Wittig and Wittig–Horner Reactions under Sonication Conditions
Abstract
1. Introduction
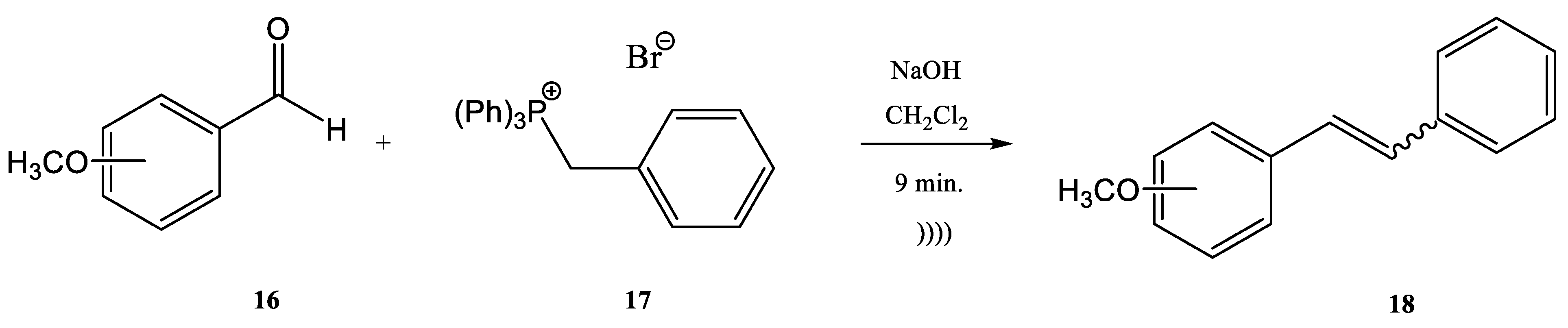
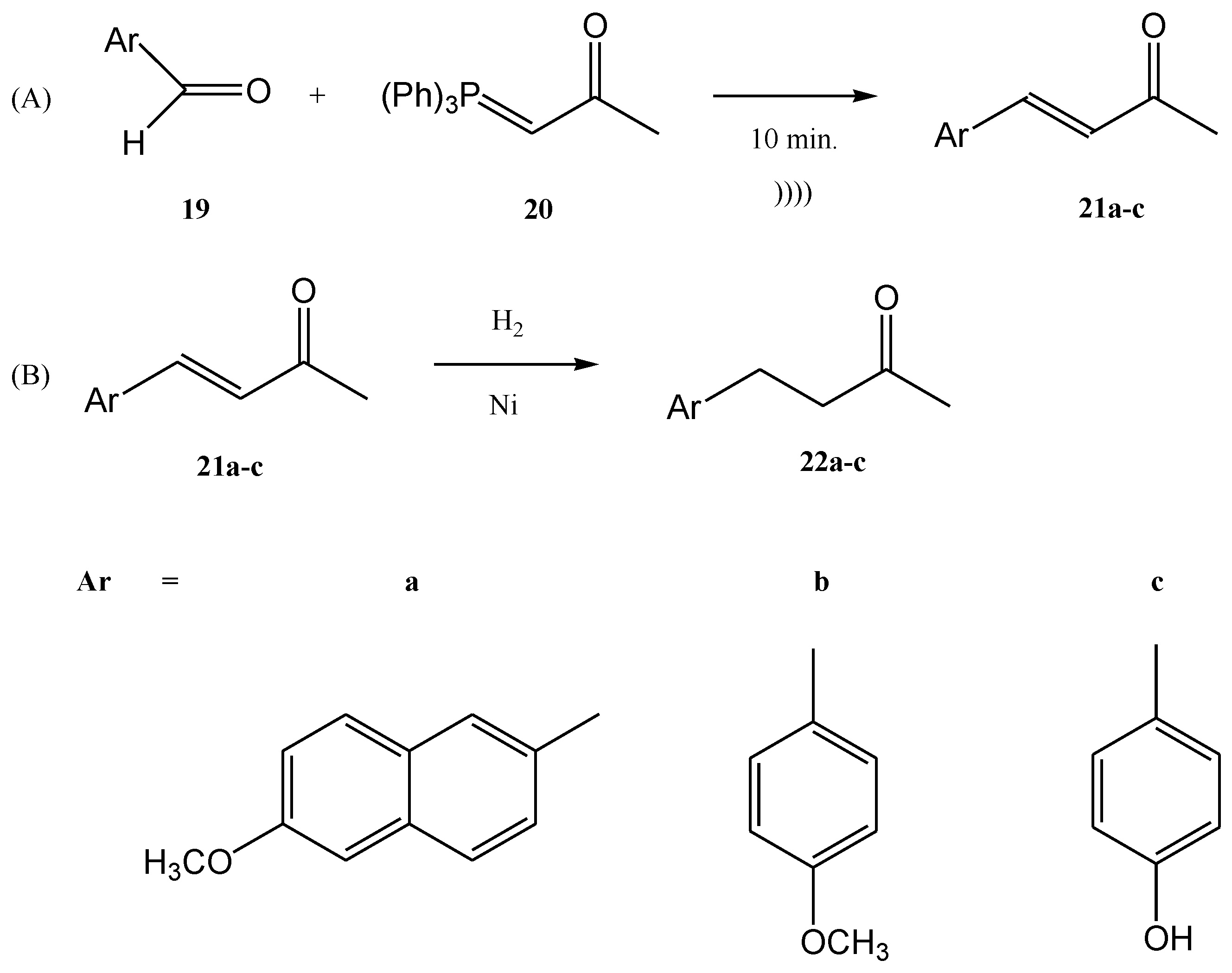
2. Wittig Reactions under Sonication Conditions
- -
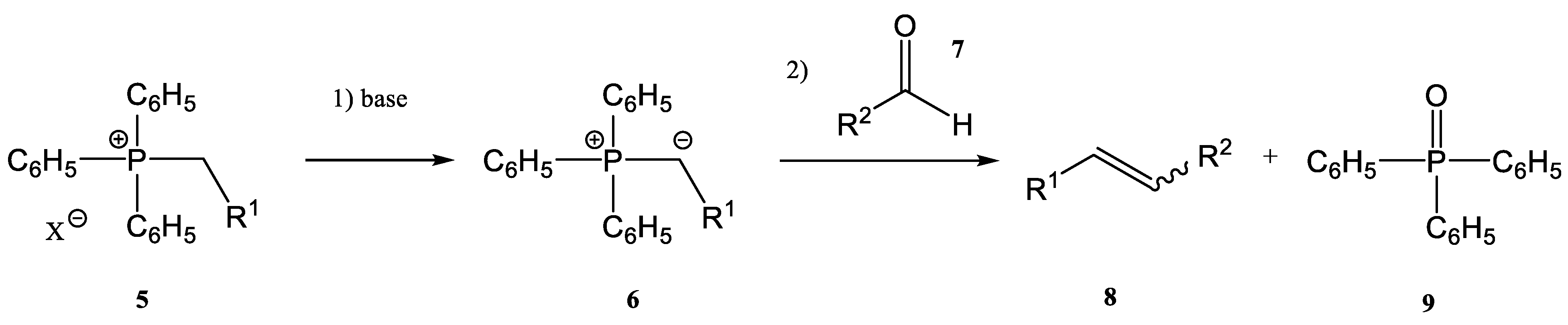
- the first step is the deprotonation reaction of a phosphonium salt (5) to obtain a phosphorous ylide (6)
- -
- the phosphorous ylide (6) reacts with a compound containing a carbonyl group, an aldehyde (7), or a ketone to give the corresponding alkene (8) and phosphine oxide (9) (Figure 2)
3. Aza-Wittig Reactions Performed under Sonication Conditions
4. Wittig–Horner Reactions Conducted Using Ultrasound
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georg, W.; Ulrich, S. Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien I. Chem. Ber. 1954, 87, 1318. [Google Scholar] [CrossRef]
- Wittig, G.; Weigmann, H.-D.; Schlosser, M. Abwandlung der Phosphylen-Methodik; zugleich III. Mitteil. über Phosphin-alkylene als olefinbildende Reagenzien. Chem. Ber. 1961, 94, 676–689. [Google Scholar] [CrossRef]
- Wittig, G.; Haag, A. Über Phosphin-alkylene als olefinbildende Reagenzien, VIII. Allenderivate aus Ketenen. Chem. Ber. 1963, 96, 1535–1543. [Google Scholar] [CrossRef]
- Horner, L.; Hoffmann, H.M.R.; Wippel, H.G. Phosphororganische verbindungen, XII. Phosphinoxyde als olefinierungsreagenzien. Chem. Ber. 1958, 91, 61–63. [Google Scholar] [CrossRef]
- Horner, L.; Hoffmann, H.M.R.; Wippel, H.G.; Klahre, G. Phosphororganische verbindungen, XX. Phosphinoxyde als olefinierungsreagenzien. Chem. Ber. 1959, 92, 2499–2505. [Google Scholar] [CrossRef]
- Nielsen, A.J. Novel Wittig and Organocatalytic Methodologies for the Synthesis of Chemotherapeutic Compounds. Ph.D. Thesis, MCmaster University, Hamilton, ON, Canada, 2019. [Google Scholar]
- Pommer, H.; Thieme, P.C. Top. Curr. Chem. Wittig Chem. 1983, 109, 165–188. [Google Scholar]
- Takeda, T. Modern Carbonyl Olefination: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Laue, T.; Plagens, A. Named Organic Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2005; p. 293. [Google Scholar]
