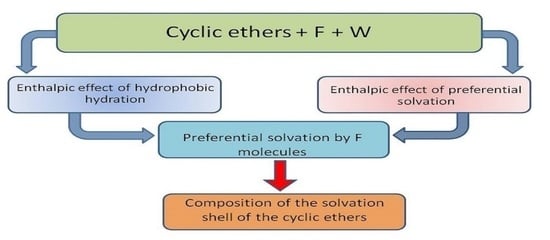
Composition of the Solvation Shell of the Selected Cyclic Ethers (1,4-Dioxane, 12-Crown-4, 15-Crown-5 and 18-Crown-6) in a Mixture of Formamide with Water at Four Temperatures
Abstract
1. Introduction
2. Results and Discussion
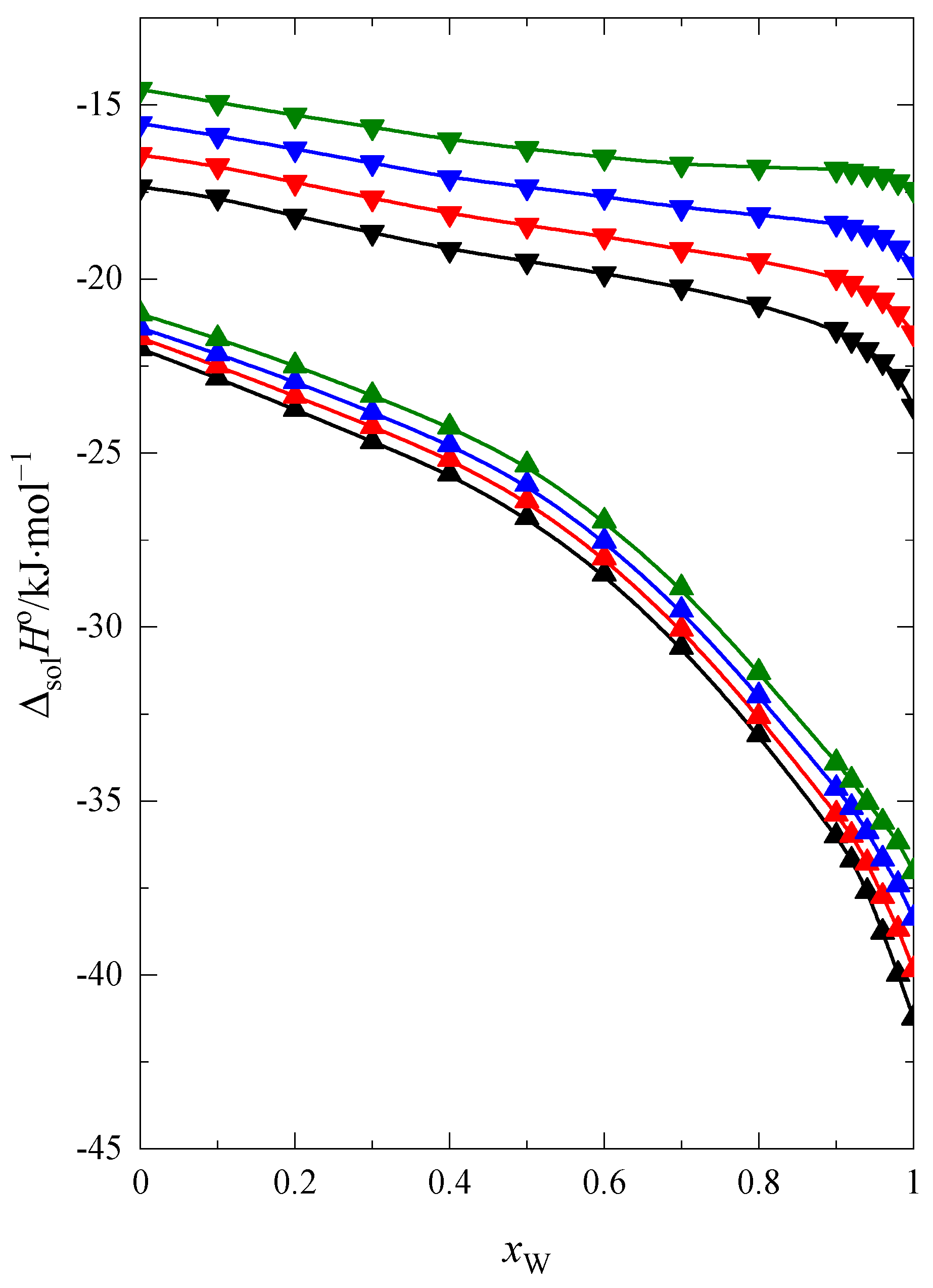
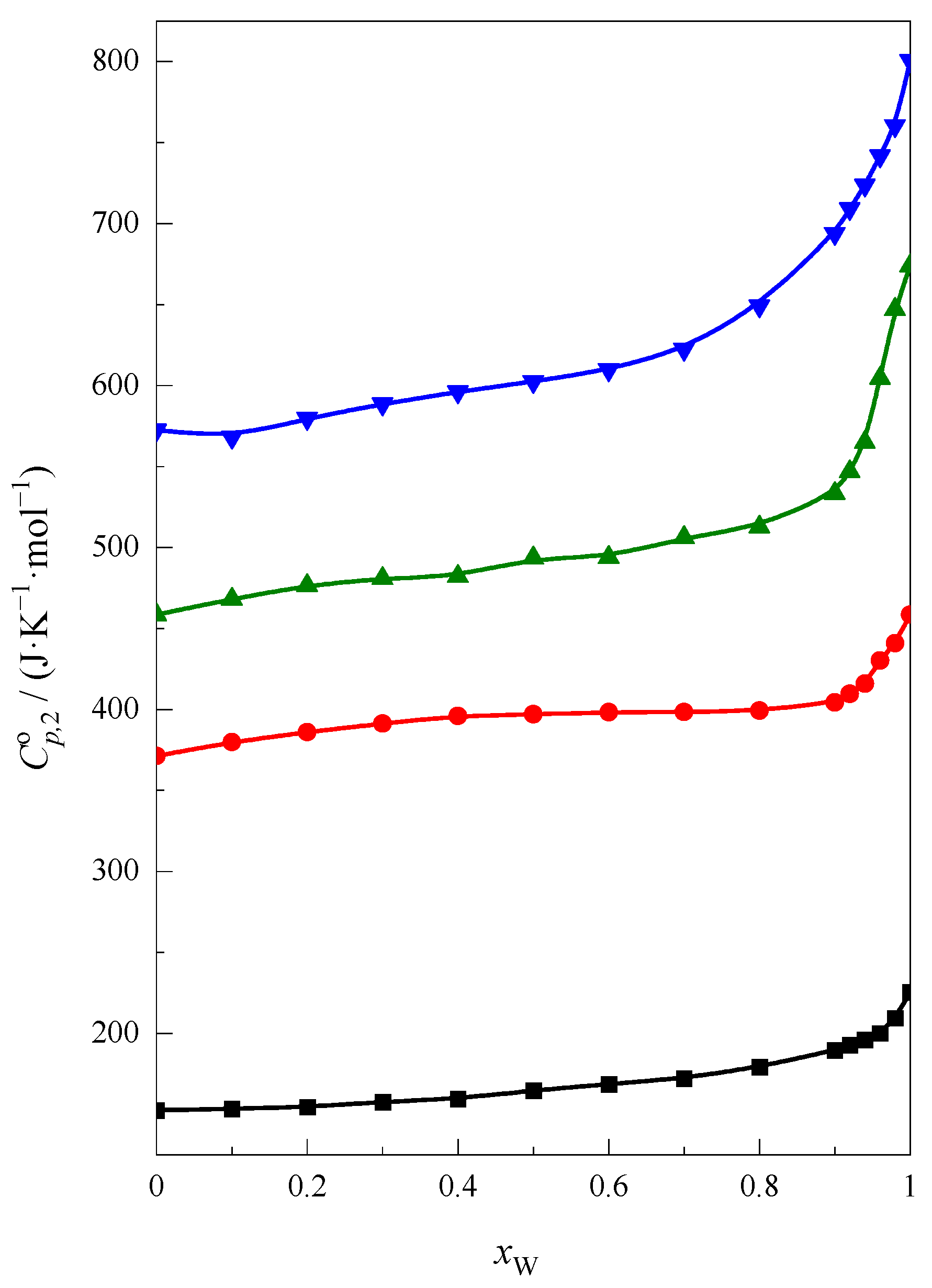
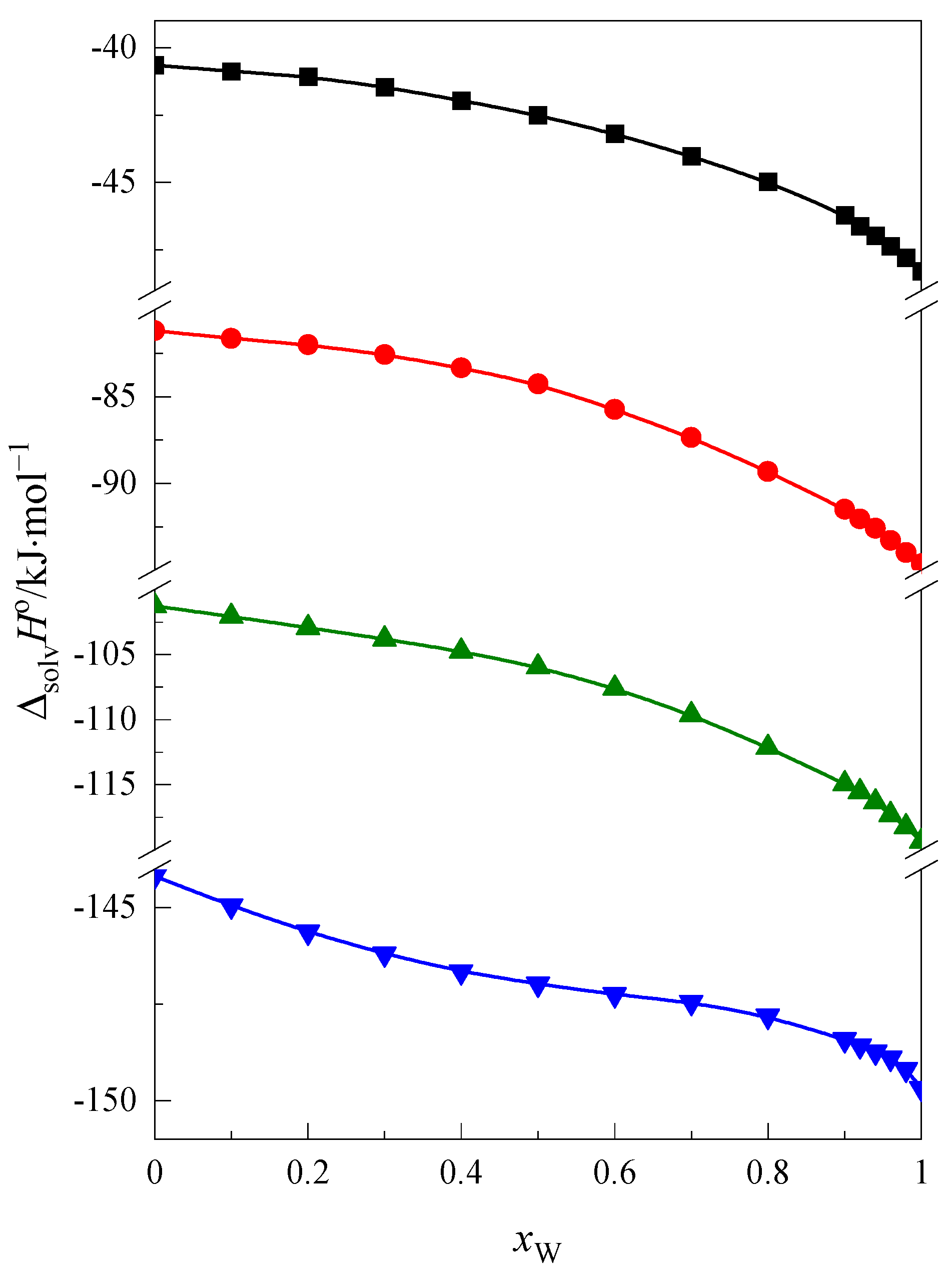
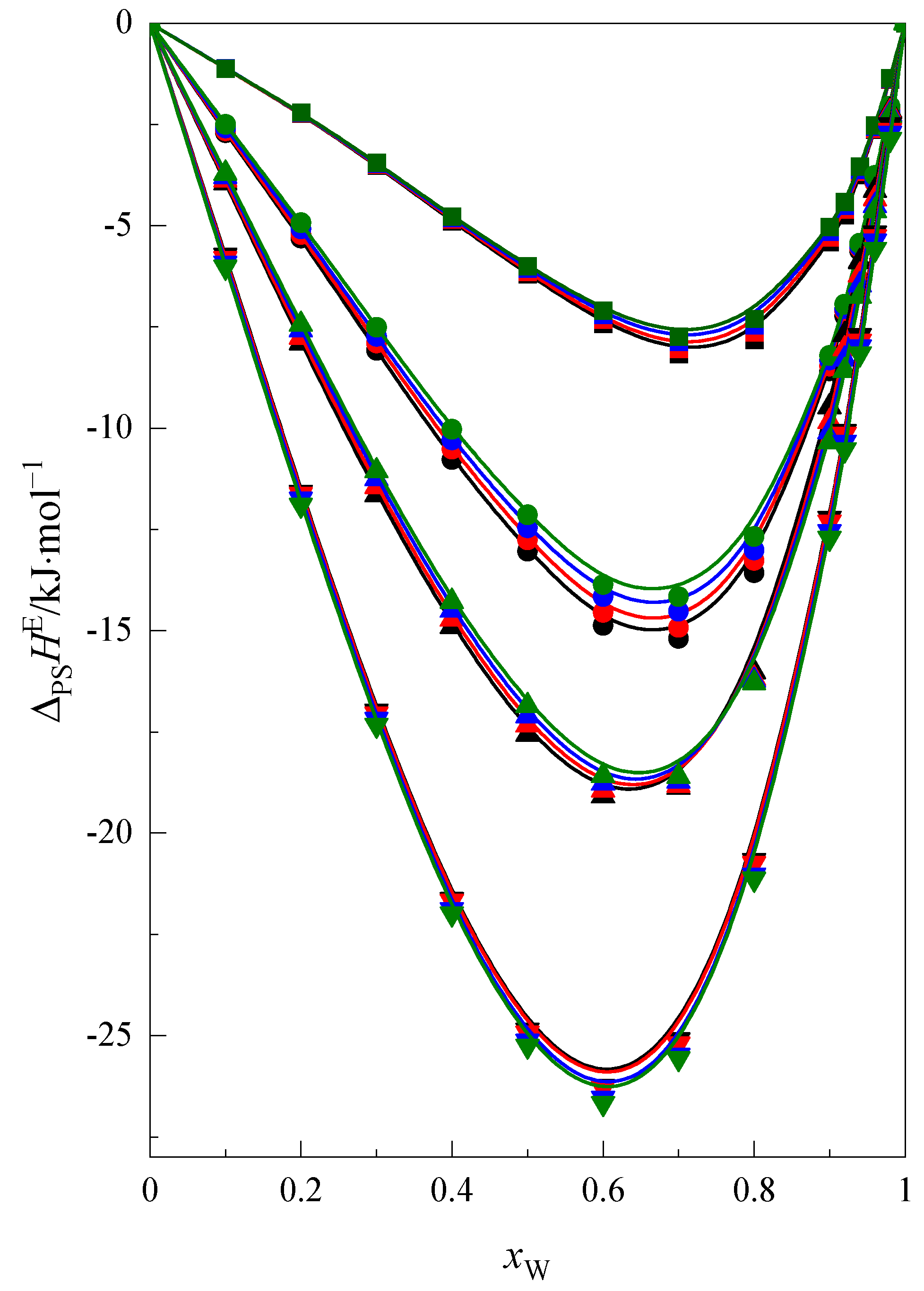
2.1. Enthalpy of Solution and Heat Capacity
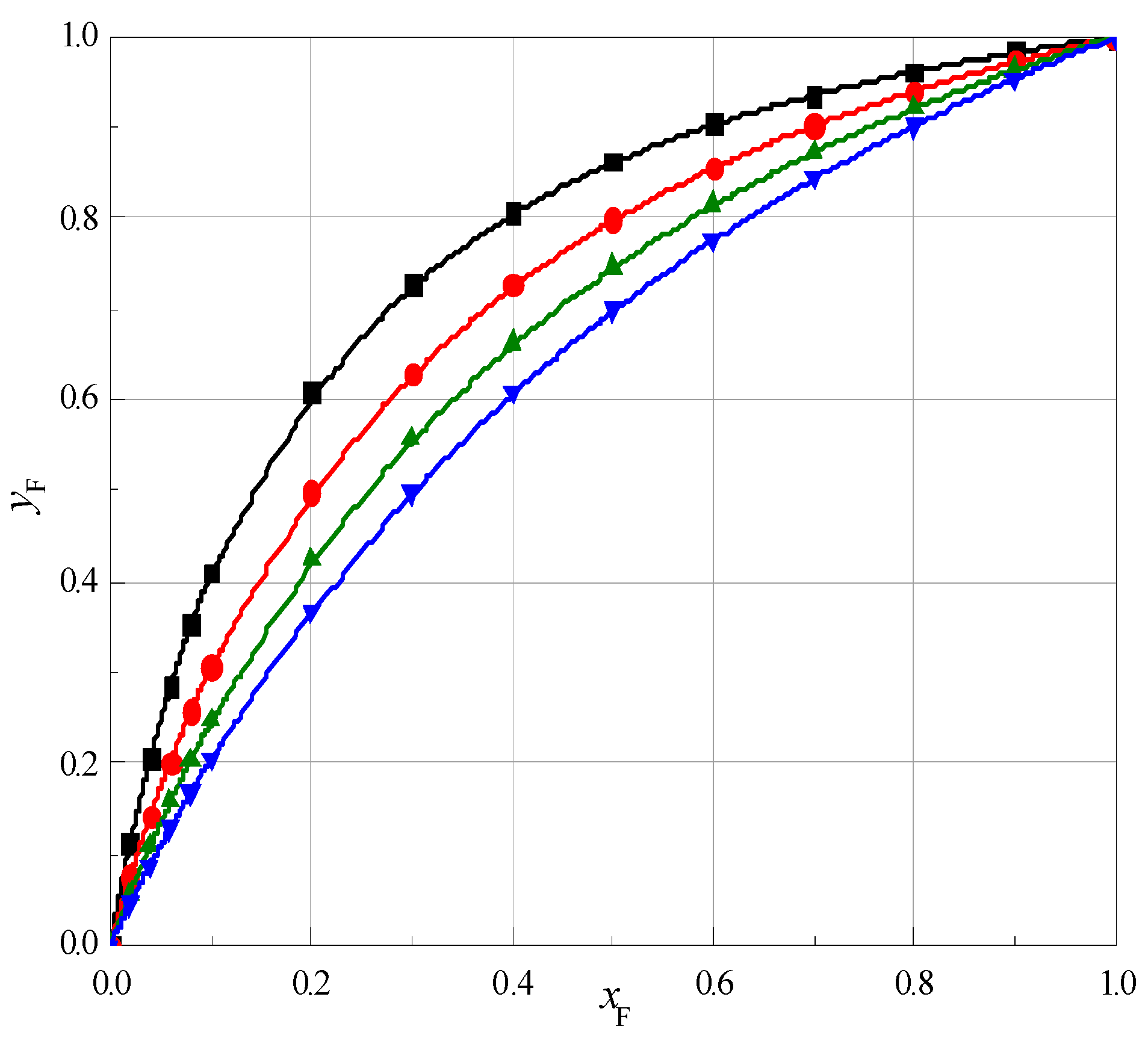
2.2. The Preferential Solvation of Cyclic Ethers
3. Experimental Section
3.1. Materials
3.2. Methods
4. Conclusions
- The exothermic enthalpic effect of the solvation process increases with the increasing of size of the cyclic ethers ring.
