Mesoporous Surface-Sulfurized Fe–Co3O4 Nanosheets Integrated with N/S Co-Doped Graphene as a Robust Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions
Abstract
1. Introduction
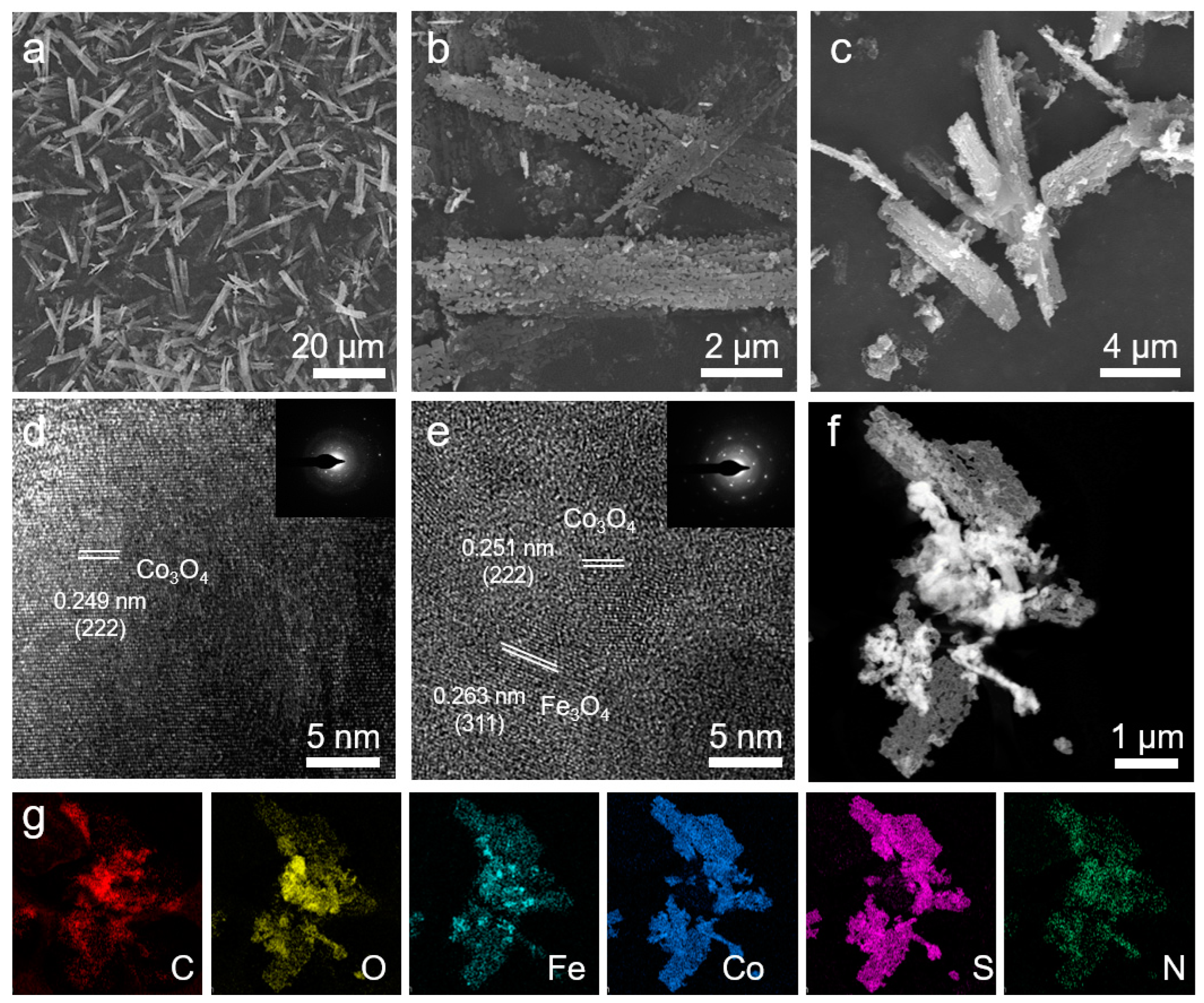
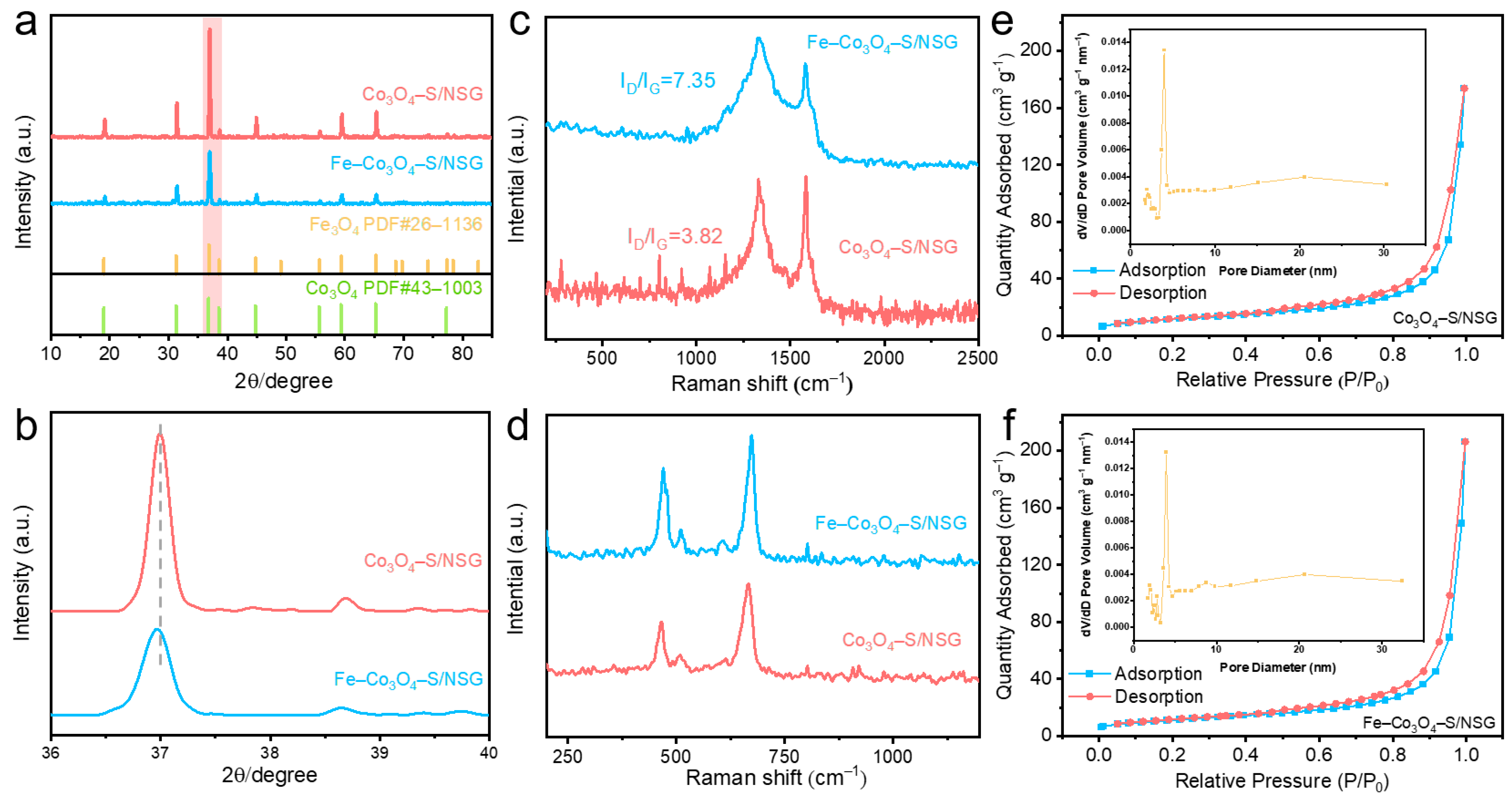
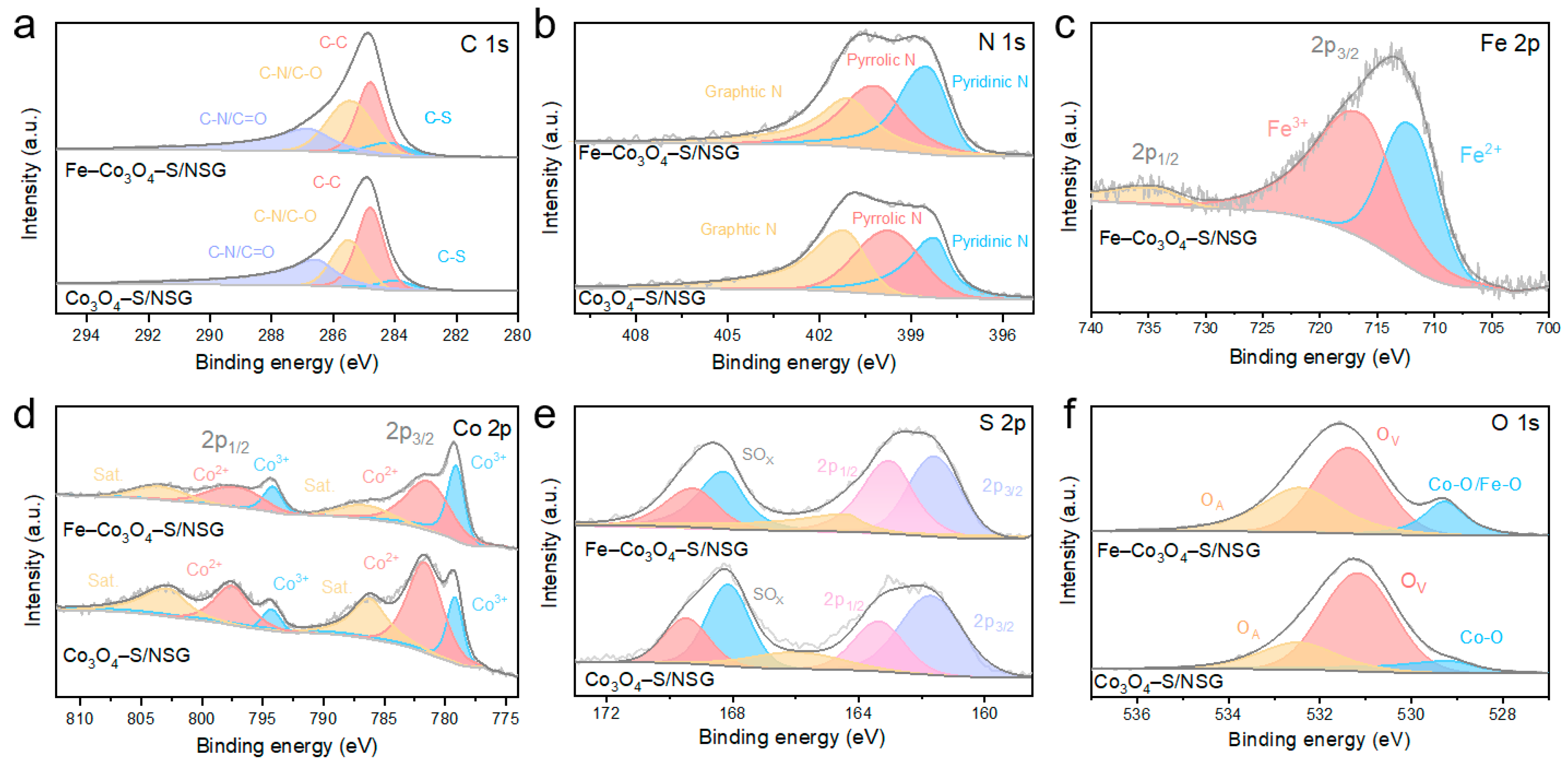
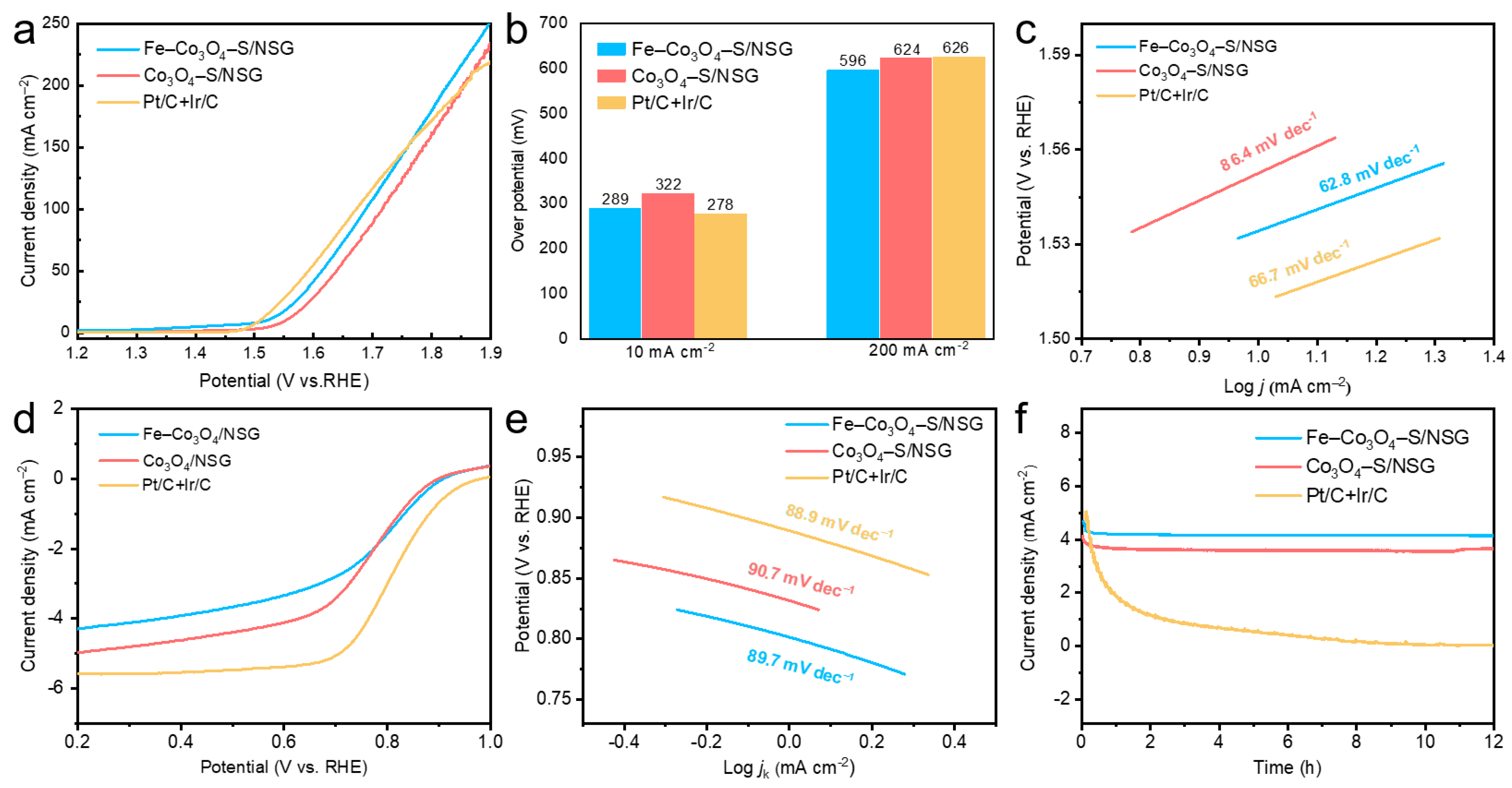
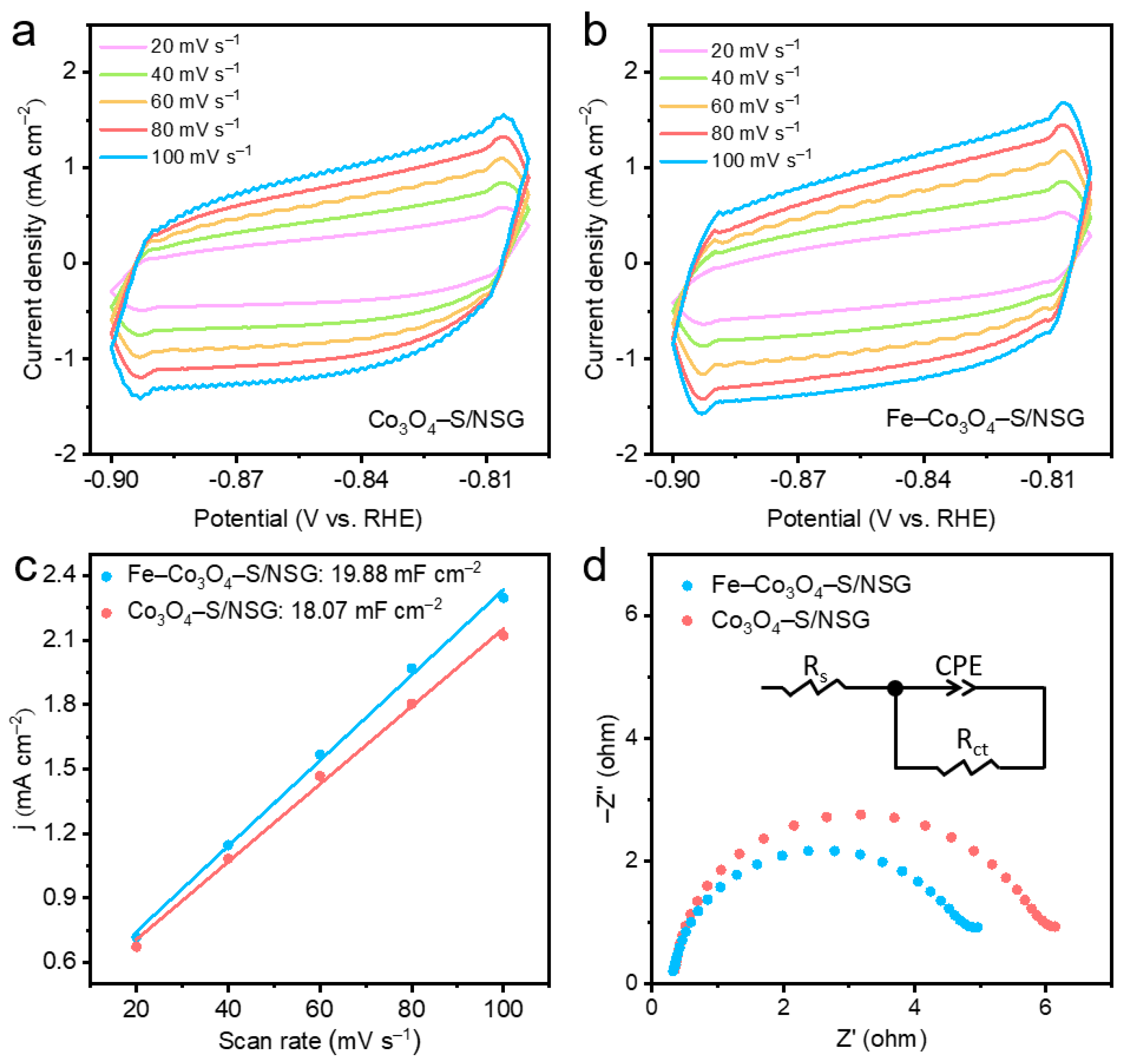
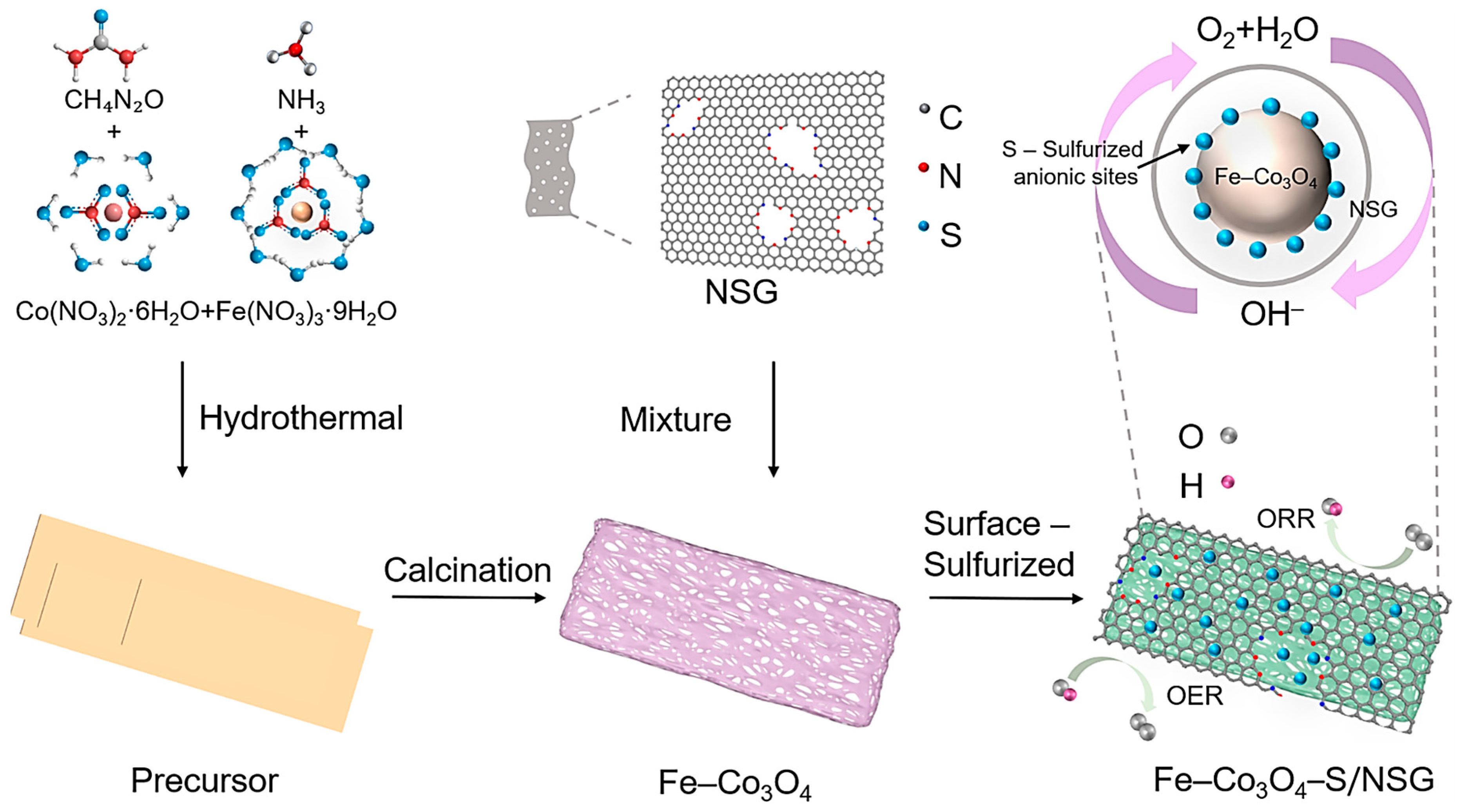
2. Results and Discussion
3. Experimental Section
3.1. Material Preparation
3.2. Material Synthesis
3.2.1. Synthesis of Co3O4 and Fe–Co3O4
3.2.2. Synthesis of NSG
3.2.3. Synthesis of Co3O4–S/NSG and Fe–Co3O4–S/NSG
3.3. Physicochemical Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Z.-Q.; Cheng, H.; Li, N.; Ma, T.Y.; Su, Y.-Z. ZnCo2O4 Quantum Dots Anchored on Nitrogen-Doped Carbon Nanotubes as Reversible Oxygen Reduction/Evolution Electrocatalysts. Adv. Mater. 2016, 28, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.-H.; Li, Y.; Wei, G.-P.; Li, Z.-Y.; Liu, Q.-Y.; Chen, H.; He, S.-G. Mutual functionalization of dinitrogen and methane mediated by heteronuclear metal cluster anions CoTaC2−. Chem. Sci. 2022, 13, 9366–9372. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Amin, H.M.A.; Mostafa, E.; Abd-El-Latif, A.A.; Iqbal, S.; Baltruschat, H. Electrodeposited Cobalt Nanosheets on Smooth Silver as a Bifunctional Catalyst for OER and ORR: In Situ Structural and Catalytic Characterization. ACS Appl. Mater. Interfaces 2022, 14, 55458–55470. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Li, Y. Two-Dimensional Organometallic Frameworks with Pyridinic Single-Metal-Atom Sites for Bifunctional ORR/OER. Adv. Funct. Mater. 2022, 32, 2207110. [Google Scholar] [CrossRef]