- Maryanoff, B.; Reitz, A.; Mutter, M.; Inners, R.; Almond, H.R., Jr.; Whittle, R.; Olofson, R. Stereochemistry and Mechanism of the Wittig Reaction. Diastereomeric Reaction Intermediates and Analysis of the Reaction Course. J. Am. Chem. Soc. 1986, 108, 7664–7678. [Google Scholar] [CrossRef]
- Reitz, A.; Nortey, S.; Jordan, A.; Mutter, M.; Maryanoff, B.E. Dramatic concentration dependence of stereochemistry in the Wittig reaction. Examination of the lithium salt effect. J. Org. Chem. 1986, 51, 3302–3308. [Google Scholar] [CrossRef]
- Vedejs, E.; Marth, C.F. Mechanism of Wittig reaction: Evidence against betaine intermediates. J. Am. Chem. Soc. 1990, 112, 3905–3909. [Google Scholar] [CrossRef]
- Byrne, P.; Gilheany, D.G. The modern interpretation of the Wittig reaction mechanism. Chem. Soc. Rev. 2013, 42, 6670–6696. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Vahideh, Z.; Mansoureh, D.; Manizheh, G. Applications of Wittig Reaction in the Total Synthesis of Natural Macrolides. Chem. Select 2020, 5, 9654–9690. [Google Scholar]
- Roman, D.; Sauer, M.; Beemelmanns, C. Applications of the Horner–Wadsworth–Emmons Olefination in Modern Natural Product Synthesis. Synthesis 2021, 53, 2713–2739. [Google Scholar]
- Maranescu, B.; Lupa, L.; Tara-Lunga Mihali, M.; Plesu, N.; Maranescu, V.; Visa, A. The corrosion inhibitor behavior of iron in saline solution by the action of magnesium carboxyphosphonate. Pure Appl. Chem. 2018, 90, 1713–1722. [Google Scholar] [CrossRef]
- Palacios, F.; Alonso, C.; Aparicio, D.; Rubiales, G.; de los Santos, J.M. The aza-Wittig reaction: An efficient tool for the construction of carbon–nitrogen double bonds. Tetrahedron 2007, 63, 523–575. [Google Scholar] [CrossRef]
- Lao, Z.; Toy, P.H. Catalytic Wittig and aza-Wittig reactions. Beilstein J. Org. Chem. 2016, 12, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Palacios, F.; Aparicio, D.; Rubiales, G.; Alonso, C.; de los Santos, J.M. Synthetic Applications of Intramolecular Aza-Wittig Reaction for the Preparation of Heterocyclic Compounds. Curr. Org. Chem. 2009, 13, 810–828. [Google Scholar] [CrossRef]
- Smith, R.; Chen, X.; Protasiewicz, J.D. A Fluorescent (E)-Poly(p-phenylenephosphaalkene) Prepared by a Phospha-Wittig Reaction. Inorg. Chem. 2003, 42, 5468–5470. [Google Scholar] [CrossRef]
