Tunable Donor–Acceptor Linear Conjugated Polymers Involving Cyanostyrylthiophene Linkages for Visible-Light-Driven Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
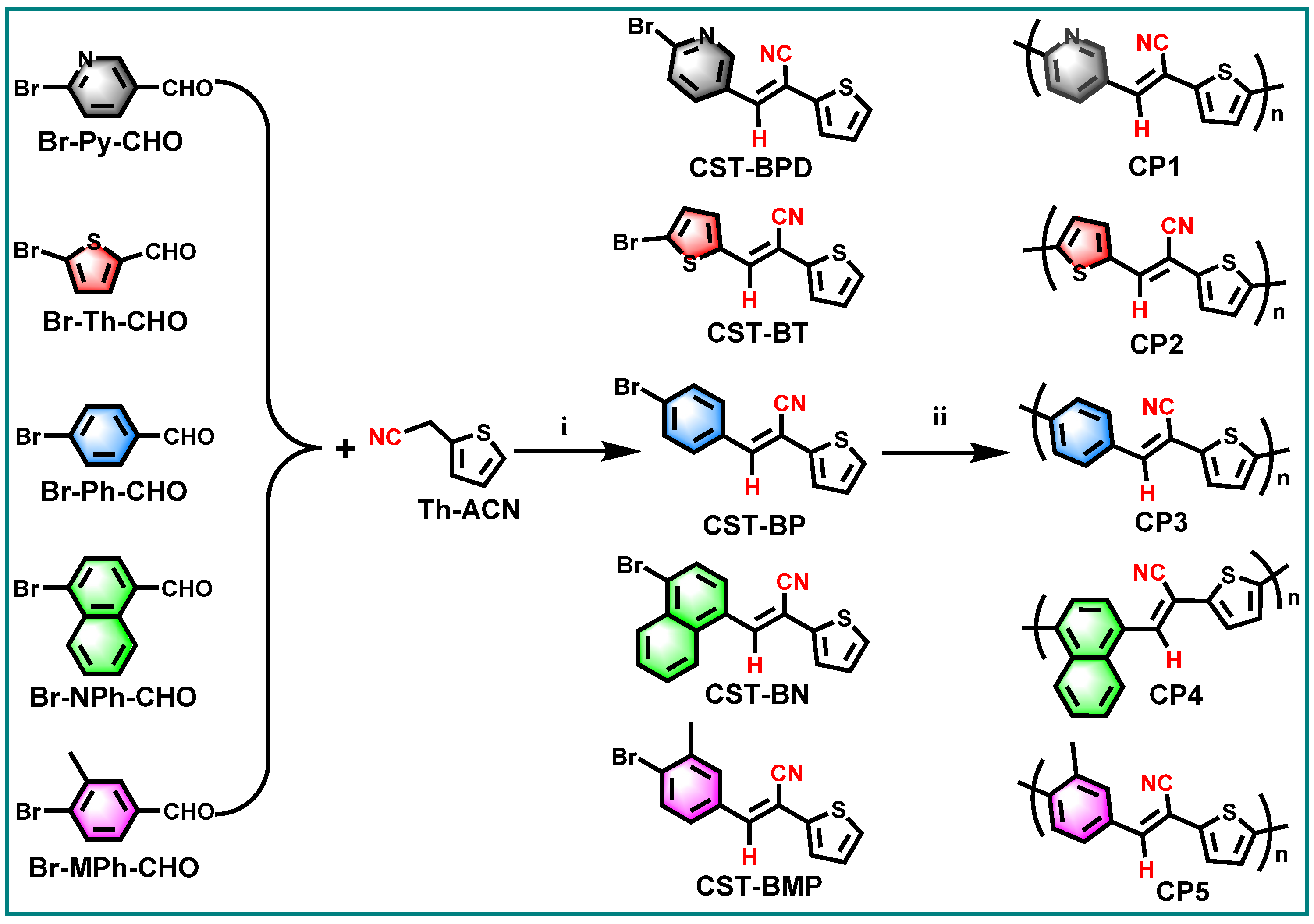
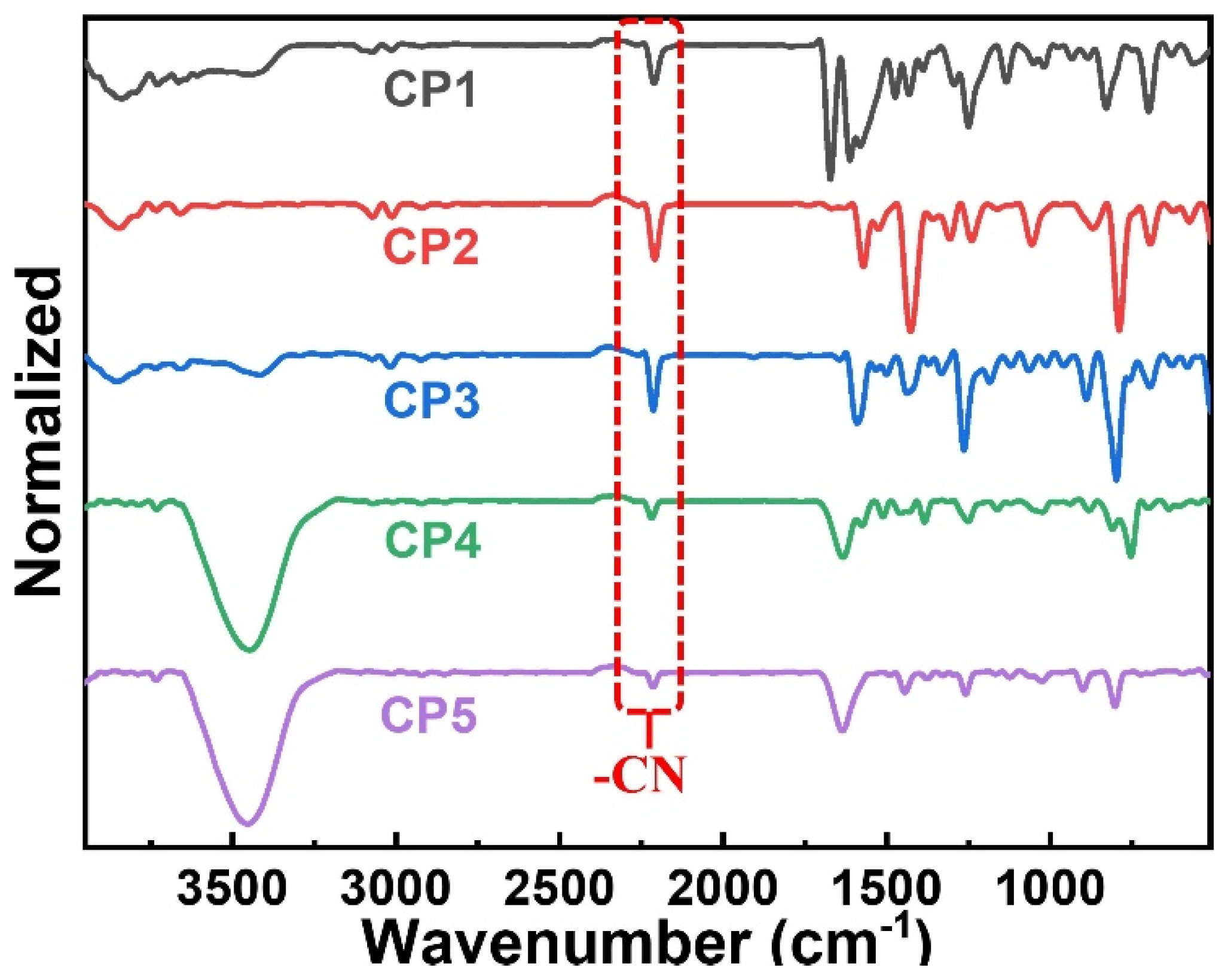
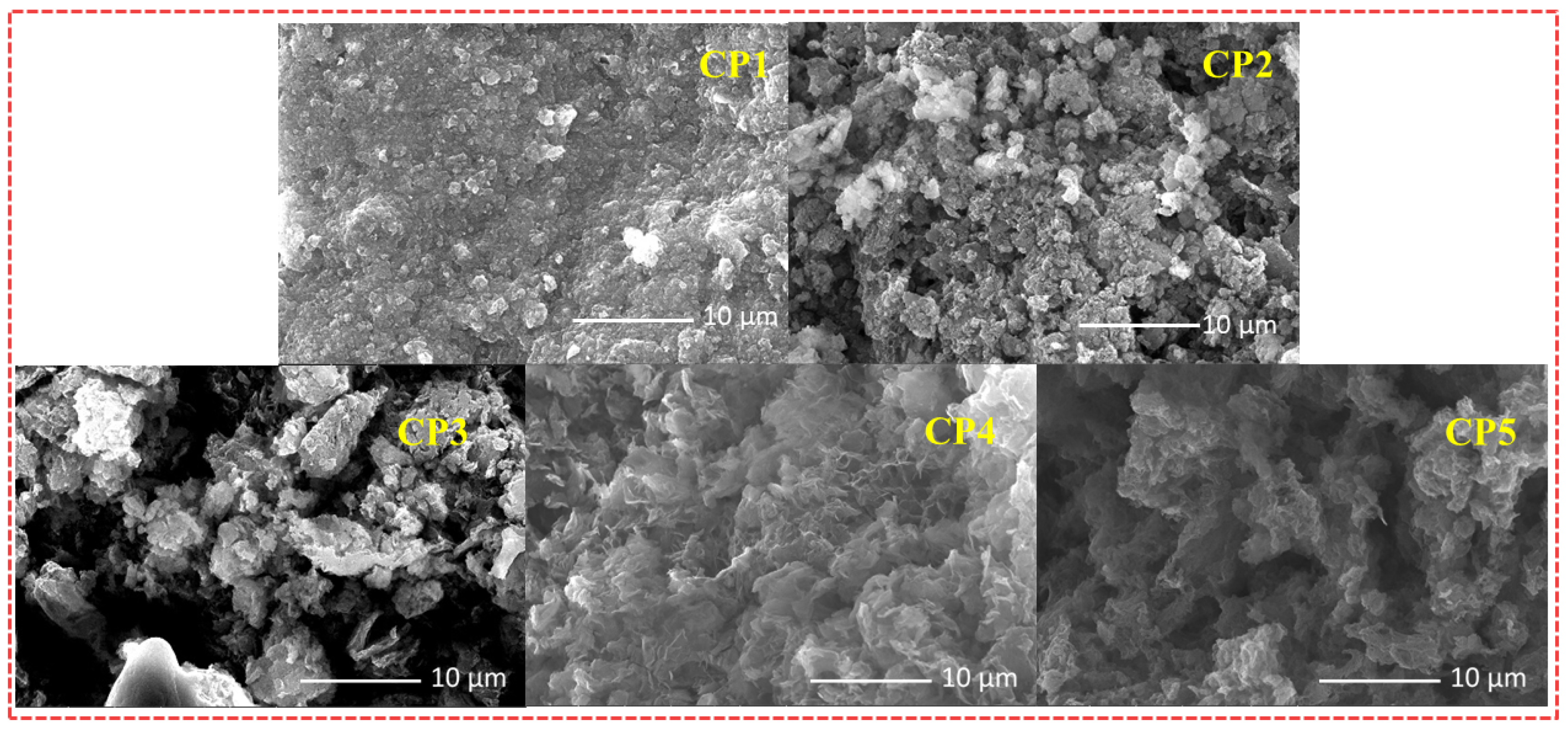
2.1. Synthesis and Characterization of the CPs
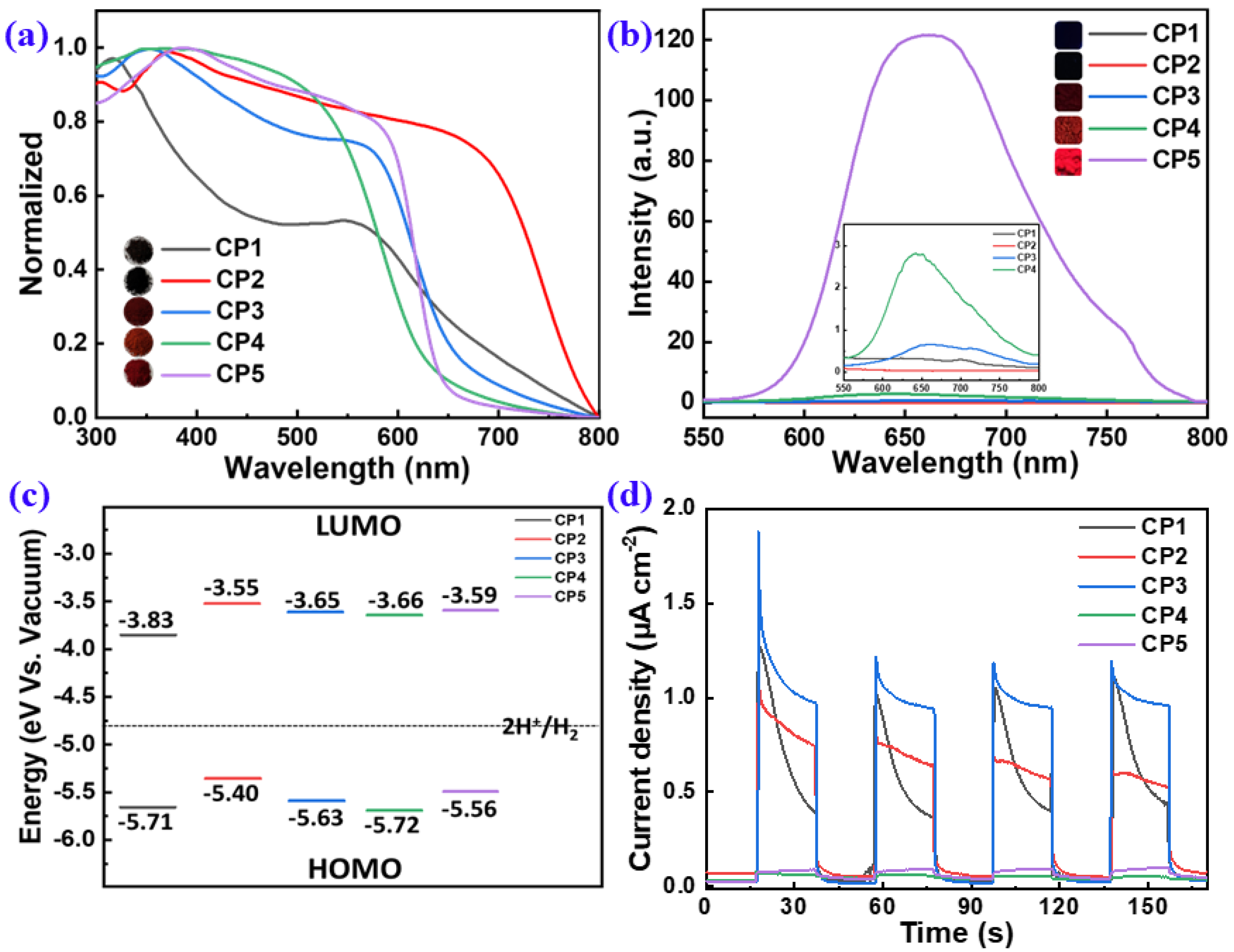
2.2. Opto-Electronic Properties of the CPs
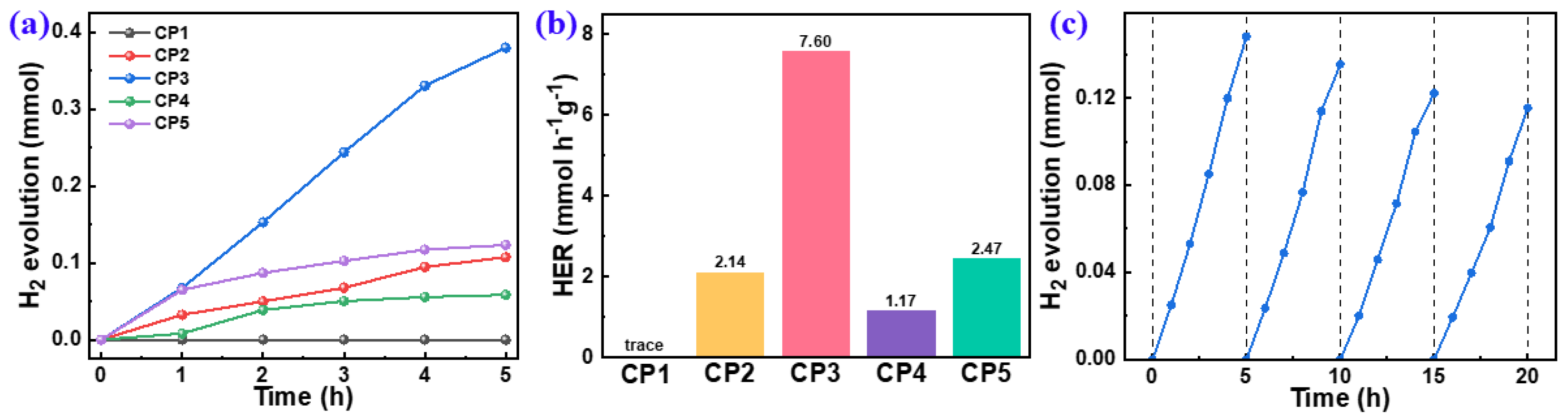
2.3. Photocatalytic Hydrogen Production Performances of the CPs
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of CST-BPD, CST-BT, CST-BP, CST-BN, and CST-BMP
3.3. Synthesis of CP1–CP5
3.4. PHP Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [PubMed]
- Liao, G.; Li, C.; Liu, S.Y.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.Y.; Fang, B.; Yang, H. Z-scheme systems: From fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, I.; Yuan, A. MoS2 as a co-catalyst for photocatalytic hydrogen production: A mini review. Molecules 2022, 27, 3289. [Google Scholar] [CrossRef]
- Banerjee, T.; Podjaski, F.; Kröger, J.; Biswal, B.P.; Lotsch, B.V. Polymer photocatalysts for solar-to-chemical energy conversion. Nat. Rev. Mater. 2021, 6, 168–190. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, L.; Liao, G.; Shen, Z.; Tan, Z.; Xing, Y.; Li, X.; Yang, K.; Chen, L.; Liu, S. Achieving an unprecedented hydrogen evolution rate by solvent exfoliated CPP-based photocatalysts. J. Mater. Chem. A 2020, 8, 5890–5899. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Sprick, R.S.; Jiang, J.X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.O.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Tan, Z.R.; Xing, Y.Q.; Cheng, J.Z.; Zhang, G.; Shen, Z.Q.; Zhang, Y.J.; Liao, G.F.; Chen, L.; Liu, S.Y. EDOT-based conjugated polymers accessed via C–H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef]
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-based covalent organic frameworks: Synthesis, photophysics and light-driven applications. Molecules 2021, 26, 7666. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Lan, Z.A.; Zhang, G.; Chen, X.; Zhang, K.A.I.; Zhang, Y.; Wang, X. Reducing the exciton binding energy of donor–acceptor-based conjugated polymers to promote charge-induced reactions. Angew. Chem. Int. Ed. 2019, 58, 10236–10240. [Google Scholar] [CrossRef]
- Sheng, Z.Q.; Xing, Y.Q.; Chen, Y.; Zhang, G.; Liu, S.Y.; Chen, L. Nanoporous and nonporous conjugated donor-acceptor polymer semiconductors for photocatalytic hydrogen production. Beilstein J. Nanotechnol. 2021, 12, 607–623. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, J.; Zhang, G.; Zhao, Z.; Liu, S.; Zhang, W.; Chen, L. Donor-acceptor type covalent organic frameworks. Chem. Eur. J. 2021, 27, 10781–10797. [Google Scholar] [CrossRef]
