Synthetic Glabridin Derivatives Inhibit LPS-Induced Inflammation via MAPKs and NF-κB Pathways in RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
2.1. Effects of Compounds on LPS-Induced NO and PGE2 Production
2.2. Effects of Compounds on LPS-Induced Cytokines
2.3. Effects of Compounds on NF-κB Signaling Pathway
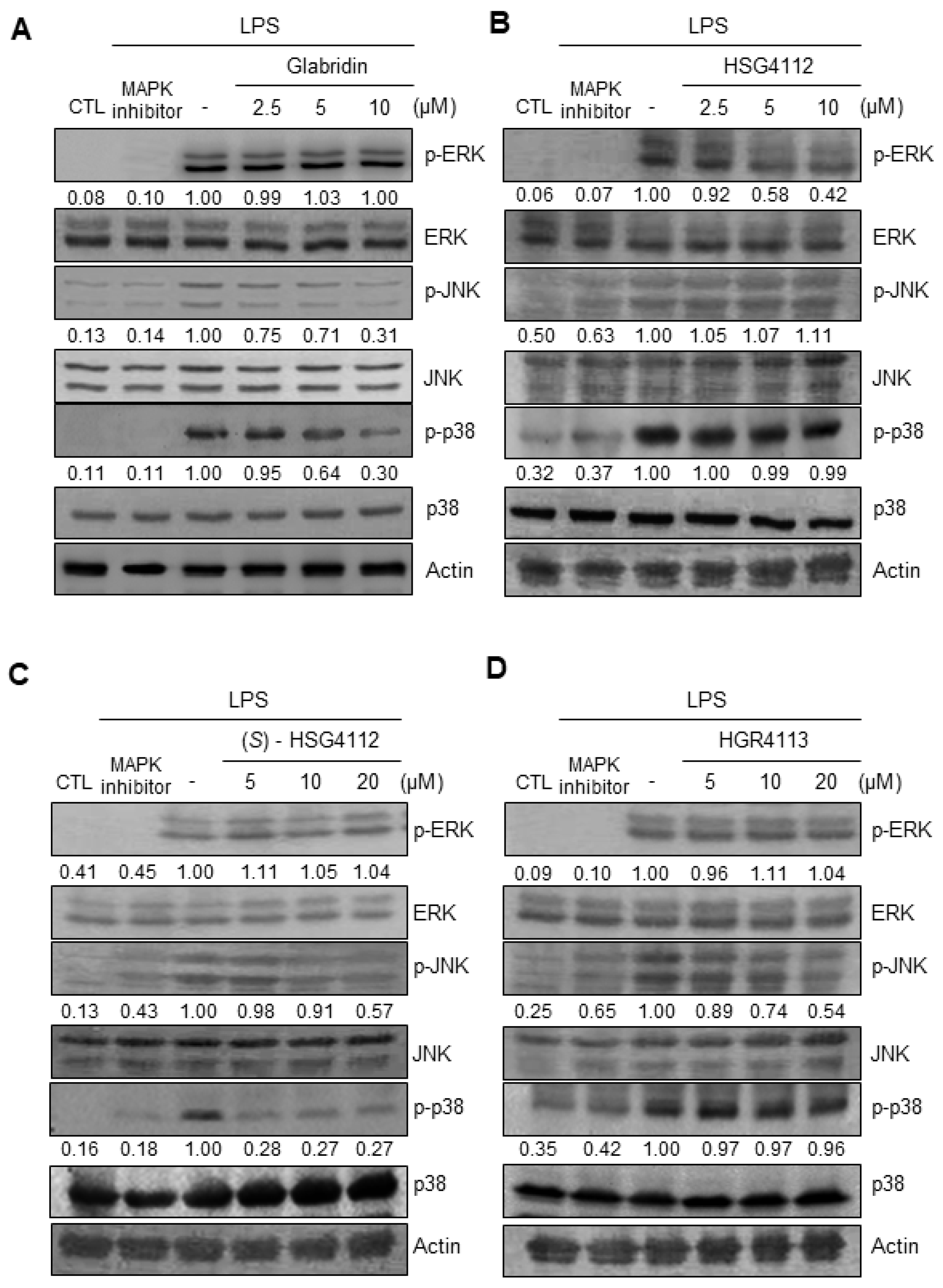
2.4. Effects of Compounds on LPS-Induced MAPK Signaling
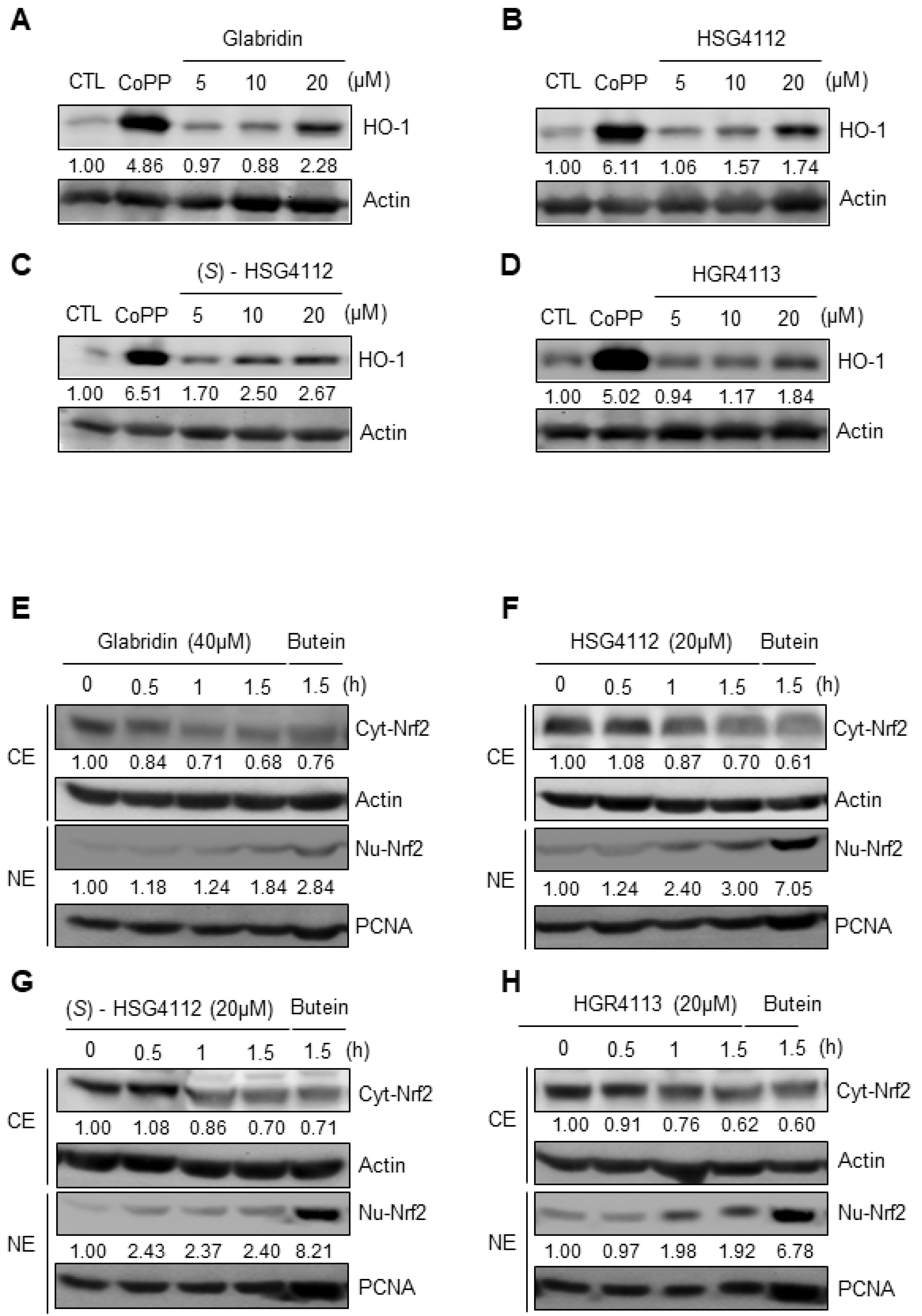
2.5. Effects of Compounds on HO-1 Induction and Nrf2 Signaling
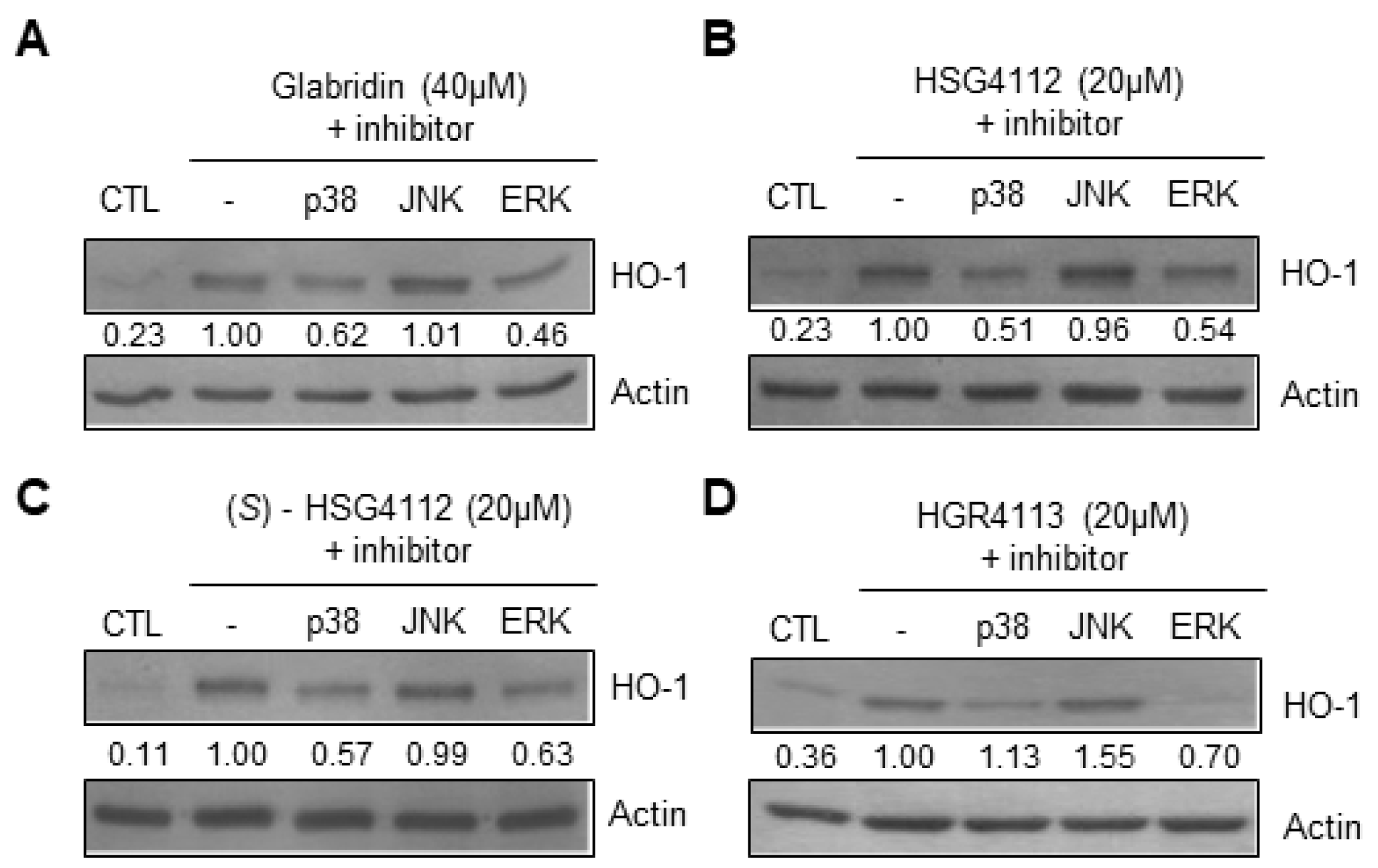
2.6. Effects of Compounds on MAPK Signaling Involved in HO-1 Induction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Viability Assay
4.3. NO Production
4.4. PGE2 Assay
4.5. Western Blot Analysis
4.6. Preparation of Cytosolic and Nuclear Fractions
4.7. DNA-Binding Activity of NF-κB
4.8. Quantitative Real-Time Reverse Transcriptase PCR (qRT-PCR) Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; Chen, L.; et al. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed Cell Death and Its Role in Inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef]
- Anderton, H.; Wicks, I.P.; Silke, J. Cell Death in Chronic Inflammation: Breaking the Cycle to Treat Rheumatic Disease. Nat. Rev. Rheumatol. 2020, 16, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef]
