Structurally Different Exogenic Brassinosteroids Protect Plants under Polymetallic Pollution via Structure-Specific Changes in Metabolism and Balance of Cell-Protective Components
Abstract
1. Introduction
- -
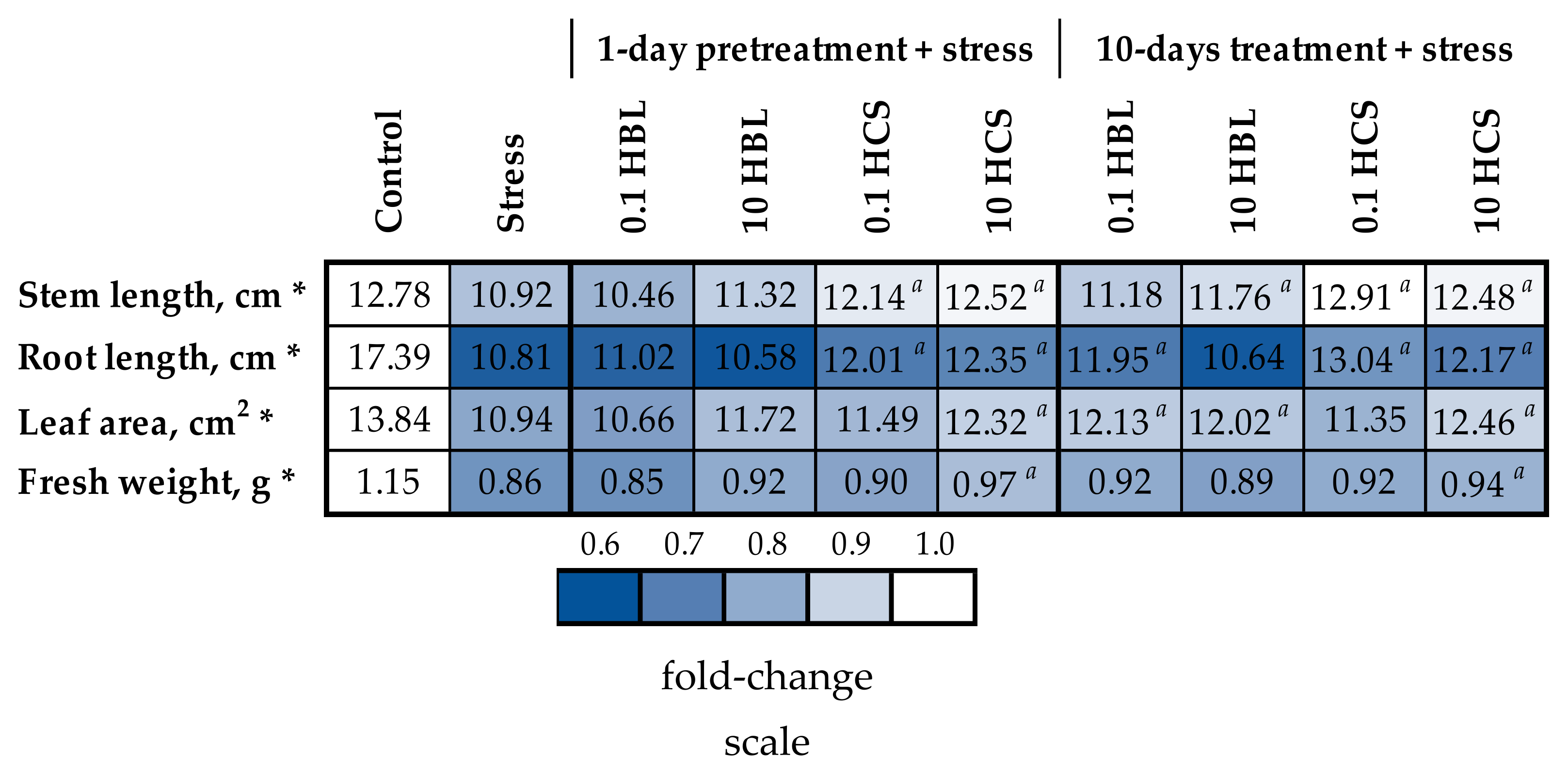
- To compare the effects of different methods of HBL and HCS application on the morphological parameters of barley plants under polymetallic stress;
- -
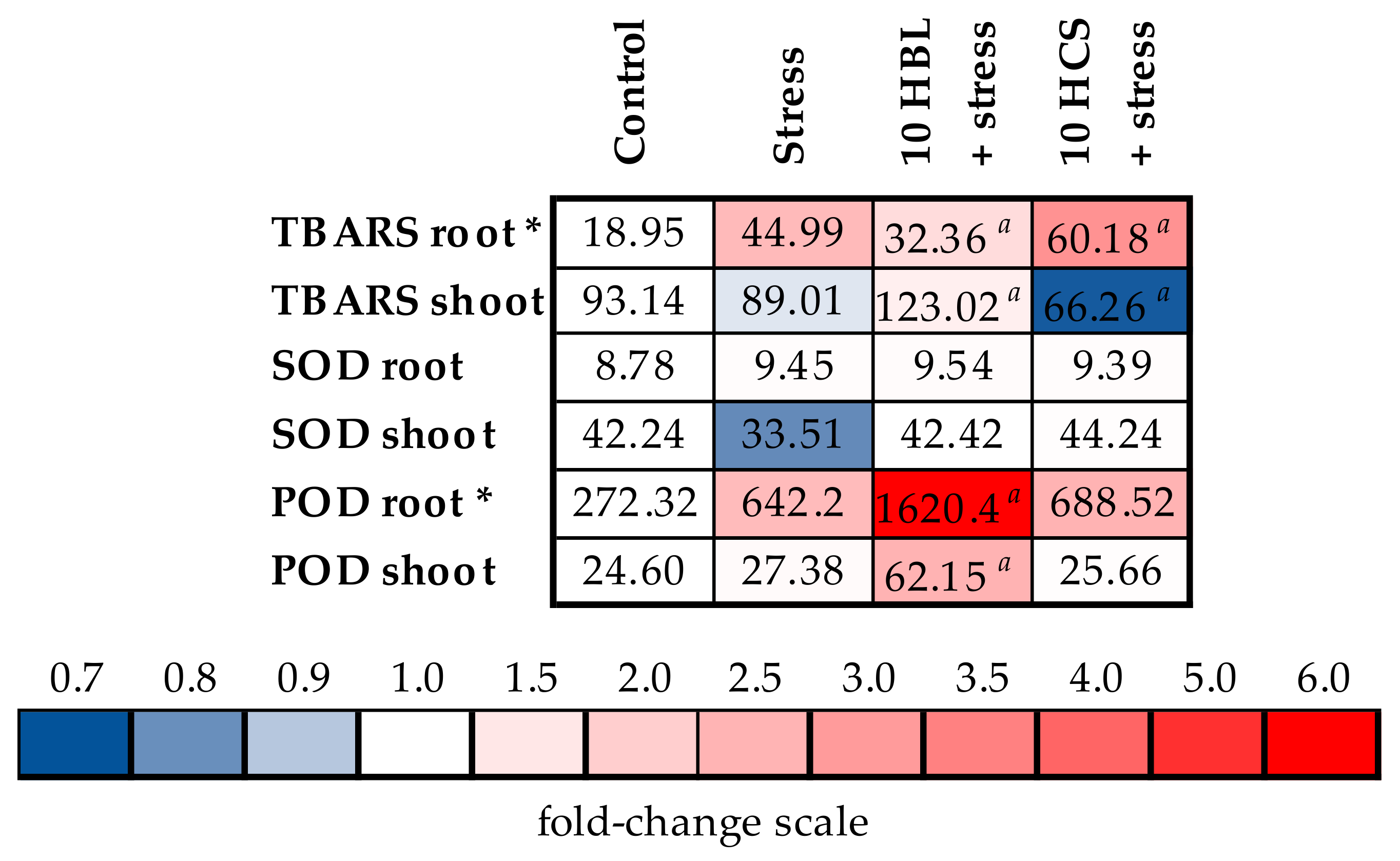