- Ye, D.N.; Zhang, Y.J.; Tan, Z.R.; Xing, Y.Q.; Chen, Z.; Qiu, J.B.; Liu, S.Y. Tunable cyano substituents in D–A conjugated polymers accessed via direct arylation for photocatalytic hydrogen production. Chem. Commun. 2022, 58, 12680–12683. [Google Scholar] [CrossRef]
- Xua, Y.; Maoa, N.; Zhang, C.; Wang, X.; Zeng, J.; Chen, Y.; Wang, F.; Jiang, J.X. Rational design of donor-π-acceptor conjugated microporous polymers for photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Lim, B.; Baeg, K.J.; Jeong, H.G.; Jo, J.; Kim, H.; Park, J.W.; Noh, Y.Y.; Vak, D.; Park, J.H.; Park, J.W.; et al. A new poly(thienylenevinylene) derivative with high mobility and oxidative stability for organic thin-film transistors and solar cells. Adv. Mater. 2009, 21, 2808–2814. [Google Scholar] [CrossRef]
- Ren, J.; Bi, P.; Zhang, J.; Liu, J.; Wang, J.; Xu, Y.; Wei, Z.; Zhang, S.; Hou, J. Molecular design revitalizes the low-cost PTV-polymer for highly efficient organic solar cells. Nat. Sci. Rev. 2021, 8, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shen, Z.; Cheng, J.; Liu, L.; Yang, K.; Wen, H.; Liu, S. C-H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent. J. Mater. Chem. A 2019, 7, 24222–24229. [Google Scholar] [CrossRef]
- Liu, H.; Tao, Y.D.; Wang, L.H.; Ye, D.N.; Huang, X.M.; Chen, N.; Li, C.Z.; Liu, S.Y. C−H direct arylation: A robust tool to tailor the π-conjugation lengths of non-fullerene acceptors. ChemSusChem 2022, 15, e202200034. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, L.; Zhang, K.; Liu, S.Y. Carbazole and diketopyrrolopyrrole-based D-A π-conjugated oligomers accessed via direct C–H arylation for opto-electronic property and performance study. Molecules 2022, 27, 9031. [Google Scholar] [CrossRef]