- Chevrie, D.; Lequeux, T.; Pommelet, J.-C. Thia-Wittig-like Reactions as a New Route for the Stereoselective Synthesis of (Z)-Fluoroalkenoates. Org. Lett. 1999, 1, 1539–1541. [Google Scholar] [CrossRef]
- Borys-Smith, A.; Rice, E.; Nichol, G.; Cowley, M.J. The Phospha-Bora-Wittig Reaction. J. Am. Chem. Soc. 2021, 143, 14065–14070. [Google Scholar] [CrossRef]
- Vedejs, E.; Peterson, M.J. Top. Stereochem; Eliel, E.L., Wilen, S.H., Eds.; Wiley: New York, NY, USA, 1994; Volume 21. [Google Scholar]
- McKenna, E.G.; Walker, B.J. The stereochemistry of Wittig reactions of ylide-anions derived from semi-stabilized phosphonium ylides. Tetrahedron Lett. 1988, 29, 485–488. [Google Scholar] [CrossRef]
- Yamataka, H.; Nagareda, K.; Ando, K.; Hanafusa, T. Relative reactivity and stereoselectivity in the Wittig reactions of substituted benzaldehydes with benzylidenetriphenylphosphorane. J. Org. Chem. 1992, 57, 2865–2869. [Google Scholar] [CrossRef]
- Ward, W.J., Jr.; McEwan, W.E. Metal ion effects in Wittig reactions. A general hypothesis for the mechanism of the Wittig reaction. J. Org. Chem. 1990, 55, 493–500. [Google Scholar] [CrossRef]
- Morodo, R.; Bianchi, P.; Monbaliu, J.-C. Continuous Flow Organophosphorus Chemistry. Eur. J. Org. Chem. 2020, 33, 5236–5277. [Google Scholar] [CrossRef]
- Drehe, M.; Simulescu, V.; Ilia, G. Progress in the development of flame retardants. Rev. Chem. Eng. 2008, 24, 263–302. [Google Scholar]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Jansa, P.; Baszczyňski, O.; Procházková, E.; Dračínský, M.; Janeba, Z. Microwave-assisted hydrolysis of phosphonatediesters: An efficient protocol for the preparation of phosphonic acids. Green Chem. 2012, 14, 2282–2288. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, Á.; Tóth, N.; Keglevich, G. Continuous Flow Alcoholysis of Dialkyl H-Phosphonates with Aliphatic Alcohols. Molecules 2018, 23, 1618. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.; Scheunemann, A.; Zur, C.; Miethchen, R. Fluorination of organic compounds with ultrasound. Ultrasonics 1996, 34, 543–546. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A.; Ilia, G.; Simon, Z.; Demadis, K.; Colodrero, R.M.P.; Rosario, M.P.; Cabeza, A.; Vallcorba, O.; Rius, J.; et al. Synthesis and structural characterization of 2-D layered copper(II) styrylphosphonate coordination polymers. J. Coord. Chem. 2014, 67, 1562–1572. [Google Scholar] [CrossRef]
