68Ga-Labeled [Thz14]Bombesin(7–14) Analogs: Promising GRPR-Targeting Agonist PET Tracers with Low Pancreas Uptake
Abstract
:1. Introduction
2. Results
2.1. Chemistry and Radiochemistry
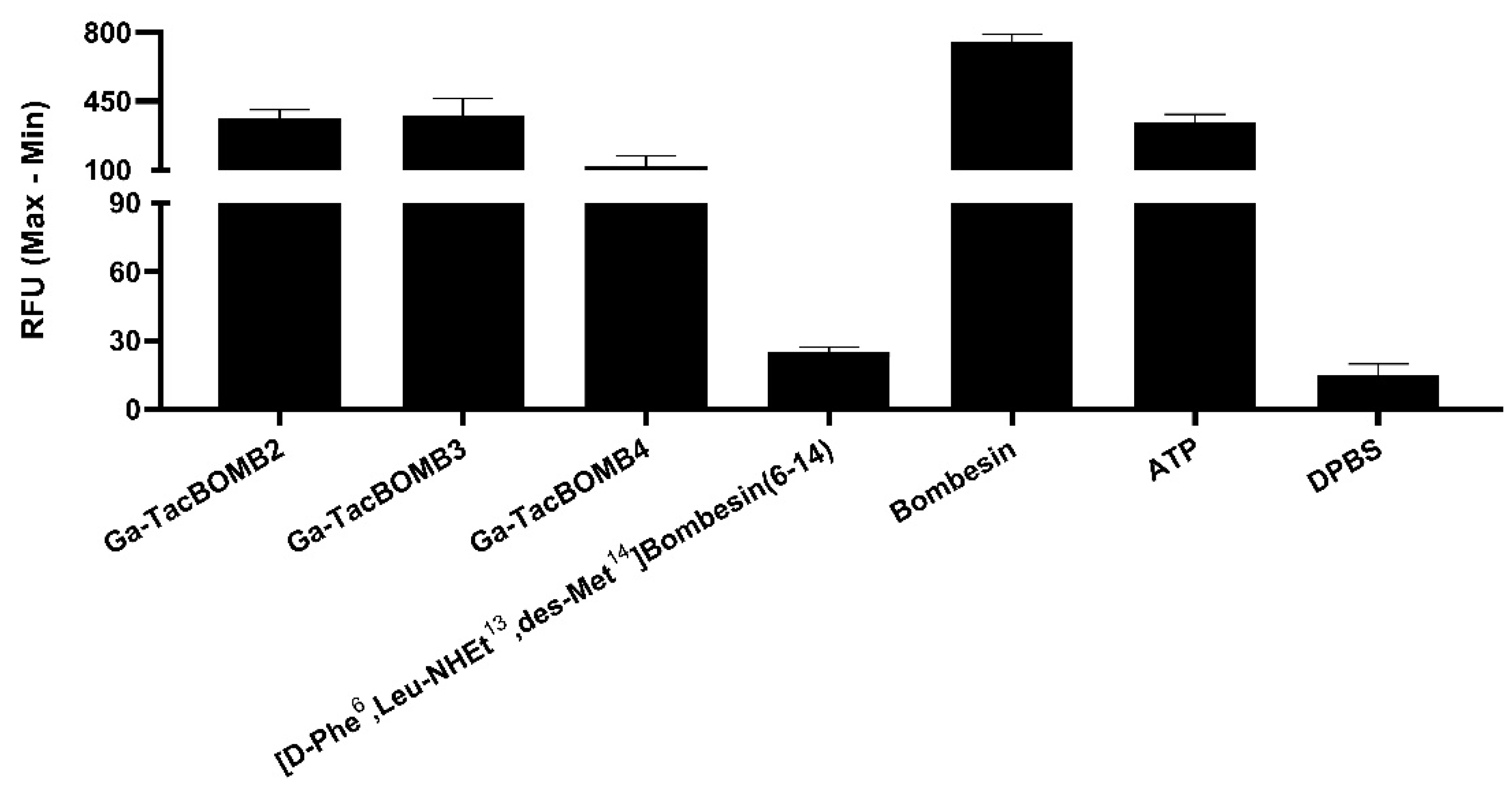
2.2. Agonist Characterization, Binding Affinity, and Hydrophilicity
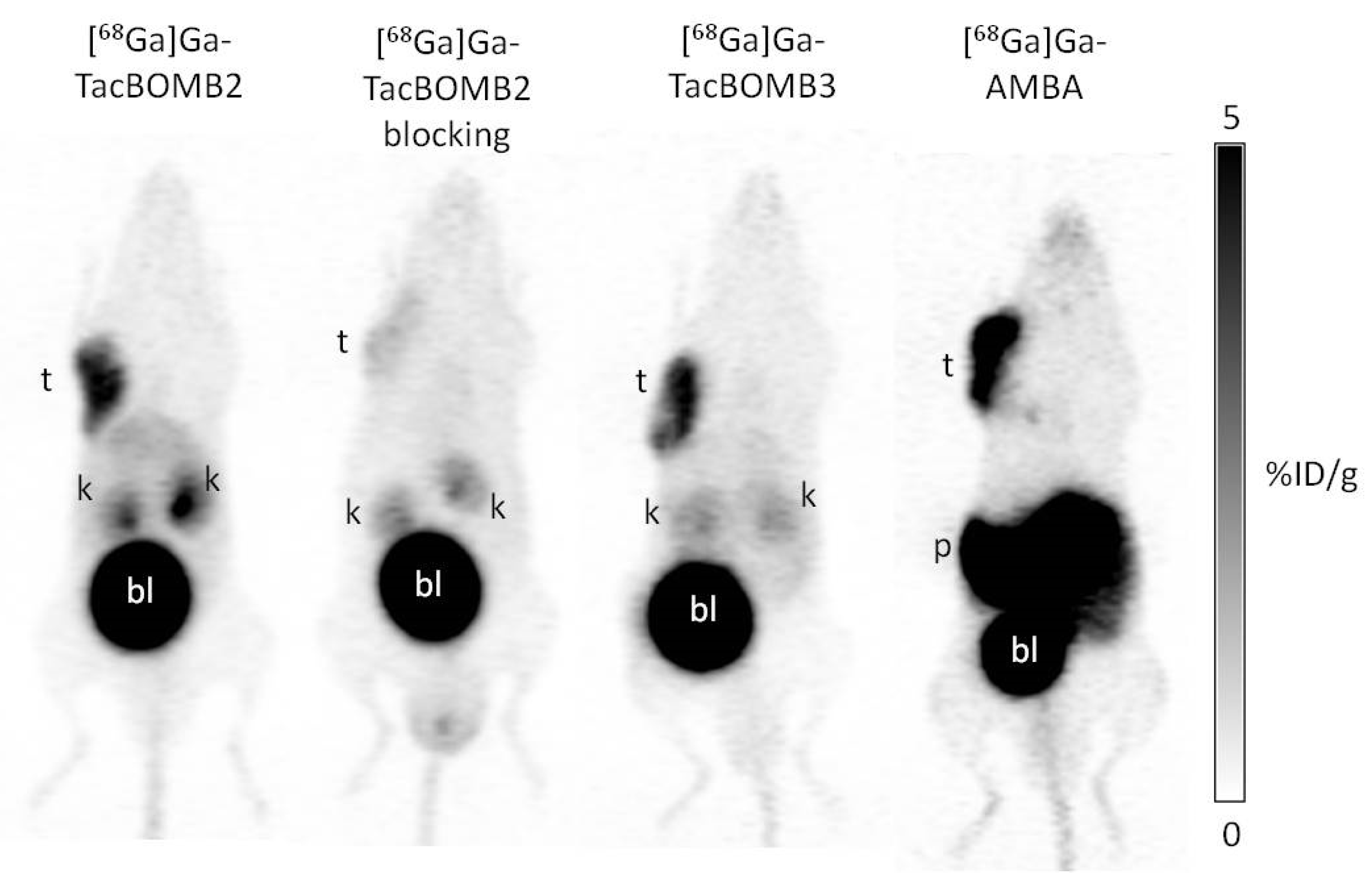
2.3. PET Imaging and Ex Vivo Biodistribution
2.4. In Vivo Stability
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Synthesis of DOTA-Conjugated Peptides
4.3. Synthesis of Nonradioactive Ga-Complexed Standards
4.4. Synthesis of 68Ga-Labeled Compounds
4.5. LogD7.4 Measurement
4.6. Cell Culture
4.7. Fluorometric Calcium Release Assay
4.8. In Vitro Competition Binding Assay
4.9. Ex Vivo Biodistribution, PET/CT Imaging and In Vivo Stability Studies
4.10. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jensen, R.; Battey, J.; Spindel, E.; Benya, R. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Bitar, K.N.; Zhu, X.-X. Expression of bombesin-receptor subtypes and their differential regulation of colonic smooth muscle contraction. Gastroenterology 1993, 105, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.C. Regulation and signaling of human bombesin receptors and their biological effects. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.B.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Hajri, A.; Koenig, M.; Balboni, G.; Damgé, C. Expression and characterization of gastrin-releasing peptide receptor in normal and cancerous pancreas. Pancreas 1996, 12, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.; Zia, F.; Venugopal, R.; Fagarasan, M.; Oie, H.; Hu, V. GRP receptors are present in non small cell lung cancer cells. J. Cell. Biochem. 1996, 63, 247–256. [Google Scholar] [CrossRef]
- Preston, S.; Woodhouse, L.; Jones-Blackett, S.; Miller, G.; Primrose, J. High-affinity binding sites for gastrin-releasing peptide on human colorectal cancer tissue but not uninvolved mucosa. Br. J. Cancer 1995, 71, 1087–1089. [Google Scholar] [CrossRef] [Green Version]
- Gugger, M.; Reubi, J.C. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am. J. Pathol 1999, 155, 2067–2076. [Google Scholar] [CrossRef] [Green Version]