- Kanterman, J.; Sade-Feldman, M.; Baniyash, M. New Insights into Chronic Inflammation-Induced Immunosuppression. Semin. Cancer Biol. 2012, 22, 307–318. [Google Scholar] [CrossRef]
- Yang, Y.-Z.; Tang, Y.-Z.; Liu, Y.-H. Wogonoside Displays Anti-Inflammatory Effects through Modulating Inflammatory Mediator Expression Using RAW264. 7 Cells. J. Ethnopharmacol. 2013, 148, 271–276. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Li, D.-L.; Bi, X.-Y.; Sun, L.; Yu, X.-J.; Fang, H.-L.; Miao, Y.; Zhao, M.; He, X.; Liu, J.-J. Acetylcholine Inhibits LPS-Induced MMP-9 Production and Cell Migration via the A7 NAChR-JAK2/STAT3 Pathway in RAW264. 7 Cells. Cell. Physiol. Biochem. 2015, 36, 2025–2038. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-Inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Zhu, M.; Lai, J.; Wu, Z. Pharmacological Properties of Glabridin (a Flavonoid Extracted from Licorice): A Comprehensive Review. J. Funct. Foods 2021, 85, 104638. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza Spp. and Its Bioactive Constituents: Update and Review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef]
- Ao, M.; Shi, Y.; Cui, Y.; Guo, W.; Wang, J.; Yu, L. Factors Influencing Glabridin Stability. Nat. Prod. Commun. 2010, 5, 1907–1912. [Google Scholar] [CrossRef]
- Bae, I.Y.; Choi, M.S.; Ji, Y.S.; Yoo, S.-K.; Kim, K.; Yoo, H.H. Species Differences in Stereoselective Pharmacokinetics of HSG4112, A New Anti-Obesity Agent. Pharmaceutics 2020, 12, 127. [Google Scholar] [CrossRef]