- Molecules of 1,4-dioxane, 12C4 15C5 and 18C6 are preferentially solvated by the molecules of formamide in the mixture of water and formamide.
- The exothermic enthalpic effect of preferential solvation process decreases with increasing temperature for the cyclic ethers (1,4-dioxane, 12C4, 15C5 and 18C6).
- The total number of W and F molecules in the solvation sphere of the molecules of cyclic ethers increases with increasing of ring size of ethers and does not depend on temperature.
- The mole fraction of formamide present in the solvation shell of cyclic ethers is higher than that in the F + W mixture.
- The 18C6 molecules most probably form complexes with formamide molecules in the solution of the F + W mixture. The same is true for 12C4 and 15C5, but to a lesser extent.
- The model of preferential solvation, proposed by Covington et al. and modified by Balk and Somsen can be successfully applied to quantitative analysis of systems in which dissolved molecules do not form complexes with solvent molecules. In the case when the solute molecules form complexes with the solvent molecules and are additionally preferentially solvated by the mixed solvent molecules, this model can be used but only for qualitative analysis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parajó, J.J.; Otero-Mato, J.M.; Lobo Ferreira, A.I.M.C.; Varela, L.M.; Santos, L.M.N.B.F. Enthalpy of solvation of alkali metal salts in a protic ionic liquid: Effect of cation charge and size. J. Mol. Liq. 2022, 360, 119228. [Google Scholar] [CrossRef]
- Rakipov, I.T.; Semenov, K.N.; Petrov, A.A.; Akhmadiyarov, A.A.; Khachatrian, A.A.; Fakhurtdinova, A.R.; Solomonov, B.N. Thermochemistry of solution, solvation and hydrogen bonding of linear and cyclic ethers in solvents. Thermochim. Acta 2021, 700, 178932. [Google Scholar] [CrossRef]
- Kuz’Mina, I.A.; Kovanova, M.A.; Udalova, A.S. Solvation of dibenzo-18-crown-6 ether in water-aprotic solvents. J. Mol. Liq. 2022, 367, 120393. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Volynkin, V.A.; Panyushkin, V.T.; Lindt, D.A.; Pham, T.L.; Nguyen, T.T.H.; Le, T.M.H.; Alister, D.A.; Kabirov, D.N.; Kuranova, N.N.; et al. Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach. Molecules 2021, 26, 4408. [Google Scholar] [CrossRef]
- Kamiyama, T.; Liu, H.L.; Kimura, T. Preferential solvation of lysozyme by dimethyl sulfoxide in binary solutions of water and dimethyl sulfoxide. J. Therm. Anal. Calorim. 2009, 95, 353–359. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Duarte, A.R.C.; Raeissi, S. Viscosity Investigations on the Binary Systems of (1 ChCl:2 Ethylene Glycol) DES and Methanol or Ethanol. Molecules 2021, 26, 5513. [Google Scholar] [CrossRef] [PubMed]
- Rakipov, I.T.; Petrov, A.A.; Akhmadiyarov, A.A.; Khachatrian, A.A.; Mukhametzyanov, T.A.; Solomonov, B.N. Thermochemistry of Solution, Solvation, and Hydrogen Bonding of Cyclic Amides in Proton Acceptor and Donor Solvents. Amide Cycle Size Effect. Molecules 2021, 26, 1411. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 2008, 140, 61–67. [Google Scholar] [CrossRef]
- Kalidas, C.; Raghunath, R. Preferential solvation of silver(I)-cryptand-2,2,2 perchlorate complex in water + acetonitrile and methanol + acetonitrile mixtures. J. Electroanal. Chem. 1995, 389, 79–83. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential solvation of ions in mixed solvents. 6: Univalent anions in aqueous organic solvents according to the inverse Kirkwood-Buff integral (IKBI) approach. J. Chem. Thermodyn. 2007, 39, 1338–1345. [Google Scholar] [CrossRef]
- Ben-Naim, A. Preferential solvation in two- and in three-component systems. Pure Appl. Chem. 1990, 62, 25–34. [Google Scholar] [CrossRef]
- Sun, H.; Du, C. Dissolution of 5-azacytidine in aqueous solution of alcohols at various temperatures: Preferential solvation and thermodynamic analysis. J. Chem. Thermodyn. 2023, 178, 106945. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, W.; Zhao, H.; Li, W.; Han, G.; Farajtabar, A. Solubility, solvent effect, preferential solvation and DFT computations of 5-nitrosalicylic acid in several aqueous blends. J. Chem. Thermodyn. 2023, 177, 106936. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Song, F.; Wang, J. Inter-/intra-molecular interactions, preferential solvation, and dissolution and transfer property for tirofiban in aqueous co-solvent mixtures. J. Mol. Liq. 2022, 361, 119665. [Google Scholar] [CrossRef]
