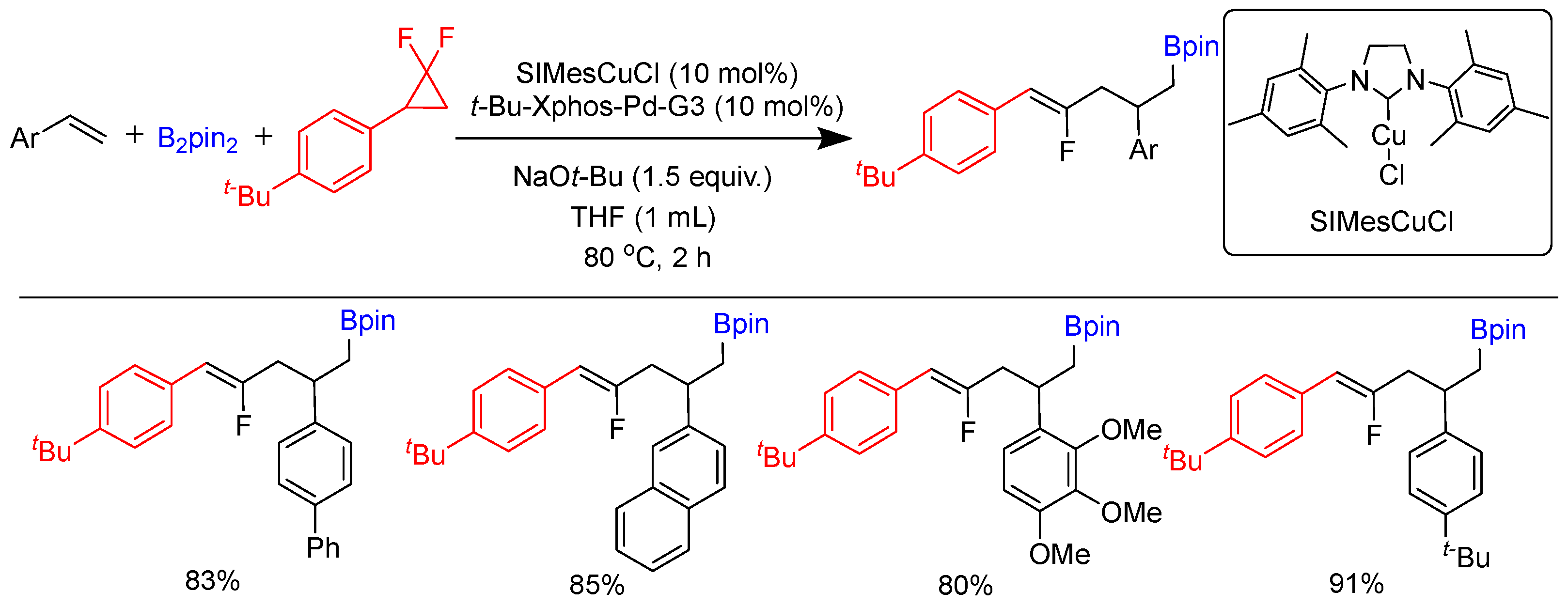
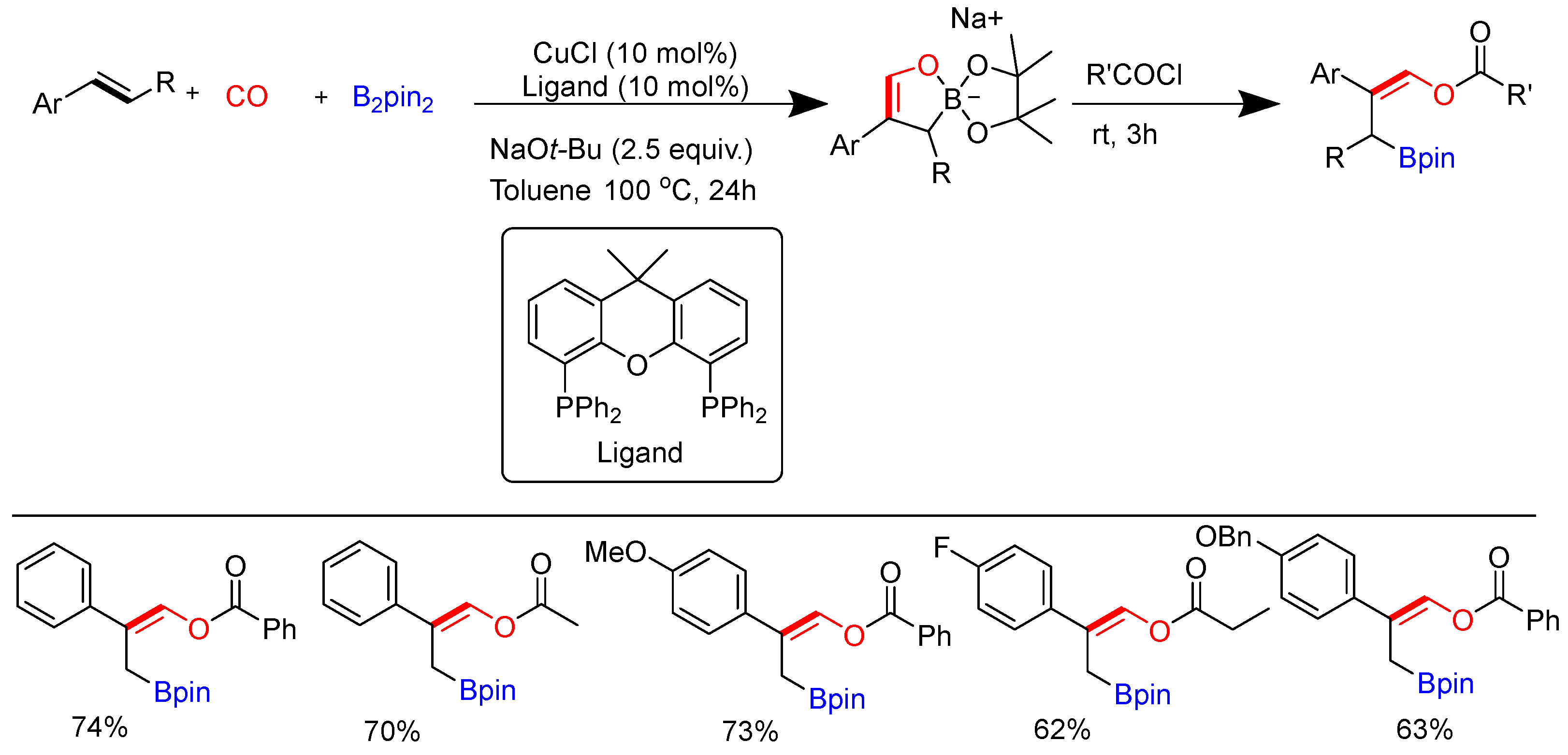
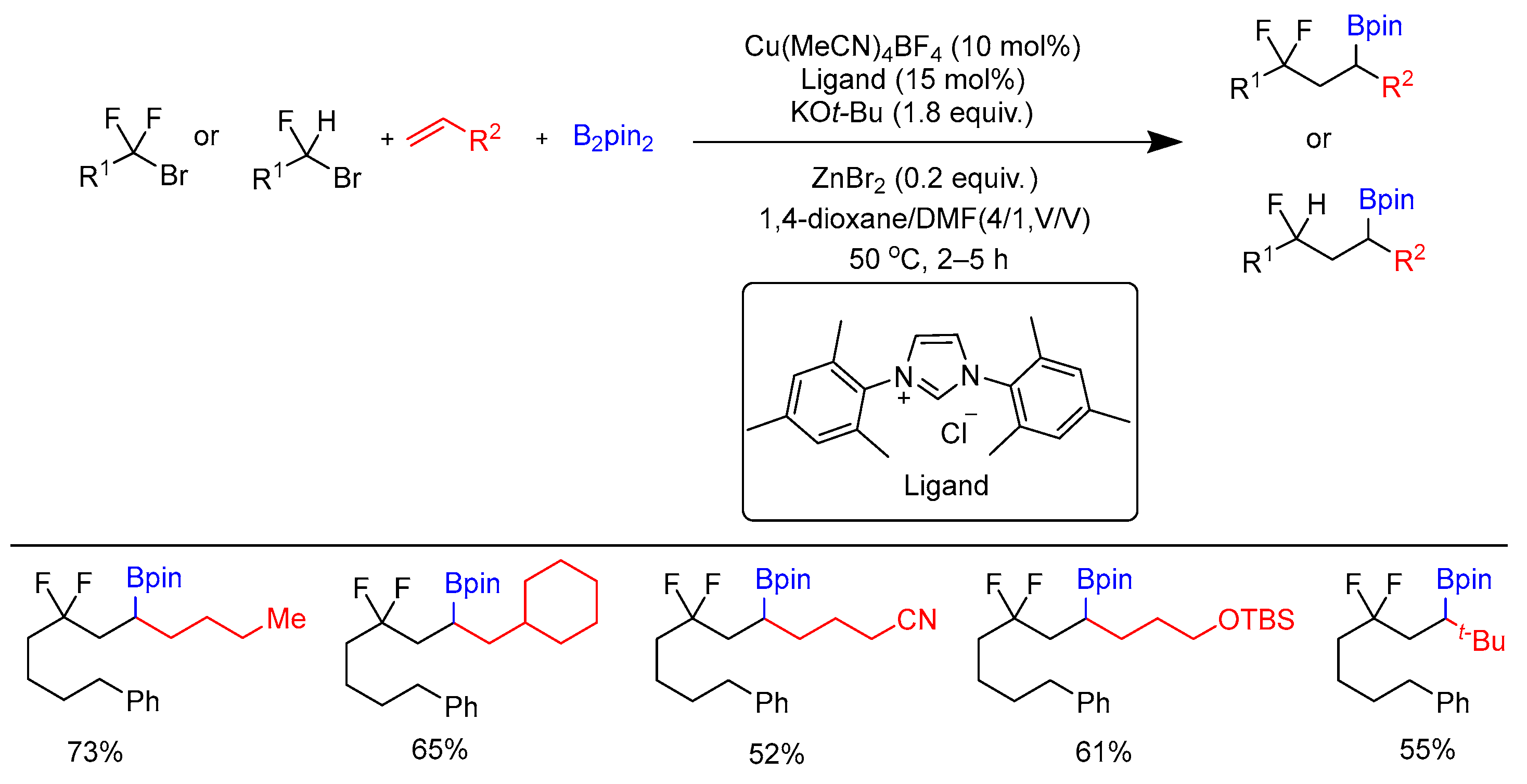

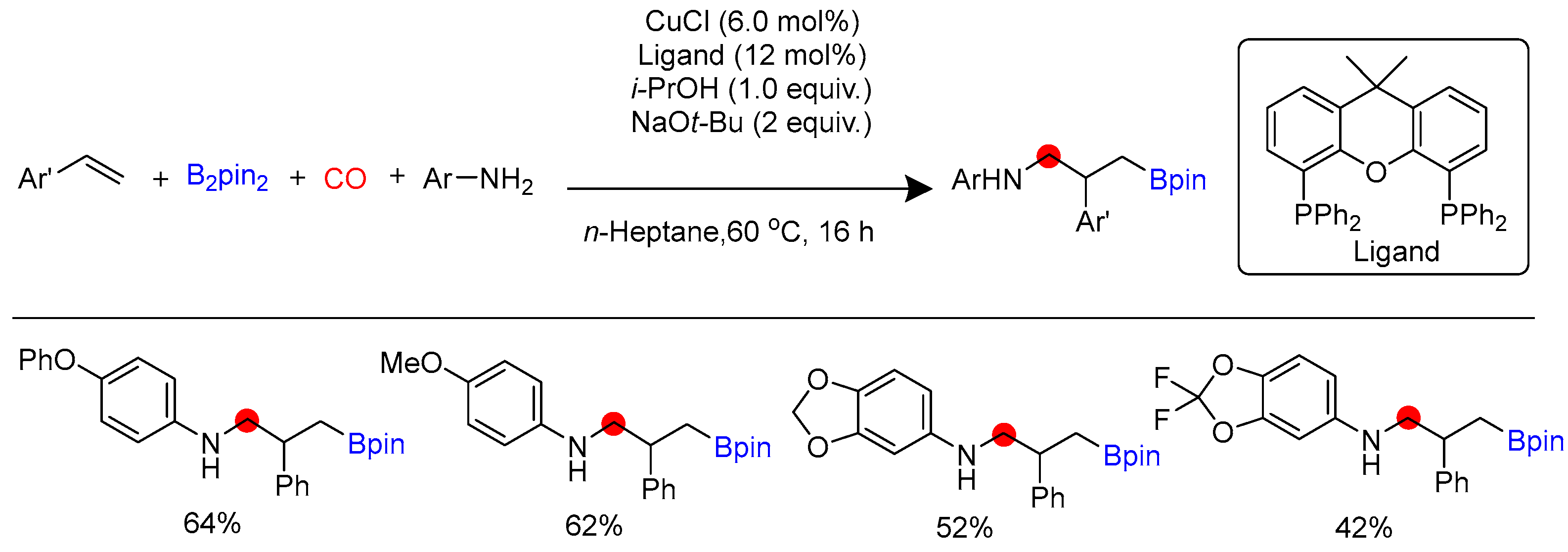
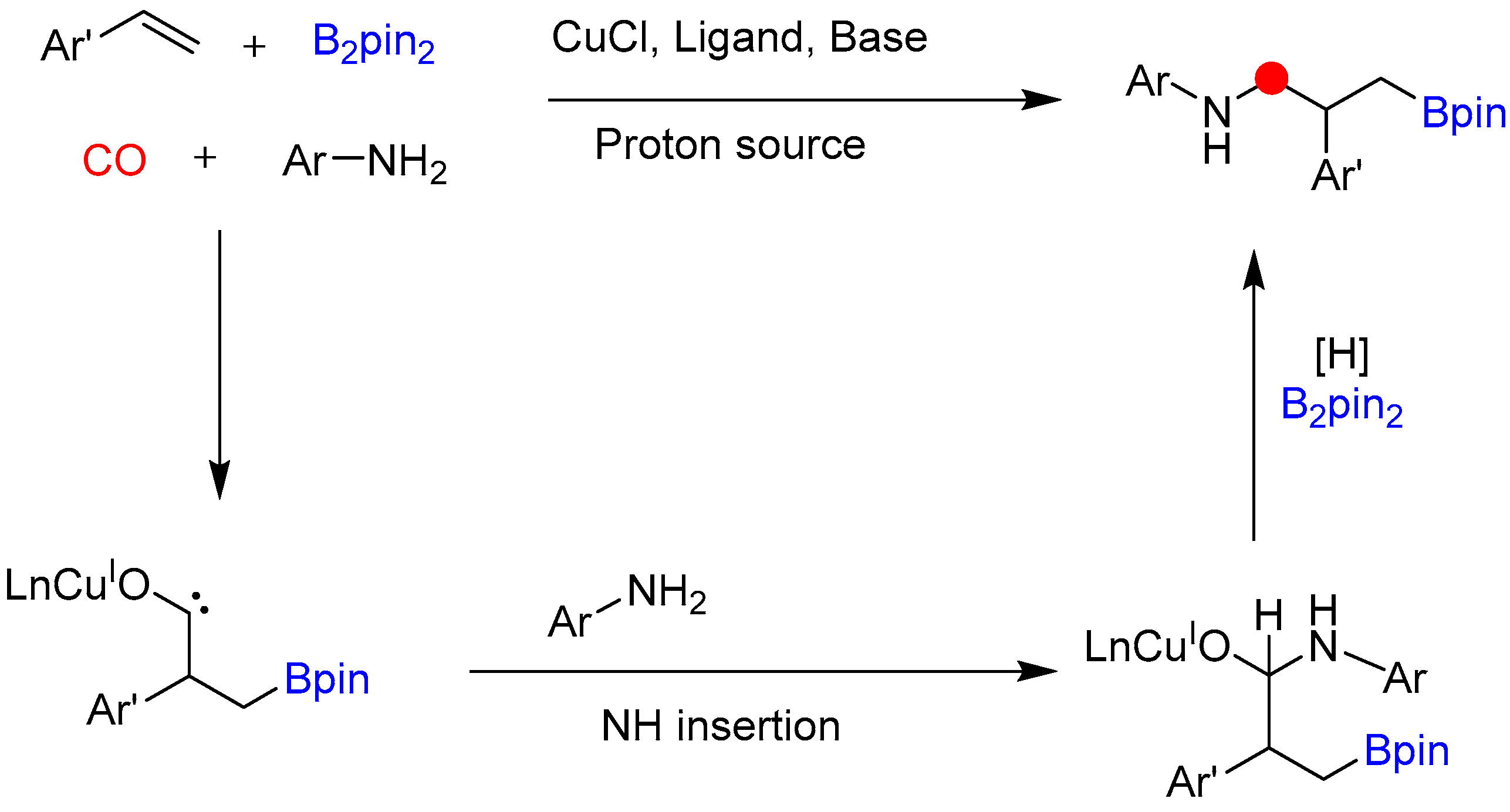
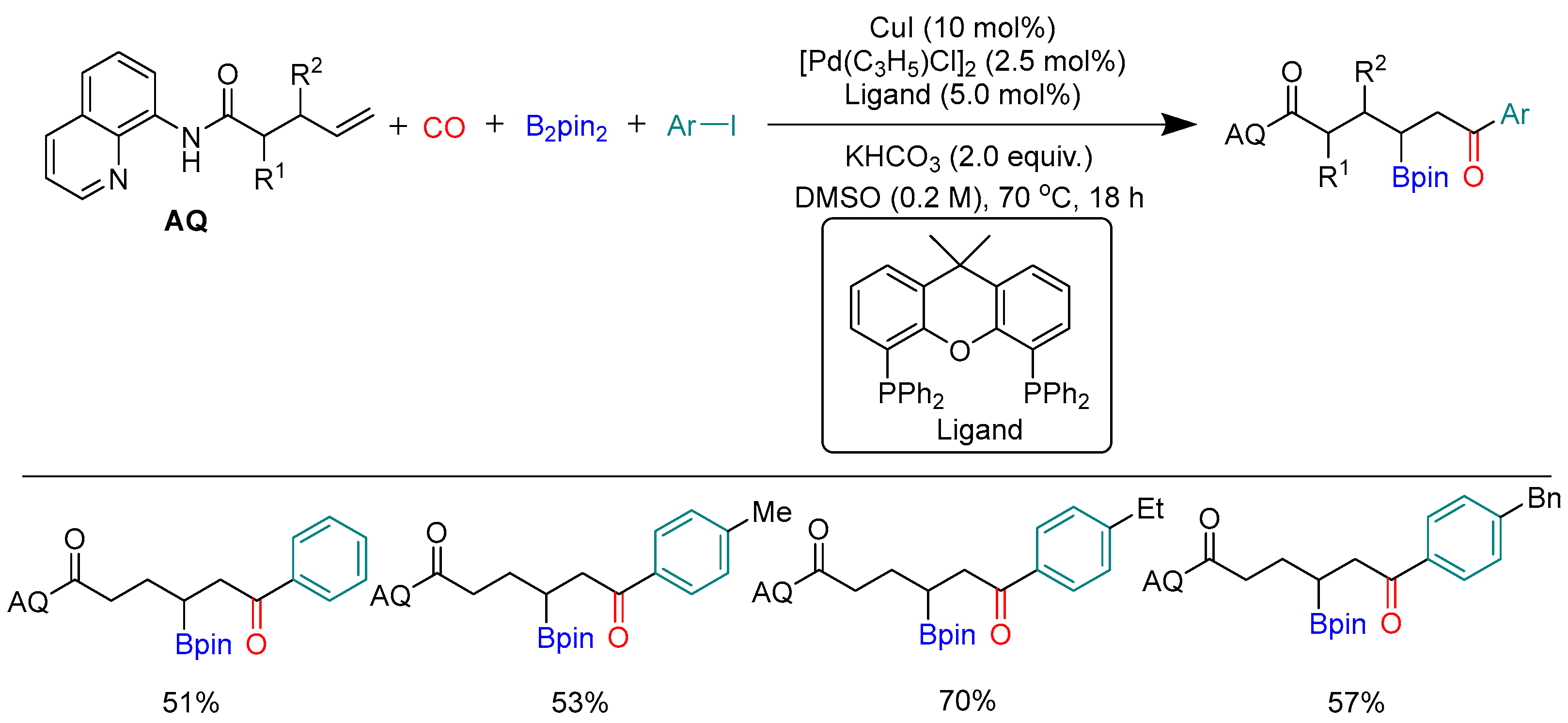