- Rao, P.; Wu, D.; Wang, T.-J.; Li, J.; Deng, P.; Chen, Q.; Shen, Y.; Chen, Y.; Tian, X. Single atomic cobalt electrocatalyst for efficient oxygen reduction reaction. eScience 2022, 2, 399–404. [Google Scholar] [CrossRef]
- Wei, X.; Cao, S.; Xu, H.; Jiang, C.; Wang, Z.; Ouyang, Y.; Lu, X.; Dai, F.; Sun, D. Novel Two-Dimensional Metal Organic Frameworks: High-Performance Bifunctional Electrocatalysts for OER/ORR. ACS Mater. Lett. 2022, 4, 1991–1998. [Google Scholar] [CrossRef]
- Nan, H.X.; Su, Y.Q.; Tang, C.; Cao, R.; Li, D.; Yu, J.; Liu, Q.B.; Deng, Y.J.; Tian, X.L. Engineering the electronic and strained interface for high activity of PdMcore@Pt-monolayer electrocatalysts for oxygen reduction reaction. Sci. Bull. 2020, 65, 1396–1404. [Google Scholar] [CrossRef]
- Gong, Y.; Ding, W.; Li, Z.; Su, R.; Zhang, X.; Wang, J.; Zhou, J.; Wang, Z.; Gao, Y.; Li, S.; et al. Inverse Spinel Cobalt–Iron Oxide and N-Doped Graphene Composite as an Efficient and Durable Bifuctional Catalyst for Li–O2 Batteries. ACS Catal. 2018, 8, 4082–4090. [Google Scholar] [CrossRef]
- Liu, W.; Rao, D.; Bao, J.; Xu, L.; Lei, Y.; Li, H. Strong coupled spinel oxide with N-rGO for high-efficiency ORR/OER bifunctional electrocatalyst of Zn-air batteries. J. Energy Chem. 2021, 57, 428–435. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.L.; Kong, L.; Chen, S.M.; Zhang, S.J. Neuron-Mimic Smart Electrode: A Two Dimensional Multiscale Synergistic Strategy for Densely Packed and High-Rate Lithium Storage. ACS Nano 2019, 13, 9148–9160. [Google Scholar] [CrossRef] [PubMed]
