BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway
Abstract
1. Introduction
2. Results
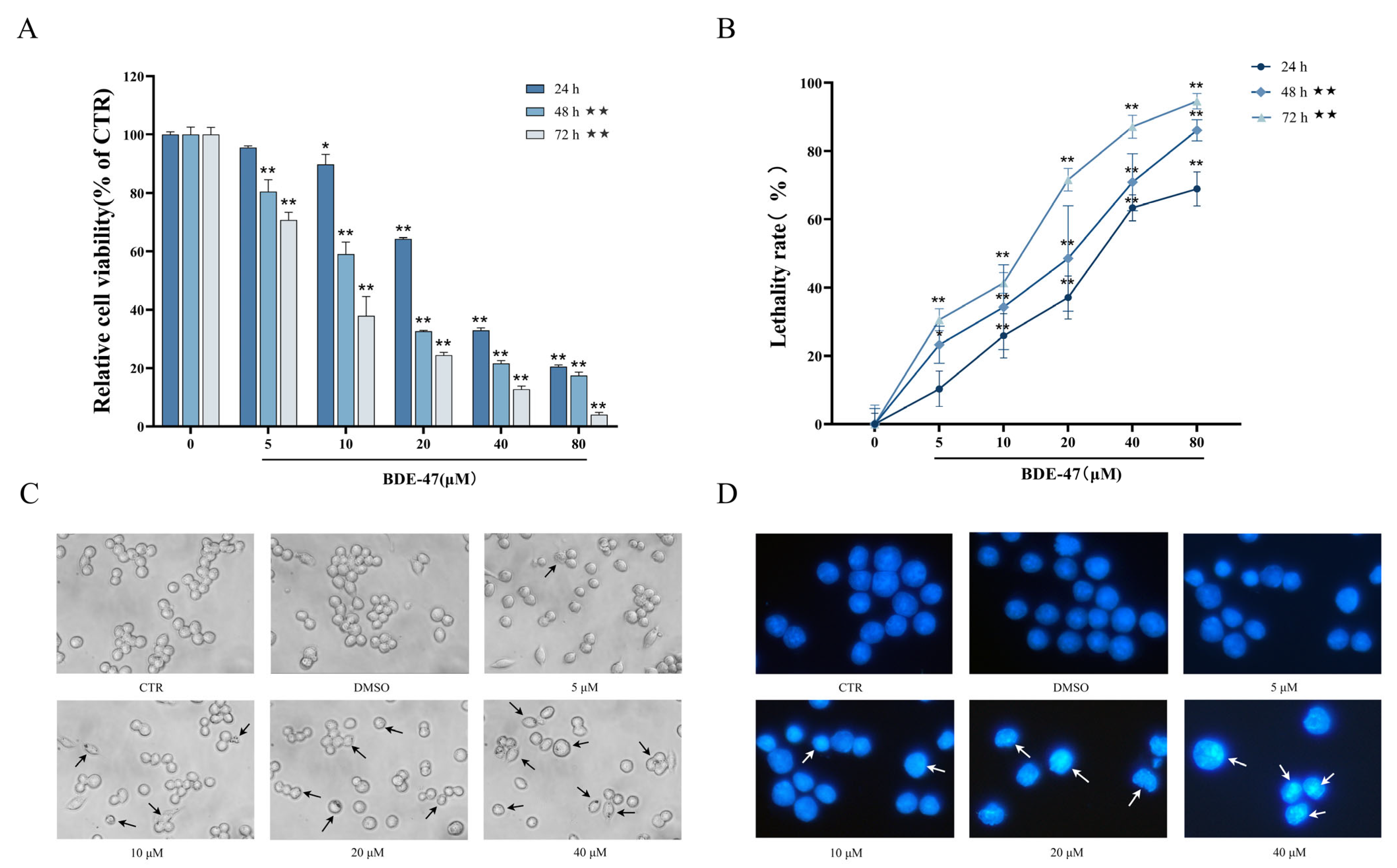
2.1. Effects of BDE-47 on Cell Viability and Morphology
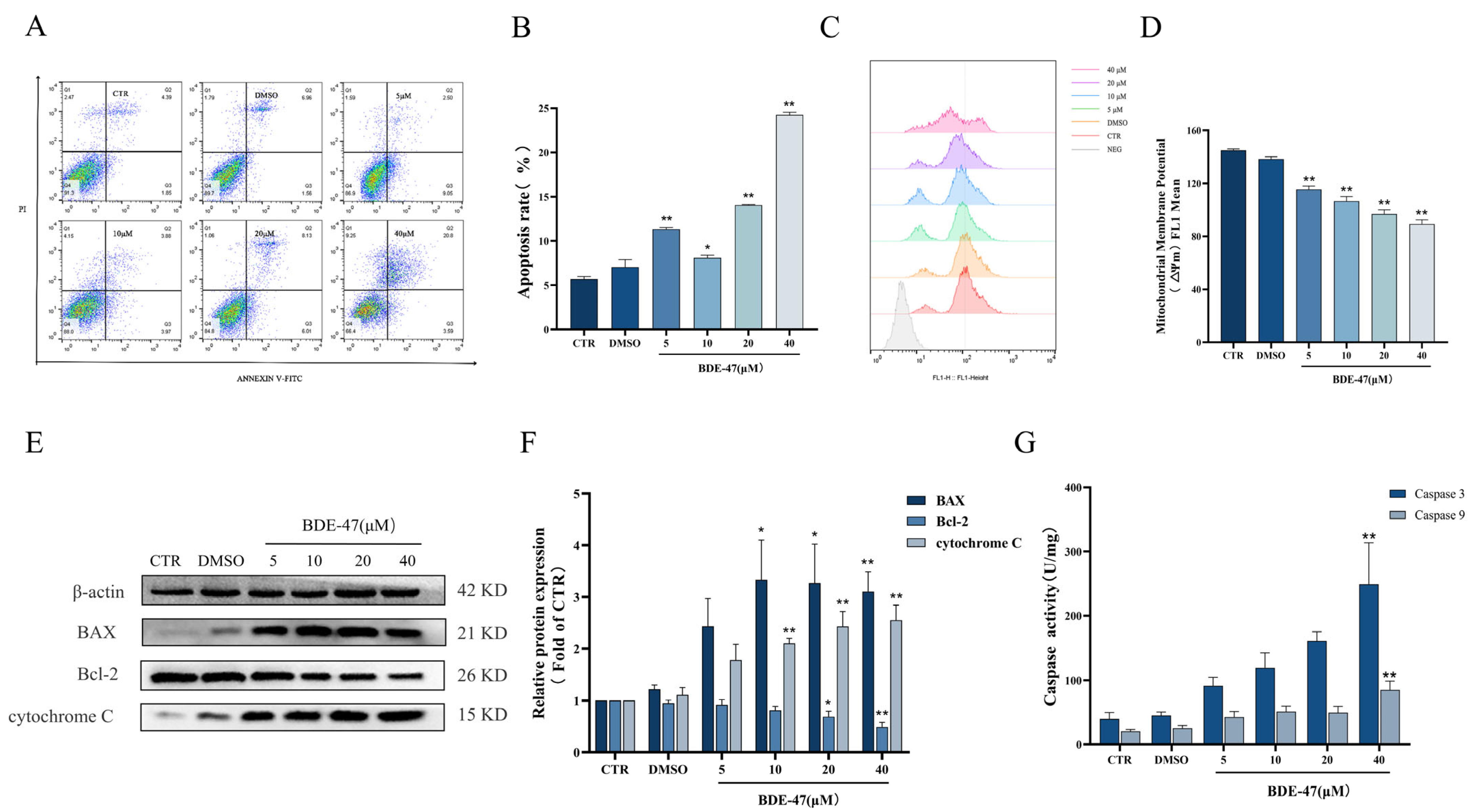
2.2. BDE-47-Induced Apoptosis in RAW264.7 Cells Is Mediated by the Mitochondrial Pathway
2.3. BDE-47 Alters Immune Function of RAW264.7 Cells
2.4. BDE-47 Increases the Level of Reactive Oxygen Species in RAW264.7 Cells and the Regulation of Oxidative Stress Genes
2.5. Role of ROS in BDE-47-Induced Apoptosis and Immune Impairment in RAW264.7 Cells
2.5.1. Effect of Antioxidant NAC Pretreatment on BDE-47-Induced Apoptosis and Cellular Immune Function on RAW264.7 Cells
2.5.2. Effect of Inducer BSO Pretreatment on Apoptosis and Cellular Immune Function after BDE-47 Exposure
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Experimental Design
4.3. Cytotoxicity Evaluation
4.4. Morphology Observation
4.5. Apoptosis Detection
4.6. Detection of Mitochondrial Membrane Potential (MMP)
4.7. Examination of ROS Level
4.8. Caspase Enzymatic Activity
4.9. Western Blotting
4.10. Phagocytic Capacity
4.11. Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR) Analysis
4.12. Cytokine Secretion Levels and NO Production
4.13. Cell Phenotype
4.14. RNA-Seq and Analysis of the Oxidative Stress Regulatory Network
4.15. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Costa, L.G.; Giordano, G. Polybrominated Diphenyl Ethers. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 1032–1034. [Google Scholar]
- Li, K.; Fu, S. Polybrominated Diphenyl Ethers (PBDEs) in House Dust in Beijing, China. Bull. Environ. Contam. Toxicol. 2013, 91, 382–385. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Polybrominated diphenyl ethers in the environmental systems: A review. J. Environ. Health Sci. Eng. 2021, 19, 1229–1247. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Song, J.; Ren, Z.; Yuan, H.; Yan, H.; Zhang, J.; Pei, Z.; He, Z. Environmental Characteristics of Polybrominated Diphenyl Ethers in Marine System, with Emphasis on Marine Organisms and Sediments. Biomed. Res. Int. 2016, 2016, 1317232. [Google Scholar] [CrossRef] [PubMed]
- RA, H. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [Google Scholar]
- Sharkey, M.; Harrad, S.; Abou-Elwafa Abdallah, M.; Drage, D.S.; Berresheim, H. Phasing-out of legacy brominated flame retardants: The UNEP Stockholm Convention and other legislative action worldwide. Environ. Int. 2020, 144, 106041. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Venkateswarlu, K.; Kakarla, D.; Megharaj, M. Inevitable human exposure to emissions of polybrominated diphenyl ethers: A perspective on potential health risks. Environ. Pollut. 