- Preston, S.R.; Woodhouse, L.F.; Gokhale, J.; Miller, G.V.; Primrose, J.N. Characterization of a bombesin/gastrin-releasing peptide receptor on a human gastric-cancer cell line. Int. J. Cancer 1994, 57, 734–741. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- Roesler, R.; Henriques, J.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. Curr. Drug Targets CNS Neurol. Disord. 2006, 5, 197–204. [Google Scholar]
- Shimoda, J. Effects of bombesin and its antibody on growth of human prostatic carcinoma cell lines. Nihon Hinyokika Gakkai zasshi. Jpn. J. Urol. 1992, 83, 1459–1468. [Google Scholar]
- Qin, Y.; Ertl, T.; Cai, R.-Z.; Halmos, G.; Schally, A.V. Inhibitory effect of bombesin receptor antagonist RC-3095 on the growth of human pancreatic cancer cells in vivo and in vitro. Cancer Res. 1994, 54, 1035–1041. [Google Scholar] [PubMed]
- Varvarigou, A.; Bouziotis, P.; Zikos, C.; Scopinaro, F.; De Vincentis, G. Gastrin-releasing peptide (GRP) analogues for cancer imaging. Cancer Biother. Radiopharm. 2004, 19, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.; Prasad, V.; Mutloka, N.; Frischknecht, M.; Maecke, H.; Reubi, J. Molecular imaging of bombesin receptors in various tumors by Ga-68 AMBA PET/CT: First results. J. Nucl. Med. 2007, 48, 79P. [Google Scholar]
- Kähkönen, E.; Jambor, I.; Kemppainen, J.; Lehtiö, K.; Grönroos, T.J.; Kuisma, A.; Luoto, P.; Sipilä, H.J.; Tolvanen, T.; Alanen, K. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef] [Green Version]
- Stoykow, C.; Erbes, T.; Maecke, H.R.; Bulla, S.; Bartholomä, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics 2016, 6, 1641. [Google Scholar] [CrossRef]
- Baratto, L.; Song, H.; Duan, H.; Hatami, N.; Bagshaw, H.; Buyyounouski, M.; Hancock, S.; Shah, S.A.; Srinivas, S.; Swift, P.; et al. PSMA- and GRPR-targeted PET: Results from 50 patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2021, 62, 1545–1549. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 123–135. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: Preclinical and first clinical results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef]
- Marsouvanidis, P.J.; Maina, T.; Sallegger, W.; Krenning, E.P.; de Jong, M.; Nock, B.A. 99mTc radiotracers based on human GRP (18–27): Synthesis and comparative evaluation. J. Nucl. Med. 2013, 54, 1797–1803. [Google Scholar] [CrossRef] [Green Version]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reile, H.; Cai, R.; Armatis, P.; Schally, A. New antagonists of bombesin gastrin-releasing Peptide with C-terminal Leu-ψ(CH2N)-Tac-NH2. Int. J. Oncol. 