- To study the influence of brassinosteroids on the photosynthetic apparatus and the antioxidant system of plants;
- -
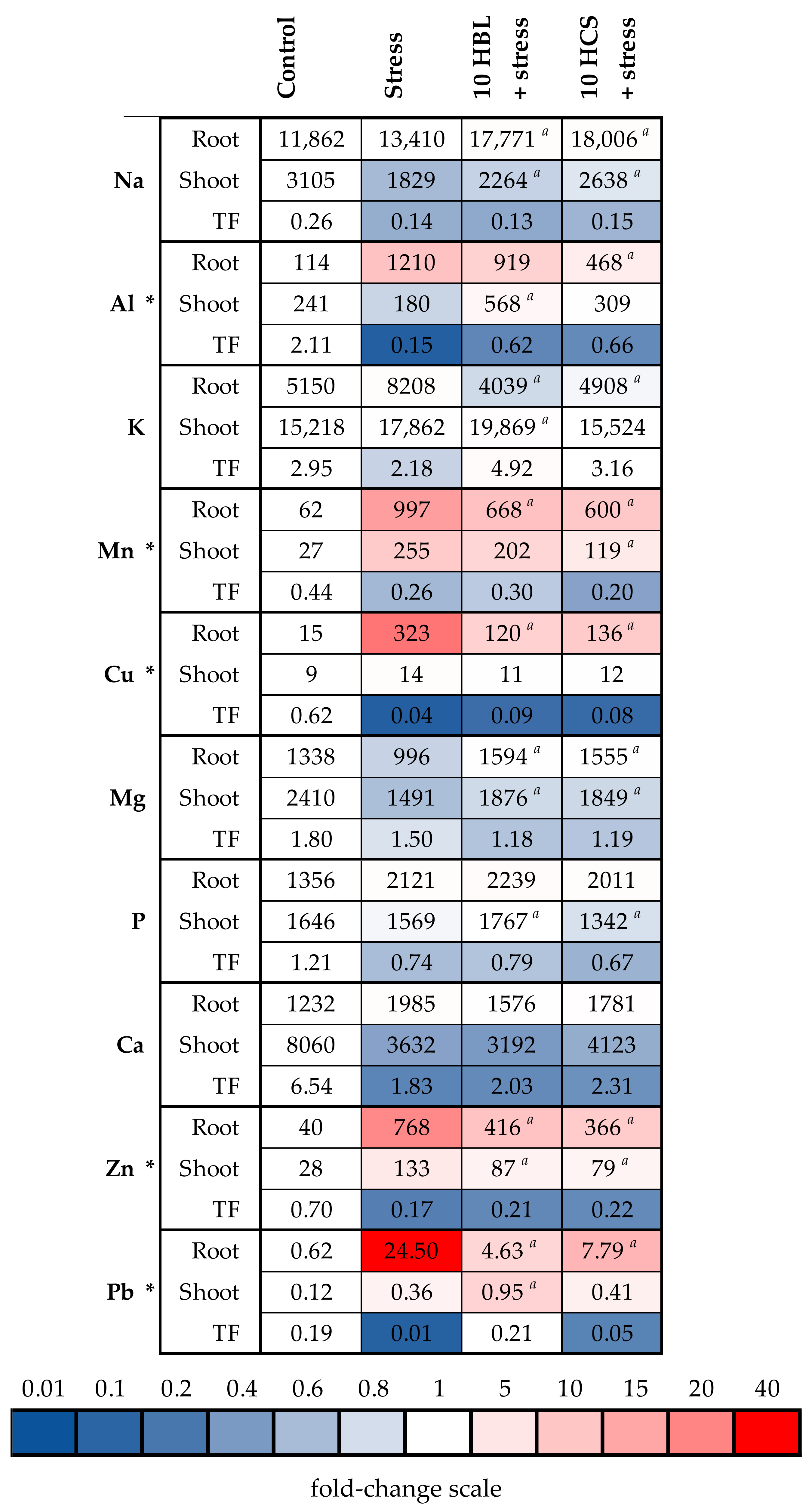
- To study how hormones influence the accumulation of toxic and essential elements in the roots and shoots of barley plants and the functioning of some systems of detoxification of excessive elements;
- -
- To reveal the effect of polymetallic stress on the accumulation of endogenous B-lactones and B-ketones.
2. Results
2.1. Plant Growth and Morphology
2.2. Plant Photosynthetic Apparatus