2020, 266, 115240. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A.; Lehman, D.C.; Salamova, A.; Venier, M. Temporal environmental hysteresis: A definition and implications for polybrominated diphenyl ethers. Sci. Total Environ. 2021, 753, 141849. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Cheng, J.; Tang, Z.; Yin, H.; Zhang, M. Global distribution and trends of polybrominated diphenyl ethers in human blood and breast milk: A quantitative meta-analysis of studies published in the period 2000–2019. J. Environ. Manag. 2021, 280, 111696. [Google Scholar] [CrossRef] [PubMed]
- Bramwell, L.; Glinianaia, S.V.; Rankin, J.; Rose, M.; Fernandes, A.; Harrad, S.; Pless-Mulolli, T. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environ. Int. 2016, 92–93, 680–694. [Google Scholar] [CrossRef]
- Sun, M.H.; Li, X.H.; Xu, Y.; Xu, Y.; Sun, S.C. Exposure to PBDE47 affects mouse oocyte quality via mitochondria dysfunction-induced oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2020, 198, 110662. [Google Scholar] [CrossRef]
- Chen, H.; Streifel, K.M.; Singh, V.; Yang, D.; Mangini, L.; Wulff, H.; Lein, P.J. BDE-47 and BDE-49 Inhibit Axonal Growth in Primary Rat Hippocampal Neuron-Glia Co-Cultures via Ryanodine Receptor-Dependent Mechanisms. Toxicol. Sci. 2017, 156, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, X.; Zhou, B.; Xu, N.; Wang, Y. Mechanism of Deca-BDE-induced apoptosis in Neuro-2a cells: Role of death-receptor pathway and reactive oxygen species-mediated mitochondrial pathway. J. Environ. Sci. 2016, 46, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Pallocca, G.; Grinberg, M.; Henry, M.; Frickey, T.; Hengstler, J.G.; Waldmann, T.; Sachinidis, A.; Rahnenfuhrer, J.; Leist, M. Identification of transcriptome signatures and biomarkers specific for potential developmental toxicants inhibiting human neural crest cell migration. Arch. Toxicol. 2016, 90, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Parker, M.; Brown, S.E.; Cevik, S.E.; Guo, L.W.; Jensen, J.; Olmsted, A.; Portman, D.; Wu, H.; Suvorov, A. Perinatal exposure to 2,2′,4′4′ -Tetrabromodiphenyl ether induces testicular toxicity in adult rats. Toxicology 2017, 389, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Lu, C.; Zhu, C.; Qian, B.; Li, Z.; Liu, C.; Shao, J.; Yan, J. The role of lysosomes in BDE 47-mediated activation of mitochondrial apoptotic pathway in HepG2 cells. Chemosphere 2015, 124, 10–21. [Google Scholar] [CrossRef]
- Tang, S.; Liu, H.; Yin, H.; Liu, X.; Peng, H.; Lu, G.; Dang, Z.; He, C. Effect of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) and its metabolites on cell viability, oxidative stress, and apoptosis of HepG2. Chemosphere 2018, 193, 978–988. [Google Scholar] [CrossRef]