1995, 7, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Reile, H.; Armatis, P.; Schally, A.V. Potent bombesin antagonists with C-terminal Leu-ψ(CH2-N)-Tac-NH2 or its derivatives. Proc. Natl. Acad. Sci. USA 1994, 91, 12664–12668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungwirth, A.; Pinski, J.; Galvan, G.; Halmos, G.B.; Szepeshazi, K.R.; Gai, R.; Groot, K.; Vadillo-Buenfil, M.; Schally, A.V. Inhibition of growth of androgen-independent DU-145 prostate cancer in vivo by luteinising hormone-releasing hormone antagonist Cetrorelix and bombesin antagonists RC-3940-II and RC-3950-II. Eur. J. Cancer 1997, 33, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Koppán, M.; Halmos, G.; Arencibia, J.M.; Lamharzi, N.; Schally, A.V. Bombesin/gastrin-releasing peptide antagonists RC-3095 and RC-3940-II inhibit tumor growth and decrease the levels and mRNA expression of epidermal growth factor receptors in H-69 small cell lung carcinoma. Cancer 1998, 83, 1335–1343. [Google Scholar] [CrossRef]
- Shirahige, Y.; Cai, R.-Z.; Szepeshazi, K.; Halmos, G.; Pinski, J.; Groot, K.; Schally, A. Inhibitory effect of bombesin/gastrin-releasing peptide (GRP) antagonists RC-3950-II and RC-3095 on MCF-7 MIII human breast cancer xenografts in nude mice. Biomed. Pharmacother. 1994, 48, 465–472. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Merkens, H.; Zeisler, J.; Zhang, C.; Roxin, A.; Tan, R.; Bénard, F.; Lin, K.-S. 68Ga-Labeled [Leu13ψThz14] Bombesin (7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake. Molecules 2022, 27, 3777. [Google Scholar] [CrossRef]
- Ghosh, A.; Woolum, K.; Kothandaraman, S.; Tweedle, M.F.; Kumar, K. Stability Evaluation and Stabilization of a Gastrin-Releasing Peptide Receptor (GRPR) Targeting Imaging Pharmaceutical. Molecules 2019, 24, 2878. [Google Scholar] [CrossRef] [Green Version]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.-L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1, 4, 7, 10-Tetraazacyclododecane-1, 4, 7, 10-Tetraacetic Acid–Conjugated Bombesin-Based Radioantagonist for the Labeling with Single-Photon Emission Computed Tomography, Positron Emission Tomography, and Therapeutic RadionuclidesDOTA-Conjugated Bombesin Antagonist. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar]