- Lupa, L.; Maranescu, B.; Visa, A. Equilibrium and kinetic studies of chromium ions adsorption on Co (II)-based phosphonate metal organic frameworks. Sep. Sci. Technol. 2018, 53, 1017–1026. [Google Scholar] [CrossRef]
- Gheonea, R.; Mak, C.; Crasmareanu, E.; Simulescu, V.; Plesu, N.; Ilia, G. Surface modification of SnO2 with phosphonic acids, Hindawi Publishing Corporation. J. Chem. 2017, 2017, 2105938. [Google Scholar] [CrossRef]
- McNulty, J.; McLeod, D.; Das, P.; Zepeda-Velázquez, C. Wittig Reactions of Trialkylphosphine-derived Ylides: New Directions and Applications in Organic Synthesis. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 619–632. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Pfaltz, A.; Yamamoto, H. Comprehensive Asymmetric Catalysis: Supplement 1 and 2; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms and Structure 2007, 6th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Asymmetric organocatalysis: From infancy to adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660. [Google Scholar] [CrossRef]
- Notz, W.; Tanaka, F.; Barbas, C.F. Enamine-based organocatalysis with proline and diamines: The development of direct catalytic asymmetric Aldol, Mannich, Michael, and Diels-alder reactions. Acc. Chem. Res. 2004, 37, 580–591. [Google Scholar] [CrossRef]
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis—After the gold rush. Chem. Soc. Rev. 2008, 38, 2178–2189. [Google Scholar] [CrossRef]
- Dalko, P.I. Enantioselective Organocatalysis: Reaction and Experimental Procedures; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Vedejs, E.; Marth, C.F.; Ruggeri, R. Substituent effects and the Wittig mechanism: The case of stereospecific oxaphosphetane decomposition. J. Am. Chem. Soc. 1988, 110, 3940–3948. [Google Scholar] [CrossRef]