- Shan, Q.; Zheng, G.H.; Han, X.R.; Wen, X.; Wang, S.; Li, M.Q.; Zhuang, J.; Zhang, Z.F.; Hu, B.; Zhang, Y.; et al. Troxerutin Protects Kidney Tissue against BDE-47-Induced Inflammatory Damage through CXCR4-TXNIP/NLRP3 Signaling. Oxid. Med. Cell. Longevity. 2018, 2018, 9865495. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, H.; Zeng, X.; Zhang, C.; Chen, D. The PBDE-209 exposure during pregnancy and lactation impairs immune function in rats. Mediat. Inflamm. 2012, 2012, 692467. [Google Scholar] [CrossRef]
- Fair, P.A.; Stavros, H.C.; Mollenhauer, M.A.; DeWitt, J.C.; Henry, N.; Kannan, K.; Yun, S.H.; Bossart, G.D.; Keil, D.E.; Peden-Adams, M.M. Immune function in female B(6)C(3)F(1) mice is modulated by DE-71, a commercial polybrominated diphenyl ether mixture. J. Immunotoxicol. 2012, 9, 96–107. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Liu, Z.; Khanniche, A.; Hu, Q.; Feng, Y.; Ye, W.; Yang, J.; Wang, S.; Zhou, L.; et al. Long-term exposure to decabrominated diphenyl ether impairs CD8 T-cell function in adult mice. Cell. Mol. Immunol. 2014, 11, 367–376. [Google Scholar] [CrossRef]
- Fowles, J.; Fairbrother, A.; Baecher-Steppan, L.; Kerkvliet, N. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 1994, 86, 49–61. [Google Scholar] [CrossRef]
- Arkoosh, M.R.; Boylen, D.; Dietrich, J.; Anulacion, B.F.; Bravo, C.F.; Johnson, L.L.; Loge, F.J.; Collier, T.K. Disease susceptibility of salmon exposed to polybrominated diphenyl ethers (PBDEs). Aquat. Toxicol. 2010, 98, 51–59. [Google Scholar] [CrossRef]
- Cary, T.L.; Ortiz-Santaliestra, M.E.; Karasov, W.H. Immunomodulation in post-metamorphic northern leopard frogs, Lithobates pipiens, following larval exposure to polybrominated diphenyl ether. Environ. Sci. Technol. 2014, 48, 5910–5919. [Google Scholar] [CrossRef]
- Koike, E.; Yanagisawa, R.; Takigami, H.; Takano, H. Brominated flame retardants stimulate mouse immune cells in vitro. J. Appl. Toxicol. 2013, 33, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Mynster Kronborg, T.; Frohnert Hansen, J.; Nielsen, C.H.; Ramhoj, L.; Frederiksen, M.; Vorkamp, K.; Feldt-Rasmussen, U. Effects of the Commercial Flame Retardant Mixture DE-71 on Cytokine Production by Human Immune Cells. PLoS One 2016, 11, e0154621. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Y.; Wan, B.; Guo, L.H.; Zhao, L.; Yang, Y. In vitro immune toxicity of polybrominated diphenyl ethers on murine peritoneal macrophages: Apoptosis and immune cell dysfunction. Chemosphere 2015, 120, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, X.; Gu, C.; Sun, C.; Li, M. Bioaccessibility of BDE 47 in a simulated gastrointestinal system and its metabolic transformation mechanisms in Caco-2 cells. Chemosphere 2019, 214, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Harju, M.; Herzke, D.; Anker-Nilssen, T.; Christensen-Dalsgaard, S.; Langset, M.; Gabrielsen, G.W. Ingested plastics in northern fulmars (Fulmarus glacialis): A pathway for polybrominated diphenyl ether (PBDE) exposure? Sci. Total Environ. 2021, 778, 146313. [Google Scholar] [CrossRef] [PubMed]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tani, H.; Roy, S.R.; et al. PBDE: Structure-activity studies for the inhibition of hepatitis C virus NS3 helicase. Molecules 2014, 19, 4006–4020. [Google Scholar] [CrossRef]
- Wei, R.G.; Zhao, Y.X.; Liu, P.Y.; Qin, Z.F.; Yan, S.S.; Li, Y.; Qin, X.F.; Xia, X.J.; Xu, X.B.; Yan, M.C. Determination of environmentally relevant exposure concentrations of polybrominated diphenyl ethers for in vitro toxicological studies. Toxicol. In Vitro 2010, 24, 1078–1085. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Scherlinger, M.; Tsokos, G.C. Reactive oxygen species: The Yin and Yang in (auto-)immunity. Autoimmun. Rev. 2021, 20, 102869. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Xu, W.; Cheng, J.; Zhang, C.; Gao, J.; Li, Z.; Tao, L.; Zhang, Y. Immunotoxicity induced by Ivermectin is associated with NF-kappaB signaling pathway on macrophages. Chemosphere 2022, 289, 133087. [Google Scholar] [CrossRef]
- Sun, S.; Shi, W.; Tang, Y.; Han, Y.; Du, X.; Zhou, W.; Hu, Y.; Zhou, C.; Liu, G. Immunotoxicity of petroleum hydrocarbons and microplastics alone or in combination to a bivalve species: Synergic impacts and potential toxication mechanisms. Sci. Total Environ. 2020, 728, 138852. [Google Scholar] [CrossRef] [PubMed]
- Naasri, S.; Helali, I.; Aouni, M.; Mastouri, M.; Harizi, H. N-acetylcysteine reduced the immunotoxicity effects induced in vitro by azoxystrobin and iprodione fungicides in mice. Environ. Toxicol. 2021, 36, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Frouin, H.; Lebeuf, M.; Hammill, M.; Masson, S.; Fournier, M. Effects of individual polybrominated diphenyl ether (PBDE) congeners on harbour seal immune cells in vitro. Mar. Pollut. Bull. 2010, 60, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflugers. Arch. 2017, 469, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Netea, M.G. Adaptive Characteristics of Innate Immune Responses in Macrophages. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef]