- Kirsanova, M.A.; Okatenko, V.D.; Aksyonov, D.A.; Forslund, R.P.; Mefford, J.T.; Stevenson, K.J.; Abakumov, A.M. Bifunctional OER/ORR catalytic activity in the tetrahedral YBaCo4O7.3 oxide. J. Mater. Chem. A 2019, 7, 330–341. [Google Scholar] [CrossRef]
- Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M.T.; Croft, M.; Gopalakrishnan, J.; Peña, M.A.; Hadermann, J.; Greenblatt, M.; et al. La1.5Sr0.5NiMn0.5Ru0.5O6 Double Perovskite with Enhanced ORR/OER Bifunctional Catalytic Activity. ACS Appl. Mater. Interfaces 2019, 11, 21454–21464. [Google Scholar] [CrossRef] [PubMed]
- Tran-Phu, T.; Daiyan, R.; Leverett, J.; Fusco, Z.; Tadich, A.; Di Bernardo, I.; Kiy, A.; Truong, T.N.; Zhang, Q.; Chen, H.; et al. Understanding the activity and stability of flame-made Co3O4 spinels: A route towards the scalable production of highly performing OER electrocatalysts. Chem. Eng. J. 2022, 429, 132180. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, Y.-H.; Gao, X.-T.; Guo, B.; Ma, J.-L.; Zhang, Y.; Bai, F.-Y.; Dong, Y.-W.; Zhao, Z. Prussian-blue-analog derived hollow Co3O4/NiO decorated CeO2 nanoparticles for boosting oxygen evolution reaction. J. Alloys Compd. 2022, 914, 165344. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Liu, G.; Zhang, P.; Zhu, W.; Xia, J.; Li, H. Biomass willow catkin-derived Co3O4/N-doped hollow hierarchical porous carbon microtubes as an effective tri-functional electrocatalyst. J. Mater. Chem. A 2017, 5, 20170–20179. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Zang, W.; Li, X.; Chen, D.; Kou, Z.; Mu, S.; Wang, J. Nanoframes of Co3O4–Mo2N Heterointerfaces Enable High-Performance Bifunctionality toward Both Electrocatalytic HER and OER. Adv. Funct. Mater. 2022, 32, 2107382. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Pan, F.; Zhu, Z.; Sun, H.; Tang, Y.; Fu, G. Plasma-induced Mo-doped Co3O4 with enriched oxygen vacancies for electrocatalytic oxygen evolution in water splitting. Carbon Energy, 2022; in press. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Wu, D.; Yang, J. Optimizing the electronic structure of cobalt via synergized oxygen vacancy and Co-N-C to boost reversible oxygen electrocatalysis for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2020, 278, 119300. [Google Scholar] [CrossRef]
- Lu, X.F.; Zhang, S.L.; Shangguan, E.; Zhang, P.; Gao, S.; Lou, X.W. Nitrogen-Doped Cobalt Pyrite Yolk–Shell Hollow Spheres for Long-Life Rechargeable Zn–Air Batteries. Adv. Sci. 2020, 7, 2001178. [Google Scholar] [CrossRef]
- Hao, J.; Yang, W.; Peng, Z.; Zhang, C.; Huang, Z.; Shi, W. A Nitrogen Doping Method for CoS2 Electrocatalysts with Enhanced Water Oxidation Performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Ren, J.-T.; Ying, Y.-D.; Liu, Y.-P.; Li, W.; Yuan, Z.-Y. Charge redistribution caused by sulfur doping of bimetal FeCo phosphides supported on heteroatoms-doped graphene for Zn-air batteries with stable cycling. J. Energy Chem. 2022, 71, 619–630. [Google Scholar] [CrossRef]
- Gu, J.; Magagula, S.; Zhao, J.; Chen, Z. Boosting ORR/OER Activity of Graphdiyne by Simple Heteroatom Doping. Small Methods 2019, 3, 1800550. [Google Scholar] [CrossRef]
- Zhu, J.; Tu, W.; Pan, H.; Zhang, H.; Liu, B.; Cheng, Y.; Deng, Z.; Zhang, H. Self-Templating Synthesis of Hollow Co3O4 Nanoparticles Embedded in N,S-Dual-Doped Reduced Graphene Oxide for Lithium Ion Batteries. ACS Nano 2020, 14, 5780–5787. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Wu, D.; Liu, X.; Cao, L.; Zhang, W.; Chen, H.; Liu, T.; Liu, D.; Chen, T.; et al. The in situ formation of defective CoOOH catalysts from semi-oxidized Co for alkaline oxygen evolution reaction. J. Mater. Chem. A 2022, 10, 20011–20017. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, S.; Zhu, C.; Shi, J.; Su, C.; Yang, J.; Wang, M.; Li, J.; Li, J.; Liu, P.; et al. Rational construction of high-active Co3O4 electrocatalysts for oxygen evolution reaction. Nano Res. 2023, 16, 624–633. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Liu, Q.; You, W.; Che, R. Interface compatibility engineering of Multi-shell Fe@C@TiO2@MoS2 heterojunction expanded microwave absorption bandwidth. Chem. Eng. J. 2022, 429, 132191. [Google Scholar] [CrossRef]
- Lu, L.; Hao, Q.; Lei, W.; Xia, X.; Liu, P.; Sun, D.; Wang, X.; Yang, X. Well-Combined Magnetically Separable Hybrid Cobalt Ferrite/Nitrogen-Doped Graphene as Efficient Catalyst with Superior Performance for Oxygen Reduction Reaction. Small 2015, 11, 5833–5843. [Google Scholar] [CrossRef]
- Pampel, J.; Fellinger, T.-P. Opening of Bottleneck Pores for the Improvement of Nitrogen Doped Carbon Electrocatalysts. Adv. Energy Mater. 2016, 6, 1502389. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Lu, W.-T.; Wang, C.-Y.; Li, Y.-K.; Ding, C.; Gu, J.; Zheng, X.-S.; Cao, F.-F. Co Nanoparticles/Co, N, S Tri-doped Graphene Templated from In-Situ-Formed Co, S Co-doped g-C3N4 as an Active Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 28566–28576. [Google Scholar] [CrossRef]