- Choi, L.S.; Jo, I.G.; Kang, K.S.; Im, J.H.; Kim, J.; Kim, J.; Chung, J.W.; Yoo, S.-K. Discovery and Preclinical Efficacy of HSG4112, a Synthetic Structural Analog of Glabridin, for the Treatment of Obesity. Int. J. Obes. 2021, 45, 130–142. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK Signalling Pathways as Molecular Targets for Anti-Inflammatory Therapy—From Molecular Mechanisms to Therapeutic Benefits. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Gottschalk, R.A.; Martins, A.J.; Angermann, B.R.; Dutta, B.; Ng, C.E.; Uderhardt, S.; Tsang, J.S.; Fraser, I.D.C.; Meier-Schellersheim, M.; Germain, R.N. Distinct NF-ΚB and MAPK Activation Thresholds Uncouple Steady-State Microbe Sensing from Anti-Pathogen Inflammatory Responses. Cell Syst. 2016, 2, 378–390. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Lee, T.H.; Shank, J.; Cusson, N.; Kelliher, M.A. The Kinase Activity of Rip1 Is Not Required for Tumor Necrosis Factor-α-Induced IκB Kinase or P38 MAP Kinase Activation or for the Ubiquitination of Rip1 by Traf2. J. Biol. Chem. 2004, 279, 33185–33191. [Google Scholar] [CrossRef]
- Karin, M. Mitogen Activated Protein Kinases as Targets for Development of Novel Anti-Inflammatory Drugs. Ann. Rheum Dis. 2004, 63, ii62–ii64. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Qu, Y.; Wang, H. Cortisol Modulates Inflammatory Responses in LPS-Stimulated RAW264. 