- Lundgren, M.; Darnerud, P.O.; Blomberg, J.; Friman, G.; Ilback, N.G. Polybrominated diphenyl ether exposure suppresses cytokines important in the defence to coxsackievirus B3 infection in mice. Toxicol. Lett. 2009, 184, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Siebert, U.; McLachlan, M.; Bruhn, R.; Thron, K.; Failing, K.; Müller, G.; Baumgärtner, W. Investigations of the potential influence of environmental contaminants on the thymus and spleen of harbor porpoises (Phocoena phocoena). Environ. Sci. Technol. 2005, 39, 3933–3938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, H.; Li, Y.; Liu, Q.; Lu, K.; Zhu, X.; Wang, Y. Transcriptome and biochemical analyses of rainbow trout (Oncorhynchus mykiss) RTG-2 gonadal cells in response to BDE-47 stress indicates effects on cell proliferation. Aquat. Toxicol. 2022, 245, 106108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, X.; Zhou, B.; Zhou, Z.; Xu, N.; Wang, Y. A ROS-mediated mitochondrial pathway and Nrf2 pathway activation are involved in BDE-47 induced apoptosis in Neuro-2a cells. Chemosphere 2017, 184, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Jiang, Y.; Lei, Y.; Lu, L.; Zhou, J.; Huang, H.; Fang, D.; Tao, G. Effect on metabolic enzymes and thyroid receptors induced by BDE-47 by activation the pregnane X receptor in HepG2, a human hepatoma cell line. Toxicol. In Vitro 2014, 28, 1377–1385. [Google Scholar] [CrossRef]
- Yang, C.; Wong, C.M.; Wei, J.; Chung, A.C.K.; Cai, Z. The brominated flame retardant BDE 47 upregulates purine metabolism and mitochondrial respiration to promote adipocyte differentiation. Sci. Total Environ. 2018, 644, 1312–1322. [Google Scholar] [CrossRef]
- Dong, L.; Li, P.; Yang, K.; Liu, L.; Gao, H.; Zhou, G.; Zhao, Q.; Xia, T.; Wang, A.; Zhang, S. Promotion of mitochondrial fusion protects against developmental PBDE-47 neurotoxicity by restoring mitochondrial homeostasis and suppressing excessive apoptosis. Theranostics 2020, 10, 1245–1261. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Zhao, C.; Zhou, Q.; Zhang, J. Interaction of BDE-47 with nuclear receptors (NRs) based on the cytotoxicity: In vitro investigation and molecular interaction. Ecotoxicol. Environ. Saf. 2021, 208, 111390. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Rao, Q.; Li, X.; Ruan, Z.; Yang, J. BDE-47 induces nephrotoxicity through ROS-dependent pathways of mitochondrial dynamics in PK15 cells. Ecotoxicol. Environ. Saf. 2021, 222, 112549. [Google Scholar] [CrossRef]
- Park, H.R.; Loch-Caruso, R. Protective effect of nuclear factor E2-related factor 2 on inflammatory cytokine response to brominated diphenyl ether-47 in the HTR-8/SVneo human first trimester extravillous trophoblast cell line. Toxicol. Appl. Pharmacol. 2014, 281, 67–77. [Google Scholar] [CrossRef]
- Liang, L.; Pan, Y.; Bin, L.; Liu, Y.; Huang, W.; Li, R.; Lai, K.P. Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere 2022, 291 Pt 2, 132892. [Google Scholar] [CrossRef]