- Riccaboni, M.; La Porta, E.; Martorana, A.; Attanasio, R. Effect of phase transfer chemistry, segmented fluid flow, and sonication on the synthesis of cinnamic esters. Tetrahedron 2010, 66, 4032–4039. [Google Scholar] [CrossRef]
- Noro, T.; Miyase, T.; Kuroyanagi, M.; Ueno, A.; Fukushima, S. Monoamine oxidase inhibitor from the rhizomes of Kaempferia galanga L. Chem. Pharm. Bull. 1983, 31, 2708–2711. [Google Scholar] [CrossRef]
- Fujimura, O.; Honma, T. Olefination of aldehydes by ethyl diazoacetate catalyzed by a ruthenium(II) complex. Tetrahedron Lett. 1998, 39, 625–626. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Laera, S.; Cioffi, N. Pd Nanoparticles Catalyzed Stereospecific Synthesis of β-Aryl Cinnamic Esters in Ionic Liquids. J. Org. Chem. 2003, 68, 2929–2933. [Google Scholar] [CrossRef]
- Ahmed-Omer, B.; Barrow, D.; Wirth, T. Heck reactions using segmented flow conditions. Tetrahedron Lett. 2009, 50, 3352–3355. [Google Scholar] [CrossRef]
- Schwalbe, T.; Autze, V.; Hohmann, M.; Stirner, W. Novel innovation systems for a cellular approach to continuous process chemistry from discovery to market. Org. Process Res. Dev. 2004, 8, 440–454. [Google Scholar] [CrossRef]
- Frattini, S.; Quai, M.; Cereda, E. Kinetic study of microwave-assisted Wittig reaction of stabilized ylides with aromatic aldehydes. Tetrahedron Lett. 2001, 42, 6827–6829. [Google Scholar] [CrossRef]
- El-Batta, A.; Jiang, C.; Zhao, W.; Annes, R.; Cooksy, A. Bergdahl, Wittig Reactions in Water Media Employing Stabilized Ylides with Aldehydes. Synthesis of α,β-Unsaturated Esters from Mixing Aldehydes, α-Bromoesters, and Ph3P in Aqueous NaHCO3. J. Org. Chem. 2007, 72, 5244–5259. [Google Scholar] [CrossRef] [PubMed]
- Nonnenmacher, A.; Mayer, R.; Plieninger, H. Application of high-pressure on Wittig reactions with resonance stabilized ylides. Liebigs Ann. Chem. 1983, 23, 2135–2140. [Google Scholar] [CrossRef]
- Boulaire, V.L.; Grèe, R. Wittig reactions in the ionic solvent [bmim][BF4]. Chem. Commun. 2000, 22, 2195–2196. [Google Scholar] [CrossRef]