7 Cells via the NF-ΚB and MAPK Pathways. BMC Vet. Res. 2018, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Cheong, S.H.; Lee, K.J.; Sok, D.E.; Kim, M.R. Anti-Inflammatory Effects of Ribes Diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-ΚB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model. Antioxidants 2020, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kwon, O.K.; Ryu, H.W.; Paik, J.H.; Paryanto, I.; Yuniato, P.; Choi, S.H.; Oh, S.R.; Ahn, K.S. Anti-Inflammatory Effects of Passiflora foetida L. In LPS-Stimulated RAW264.7 Macrophages. Int. J. Mol. Med. 2018, 41, 3709–3716. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; May, M.J. The IκB Kinase Complex: Master Regulator of NF-ΚB Signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. Delivering Carbon Monoxide from a Prorous Material with an Entrapped Photoactive Manganese Carbonyl. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Shih, R.-H.; Yang, C.-M. Induction of Heme Oxygenase-1 Attenuates Lipopolysaccharide-Induced Cyclooxygenase-2 Expression in Mouse Brain Endothelial Cells. J. Neuroinflammation 2010, 7, 86. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Zhang, D.D. Phosphorylation of Nrf2 at Multiple Sites by MAP Kinases Has a Limited Contribution in Modulating the Nrf2-Dependent Antioxidant Response. PLoS ONE 2009, 4, e6588. [Google Scholar] [CrossRef]