- Cong, Y.; Du, C.; Xing, K.; Bian, Y.; Li, X.; Wang, M. Research on dissolution of actarit in aqueous mixtures: Solubility determination and correlation, preferential solvation, solvent effect and thermodynamics. J. Mol. Liq. 2022, 358, 119141. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Kosiorowska, M.A. Effect of Temperature on the Process of Hydrophobic Hydration. Part I. Hydrophobic Hydration of 1,4-Dioxane and 12-Crown-4 Ethers. J. Chem. Eng. Data 2010, 55, 2776–2780. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Kosiorowska, M.A.; Wasiak, M. Effect of Temperature on the Process of Hydrophobic Hydration. Part II. Hydrophobic Hydration of 15-Crown-5 and 18-Crown-6 Ethers. J. Chem. Eng. Data 2010, 55, 5138–5143. [Google Scholar] [CrossRef]
- Jóźwiak, M. The effect of properties of water-organic solvent mixtures on the solvation enthalpy of 12-crown-4, 15-crown-5, 18-crown-6 and benzo-15-crown-55 ethers at 298.15 K. Thermochim. Acta 2004, 417, 31–41. [Google Scholar] [CrossRef]
- Nayak, J.N.; Aralaguppi, M.I.; Naidu, B.V.K.; Aminabhavi, T.M. Thermodynamic Properties of Water + Tetrahydrofuran and Water + 1,4-Dioxane Mixtures at (303.15, 313.15, and 323.15) K. J. Chem. Eng. Data 2004, 49, 468–474. [Google Scholar] [CrossRef]
- Junk, P.C. Crown ethers as stabilising ligands for oxonium ions. New J. Chem. 2008, 32, 762–773. [Google Scholar] [CrossRef]
- Hilderbrand, A.E.; Myung, S.; Clemmer, D.E. Exploring Crown Ethers as Shift Reagents for Ion Mobility Spectrometry. Anal. Chem. 2006, 78, 6792–6800. [Google Scholar] [CrossRef]
- Sisson, A.L.; Shah, M.R.; Bhosale, S.; Matile, S. Synthetic ion channels and pores (2004–2005). Chem. Soc. Rev. 2006, 35, 1269–1286. [Google Scholar] [CrossRef]
- Suzumura, A.; Paul, D.; Sugimoto, H.; Shinoda, S.; Julian, R.R.; Beauchamp, J.L.; Teraoka, J.; Tsukube, H. Cytochrome c-Crown Ether Complexes as Supramolecular Catalysts: Cold-Active Synzymes for Asymmetric Sulfoxide Oxidation in Methanol. Inorg. Chem. 2005, 44, 904–910. [Google Scholar] [CrossRef]
- Jamali, S.H.; Ramdin, M.; Becker, T.M.; Rinwa, S.K.; Buijs, W.; Vlugt, T.J.H. Thermodynamic and Transport Properties of Crown-Ethers: Force Field Development and Molecular Simulations. J. Phys. Chem. B 2017, 121, 8367–8376. [Google Scholar] [CrossRef]
- Ullah, F.; Khan, T.A.; Iltaf, J.; Anwar, S.; Khan, M.F.A.; Khan, M.R.; Ullah, S.; Rehman, M.F.U.; Mustaqeem, M.; Kotwica-Mojzych, K.; et al. Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Appl. Sci. 2022, 12, 1102. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Łudzik, K.; Cokot, M.; Jóźwiak, A.; Kłys, A. Solvation enthalpy of selected glymes in the mixtures of N,N-dimethylformamide with propan-1-ol or methanol at 298.15 K. The solvent contribution to the solvation enthalpy of glymes. J. Mol. Liq. 2020, 314, 113733. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Urban, A.; Tyczyńska, M. Effect of properties of N,N-dimethylformamide + propan-1-ol mixtures on the solution enthalpy of selected cyclic ethers in these mixtures at 298.15 K. The contribution of solvent to the enthalpy of solvation of cyclic ethers. J. Mol. Liq. 2021, 321, 114754. [Google Scholar] [CrossRef]
- Pullman, A.; Berthod, H.; Giessner-Prettre, C.; Hinton, J.F.; Harpool, D. Hydrogen bonding in pure and aqueous formamide. J. Am. Chem. Soc. 1978, 100, 3991–3994. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents, 4th ed.; Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Shedlovskiy, D.; Shcherbik, N.; Pestov, D.G. One-step hot formamide extraction of RNA from Saccharomyces cerevisiae. RNA Biol. 2017, 14, 1722–1726. [Google Scholar] [CrossRef]
- Höhn, A.; Staff, U. Formamide. In Kirk-Othmer encyclopedia of chemical technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–10. ISBN 978-0-47-148494-3. [Google Scholar] [CrossRef]
- Brocos, P.; Calvo, E.; Bravo, R.; Pintos, M.; Amigo, A.; Roux, A.H.; Roux-Desgranges, G. Heat Capacities, Excess Enthalpies, and Volumes of Mixtures Containing Cyclic Ethers. 3. Binary Systems {Tetrahydrofuran, Tetrahydropyran, 1,4-Dioxane, or 1,3-Dioxolane + Cyclohexane or Toluene}. J. Chem. Eng. Data 1999, 44, 67–72. [Google Scholar] [CrossRef]
- Briggner, L.-E.; Wadsö, I. Some thermodynamic properties of crown ethers in aqueous solution. J. Chem. Thermodyn. 1990, 22, 143–148. [Google Scholar] [CrossRef]