2.3. Oxidative Stress and Antioxidant Enzymes
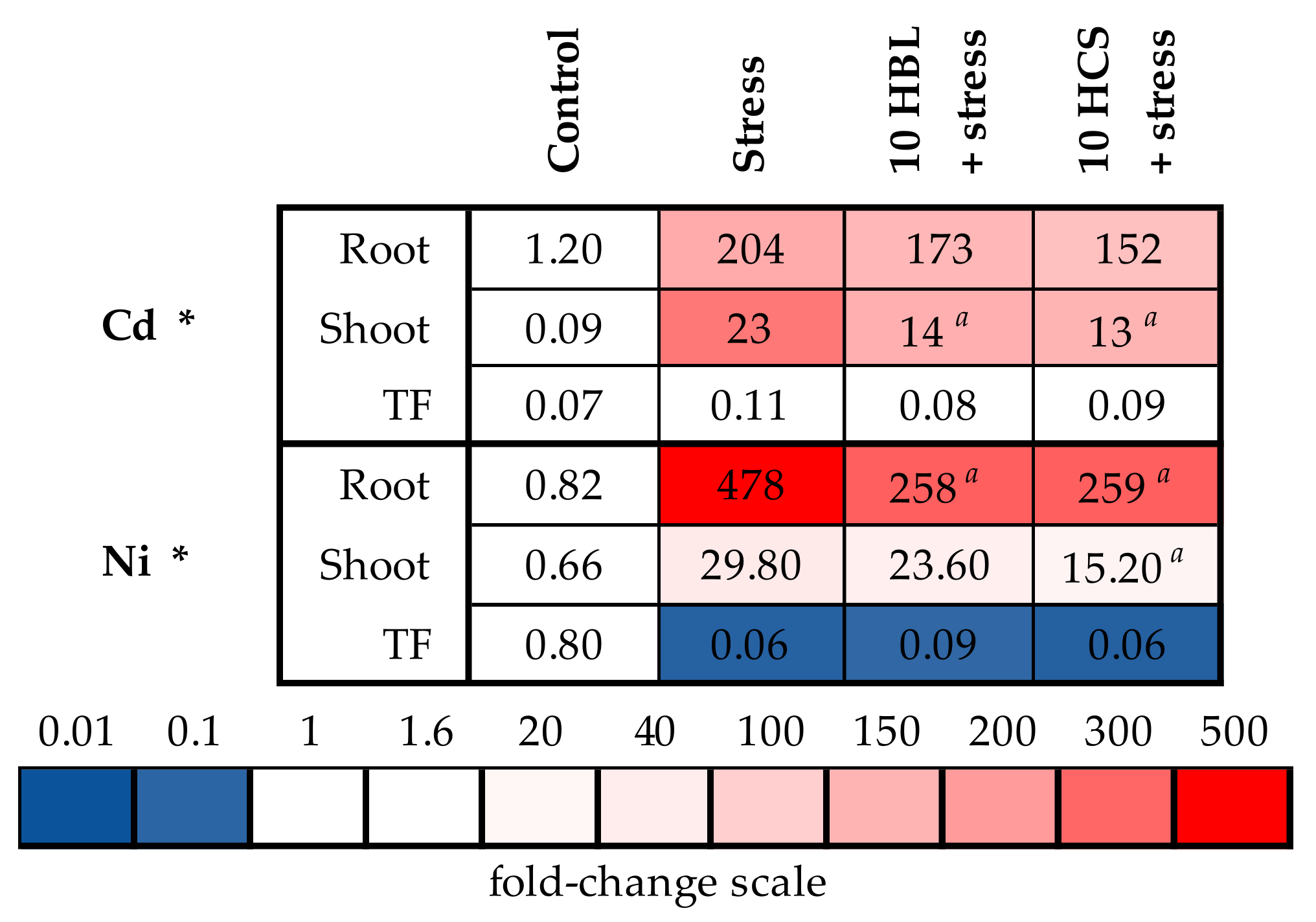
2.4. Accumulation of Mineral Elements
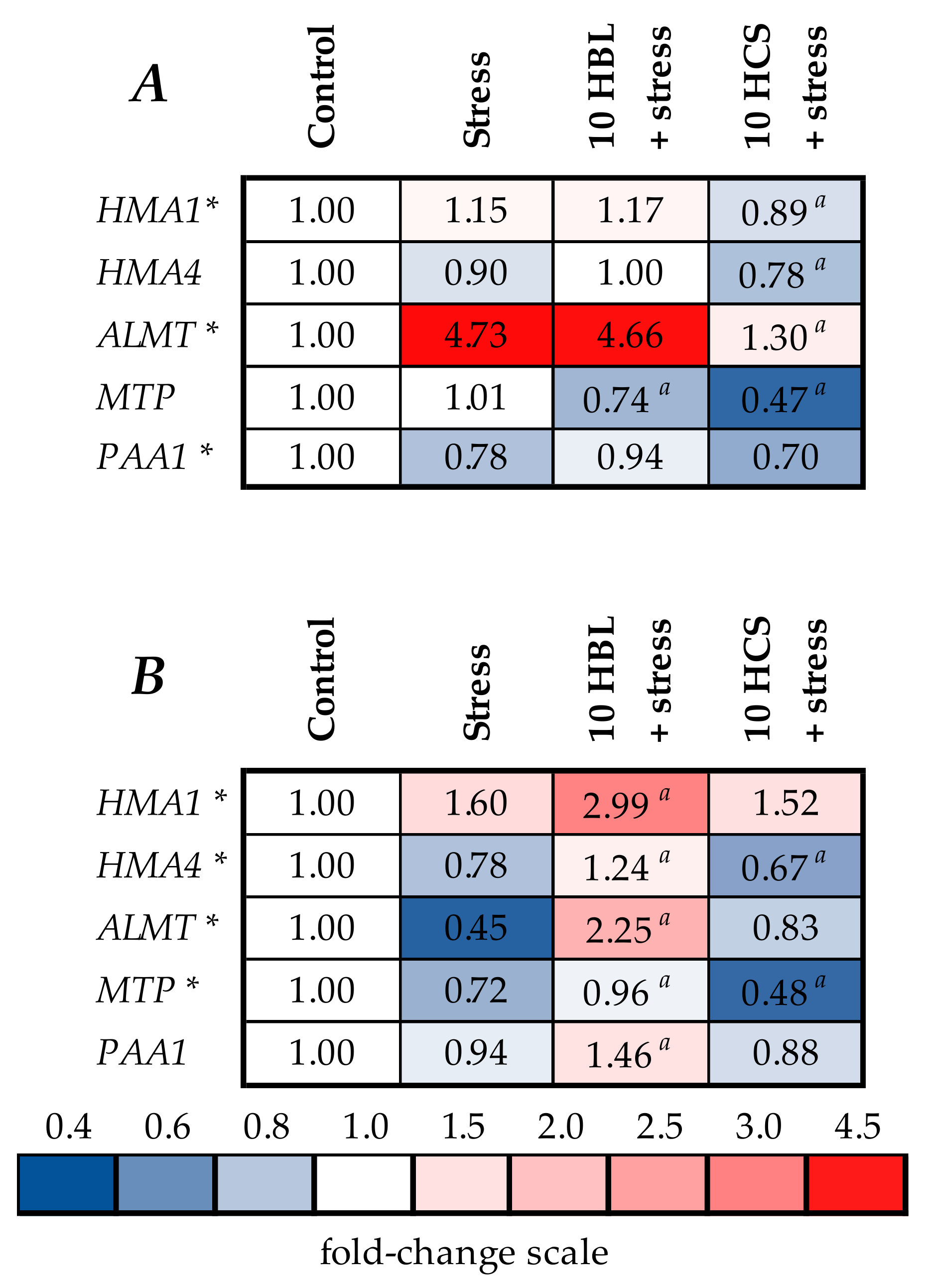
2.5. Expression of Genes of Metal Detoxification
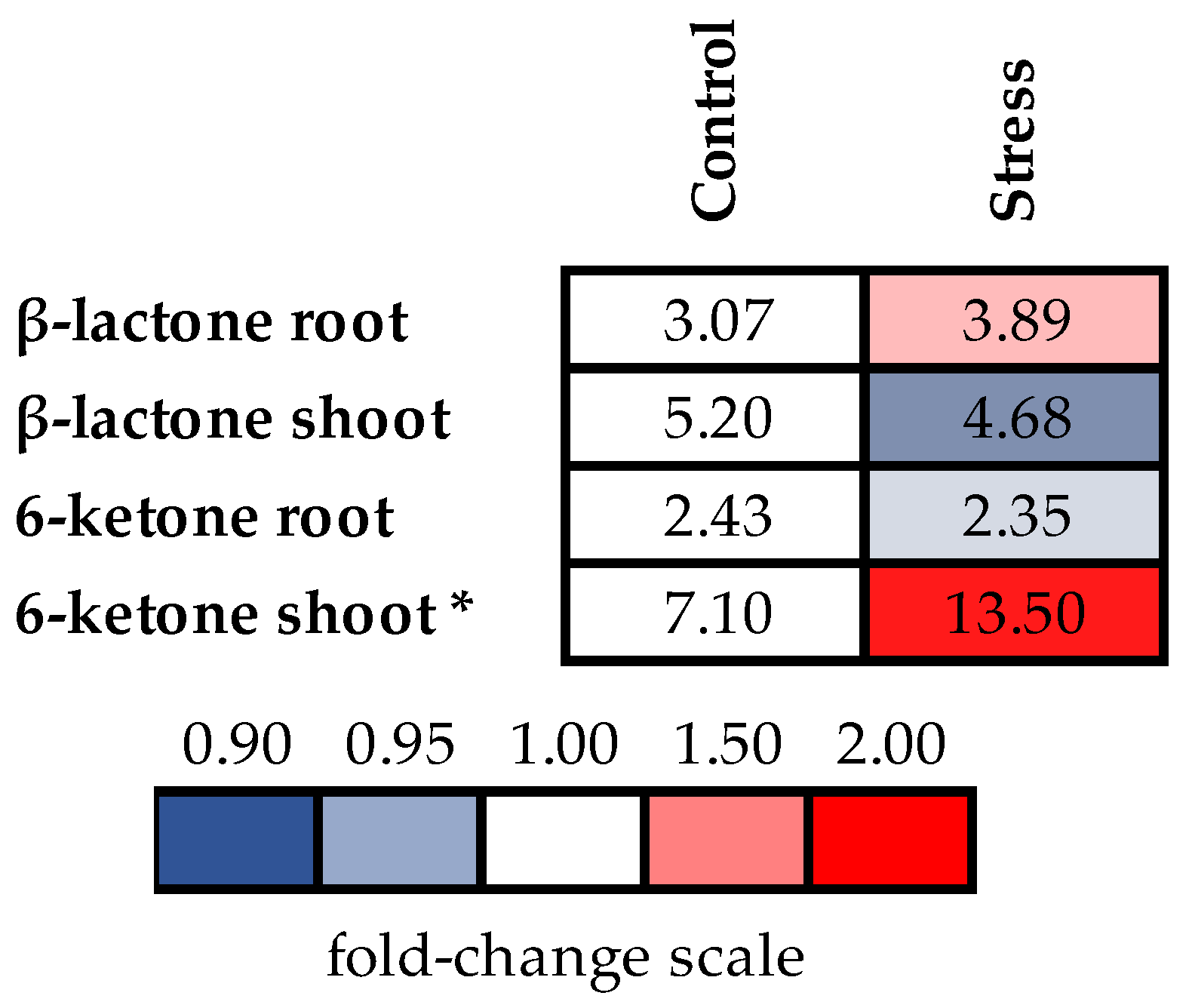
2.6. Accumulation of Endogenous Brassinosteroids
3. Discussion
4. Materials and Methods
- Control variant with the standard nutrient medium.
- Polymetallic stress by the addition of excessive metal ions to the medium. The effective concentrations of heavy metals (Mn2+, Cd2+, Cu2+, Ni2+, Zn2+, and Pb2+) and aluminum (Al3+) were selected on the basis of the typical concentrations of these ions in soil solutions of industrially polluted acidic soils [1,17] and on the basis of the results of our previous experiments [28,29], see Table 1.
- Pretreatment with 0.1 nM HBL for 1 day by addition to the nutrient medium with the following 10-day polymetallic stress.
- Pretreatment with 10 nM HBL for 1 day with the following 10-day polymetallic stress.
- Pretreatment with 0.1 nM HCS for 1 day with the following 10-day polymetallic stress.
- Pretreatment with 10 nM HCS for 1 day with the following 10-day polymetallic stress.
- Simultaneous treatment with 0.1 nM HBL added to the nutrient medium and with polymetallic stress.
- Simultaneous treatment with 10 nM HBL and with polymetallic stress.
- Simultaneous treatment with 0.1 nM HCS and with polymetallic stress.
- Simultaneous treatment with 10 nM HCS and with polymetallic stress.