- Lee, D.-S.; Jeong, G.-S. Butein Provides Neuroprotective and Anti-Neuroinflammatory Effects through Nrf2/ARE-Dependent Haem Oxygenase 1 Expression by Activating the PI3K/Akt Pathway. Br. J. Pharmacol. 2016, 173, 2894–2909. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, J. Anti-Inflammatory Activity of Butein and Luteolin Through Suppression of NFκB Activation and Induction of Heme Oxygenase-1. J. Med. Food 2015, 18, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in Health and Disease: A Comprehensive Review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Liu, G.; Yang, D.; Dong, Y.; Zhou, L. Glabridin Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting P38MAPK/ERK Signaling Pathway. Oncotarget 2017, 8, 18935. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE Pathway Activation, HO-1 and NQO1 Induction by Polychlorinated Biphenyl Quinone Is Associated with Reactive Oxygen Species and PI3K/AKT Signaling. Chem. Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Simmler, C.; Pauli, G.F.; Chen, S.N. Phytochemistry and Biological Properties of Glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Pharmacologically Active Compounds in the Environment and Their Chirality. Chem. Soc. Rev. 2010, 39, 4466–4503. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Cho, I.J.; Han, M.H.; Lee, C.W.; Park, S.-K.; Kim, H.M. Glabridin, an Isoflavan from Licorice Root, Inhibits Inducible Nitric-Oxide Synthase Expression and Improves Survival of Mice in Experimental Model of Septic Shock. J. Pharmacol. Exp. Ther. 2005, 312, 1187–1194. [Google Scholar] [CrossRef]
- Boaru, S.G.; Borkham-Kamphorst, E.; van de Leur, E.; Lehnen, E.; Liedtke, C.; Weiskirchen, R. NLRP3 Inflammasome Expression Is Driven by NF-ΚB in Cultured Hepatocytes. Biochem. Biophys. Res. Commun. 2015, 458, 700–706. [Google Scholar] [CrossRef]
- Manzoor, Z.; Koh, Y.S. Mitogen-Activated Protein Kinases in Inflammation. J. Bacteriol. Virol. 2012, 42, 189–195. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional Roles of P38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, H.J.; Choi, M.S.; Son, D.J.; Song, H.S.; Song, M.J.; Lee, J.M.; Han, S.B.; Kim, Y.; Hong, J.T. JNK Pathway Is Involved in the Inhibition of Inflammatory Target Gene Expression and NF-KappaB Activation by Melittin. J. Inflamm. 2008, 5, 7. [Google Scholar] [CrossRef]
- Kim, H.K. Role of ERK/MAPK Signalling Pathway in Anti-Inflammatory Effects of Ecklonia Cava in Activated Human Mast Cell Line-1 Cells. Asian Pac. J. Trop. Med. 2014, 7, 703–708. [Google Scholar] [CrossRef]