- Chatalic, K.L.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.-A.; Martinez, J.; van Gent, D.C. In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances PET imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansi, R.; Fleischmann, A.; Mäcke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers—From basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gao, H.; Zhou, Y.; Ma, Y.; Quan, Q.; Lang, L.; Chen, K.; Niu, G.; Yan, Y.; Chen, X. 18F-labeled GRPR agonists and antagonists: A comparative study in prostate cancer imaging. Theranostics 2011, 1, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leban, J.J.; Kull Jr, F.C.; Landavazo, A.; Stockstill, B.; McDermed, J.D. Development of potent gastrin-releasing peptide antagonists having a D-Pro-ψ (CH2NH)-Phe-NH2 C terminus. Proc. Natl. Acad. Sci. USA 1993, 90, 1922–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leban, J.J.; Landavazo, A.; McDermed, J.D.; Diliberto Jr, E.J.; Jansen, M.; Stockstill, B.; Kull, F.C., Jr. Potent Gastrin-Releasing Peptide (GRP) Antagonists Derived from GRP (19–27) with a C-Terminal DProψ[CH2NH]Phe-NH2 and N-Terminal Aromatic Residues. J. Med. Chem. 1994, 37, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Lantry, L.E.; Cappelletti, E.; Maddalena, M.E.; Fox, J.S.; Feng, W.; Chen, J.; Thomas, R.; Eaton, S.M.; Bogdan, N.J.; Arunachalam, T. 177Lu-AMBA: Synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J. Nucl. Med. 2006, 47, 1144–1152. [Google Scholar] [PubMed]
- Cagnolini, A.; Chen, J.; Ramos, K.; Skedzielewski, T.M.; Lantry, L.E.; Nunn, A.D.; Swenson, R.E.; Linder, K.E. Automated synthesis, characterization and biological evaluation of [68Ga]Ga-AMBA, and the synthesis and characterization of natGa-AMBA and [67Ga]Ga-AMBA. Appl. Radiat. Isot. 2010, 68, 2285–2292. [Google Scholar] [CrossRef]
- Lin, K.-S.; Pan, J.; Amouroux, G.; Turashvili, G.; Mesak, F.; Hundal-Jabal, N.; Pourghiasian, M.; Lau, J.; Jenni, S.; Aparicio, S. In vivo radioimaging of bradykinin receptor B1, a widely overexpressed molecule in human cancer. Cancer Res. 2015, 75, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Amouroux, G.; Pan, J.; Jenni, S.; Zhang, C.; Zhang, Z.; Hundal-Jabal, N.; Colpo, N.; Liu, Z.; Benard, F.; Lin, K.-S. Imaging bradykinin B1 receptor with 68Ga-labeled [des-Arg10] Kallidin derivatives: Effect of the linker on biodistribution and tumor uptake. Mol. Pharm. 2015, 12, 2879–2888. [Google Scholar] [CrossRef]