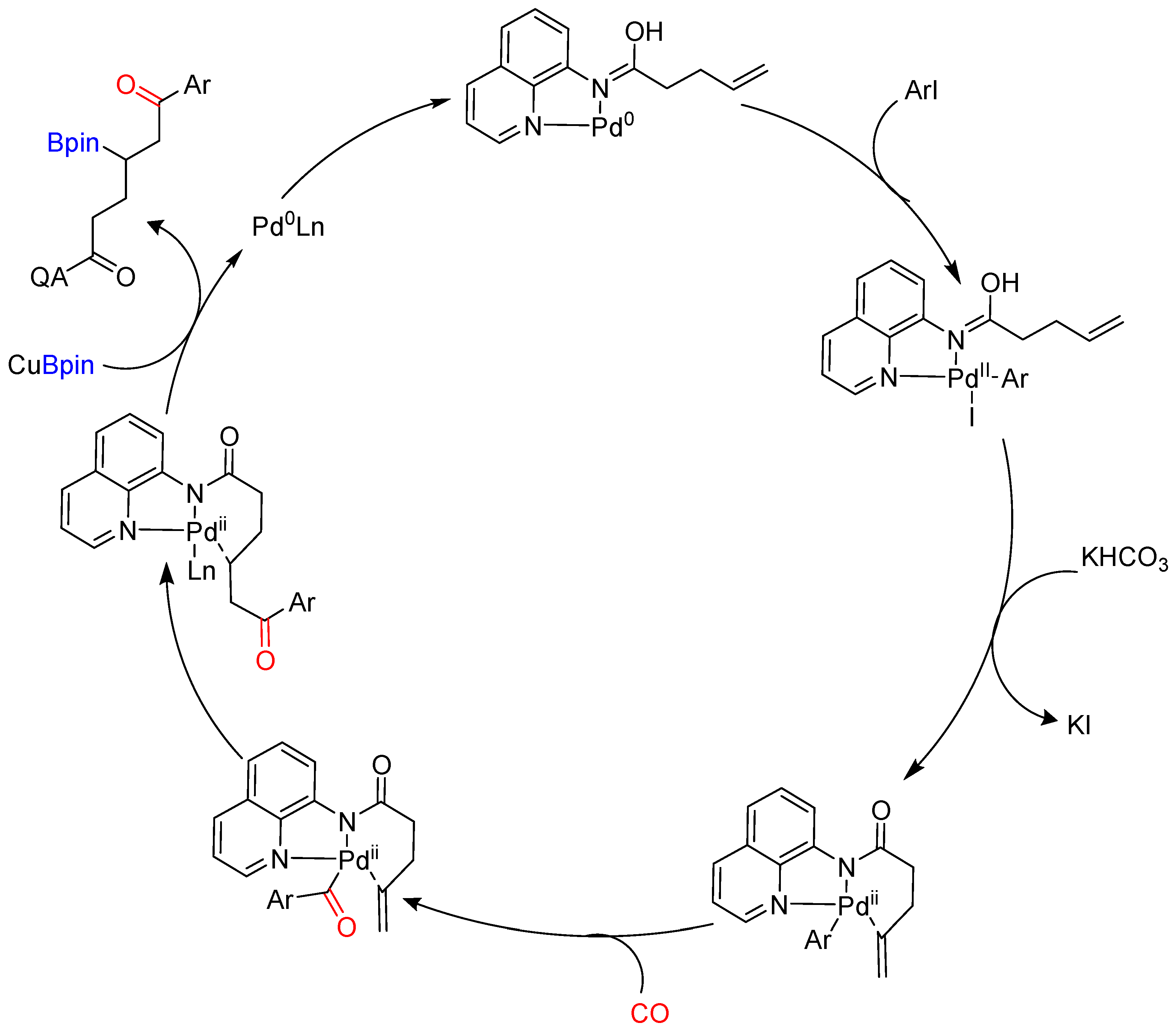

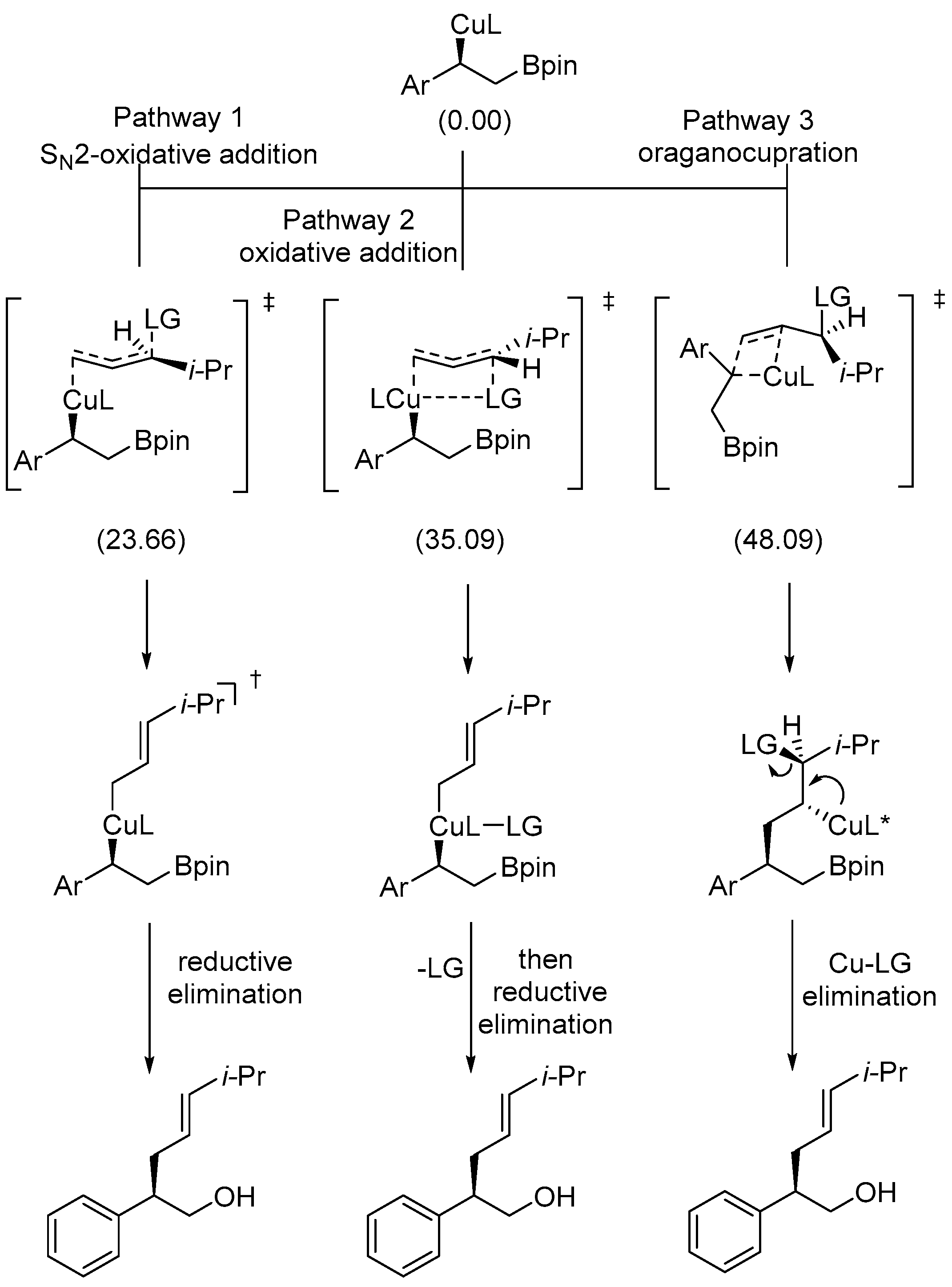
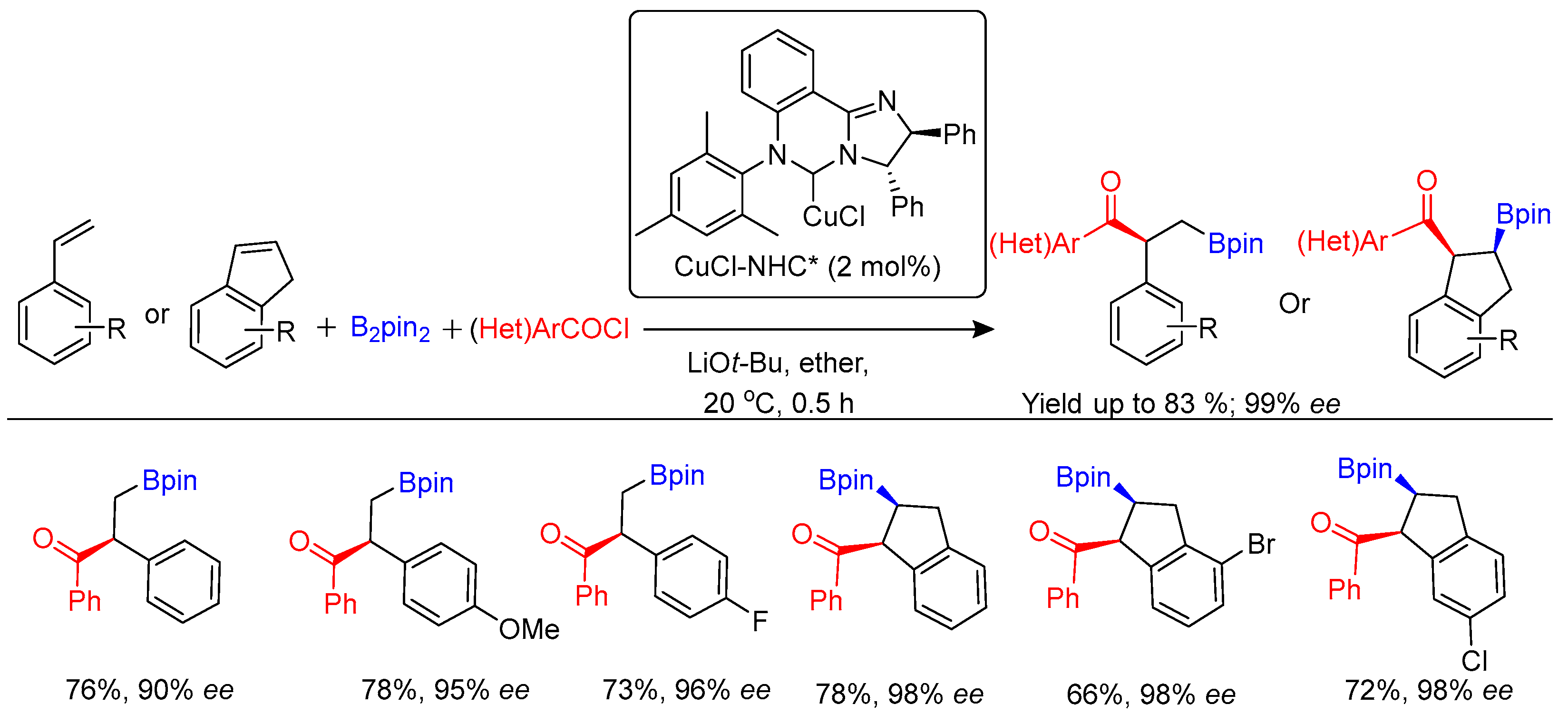


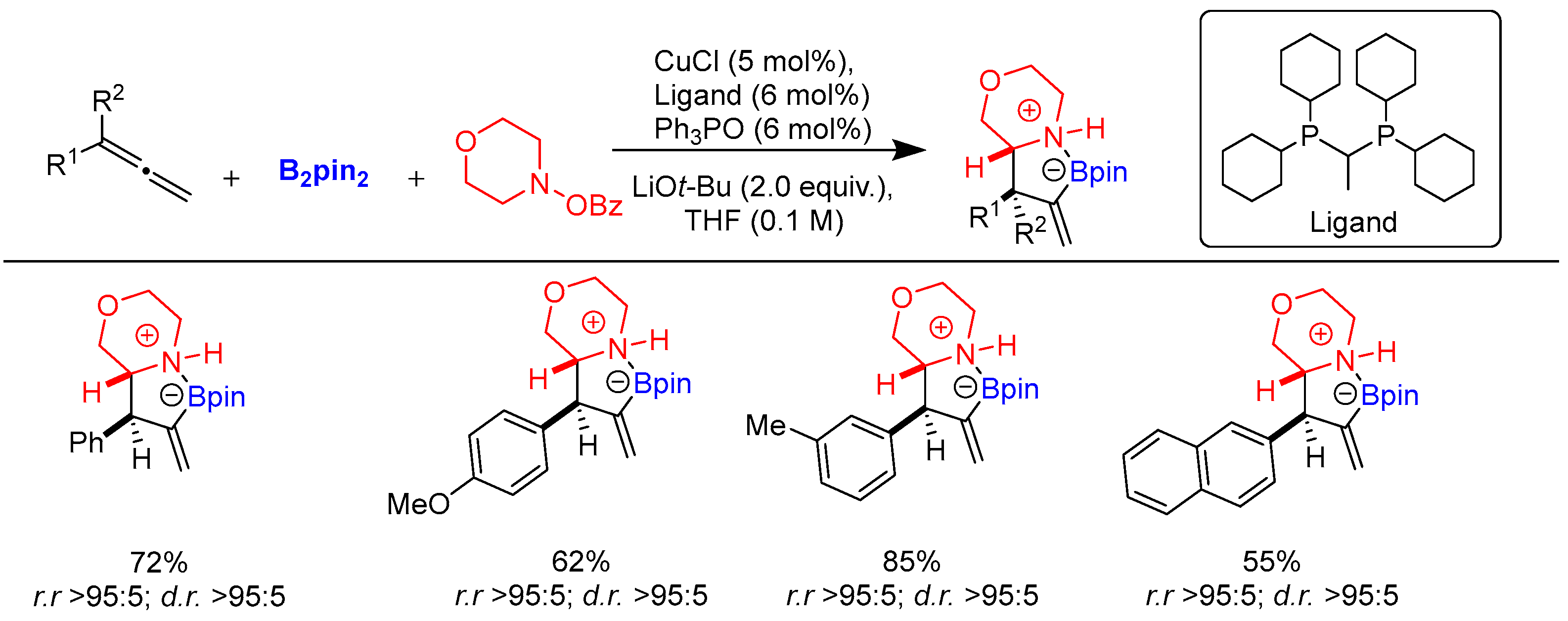
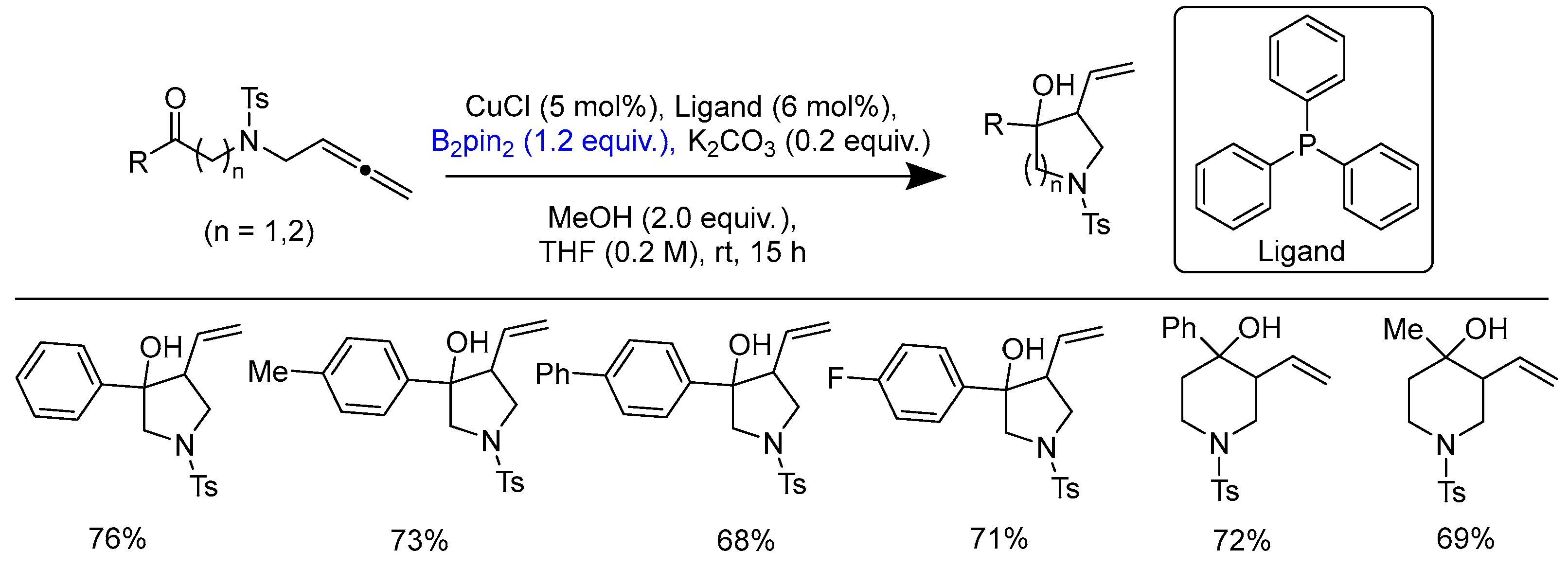