- Siemens. SHELXTL Reference Manual, 5th ed.; Siemens Energy & Automation Inc.: Madison, WI, USA, 1994. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01) I; Gaussian: Wallingford, CT, USA, 2013. [Google Scholar]
- Jacquemin, D.; Perpe`te, E.A.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D.G. On the performances of the m06 family of density functionals for electronic excitation energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Zhang, W.; Huang, J.; Wang, Q.; Shi, K.; Chen, Z.; Zhou, Y.; Wang, L.; Yu, G. Cyanostyrylthiophene-based ambipolar conjugated polymers: Synthesis, properties, and analyses of backbone fluorination effect. Macromolecules 2018, 51, 966–976. [Google Scholar] [CrossRef]
- Kim, G.Y.; Choi, M.C.; Song, M.; Jin, S.H.; Liaw, D.J.; Wu, H.Y.; Huang, Y.C.; Ha, C.S. Synthesis and characterization of new donor–acceptor type copolymers based on fluorene derivatives for photovoltaic solar cells. J. Nanosci. Nanotechnol. 2012, 12, 5735–5741. [Google Scholar] [CrossRef]
- Garcias-Morales, C.; Romero-Borja, D.; Maldonado, J.; Roa, A.E.; Rodríguez, M.; García-Merinos, J.P.; Ariza-Castolo, A. Small molecules derived from thieno[3,4-c]pyrrole-4,6-dione (tpd) and their use in solution processed organic solar cells. Molecules 2017, 22, 1607. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, B.; Li, Q.; Fang, H.; Yu, M. Synthesis and photophysical properties of novel biphenyl derivatives containing furan and thiophene groups. J. Chem. Res. 2010, 34, 653–655. [Google Scholar] [CrossRef]
- Wu, H.; Fang, R.; Tao, J.; Wang, D.; Qiao, X.; Yang, X.; Hartl, F.; Li, H. Diacenaphthylene-fused benzo[1,2-b:4,5-b']dithiophenes: Polycyclic heteroacenes containing full-carbon five-membered aromatic rings. Chem. Commun. 2017, 53, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Huang, Y. Polymer composites of carbon nitride and poly(3-hexylthiophene) to achieve enhanced hydrogen production from water under visible light. Chem. Commun. 2011, 47, 4168–4170. [Google Scholar] [CrossRef]
- Chen, J.; Dong, C.L.; Zhao, D.; Huang, Y.C.; Wang, X.X.; Samad, L.; Dang, L.N.; Shearer, M.; Shen, S.H.; Guo, L.J. Molecular design of polymer heterojunctions for efficient solar–hydrogen conversion. Adv. Mater. 2017, 29, 1606198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Wang, X.P.; Xiao, J.; Wang, S.Y.; Huang, D.K.; Ding, X.; Xiang, Y.G.; Chen, H. Synthesis of 1,4-diethynylbenzene-based conjugated polymer photocatalysts and their enhanced visible/near-infrared-light-driven hydrogen production activity. J. Catal. 2017, 350, 64–71. [Google Scholar] [CrossRef]
- Wang, X.P.; Chen, B.; Dong, W.B.; Zhang, X.H.; Li, Z.B.; Xiang, Y.G.; Chen, H. Hydrophilicity-controlled conjugated microporous polymers for enhanced visible-light-driven photocatalytic H2 evolution. Macromol. Rapid. Commun. 2018, 40, 1800494. [Google Scholar] [CrossRef]