- Koga, Y.; Tsurumaki, H.; Aoki-Saito, H.; Sato, M.; Yatomi, M.; Takehara, K.; Hisada, T. Roles of Cyclic AMP Response Element Binding Activation in the ERK1/2 and P38 MAPK Signalling Pathway in Central Nervous System, Cardiovascular System, Osteoclast Differentiation and Mucin and Cytokine Production. Int. J. Mol. Sci. 2019, 20, 1346. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S. LPS-induced Proinflammatory Cytokine Expression in Human Airway Epithelial Cells and Macrophages via NF-κB, STAT3 or AP-1 Activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Choi, E.M.; Suh, K.S.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Chon, S. Glabridin Alleviates the Toxic Effects of Methylglyoxal on Osteoblastic MC3T3-E1 Cells by Increasing Expression of the Glyoxalase System and Nrf2/HO-1 Signaling and Protecting Mitochondrial Function. J. Agric. Food Chem. 2016, 64, 226–235. [Google Scholar] [CrossRef]
- Belinky, P.A.; Aviram, M.; Fuhrman, B.; Rosenblat, M.; Vaya, J. The Antioxidative Effects of the Isoflavan Glabridin on Endogenous Constituents of LDL during Its Oxidation. Atherosclerosis 1998, 137, 49–61. [Google Scholar] [CrossRef]
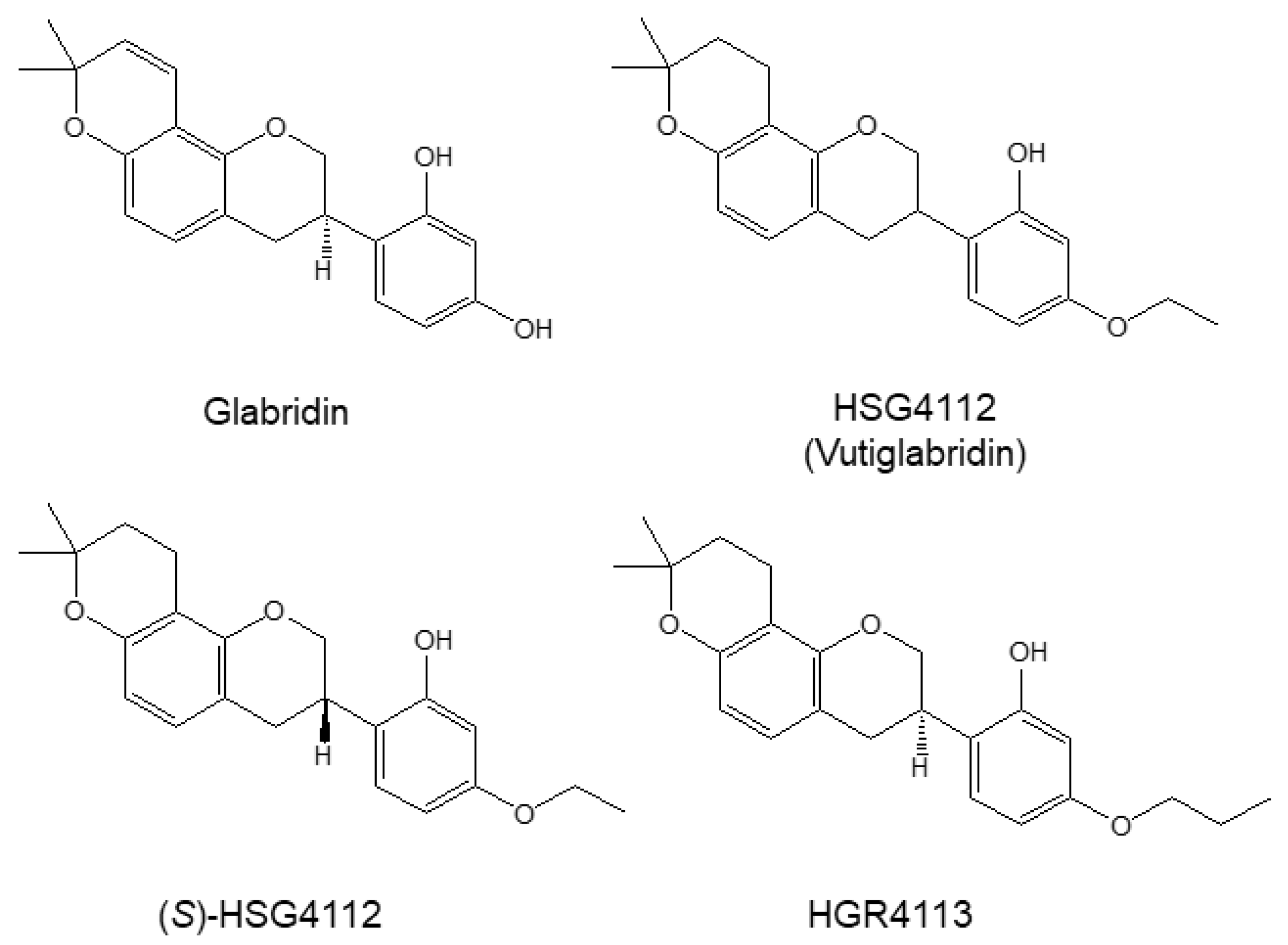
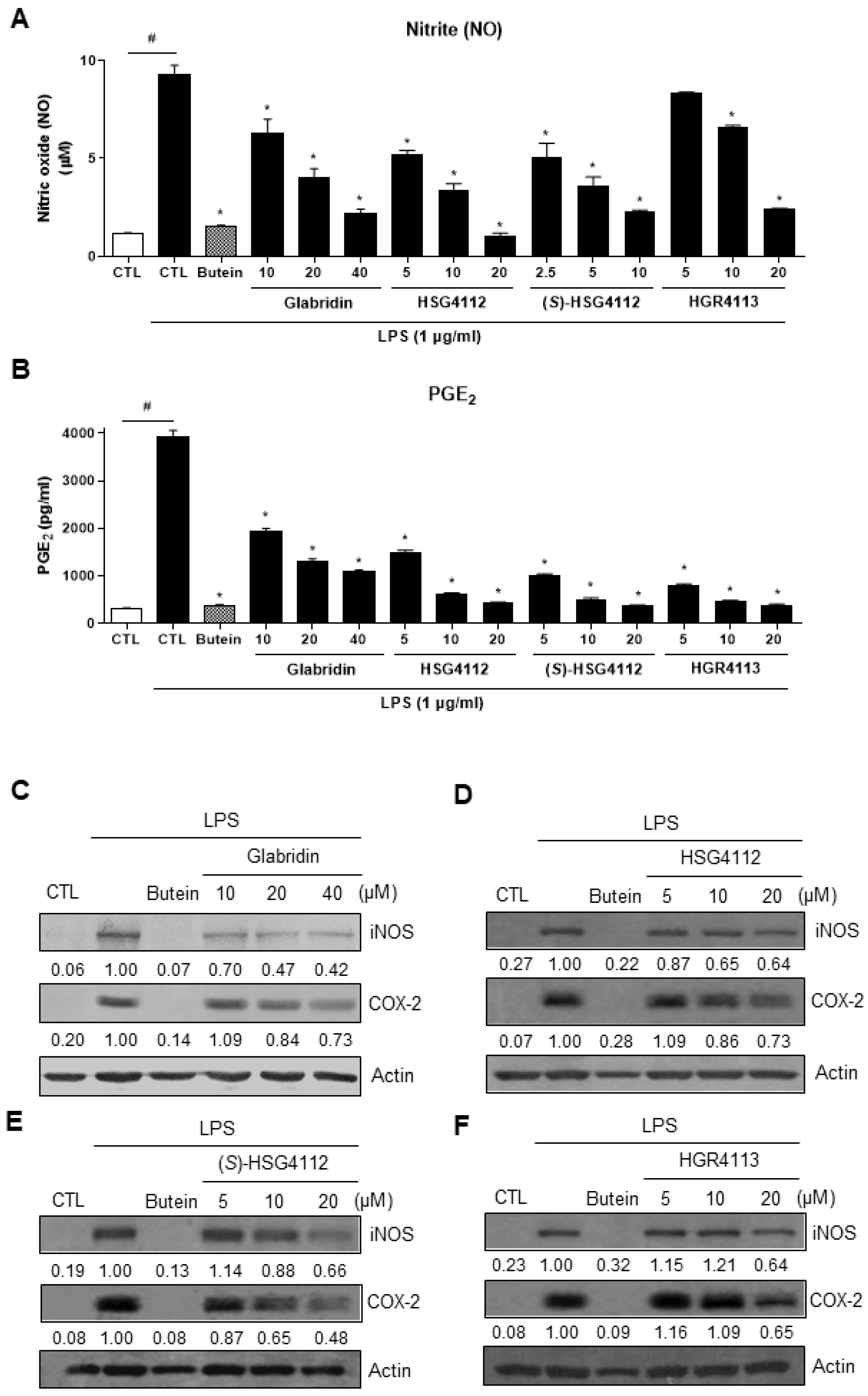
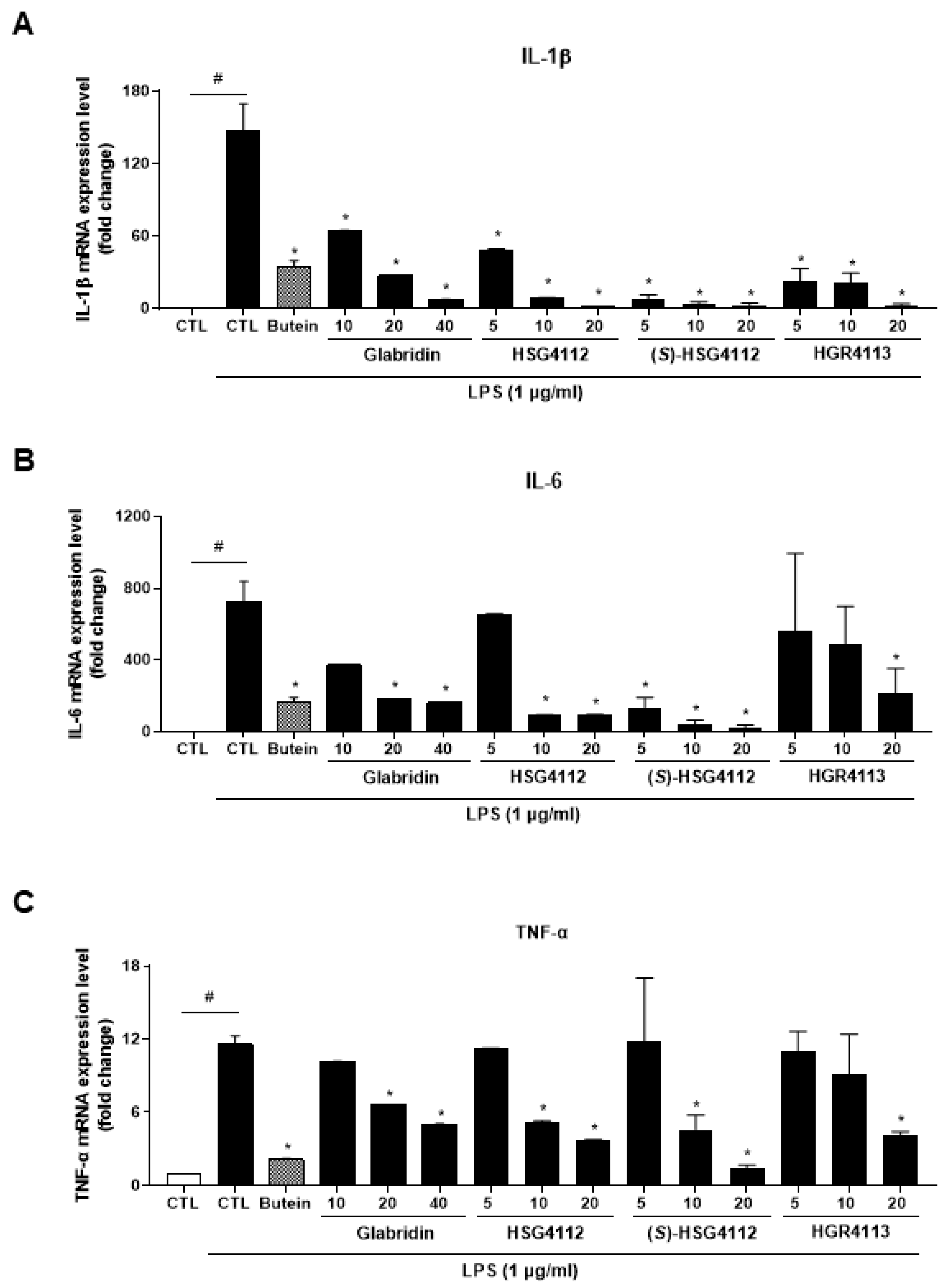
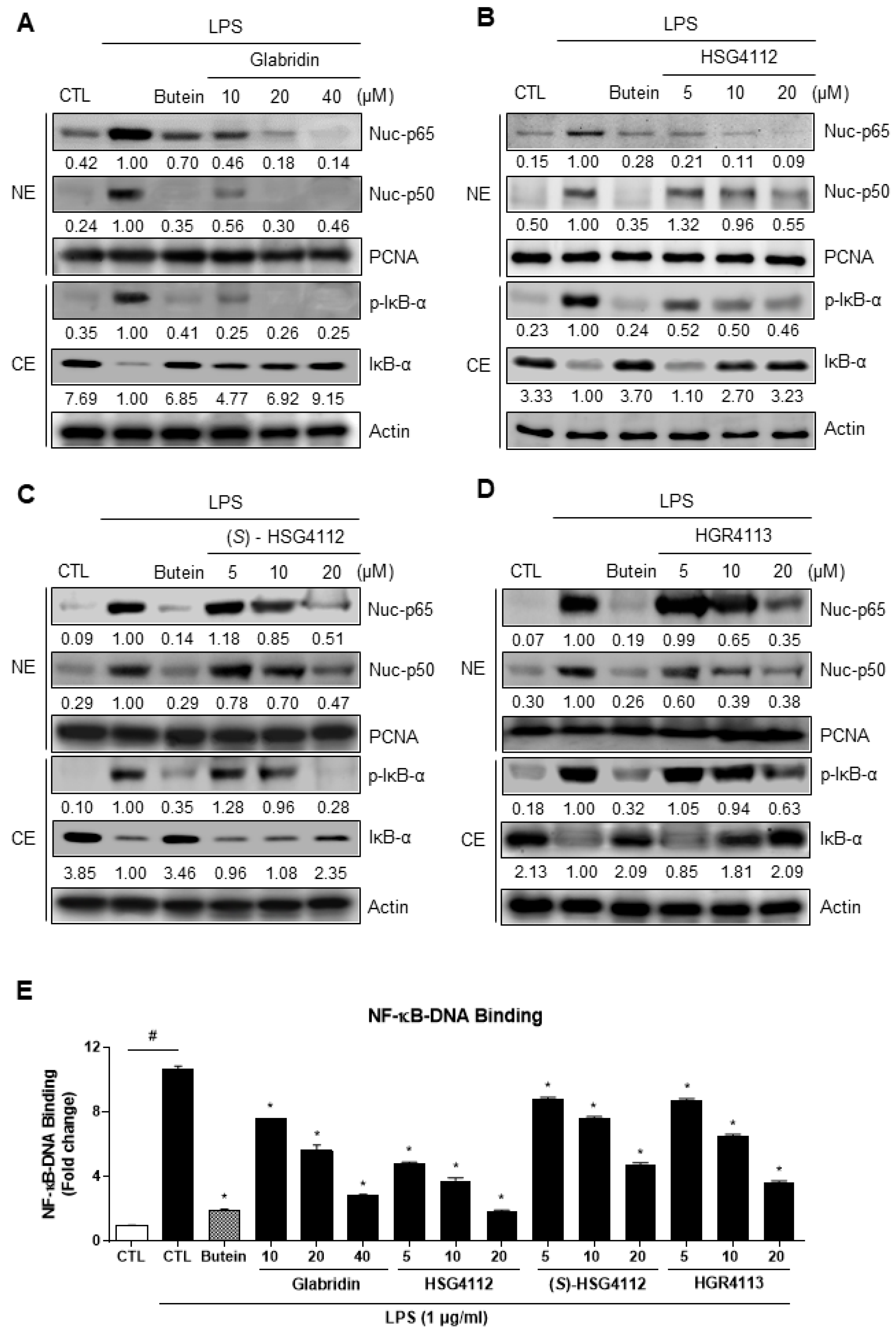

| Compound | NO (IC50, μM) | PGE2 (IC50, μM) | Cytokine mRNA Expression Inhibition | NF-κB Pathway Inhibition | MAPK Pathway Inhibition | HO-1 Induction by MAPK |
|---|---|---|---|---|---|---|
| Glabridin | 9.36 | 7.09 | IL-1β, IL-6, TNF-α | p-IκB-α | JNK, p38 | p38, ERK |