- Lin, K.-S.; Amouroux, G.; Pan, J.; Zhang, Z.; Jenni, S.; Lau, J.; Liu, Z.; Hundal-Jabal, N.; Colpo, N.; Bénard, F. Comparative studies of three 68Ga-labeled [Des-Arg10] kallidin derivatives for imaging bradykinin B1 receptor expression with PET. J. Nucl. Med. 2015, 56, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.; Rousseau, E.; Zhang, Z.; Uribe, C.F.; Kuo, H.-T.; Zeisler, J.; Zhang, C.; Kwon, D.; Lin, K.-S.; Bénard, F. Positron emission tomography imaging of the gastrin-releasing peptide receptor with a novel bombesin analogue. ACS Omega 2019, 4, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Bratanovic, I.J.; Zhang, C.; Zhang, Z.; Kuo, H.T.; Colpo, N.; Zeisler, J.; Merkens, H.; Uribe, C.; Lin, K.S.; Bénard, F. A Radiotracer for Molecular Imaging and Therapy of Gastrin-Releasing Peptide Receptor–Positive Prostate Cancer. J. Nucl. Med. 2022, 63, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.-S.; Benard, F. Effects of linker modification on tumor-to-kidney contrast of 68Ga-labeled PSMA-targeted imaging probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Bratanovic, I.J.; Zhang, Z.; Kuo, H.-T.; Merkens, H.; Zeisler, J.; Zhang, C.; Tan, R.; Bénard, F.; Lin, K.-S. 68Ga-Labeled [Thz14]Bombesin(7–14) Analogs: Promising GRPR-Targeting Agonist PET Tracers with Low Pancreas Uptake. Molecules 2023, 28, 1977. https://doi.org/10.3390/molecules28041977
Wang L, Bratanovic IJ, Zhang Z, Kuo H-T, Merkens H, Zeisler J, Zhang C, Tan R, Bénard F, Lin K-S. 68Ga-Labeled [Thz14]Bombesin(7–14) Analogs: Promising GRPR-Targeting Agonist PET Tracers with Low Pancreas Uptake. Molecules. 2023; 28(4):1977. https://doi.org/10.3390/molecules28041977
Chicago/Turabian StyleWang, Lei, Ivica Jerolim Bratanovic, Zhengxing Zhang, Hsiou-Ting Kuo, Helen Merkens, Jutta Zeisler, Chengcheng Zhang, Ruiyan Tan, François Bénard, and Kuo-Shyan Lin. 2023. "68Ga-Labeled [Thz14]Bombesin(7–14) Analogs: Promising GRPR-Targeting Agonist PET Tracers with Low Pancreas Uptake" Molecules 28, no. 4: 1977. https://doi.org/10.3390/molecules28041977
APA StyleWang, L., Bratanovic, I. J., Zhang, Z., Kuo, H.-T., Merkens, H., Zeisler, J., Zhang, C., Tan, R., Bénard, F., & Lin, K.-S. (2023). 68Ga-Labeled [Thz14]Bombesin(7–14) Analogs: Promising GRPR-Targeting Agonist PET Tracers with Low Pancreas Uptake. Molecules, 28(4), 1977. https://doi.org/10.3390/molecules28041977