- Control variant with the standard nutrient medium.
- Polymetallic stress by the addition of excessive metal ions to the medium.
- Simultaneous treatment with 10 nM HBL was added to the nutrient medium and polymetallic stress.
- Simultaneous treatment with 10 nM HCS was added to the nutrient medium and polymetallic stress.
4.1. Physiological and Biochemical Analyses
4.1.1. Determination of Growth Parameters
4.1.2. Determination of the Lipid Peroxidation Level
4.1.3. Determination of the Photosynthetic Pigments Content
4.1.4. Determination of Photosynthetic Activity
4.1.5. Determination of the Activity of Antioxidant Enzymes
4.2. Analysis of Elemental Composition of Shoots and Roots
4.2.1. Determination of the Endogenous Content of Brassinosteroids
4.2.2. RNA Isolation and cDNA Synthesis
4.2.3. Identification of Candidate PCR Reference and Target Genes and Primer Design
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| BS | brassinosteroid |
| ETR | electron transport rate |
| Fv/Fm | maximal quantum yields of PSII |
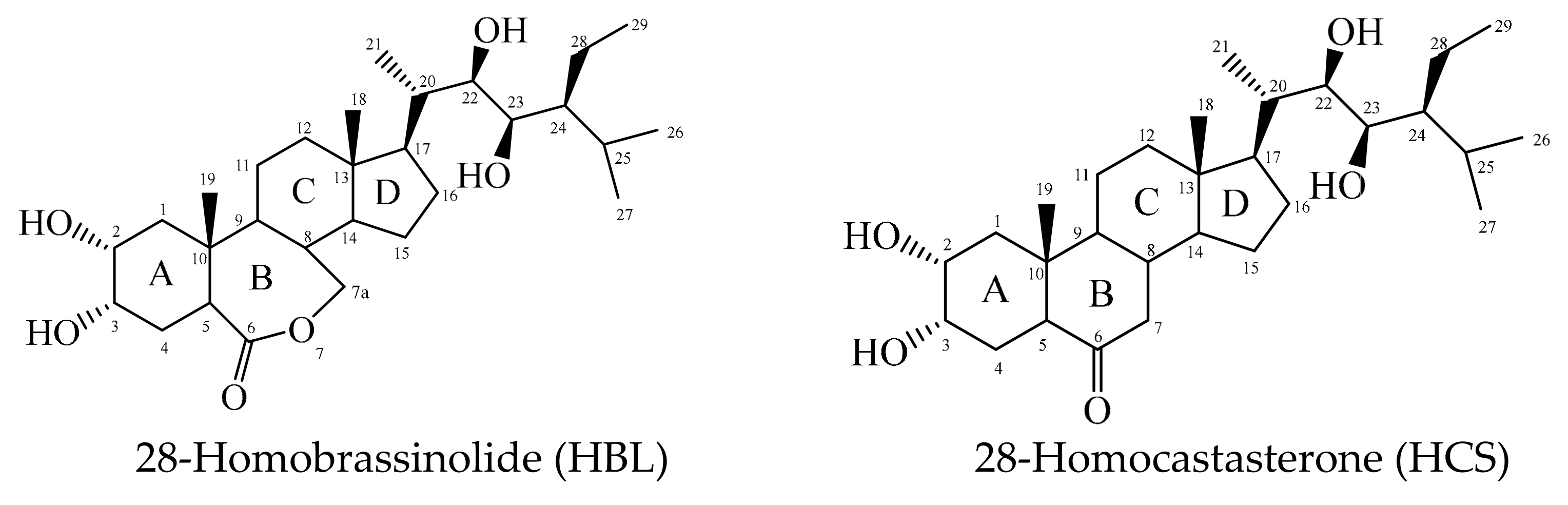
| HBL | 28-homobrassinolide |
| HCS | 28-homocastasterone |
| POD | peroxidase |
| qL | coefficients of photochemical quenching based on the “lake” model |
| qP | coefficients of photochemical quenching based on the “puddle” model |
| SOD | superoxide dismutase |
| TBARS | complex between thiobarbituric acid and thiobarbituric acid-reactive substances |
| TF | translocation factor |
| Y(NO) | quantum yields of nonregulated energy dissipation |
| Y(NPQ) | quantum yields of and regulated energy dissipation |
| Y(II) | effective quantum yields of PSII |
References
- Kopittke, P.M.; Blamey, F.P.C.; Asher, C.J.; Menzies, N.W. Trace metal phytotoxicity in solution culture: A review. J. Exp. Bot. 2010, 61, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Villiers, F.; Jourdain, A.; Bastien, O.; Leonhardt, N.; Fujioka, S.; Tichtincky, G.; Parcy, F.; Bourguignon, J.; Hugouvieux, V. Evidence for functional interaction between brassinosteroids and cadmium response in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Plant Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Blamey, F.P.C. Theoretical and experimental assessment of nutrient solution composition in short-term studies of aluminum rhizotoxicity. Plant Soil 2016, 406, 311–326. [Google Scholar] [CrossRef]
- Gomes, M.M.A. Physiological effects related to brassinosteroid application in plants. In Brassinosteroids: A Class of Plant Hormone; Springer: Dordrecht, The Netherlands, 2011; pp. 193–242. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculik, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.U.; Dong, Z. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Rao, X.; Dixon, R.A. Brassinosteroid mediated cell wall remodeling in grasses under abiotic stress. Front. Plant Sci. 2017, 8, 8061143. [Google Scholar] [CrossRef]
- Vriet, C.; Russinova, E.; Reuzeau, C. Boosting crop yields with plant steroids. Plant Cell 2012, 24, 842–857. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Xu, H.; Zeng, W.; Yu, R.; Wu, X.; Li, S.; Liu, Y.; Li, J. The potential role of brassinosteroids (BRs) in alleviating antimony (Sb) stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 51–59. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Danilova, E.D.; Khripach, V.A.; Zhabinskyi, V.N.; Kuznetsov, V.V.; Efimova, M.V. Ability of lactone- and ketone-containing brassinosteroids to induce priming in rapeseed plants to salt stress. Russ. J. Plant Physiol. 2021, 68, 499–509. [Google Scholar] [CrossRef]