- Su, R.; Jin, X.; Zhang, W.; Li, Z.; Liu, X.; Ren, J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere 2017, 167, 444–453. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Q.; Xie, S.; Xu, J.; Huang, B.; Wu, Y.; Xia, D. Cypermethrin Induces Macrophages Death through Cell Cycle Arrest and Oxidative Stress-Mediated JNK/ERK Signaling Regulated Apoptosis. Int. J. Mol. Sci. 2016, 17, 885. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, L.; Fang, W.; Zhang, X.; Zhong, Y. Perfluorooctanoic acid-induced immunotoxicity via NF-kappa B pathway in zebrafish (Danio rerio) kidney. Fish Shellfish Immunol. 2021, 113, 9–19. [Google Scholar] [CrossRef]
- Zamani, E.; Shaki, F.; AbedianKenari, S.; Shokrzadeh, M. Acrylamide induces immunotoxicity through reactive oxygen species production and caspase-dependent apoptosis in mice splenocytes via the mitochondria-dependent signaling pathways. Biomed. Pharmacother. 2017, 94, 523–530. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Mitochondrial function in immune cells in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165845. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Wu, C.; Zhou, C.; Zhang, J.; Zhu, Q.; Shen, T. Bisphenol A promotes macrophage proinflammatory subtype polarization via upregulation of IRF5 expression in vitro. Toxicol. In Vitro 2019, 60, 97–106. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, H.; Yang, T.; Rui, W.; Liu, F.; Zhang, F.; Zhao, Y.; Ding, W. Direct effects of airborne PM2.5 exposure on macrophage polarizations. Biochim. Biophys. Acta 2016, 1860, 2835–2843. [Google Scholar] [CrossRef]
- Virag, L.; Jaen, R.I.; Regdon, Z.; Bosca, L.; Prieto, P. Self-defense of macrophages against oxidative injury: Fighting for their own survival. Redox Biol. 2019, 26, 101261. [Google Scholar] [CrossRef]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukocyte Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Costa Carvalho, J.; Brito, A. The M1/M2 Pattern and the Oxidative Stress are Modulated by Low-Level Laser in Human Macrophage. J. Clin. Cell. Immunol. 2016, 7, 387. [Google Scholar]
- Hou, L.; Zhou, X.; Gan, F.; Liu, Z.; Zhou, Y.; Qian, G.; Huang, K. Combination of Selenomethionine and N-Acetylcysteine Alleviates the Joint Toxicities of Aflatoxin B1 and Ochratoxin A by ERK MAPK Signal Pathway in Porcine Alveolar Macrophages. J. Agric. Food. Chem. 2018, 66, 5913–5923. [Google Scholar] [CrossRef]