- Li, Y.-W.; Zhang, W.-J.; Li, J.; Ma, H.-Y.; Du, H.-M.; Li, D.-C.; Wang, S.-N.; Zhao, J.-S.; Dou, J.-M.; Xu, L. Fe-MOF-Derived Efficient ORR/OER Bifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 44710–44719. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Zhang, B.; Zhang, Y.; Ma, X.Y.D.; Yan, T.; Liu, J.; Che, B.; Huang, Y.; Lu, X. FeCo/FeCoNi/N-doped carbon nanotubes grafted polyhedron-derived hybrid fibers as bifunctional oxygen electrocatalysts for durable rechargeable zinc–air battery. Appl. Catal. B Environ. 2019, 254, 26–36. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Lu, Q.; Chen, B.; Chen, J.; Huang, Y.; Ma, Q.; Tan, C.; Yang, J.; Cao, X.; et al. Self-Assembly of Single-Layer CoAl-Layered Double Hydroxide Nanosheets on 3D Graphene Network Used as Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 7640–7645. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, J.; Wu, Q.; Li, Q.; Wang, H.-g.; Li, Y.; Duan, Q. In Situ Coupling Strategy for Anchoring Monodisperse Co9S8 Nanoparticles on S and N Dual-Doped Graphene as a Bifunctional Electrocatalyst for Rechargeable Zn–Air Battery. Nano-Micro Lett. 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, L.; Lian, Y.; Yang, W.; Lin, L.; Chen, Y.; Xu, J.; Wang, D.; Yang, X.; Rümmerli, M.H.; et al. Topotactically Transformed Polygonal Mesopores on Ternary Layered Double Hydroxides Exposing Under-Coordinated Metal Centers for Accelerated Water Dissociation. Adv. Mater. 2020, 32, 2006784. [Google Scholar] [CrossRef]
- Cai, Z.; Bi, Y.; Hu, E.; Liu, W.; Dwarica, N.; Tian, Y.; Li, X.; Kuang, Y.; Li, Y.; Yang, X.-Q.; et al. Single-Crystalline Ultrathin Co3O4 Nanosheets with Massive Vacancy Defects for Enhanced Electrocatalysis. Adv. Energy Mater. 2018, 8, 1701694. [Google Scholar] [CrossRef]