- Kovtun, I.S.; Kukharenko, N.E.; Kuznetsov, V.V.; Khripach, V.A.; Efimova, M.V. Effect of lactone- and ketone-containing brassinosteroids on photosynthetic activity of barley leaves during aging. Russ. J. Plant Physiol. 2021, 68, 440–450. [Google Scholar] [CrossRef]
- Blamey, F.P.C.; Hernandez-Soriano, M.C.; Cheng, M.; Tang, C.; Paterson, D.J.; Lombi, E.; Wang, W.H.; Scheckel, K.G.; Kopittke, P.M. Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiol. 2015, 169, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, P.; Menzies, N.W.; Kopittke, P.M. Defining appropriate methods for studying toxicities of trace metals in nutrient solutions. Ecotoxicol. Environ. Saf. 2018, 147, 872–880. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Pal, M.; Janda, T.; Szalai, G. Interactions between plant hormones and thiol-related heavy metal chelators. Plant Growth Regul. 2018, 85, 173–185. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef]
- Kaur, N.; Pati, P.K. Harnessing the potential of brassinosteroids in abiotic stress tolerance in plants. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 407–423. [Google Scholar] [CrossRef]
- Campbell, D.A.; Tyystjärvi, E. Parameterization of photosystem II photoinactivation and repair. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 258–265. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuine, S.; Triantaphylides, C.; Ravanat, J.L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhardwaj, R. Effect of 24-epibrassinolide on seed germination, seedling growth and heavy metal uptake in Brassica junceae L. Gen. Appl. Plant Physiol. 2007, 33, 59–73. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, P.; Arora, H.K.; Arora, N. 28-Homobrassinolide regulated Mn-uptake and growth of Brassica juncea L. Can. J. Pure Appl. Sci. 2008, 2, 149–154. [Google Scholar]
- Ramakrishna, B.; Rao, S.S.R. Preliminary studies on the involvement of glutathione metabolism and redox status against zinc toxicity in radish seedlings by 28-homobrassinolide. Environ. Exp. Bot. 2013, 96, 52–58. [Google Scholar] [CrossRef]
- Marschner, H. (Ed.) Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; 651p. [Google Scholar] [CrossRef]
- Danilova, E.D.; Kuznetsov, V.V.; Efimova, M.V.; Zlobin, I.E. Exogenic melatonin reduces the toxic effect of polymetallic stress on barley plants. Dokl. Biochem. Biophys. 2021, 499, 228–232. [Google Scholar] [CrossRef]
- Danilova, E.D.; Litvinovskaya, R.P.; Zlobin, I.E.; Kolomeichuk, L.V.; Murgan, O.K.; Sauchuk, A.L.; Khripach, V.A.; Kuznetsov, V.V.; Efimova, M.V. Polymetallic stress changes the endogenous status of brassinosteroids and reduces the effectiveness of photochemical reactions photosystem II in barley plants. Dokl. Biochem. Biophys. 2022, 504, 123–127. [Google Scholar] [CrossRef]
- Anikiev, V.V.; Kutuzov, F.F. Novyj sposob opredelenija ploshhadi listovoj poverhnosti u zlakov [A new method for leaf area estimation in cereals]. Russ. J. Plant Physiol. 1961, 8, 375–377. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Efimova, M.V.; Khripach, V.A.; Boyko, E.V.; Malofii, M.K.; Kolomeichuk, L.V.; Murgan, O.K.; Vidershpun, A.N.; Mukhamatdinova, E.A.; Kuznetsov, V.V. The priming of potato plants induced by brassinosteroids reduces oxidative stress and increases salt tolerance. Dokl. Biol. Sci. 2018, 478, 33–36. [Google Scholar] [CrossRef]
- Esen, A. A simple method for quantitative, semiquantitative, and qualitative assay of protein. Anal. Biochem. 1978, 89, 264–273. [Google Scholar] [CrossRef]
- Pradko, A.G.; Litvinovskaya, R.P.; Sauchuk, A.L.; Drach, S.V.; Baranovsky, A.V.; Zhabinskii, V.N.; Mirantsova, T.V.; Khripach, V.A. A new ELISA for quantification of brassinosteroids in plants. Steroids 2015, 97, 78–86. [Google Scholar] [CrossRef]