| HSG4112 | 6.79 | 3.55 | ERK | |||
| (S)-HSG4112 | 3.85 | 2.37 | JNK, p38 | |||
| HGR4113 | 11.32 | 1.64 | JNK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Choi, L.S.; Jeon, H.J.; Lee, H.M.; Kim, S.H.; Kim, K.-W.; Ko, W.; Oh, H.; Park, H.S. Synthetic Glabridin Derivatives Inhibit LPS-Induced Inflammation via MAPKs and NF-κB Pathways in RAW264.7 Macrophages. Molecules 2023, 28, 2135. https://doi.org/10.3390/molecules28052135
Shin J, Choi LS, Jeon HJ, Lee HM, Kim SH, Kim K-W, Ko W, Oh H, Park HS. Synthetic Glabridin Derivatives Inhibit LPS-Induced Inflammation via MAPKs and NF-κB Pathways in RAW264.7 Macrophages. Molecules. 2023; 28(5):2135. https://doi.org/10.3390/molecules28052135
Chicago/Turabian StyleShin, Jaejin, Leo Sungwong Choi, Hyun Ju Jeon, Hyeong Min Lee, Sang Hyo Kim, Kwan-Woo Kim, Wonmin Ko, Hyuncheol Oh, and Hyung Soon Park. 2023. "Synthetic Glabridin Derivatives Inhibit LPS-Induced Inflammation via MAPKs and NF-κB Pathways in RAW264.7 Macrophages" Molecules 28, no. 5: 2135. https://doi.org/10.3390/molecules28052135
APA StyleShin, J., Choi, L. S., Jeon, H. J., Lee, H. M., Kim, S. H., Kim, K.-W., Ko, W., Oh, H., & Park, H. S. (2023). Synthetic Glabridin Derivatives Inhibit LPS-Induced Inflammation via MAPKs and NF-κB Pathways in RAW264.7 Macrophages. Molecules, 28(5), 2135. https://doi.org/10.3390/molecules28052135