- Cabani, S.; Gianni, P.; Mollica, V.; Lepori, L. Group Contributions to the Thermodynamic Properties of Non-Ionic Organic Solutes in Dilute Aqueous Solution. J. Solut. Chem. 1981, 10, 563–595. [Google Scholar] [CrossRef]
- Vögtle, F.; Müller, W.M.; Weber, E. Komplexe zwischen Neutralmolekülen, VII. Kronenether als Wirtssubstanzen für organische Gastmoleküle. Chem. Ber. 1980, 113, 1130–1137. [Google Scholar] [CrossRef]
- Watson, W.H.; Galloy, J.; Grossie, D.A.; Voegtle, F.; Mueller, W.M. Host-guest complex chemistry. Structures of 18-crown-6 and diaza-18-crown-6 with neutral molecules. J. Org. Chem. 1984, 49, 347–353. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Popov, A.I. NMR and infrared studies of the complexation reaction of 18-crown-6 with some organic solvents. J. Am. Chem. Soc. 1985, 107, 6168–6174. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamada, Y.; Kanamori, K.; Kudo, Y.; Takeda, Y. On the Facilitation Effect of Neutral Macrocyclic Ligands on the Ion Transfer across the Interface between Aqueous and Organic Solutions. I. Theoretical Equation of Ion-Transfer-Polarographic Current-Potential Curves and Its Experimental Verification. Bull. Chem. Soc. Jpn. 1991, 64, 1497–1508. [Google Scholar] [CrossRef]
- Kowall, T.; Geiger, A. Molecular Dynamics Simulation Study of 18-Crown-6 in Aqueous Solution. 1. Structure and Dynamics of the Hydration Shell. J. Phys. Chem. 1994, 98, 6216–6224. [Google Scholar] [CrossRef]
- Patil, K.J.; Pawar, R.B.; Patil, P.D. The studies of viscosity behaviour in aqueous 18-crown-6 solutions at 25 °C. J. Mol. Liq. 2000, 84, 223–233. [Google Scholar] [CrossRef]
- Dagade, D.; Pawar, R.; Patil, K. Viscosity Behavior of 18-Crown-6 in Aqueous and Carbon Tetrachloride Solutions at Different Temperatures and at Ambient Pressure. J. Chem. Eng. Data 2004, 49, 341–346. [Google Scholar] [CrossRef]
- Bryan, S.A.; Willis, R.R.; Moyer, B.A. Hydration of 18-crown-6 in carbon tetrachloride: Infrared spectral evidence for an equilibrium between monodentate and bidentate forms of bound water in the 1:1 crown-water adduct. J. Phys. Chem. 1990, 94, 5230–5233. [Google Scholar] [CrossRef]
- Fukuhara, K.; Tachikake, M.; Matsumoto, S.; Matsuura, H. Raman Spectroscopic Study of the Hydrates of 18-Crown-6. J. Phys. Chem. 1995, 99, 8617–8623. [Google Scholar] [CrossRef]
- Covington, A.K.; Thain, J.M. Nuclear magnetic resonance studies of preferential solvation. Part 4.—Thermodynamic treatment involving non-statistical distribution of solvated species. J. Chem. Soc. Faraday Trans. 1 1974, 70, 1879–1887. [Google Scholar] [CrossRef]
- Remerie, K.; Engberts, J.B.F.N. Preferential solvation of nonelectrolytes in mixed aqueous solvents. A quantitative approach for.beta.-disulfones in 1,4-dioxane-water, 1,3-dioxane-water and dimethoxyethane-water in terms of the Covington theory. J. Phys. Chem. 1983, 87, 5449–5455. [Google Scholar] [CrossRef]
- Balk, R.W.; Somsen, G. Preferential solvation and hydrophobic hydration of polyols in mixtures of water and N,N-dimethylformamide. J. Phys. Chem. 1985, 89, 5093–5097. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Piekarski, H. Heat of solution of 15-crown-5 ether in the mixtures of water with DMSO, DMF, DMA and HMPA at 298.15K. J. Mol. Liq. 1999, 81, 63–70. [Google Scholar] [CrossRef]
- Mastroianni, M.J.; Pikal, M.J.; Lindenbaum, S. Effect of dimethyl sulfoxide, urea, guanidine hydrochloride, and sodium chloride on hydrophobic interactions. Heats of dilution of tetrabutylammonium bromide and lithium bromide in mixed aqueous solvent systems. J. Phys. Chem. 1972, 76, 3050–3057. [Google Scholar] [CrossRef]
- Covington, A.K.; Newman, K.E. Thermodynamics of Preferential Solvation of Electrolytes in Binary Solvent Mixtures. Adv. Chem. Ser. 1976, 155, 153–196. [Google Scholar] [CrossRef]
- Piekarski, H.; Waliszewski, D. Hydration effect on urea-non-electrolyte enthalpic pair interaction coefficients. Dissolution enthalpies of urea in aqueous solution of alkoxyethanols at 298.15 K. Thermochim. Acta 1995, 258, 67–76. [Google Scholar] [CrossRef]
- Sabbah, R.; Xu, A.; Chickos, J.S.; Planas Leitão, M.L.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Wadsö, I.; Goldberg, R.N. Standards in isothermal microcalorimetry (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 1625–1639. [Google Scholar] [CrossRef]
- Paŀecz, B. The enthalpies of interaction of glycine with some amides and ureas in water at 25 °C. J. Solut. Chem. 1995, 24, 537–550. [Google Scholar] [CrossRef]