- Efimova, M.V.; Litvinovskaya, R.P.; Medvedeva, Y.V.; Murgan, O.K.; Sauchuk, A.L.; Kuznetsov, V.V.; Khripach, V.A. The endogenous brassinosteroid content and balance in potato microclones is determined by organ specificity and the variety ripening term. Dokl. Biol. Sci. 2019, 485, 33–36. [Google Scholar] [CrossRef]
- Wang, X.K.; Gong, X.; Cao, F.; Wang, Y.; Zhang, G.; Wu, F. HvPAA1 encodes a P-Type ATPase, a novel gene for cadmium accumulation and tolerance in barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2019, 20, 1732. [Google Scholar] [CrossRef]
- Tiong, J.; McDonald, G.; Genc, Y.; Shirley, N.; Langridge, P.; Huang, C.Y. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare). New Phytol. 2015, 207, 1097–1109. [Google Scholar] [CrossRef]

| Metal | Concentration, µM |
|---|---|
| Al3+ | 20 |
| Mn2+ | 50 |
| Cd2+ | 2.8 |
| Cu2+ | 2 |
| Ni2+ | 16 |
| Zn2+ | 40 |
| Pb2+ | 30 |
| Gene ID | Gene | F (5′→3′) | R (5′→3′) | Tm, °C | Amplicon Size, bp | References |
|---|---|---|---|---|---|---|
| LOC123430406 | Actin, the gene encoding the protein actin | TGGCTGACGGTGAGGACA | CGAGGGCGACCAACTATG | 61 | 121 | [38] |
| LOC123413551 | HvGAPDH, the gene encoding glyceraldehyde-3-phosphate dehydrogenase 1 | GTGAGGCTGGTGCTGATTACG | TGGTGCAGCTAGCATTTGAGAC | 61 | 198 | [39] |
| LOC123406919 | HvPAA1, encodes a specific ATPase for Cu2+/Ag2+ transfer | ATGTGCTTGGTCTTGCCA | TCCCTCGCTGTGAGAAGCTA | 53 | 194 | [38] |
| LOC123407761 | HMA1, encodes a specific ATPase for Zn2+/Cu2+/Cd2+/Pb2+ transfer | CCATGTGCATTGGCAGTAGC | AATACATGCCCGCCTTTCAA | 59 | 92 (512) | |
| LOC123401671 | HMA4, encodes a specific ATPase forZn2+/Cu2+/Cd2+/Pb2+ transfer | GACAGTGGTGGCAGGATTGAAGG | TGGTTCTTGCATCGGTCTCCTCG | 64 | 104 | |
| LOC123414343 | HvMTP1, encodes a metal resistance protein | CGCAGGATGTGGATGCTGAT | CTCCAGCACCAAAGGCAACA | 61 | 223 | [39] |
| LOC123430267 | ALMT, encodes the protein aluminum-activated malate transporter | CGGAGCTCTTTGTCGTCAGT | CATTTCCCCACACGCCATTC | 60 | 133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlobin, I.E.; Danilova, E.D.; Murgan, O.K.; Kolomeichuk, L.V.; Litvinovskaya, R.P.; Sauchuk, A.L.; Kuznetsov, V.V.; Efimova, M.V. Structurally Different Exogenic Brassinosteroids Protect Plants under Polymetallic Pollution via Structure-Specific Changes in Metabolism and Balance of Cell-Protective Components. Molecules 2023, 28, 2077. https://doi.org/10.3390/molecules28052077
Zlobin IE, Danilova ED, Murgan OK, Kolomeichuk LV, Litvinovskaya RP, Sauchuk AL, Kuznetsov VV, Efimova MV. Structurally Different Exogenic Brassinosteroids Protect Plants under Polymetallic Pollution via Structure-Specific Changes in Metabolism and Balance of Cell-Protective Components. Molecules. 2023; 28(5):2077. https://doi.org/10.3390/molecules28052077
Chicago/Turabian StyleZlobin, Ilya E., Elena D. Danilova, Ol’ga K. Murgan, Liliya V. Kolomeichuk, Raisa P. Litvinovskaya, Alina L. Sauchuk, Vladimir V. Kuznetsov, and Marina V. Efimova. 2023. "Structurally Different Exogenic Brassinosteroids Protect Plants under Polymetallic Pollution via Structure-Specific Changes in Metabolism and Balance of Cell-Protective Components" Molecules 28, no. 5: 2077. https://doi.org/10.3390/molecules28052077
APA StyleZlobin, I. E., Danilova, E. D., Murgan, O. K., Kolomeichuk, L. V., Litvinovskaya, R. P., Sauchuk, A. L., Kuznetsov, V. V., & Efimova, M. V. (2023). Structurally Different Exogenic Brassinosteroids Protect Plants under Polymetallic Pollution via Structure-Specific Changes in Metabolism and Balance of Cell-Protective Components. Molecules, 28(5), 2077. https://doi.org/10.3390/molecules28052077