- Mahboobi, S.; Sellmer, A.; Höcher, H.; Garhammer, C.; Pongratz, H.; Maier, T.; Ciossek, T.; Beckers, T. 2-Aroylindoles and 2-Aroylbenzofurans with N-Hydroxyacrylamide Substructures as a Novel Series of Rationally Designed Histone Deacetylase Inhibitors. J. Med. Chem. 2007, 50, 4405–4418. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Applications of the Wittig Reaction on the Synthesis of Natural and Natural-Analogue Heterocyclic Compounds. Eur. J. Org. Chem. 2018, 20, 2443–2457. [Google Scholar] [CrossRef]
- Skelton, V.; Greenway, G.M.; Haswell, S.J.; Styring, P.; Morgan, D.O.; Warrington, B.; Wong, S.Y.F. The preparation of a series of nitrostilbene ester compounds using micro reactor technology. Analyst 2001, 126, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Skelton, V.; Greenway, G.M.; Haswell, S.J.; Styring, P.; Morgan, D.O.; Warrington, B.H.; Wong, S.Y.F. The generation of concentration gradients using electroosmotic flow in micro reactors allowing stereoselective chemical synthesis. Analyst 2001, 126, 11–13. [Google Scholar] [CrossRef]
- Comer, E.; Organ, M.G. A Microreactor for Microwave-Assisted Capillary (Continuous Flow) Organic Synthesis. J. Am. Chem. Soc. 2005, 127, 8160–8167. [Google Scholar] [CrossRef]
- Rivas, D.F.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70. [Google Scholar] [CrossRef]
- Dong, Z.; Delacour, C.; Carogher, K.M.; Udepurkar, P.A.; Kuhn, S. Continuous Ultrasonic Reactors: Design, Mechanism and Application. Materials 2020, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Q.; Yang, C.-G.; Yang, L.-M.; Yang, L.-J. Ultrasound-assisted Wittig Reaction: A Short, Efficient Synthesis of 2-Methoxy-6-alkyl-1,4-benzoquinones. J. Chin. Chem. Soc. 2009, 56, 47–50. [Google Scholar] [CrossRef]
- Šinkovec, E.; Krajnc, M. Phase Transfer Catalyzed Wittig Reaction in the Microtube Reactor under Liquid–Liquid Slug-Flow Pattern. Org. Process Res. Dev. 2011, 15, 817–823. [Google Scholar] [CrossRef]
- Viviano, M.; Glasnov, T.N.; Reichart, B.; Tekautz, G.; Kappe, C.O. A Scalable Two-Step Continuous Flow Synthesis of Nabumetone and Related 4-Aryl-2-butanones. Org. Process Res. Dev. 2011, 15, 858–870. [Google Scholar] [CrossRef]
- Karama, U.; Al-Othman, Z.; Al-Majid, A.; Almansour, A. A Facile One-Pot Synthesis of α-Bromo-α,β-unsaturated Esters from Alcohols. Molecules 2010, 15, 3276–3280. [Google Scholar] [CrossRef]