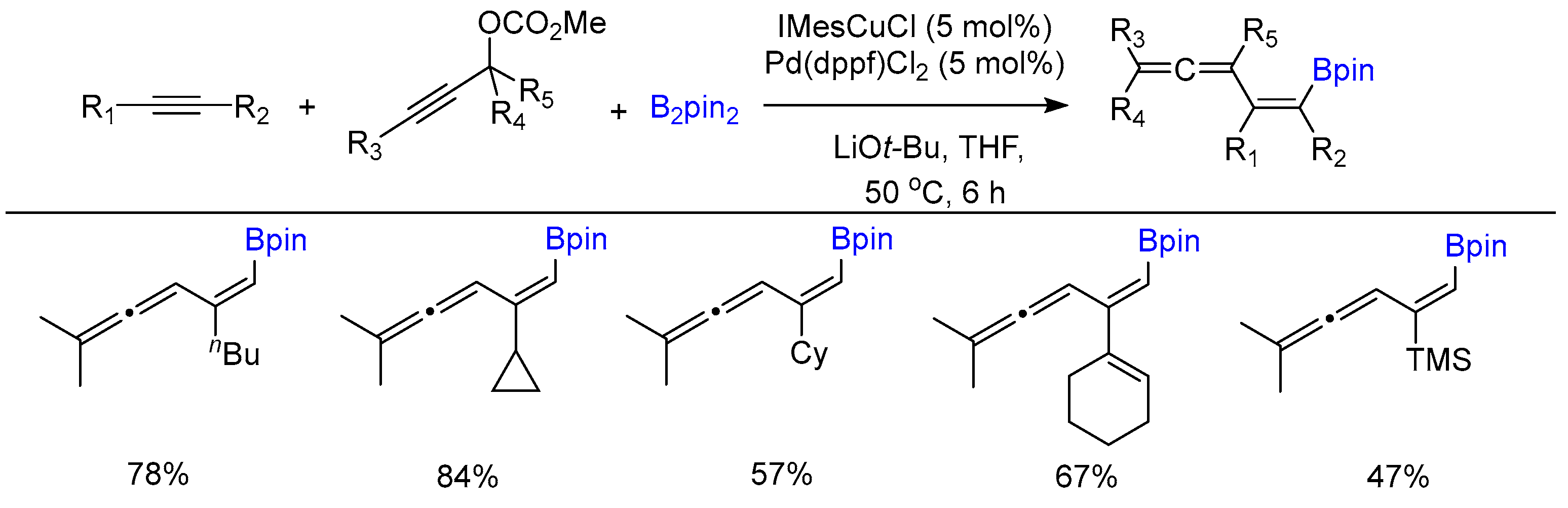
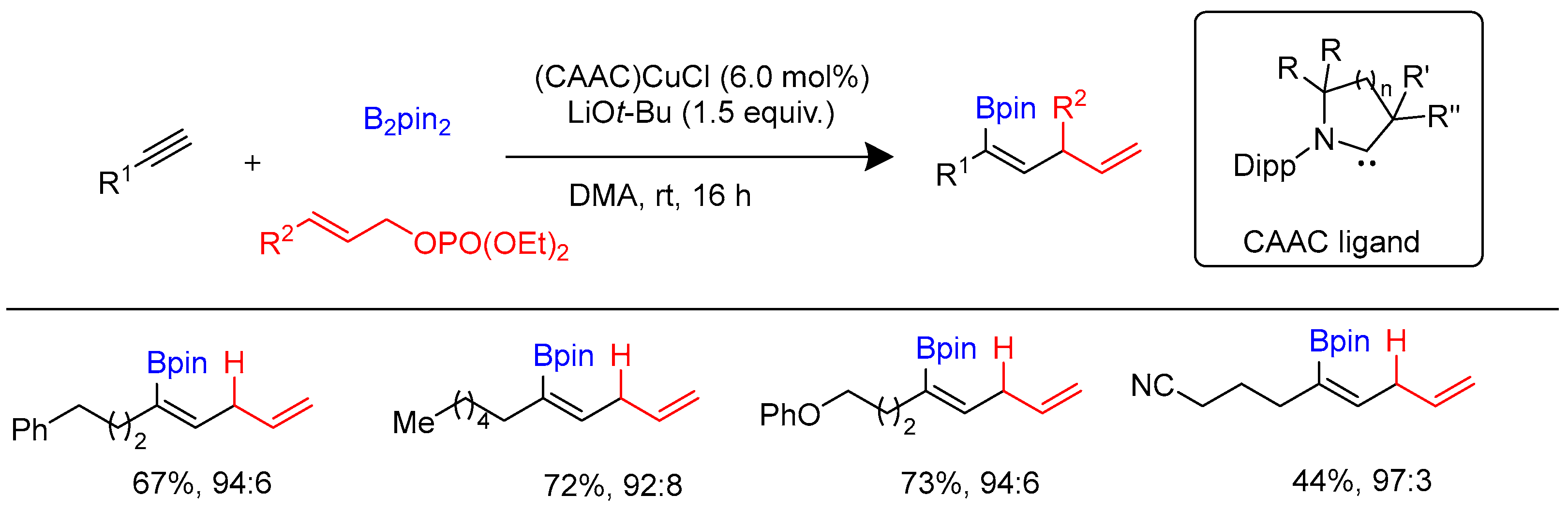
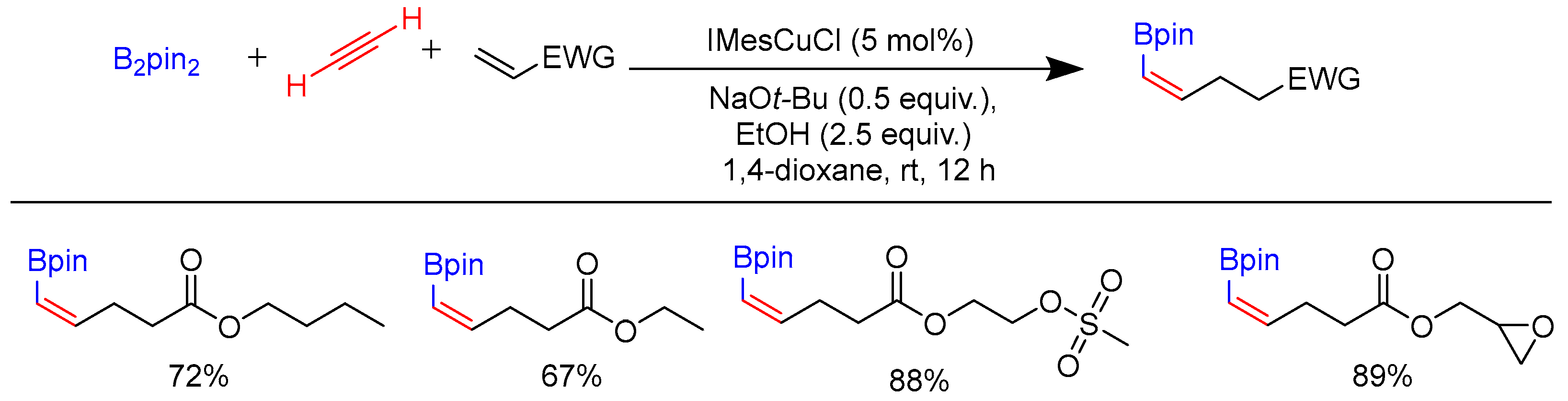


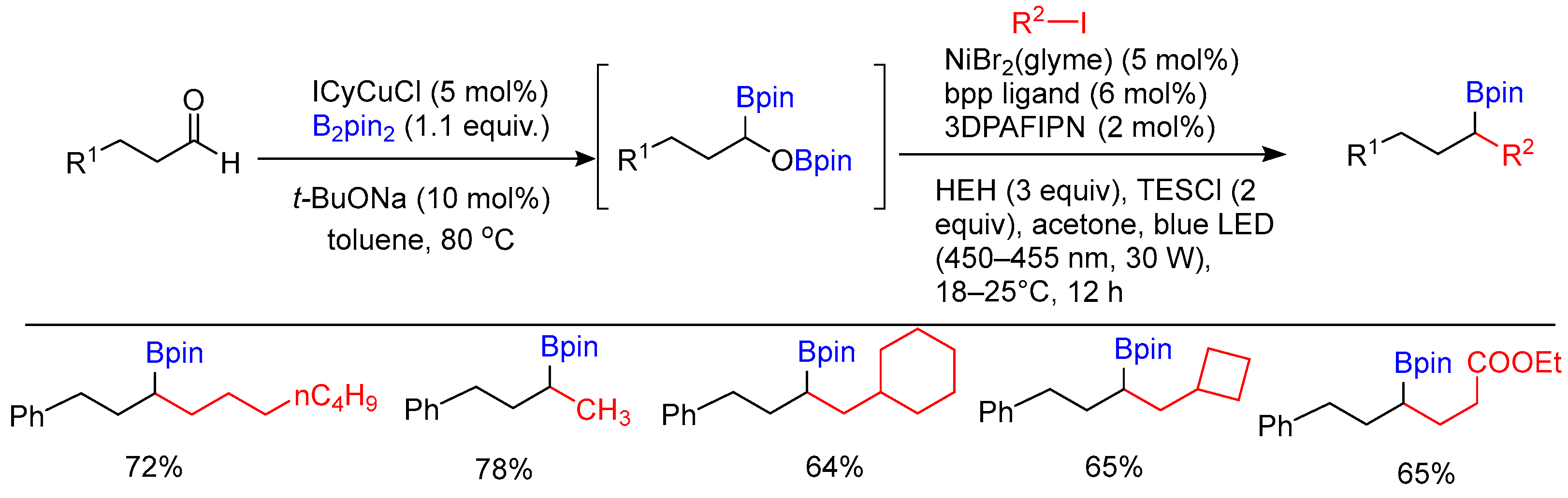
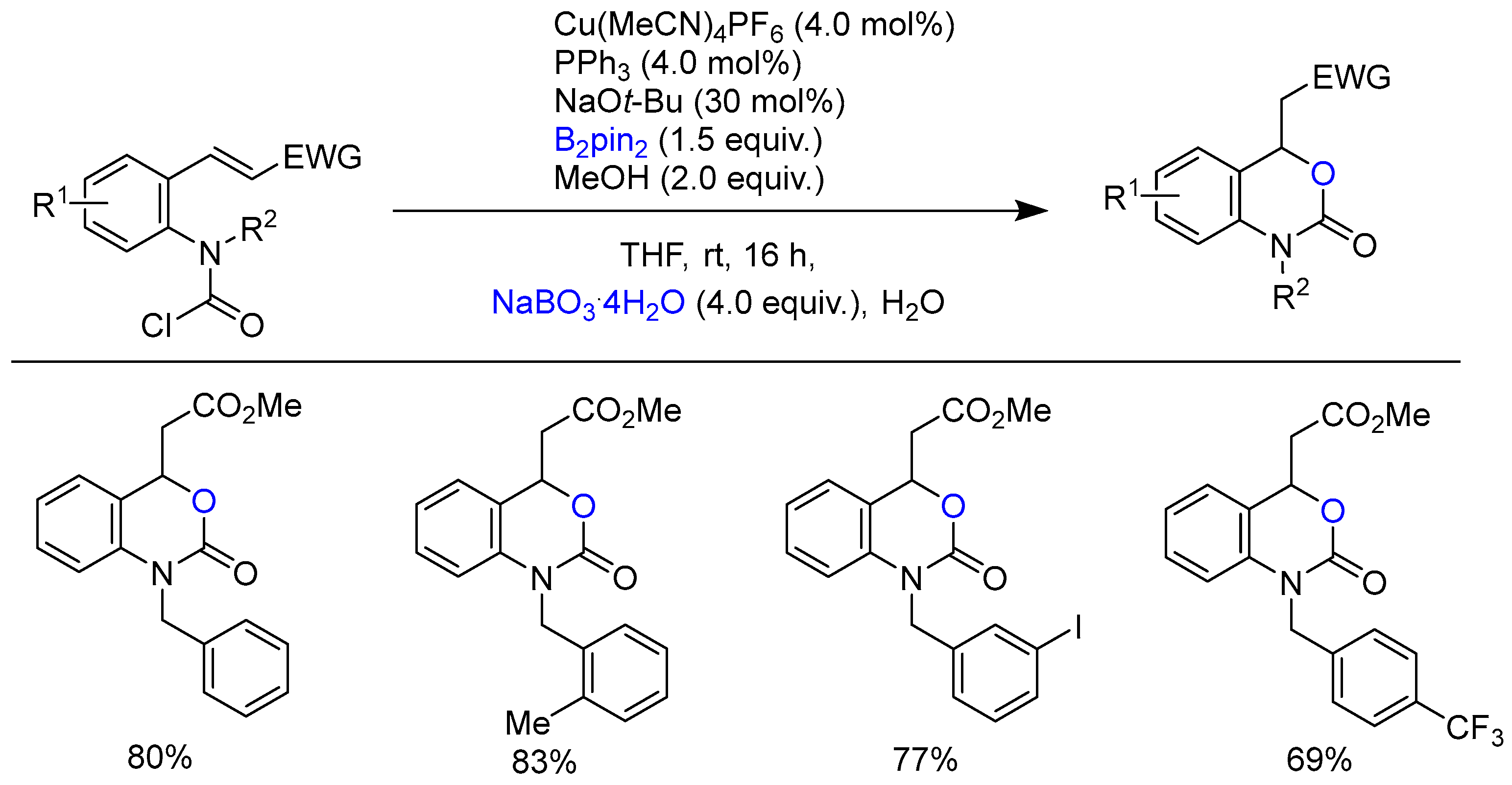
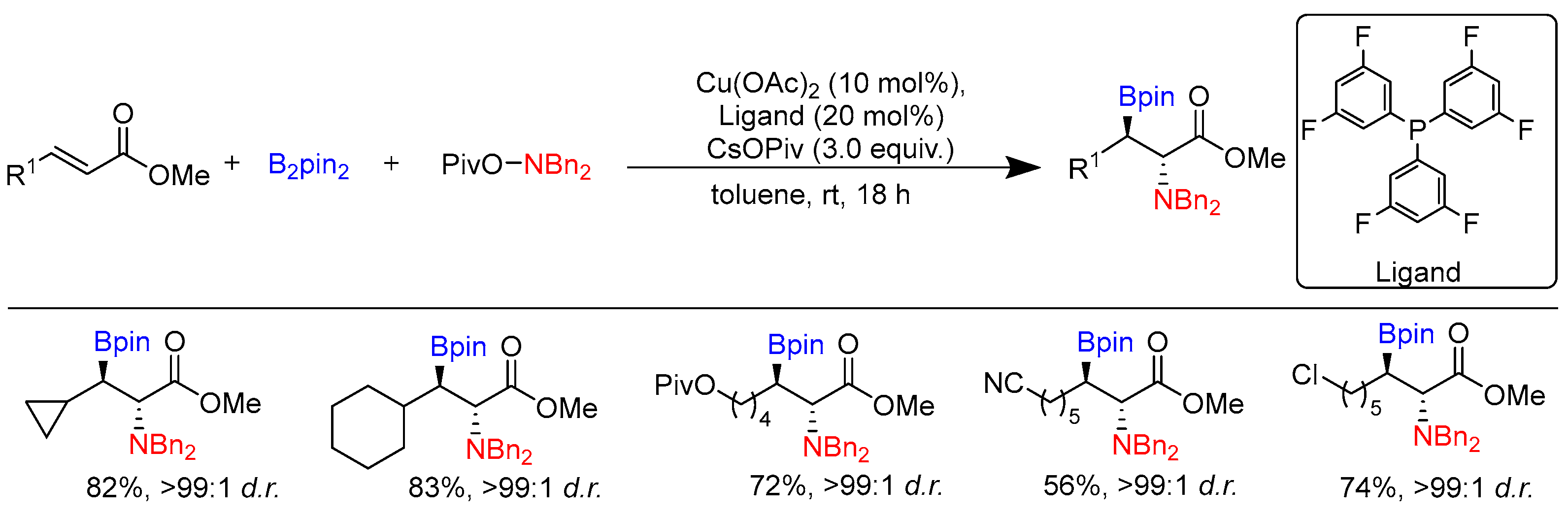