- Desnoyers, J.E.; Perron, G.; Avedikian, L.; Morel, J.-P. Enthalpies of the urea-tert-butanol-water system at 25 °C. J. Solut. Chem. 1976, 5, 631–644. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Kuz’Mina, I.A.; Sharnin, V.A.; Chernov, I.V.; Matteoli, E. The influence of the composition of an aqueous-acetone solvent on the thermodynamic characteristics of complex formation of 18-crown-6 ether with glycine. Russ. J. Phys. Chem. A 2011, 85, 948–951. [Google Scholar] [CrossRef]
| /kJ·mol−1 | |||
|---|---|---|---|
| Cyclic Ethers | at xW = 0 | at xW = 1 | |
| 1,4-dioxane | −40.65 | −48.28 | 7.63 |
| 12C4 | −81.20 | −94.63 | 13.43 |
| 15C5 | −101.26 | −119.37 | 18.11 |
| 18C6 | −144.18 | −149.68 | 5.50 |
| Cyclic Ether | T/K | r | K1/r | K | K′ = 1/K | R2 |
|---|---|---|---|---|---|---|
| 15C5 | 293.15 | 30.3 ± 0.4 | 0.36 ± 0.01 | 3.49 × 10–14 | 2.9 × 1013 | 0.9921 |
| 298.15 | 26.0 ± 0.3 | 0.34 ± 0.01 | 6.44 × 10–13 | 1.6 × 1012 | 0.9936 | |
| 303.15 | 22.9 ± 0.3 | 0.32 ± 0.01 | 4.50 × 10–12 | 2.2 × 1011 | 0.9944 | |
| 308.15 | 20.3 ± 0.2 | 0.30 ± 0.01 | 2.46 × 10–12 | 4.1 × 1010 | 0.9952 | |
| 18C6 | 293.15 | 61.3 ± 0.7 | 0.44 ± 0.01 | 1.38 × 10–22 | 7.2 × 1021 | 0.9949 |
| 298.15 | 59.5 ± 0.6 | 0.43 ± 0.01 | 1.51 × 10–22 | 6.6 × 1021 | 0.9954 | |
| 303.15 | 57.6 ± 0.6 | 0.43 ± 0.01 | 7.79 × 10–22 | 1.3 × 1021 | 0.9958 | |
| 308.15 | 55.9 ± 0.5 | 0.42 ± 0.01 | 8.55 × 10–22 | 1.2 × 1021 | 0.9967 |
| 1,4-Dioxane | 12C4 | 15C5 | 18C6 | ||
|---|---|---|---|---|---|
| a | b | c | c | c | c |
| 0.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 0.100 | 0.900 | 0.983 ± 0.000 | 0.973 ± 0.001 | 0.965 ± 0.003 | 0.954 ± 0.001 |
| 0.200 | 0.800 | 0.962 ± 0.001 | 0.942 ± 0.001 | 0.924 ± 0.005 | 0.903 ± 0.002 |
| 0.300 | 0.700 | 0.937 ± 0.001 | 0.904 ± 0.002 | 0.876 ± 0.008 | 0.844 ± 0.003 |
| 0.400 | 0.600 | 0.905 ± 0.002 | 0.858 ± 0.002 | 0.820 ± 0.012 | 0.777 ± 0.003 |
| 0.500 | 0.500 | 0.864 ± 0.003 | 0.801 ± 0.003 | 0.752 ± 0.015 | 0.699 ± 0.004 |
| 0.600 | 0.400 | 0.809 ± 0.004 | 0.729 ± 0.004 | 0.669 ± 0.017 | 0.608 ± 0.005 |
| 0.700 | 0.300 | 0.732 ± 0.005 | 0.634 ± 0.005 | 0.566 ± 0.019 | 0.499 ± 0.005 |
| 0.800 | 0.200 | 0.614 ± 0.006 | 0.502 ± 0.005 | 0.432 ± 0.019 | 0.368 ± 0.004 |
| 0.900 | 0.100 | 0.414 ± 0.007 | 0.310 ± 0.004 | 0.253 ± 0.015 | 0.205 ± 0.003 |
| 0.920 | 0.080 | 0.356 ± 0.006 | 0.260 ± 0.004 | 0.209 ± 0.013 | 0.168 ± 0.003 |
| 0.940 | 0.060 | 0.289 ± 0.006 | 0.205 ± 0.003 | 0.163 ± 0.011 | 0.129 ± 0.002 |
| 0.960 | 0.040 | 0.210 ± 0.005 | 0.144 ± 0.002 | 0.113 ± 0.008 | 0.088 ± 0.002 |
| 0.980 | 0.020 | 0.115 ± 0.003 | 0.076 ± 0.001 | 0.059 ± 0.004 | 0.045 ± 0.001 |
| 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Chemical Name | Source | Mole Fraction Purity a | Purification Method | Mass Fraction of Water b |
|---|---|---|---|---|
| Urea (U) | Sigma-Aldrich, (Poznan, Poland) | >0.995 | recrystallization from ethanol and dried under reduced pressure to constant mass | − |
| Potassium chloride (KCl) | Sigma-Aldrich, (Poznan, Poland) | >0.99 | drying under reduced pressure to constant mass | − |
| 15-crown-5 (15C5) | Sigma-Aldrich, (Poznan, Poland) | 0.98 | drying under reduced pressure | 1 × 10−3 |
| 18-crown-6 (18C6) | Sigma-Aldrich, (Poznan, Poland) | ≥0.99 | recrystallization from hexane and dried under reduced pressure | − |
| Formamide (F) | Sigma-Aldrich, (Poznan, Poland | >0.99 | drying using 4A molecular sieves and calcium oxide and distillation under reduced pressure | 3 × 10−4 |
| T = 293.15 K | T = 298.15 K | T = 303.15 K | T = 308.15 K | |||||
|---|---|---|---|---|---|---|---|---|
| a | ||||||||
| 0.000 | 2.42–2.60 | −22.00 ± 0.04 c | 2.31–2.34 | −21.69 ± 0.04 | 2.62–2.88 | −21.40 ± 0.06 | 3.62–4.62 | −21.00 ± 0.05 |
| 0.100 | 3.07–3.30 | −22.85 ± 0.04 | 2.24–2.35 | −22.50 ± 0.05 | 1.99–2.90 | −22.15 ± 0.06 | 3.80–5.36 | −21.71 ± 0.05 |
| 0.200 | 2.84–3.15 | −23.75 ± 0.08 | 2.22–2.26 | −23.36 ± 0.04 | 2.61–3.18 | −22.95 ± 0.04 | 2.87–6.70 | −22.49 ± 0.08 |
| 0.300 | 2.58–2.99 | −24.67 ± 0.05 | 2.26–2.29 | −24.24 ± 0.04 | 2.42–3.25 | −23.83 ± 0.04 | 1.57–1.62 | −23.34 ± 0.04 |
| 0.400 | 2.40–3.20 | −25.60 ± 0.06 | 2.17–2.21 | −25.18 ± 0.06 | 2.55–3.26 | −24.76 ± 0.08 | 1.46–1.50 | −24.25 ± 0.04 |
| 0.500 | 2.58–2.71 | −26.86 ± 0.06 | 2.13–2.16 | −26.37 ± 0.06 | 2.72–2.74 | −25.90 ± 0.06 | 1.05–1.63 | −25.34 ± 0.06 |