- Kim, K.; Min, K.; Go, Y.; Lee, Y.; Shim, S.E.; Lim, D.; Baeck, S.-H. FeCo alloy nanoparticles embedded in N-doped carbon supported on highly defective ketjenblack as effective bifunctional electrocatalysts for rechargeable Zn–air batteries. Appl. Catal. B Environ. 2022, 315, 121501. [Google Scholar] [CrossRef]
- Ma, Y.; Gan, L.; Li, D.; Gao, Y.; Yang, X.; Wang, K.; Lu, S.; Wu, H.; Ding, S.; Xiao, C. Rational modulation of N, P co-doped carbon nanotubes encapsulating Co3Fe7 alloy as bifunctional oxygen electrocatalysts for Zinc–Air batteries. J. Power Sources 2019, 441, 227177. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Carbon-Based Nanocages: A New Platform for Advanced Energy Storage and Conversion. Adv. Mater. 2020, 32, 1904177. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Chen, J.; Pang, Q.; Shen, P. Spinel NiCo2O4 3-D nanoflowers supported on graphene nanosheets as efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 16120–16131. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Q.; Liu, M.; Zheng, L.; Tong, F.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; et al. Surface Fluorination Engineering of NiFe Prussian Blue Analogue Derivatives for Highly Efficient Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 5142–5152. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chen, J.; Chen, K.; Wen, Z.; Huang, A. Core–Shell Carbon-Based Bifunctional Electrocatalysts Derived from COF@MOF Hybrid for Advanced Rechargeable Zn–Air Batteries. Small 2022, 18, 2202018. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Li, J.; Wang, K.; Li, Z.; Zhu, M.; Xu, Z. Structural Buffer Engineering on Metal Oxide for Long-Term Stable Seawater Splitting. Adv. Funct. Mater. 2022, 32, 2201127. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Dao, V.