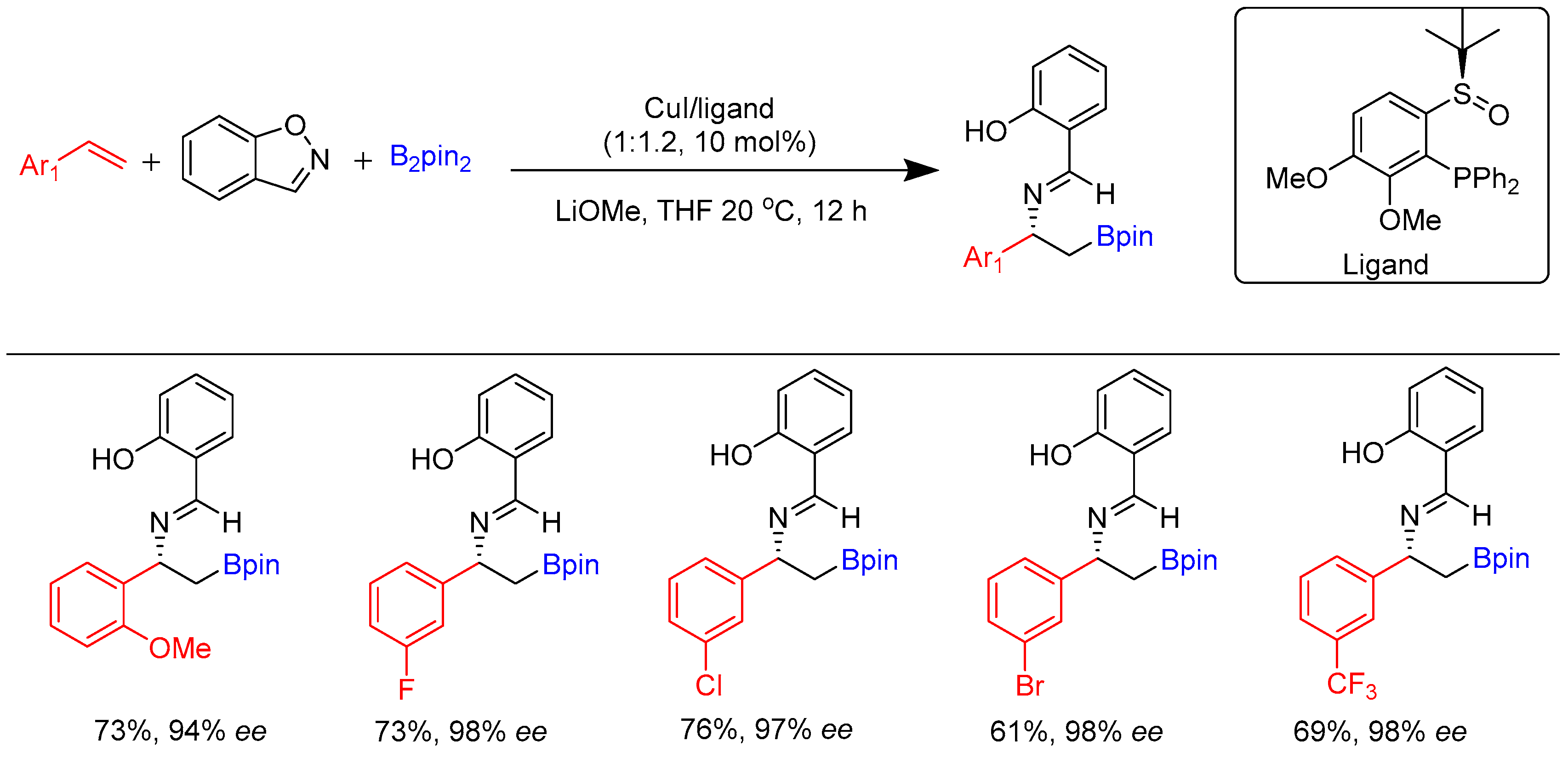
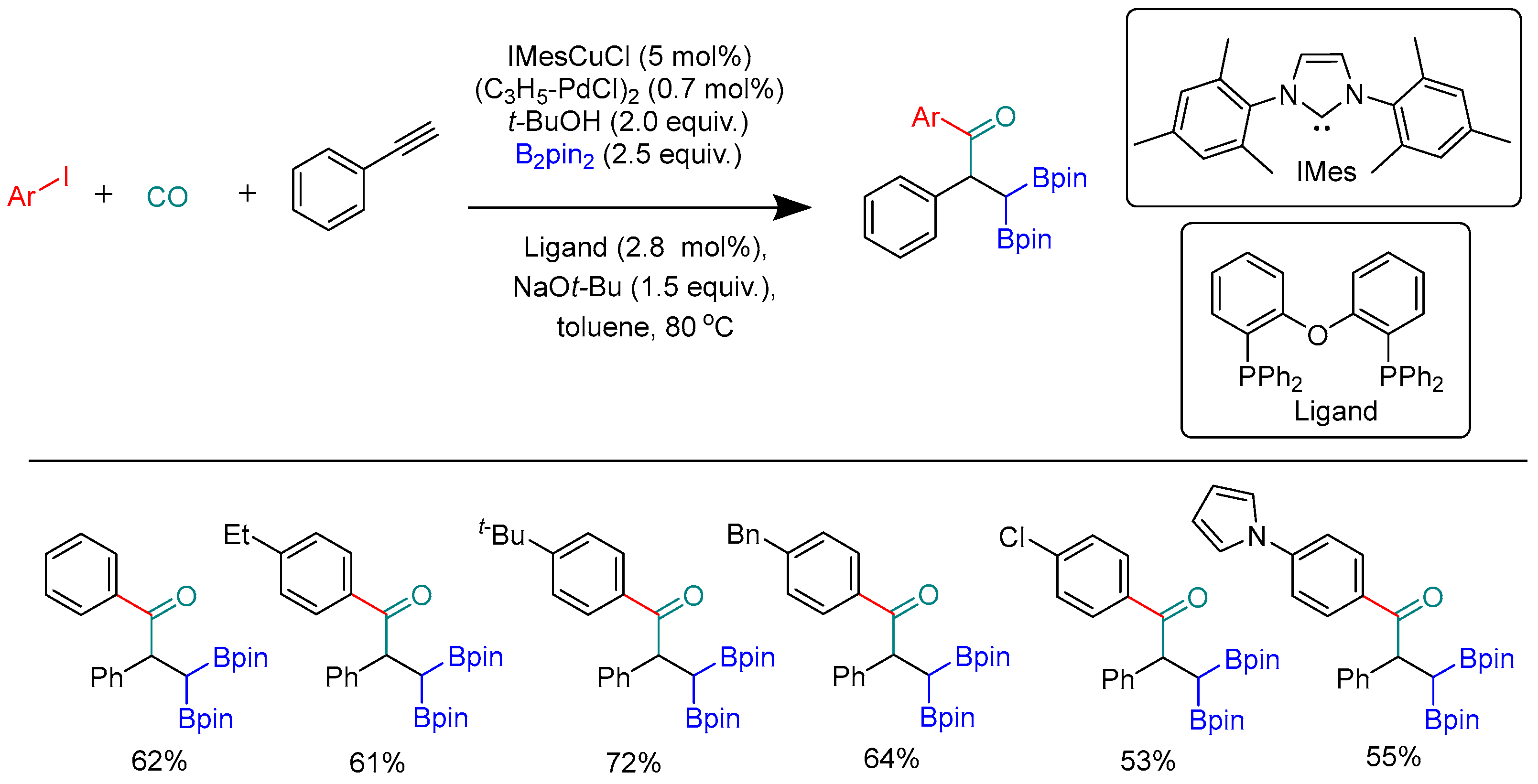

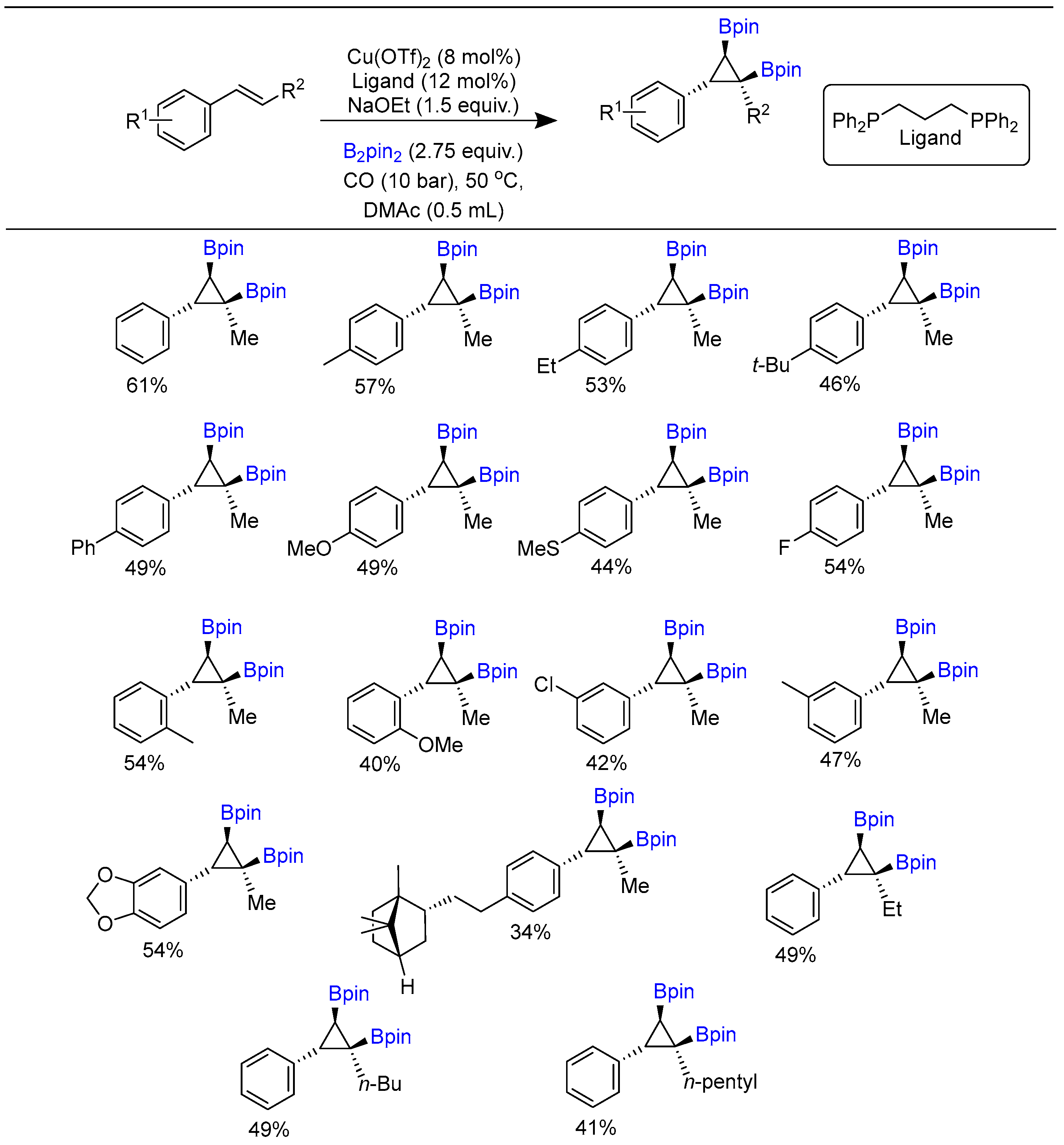
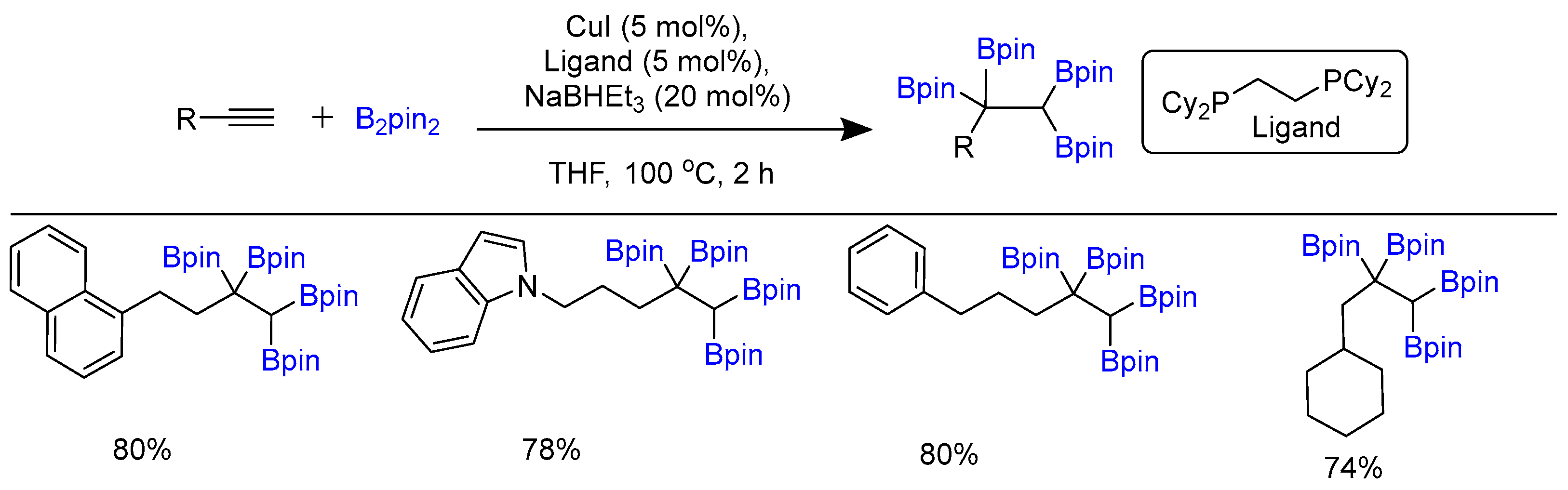

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Liang, H.; Vignesh, A.; Zhou, X.; Liu, Y.; Ke, Z. Updated Progress of the Copper-Catalyzed Borylative Functionalization of Unsaturated Molecules. Molecules 2023, 28, 2252. https://doi.org/10.3390/molecules28052252
Li B, Liang H, Vignesh A, Zhou X, Liu Y, Ke Z. Updated Progress of the Copper-Catalyzed Borylative Functionalization of Unsaturated Molecules. Molecules. 2023; 28(5):2252. https://doi.org/10.3390/molecules28052252
Chicago/Turabian StyleLi, Bingru, Huayu Liang, Arumugam Vignesh, Xiaoyu Zhou, Yan Liu, and Zhuofeng Ke. 2023. "Updated Progress of the Copper-Catalyzed Borylative Functionalization of Unsaturated Molecules" Molecules 28, no. 5: 2252. https://doi.org/10.3390/molecules28052252
APA StyleLi, B., Liang, H., Vignesh, A., Zhou, X., Liu, Y., & Ke, Z. (2023). Updated Progress of the Copper-Catalyzed Borylative Functionalization of Unsaturated Molecules. Molecules, 28(5), 2252. https://doi.org/10.3390/molecules28052252