- Dai, C.H.; Xu, S.D.; Liu, W.; Gong, X.Z.; Panahandeh-Fard, M.; Liu, Z.; Zhang, D.Q.; Xue, C.; Loh, K.P.; Liu, B. Dibenzothiophene-S,S-dioxide-based conjugated polymers: Highly efficient photocatalyts for hydrogen production from water under visible light. Small 2018, 14, 1801839. [Google Scholar] [CrossRef]
- Sachs, M.; Sprick, R.S.; Pearce, D.; Hillman, S.A.J.; Monti, A.; Guilbert, A.A.Y.; Brownbill, N.J.; Dimitrov, S.; Shi, X.Y.; Blanc, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Commun. 2018, 9, 4968. [Google Scholar] [CrossRef]
- Wang, Z.J.; Mao, N.; Zhao, Y.B.; Yang, T.J.; Wang, F.; Jiang, J.X. Building an electron push–pull system of linear conjugated polymers for improving photocatalytic hydrogen evolution efciency. Polym. Bull. 2019, 76, 3195–3206. [Google Scholar] [CrossRef]
- Bai, Y.; Woods, D.C.J.; Wilbraham, L.; Aitchison, C.M.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Hydrogen evolution from water using heteroatom substituted fluorene conjugated co-polymers. J. Mater. Chem. A 2020, 8, 8700–8705. [Google Scholar] [CrossRef]
- Chen, R.K.; Hu, P.W.; Xian, Y.X.; Hu, X.H.; Zhang, G.B. Incorporation of sequence aza-substitution and thiophene bridge in linear conjugated polymers toward highly efficient photo-catalytic hydrogenevolution. Macromol. Rapid. Commun. 2022, 43, 2100872. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xiao, Z.H.; Hu, J.H.; Gao, X.F.; Asim, M.; Pan, L.; Shi, C.X.; Zhang, X.W.; Zou, J.J. Rational design of alkynyl-based linear donor−π−acceptor conjugated polymers with accelerated exciton dissociation for photocatalysis. Macromolecules 2022, 55, 5412–5421. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, D.; Liu, L.; Zhang, Y.; Qiu, J.; Tan, Z.; Xing, Y.; Liu, S. Tunable Donor–Acceptor Linear Conjugated Polymers Involving Cyanostyrylthiophene Linkages for Visible-Light-Driven Hydrogen Production. Molecules 2023, 28, 2203. https://doi.org/10.3390/molecules28052203
Ye D, Liu L, Zhang Y, Qiu J, Tan Z, Xing Y, Liu S. Tunable Donor–Acceptor Linear Conjugated Polymers Involving Cyanostyrylthiophene Linkages for Visible-Light-Driven Hydrogen Production. Molecules. 2023; 28(5):2203. https://doi.org/10.3390/molecules28052203
Chicago/Turabian StyleYe, Dongnai, Lei Liu, Yujie Zhang, Jiabin Qiu, Zhirong Tan, Yuqin Xing, and Shiyong Liu. 2023. "Tunable Donor–Acceptor Linear Conjugated Polymers Involving Cyanostyrylthiophene Linkages for Visible-Light-Driven Hydrogen Production" Molecules 28, no. 5: 2203. https://doi.org/10.3390/molecules28052203
APA StyleYe, D., Liu, L., Zhang, Y., Qiu, J., Tan, Z., Xing, Y., & Liu, S. (2023). Tunable Donor–Acceptor Linear Conjugated Polymers Involving Cyanostyrylthiophene Linkages for Visible-Light-Driven Hydrogen Production. Molecules, 28(5), 2203. https://doi.org/10.3390/molecules28052203