| 0.600 | 3.03–3.12 | −28.48 ± 0.08 | 2.23–2.28 | −28.01 ± 0.04 | 2.73–3.10 | −27.53 ± 0.06 | 1.56–2.12 | −26.95 ± 0.06 |
| 0.700 | 2.60 –2.66 | −30.58 ± 0.04 | 2.91–3.02 | −30.06 ± 0.06 | 2.76–3.42 | −29.52 ± 0.09 | 1.82–1.85 | −28.87 ± 0.06 |
| 0.800 | 2.52–3.58 | −33.10 ± 0.04 | 1.82–1.97 | −32.57 ± 0.04 | 2.88–3.06 | −31.97 ± 0.05 | 1.53–1.91 | −31.30 ± 0.06 |
| 0.900 | 3.88 –3.89 | −36.00 ± 0.04 | 2.61–2.79 | −35.37 ± 0.04 | 2.67–2.69 | −34.64 ± 0.09 | 1.38–1.58 | −33.90 ± 0.09 |
| 0.920 | 3.16–3.30 | −36.70 ± 0.04 | 2.27–2.35 | −35.98 ± 0.06 | 1.53–1.97 | −35.18 ± 0.04 | 1.64–1.82 | −34.40 ± 0.08 |
| 0.940 | 3.47–3.59 | −37.60 ± 0.06 | 2.26–2.58 | −36.78 ± 0.05 | 1.69–1.75 | −35.88 ± 0.06 | 1.60–2.09 | −35.03 ± 0.08 |
| 0.960 | 3.38–3.43 | −38.77 ± 0.05 | 2.94–3.07 | −37.74 ± 0.05 | 1.32–1.66 | −36.67 ± 0.05 | 2.14–3.81 | −35.60 ± 0.06 |
| 0.980 | 3.18–3.81 | −39.98 ± 0.04 | 2.14–2.23 | −38.68 ± 0.04 | 1.20–1.97 | −37.40 ± 0.06 | 3.39–3.79 | −36.17 ± 0.04 |
| 1.000 | 1.16–2.48 | −41.24 ± 0.04 | 2.15–2.32 | −39.85 ± 0.02 | 1.58–3.52 | −38.37 ± 0.03 | 1.37–1.52 | −37.03 ± 0.04 |
| −40.64 [47] | ||||||||
| −39.71 [33] | ||||||||
| T = 293.15 K | T = 298.15 K | T = 303.15 K | T = 308.15 K | |||||
|---|---|---|---|---|---|---|---|---|
| a | ||||||||
| 0.00 | 1.02–1.08 | −17.35 ± 0.04 c | 1.09–1.69 | −16.43 ± 0.06 | 1.23–1.43 | −15.53 ± 0.05 | 1.13–1.71 | −14.55 ± 0.06 |
| 0.10 | 1.23–1.74 | −17.65 ± 0.04 | 1.31–1.69 | −16.75 ± 0.09 | 1.58–2.24 | −15.87 ± 0.06 | 1.14–1.27 | −14.92 ± 0.06 |
| 0.20 | 1.07–1.73 | −18.19 ± 0.08 | 1.03–2.01 | −17.22 ± 0.04 | 1.94–1.53 | −16.26 ± 0.05 | 1.00–1.43 | −15.29 ± 0.04 |
| 0.30 | 1.17–1.47 | −18.66 ± 0.06 | 2.04–2.26 | −17.67 ± 0.09 | 0.92–1.47 | −16.66 ± 0.06 | 1.03–1.53 | −15.63 ± 0.06 |
| 0.40 | 1.21–1.48 | −19.15 ± 0.05 | 1.72–2.73 | −18.12 ± 0.06 | 1.22–1.51 | −17.09 ± 0.04 | 1.01–1.58 | −16.00 ± 0.08 |
| 0.50 | 1.12–1.15 | −19.48 ± 0.04 | 1.41–1.73 | −18.46 ± 0.05 | 1.72–1.90 | −17.35 ± 0.08 | 0.78–1.48 | −16.25 ± 0.06 |
| 0.60 | 0.87–1.74 | −19.84 ± 0.06 | 1.40–1.88 | −18.77 ± 0.06 | 2.26–2.71 | −17.63 ± 0.05 | 1.52–2.51 | −16.50 ± 0.04 |
| 0.70 | 0.97–1.31 | −20.23 ± 0.06 | 1.70–2.30 | −19.15 ± 0.05 | 1.95–2.49 | −17.95 ± 0.05 | 1.42–2.11 | −16.70 ± 0.06 |
| 0.80 | 1.52–1.60 | −20.72 ± 0.04 | 1.68–2.18 | −19.46 ± 0.06 | 2.06–2.54 | −18.15 ± 0.04 | 1.08–1.40 | −16.78 ± 0.04 |
| 0.90 | 1.37–1.58 | −21.45 ± 0.04 | 1.80–2.68 | −19.94 ± 0.06 | 2.84–3.23 | −18.40 ± 0.05 | 1.17–1.31 | −16.84 ± 0.06 |
| 0.92 | 1.04–1.45 | −21.74 ± 0.05 | 2.00–2.67 | −20.12 ± 0.06 | 3.97–4.91 | −18.51 ± 0.06 | 0.98–1.81 | −16.90 ± 0.08 |
| 0.94 | 1.20–1.30 | −22.02 ± 0.08 | 2.53–3.16 | −20.39 ± 0.04 | 2.06–3.11 | −18.68 ± 0.04 | 1.54–1.92 | −16.97 ± 0.06 |
| 0.96 | 1.41–2.00 | −22.37 ± 0.05 | 1.43–1.82 | −20.60 ± 0.05 | 2.10–3.02 | −18.80 ± 0.08 | 1.44–1.92 | −17.05 ± 0.04 |
| 0.98 | 0.96–1.08 | −22.80 ± 0.04 | 1.34–1.80 | −20.99 ± 0.06 | 2.16–3.39 | −19.10 ± 0.06 | 1.41–1.77 | −17.20 ± 0.06 |
| 1.00 | 5.43–6.52 | −23.65 ± 0.02 | 1.92–2.44 | −21.55 ± 0.03 | 2.55–5.87 | −19.57 ± 0.02 | 2.48–4.64 | −17.42 ± 0.02 |
| −21.58 [17] | ||||||||
| −21.54 [33] | ||||||||
| −21.36 [55] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, M.; Trzmielak, M.A.; Wasiak, M.; Łudzik-Dychto, K. Composition of the Solvation Shell of the Selected Cyclic Ethers (1,4-Dioxane, 12-Crown-4, 15-Crown-5 and 18-Crown-6) in a Mixture of Formamide with Water at Four Temperatures. Molecules 2023, 28, 2169. https://doi.org/10.3390/molecules28052169
Jóźwiak M, Trzmielak MA, Wasiak M, Łudzik-Dychto K. Composition of the Solvation Shell of the Selected Cyclic Ethers (1,4-Dioxane, 12-Crown-4, 15-Crown-5 and 18-Crown-6) in a Mixture of Formamide with Water at Four Temperatures. Molecules. 2023; 28(5):2169. https://doi.org/10.3390/molecules28052169
Chicago/Turabian StyleJóźwiak, Małgorzata, Monika A. Trzmielak, Michał Wasiak, and Katarzyna Łudzik-Dychto. 2023. "Composition of the Solvation Shell of the Selected Cyclic Ethers (1,4-Dioxane, 12-Crown-4, 15-Crown-5 and 18-Crown-6) in a Mixture of Formamide with Water at Four Temperatures" Molecules 28, no. 5: 2169. https://doi.org/10.3390/molecules28052169
APA StyleJóźwiak, M., Trzmielak, M. A., Wasiak, M., & Łudzik-Dychto, K. (2023). Composition of the Solvation Shell of the Selected Cyclic Ethers (1,4-Dioxane, 12-Crown-4, 15-Crown-5 and 18-Crown-6) in a Mixture of Formamide with Water at Four Temperatures. Molecules, 28(5), 2169. https://doi.org/10.3390/molecules28052169