- Schweikl, H.; Birke, M.; Gallorini, M.; Petzel, C.; Bolay, C.; Waha, C.; Hiller, K.A.; Buchalla, W. HEMA-induced oxidative stress inhibits NF-kappaB nuclear translocation and TNF release from LTA- and LPS-stimulated immunocompetent cells. Dent. Mater. 2021, 37, 175–190. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Zhou, B.; Xu, N.; Zhou, Z.; Fang, K.; Wang, Y. BDE-47 and BDE-209 inhibit proliferation of Neuro-2a cells via inducing G1-phase arrest. Environ. Toxicol. Pharmacol. 2017, 50, 76–82. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, X.; Chen, H.; Wang, Y. Comparative studies of saxitoxin (STX)-induced cytotoxicity in Neuro-2a and RTG-2 cell lines: An explanation with respect to changes in ROS. Chemosphere 2018, 192, 66–74. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Chen, H.; Tang, X.; Wang, Y. Reactive oxygen species (ROS) and the calcium-(Ca2+) mediated extrinsic and intrinsic pathways underlying BDE-47-induced apoptosis in rainbow trout (Oncorhynchus mykiss) gonadal cells. Sci. Total Environ. 2019, 656, 778–788. [Google Scholar] [CrossRef]
- Santos, S.S.; Brunialti, M.K.C.; Rodrigues, L.; Liberatore, A.M.A.; Koh, I.H.J.; Martins, V.; Soriano, F.G.; Szabo, C.; Salomao, R. Effects of the PARP Inhibitor Olaparib on the Response of Human Peripheral Blood Leukocytes to Bacterial Challenge or Oxidative Stress. Biomolecules 2022, 12, 788. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, Z.; Xie, P.; Lin, K.; Chung, A.C.K.; Cai, Z. Immunotoxic Potential of Bisphenol F Mediated through Lipid Signaling Pathways on Macrophages. Environ. Sci. Technol. 2019, 53, 11420–11428. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]






| Target Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| MHC-II | GTGTGCAGACACAACTACGAGG | CTGTCACTGAGCAGACCAGAGT |
| CD40 | ACCAGCAAGGATTGCGAGGCAT | GGATGACAGACGGTATCAGTGG |
| CD80 | CCTCAAGTTTCCATGTCCAAGGC | GAGGAGAGTTGTAACGGCAAGG |
| CD86 | ACGTATTGGAAGGAGATTACAGCT | TCTGTCAGCGTTACTATCCCGC |
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Zhou, Z.-Y.; He, Y.-N.; Dong, M.-H.; Wang, Z.-N.; Chen, H.-M. BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway. Molecules 2023, 28, 2036. https://doi.org/10.3390/molecules28052036
Gao Q, Zhou Z-Y, He Y-N, Dong M-H, Wang Z-N, Chen H-M. BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway. Molecules. 2023; 28(5):2036. https://doi.org/10.3390/molecules28052036
Chicago/Turabian StyleGao, Qian, Zhong-Yuan Zhou, Ya-Ning He, Ming-Hui Dong, Zhao-Ning Wang, and Hong-Mei Chen. 2023. "BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway" Molecules 28, no. 5: 2036. https://doi.org/10.3390/molecules28052036
APA StyleGao, Q., Zhou, Z.-Y., He, Y.-N., Dong, M.-H., Wang, Z.-N., & Chen, H.-M. (2023). BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway. Molecules, 28(5), 2036. https://doi.org/10.3390/molecules28052036