-D.; Mohamed, I.M.A. Electrochemical Impedance Investigation of Dye-Sensitized Solar Cells Based on Electrospun TiO2 Nanofibers Photoanodes. Materials 2022, 15, 6175. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.M.A.; Kanagaraj, P.; Yasin, A.S.; Iqbal, W.; Liu, C. Electrochemical impedance investigation of urea oxidation in alkaline media based on electrospun nanofibers towards the technology of direct-urea fuel cells. J. Alloys Compd. 2020, 816, 152513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Wang, Y.; Liu, W.; Fan, C.; Nan, H.; Wang, J.; Yu, J. Mesoporous Surface-Sulfurized Fe–Co3O4 Nanosheets Integrated with N/S Co-Doped Graphene as a Robust Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions. Molecules 2023, 28, 2221. https://doi.org/10.3390/molecules28052221
Meng L, Wang Y, Liu W, Fan C, Nan H, Wang J, Yu J. Mesoporous Surface-Sulfurized Fe–Co3O4 Nanosheets Integrated with N/S Co-Doped Graphene as a Robust Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions. Molecules. 2023; 28(5):2221. https://doi.org/10.3390/molecules28052221
Chicago/Turabian StyleMeng, Lingxue, Yige Wang, Wenwei Liu, Chunlei Fan, Haoxiong Nan, Jiang Wang, and Jia Yu. 2023. "Mesoporous Surface-Sulfurized Fe–Co3O4 Nanosheets Integrated with N/S Co-Doped Graphene as a Robust Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions" Molecules 28, no. 5: 2221. https://doi.org/10.3390/molecules28052221
APA StyleMeng, L., Wang, Y., Liu, W., Fan, C., Nan, H., Wang, J., & Yu, J. (2023). Mesoporous Surface-Sulfurized Fe–Co3O4 Nanosheets Integrated with N/S Co-Doped Graphene as a Robust Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions. Molecules, 28(5), 2221. https://doi.org/10.3390/molecules28052221