- Maity, G.; Ghosha, A.K.; Ghosh, P.K. Green Chemistry: Synthesis of organic compounds through green approach. J. Indian Chem. Soc. 2020, 97, 2897–2902. [Google Scholar]
- Wu, L.; Yang, C.; Yang, L. Synthesis of 2-Hydroxy-5-methoxy-3-alkyl-1,4-benzoquinones. Asian J. Chem. 2009, 21, 7005–7011. [Google Scholar]
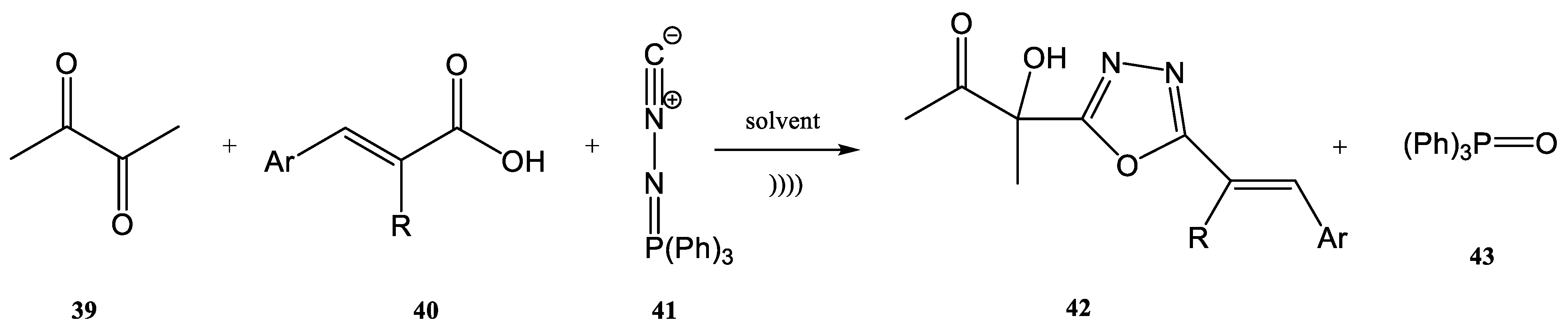
- Ramazani, A.; Zeinali Nasrabadi, F.; Karimi, Z.; Rouhani, M. Preparation of Fully Substituted 1,3,4-Oxadiazole Derivatives from N-Isocyaniminotriphenylphosphorane, (E)-Cinnamic Acids, Chloroacetone and Primary Amines. Bull. Korean Chem. Soc. 2011, 32, 2700–2704. [Google Scholar] [CrossRef]
- Simulescu, V.; Crasmareanu, E.; Ilia, G. Synthesis, Properties and Structures of Phosphorus–Nitrogen Heterocycles. Heterocycles 2011, 83, 275–291. [Google Scholar] [CrossRef]
- Ramazani, A.; Rouhani, M.; Rezaei, A.; Shajari, N.; Souldozi, A. The Reaction of (N-Isocyanimino)triphenylphosphorane with Biacetyl in the Presence of Aromatic Carboxylic Acids: Efficient One-Pot Three-Component Reaction for the Synthesis of 3-(5-Aryl-1,3,4-oxadiazol-2-yl)-3-hydroxybutan-2-one Derivatives. Helv. Chim. Acta. 2011, 94, 282–288. [Google Scholar] [CrossRef]
- Schlosser, M.; Schaub, B.; Oliveira-Neto, J.; Jeganathan, S. Enhanced cis-selectivities in olefination reactions employing ylides with modified stationary groups. Chimia 1986, 40, 246–247. [Google Scholar]
- Stolzenberg, H.; Weinberger, B.; Fehlhammer, W.P.; Puhlhofer, F.G.; Weiss, R. Free and Metal-Coordinated (N-Isocyanimino)triphenylphosphorane: X-ray Structures and Selected Reactions. Eur. J. Inorg. Chem. 2005, 21, 4263–4271. [Google Scholar] [CrossRef]
- Ramazani, A.; Ahmadi, Y.; Tarasi, R. Efficient one-pot synthesis of disubstituted 1,3,4-oxadiazole derivatives from the reaction of (N-isocyanimino)triphenylphosphorane, acetaldehyde, a secondary amine, and an electron-poor (E)-cinnamic acid. Heteroat. Chem. 2011, 22, 79–84. [Google Scholar] [CrossRef]
- Ramazani, A.; Shajari, N.; Mahyari, A.; Ahmadi, Y. A novel four-component reaction for the synthesis of disubstituted 1,3,4-oxadiazole derivatives. Mol. Divers. 2011, 15, 521–527. [Google Scholar] [CrossRef]
- Ramazani, A.; Rouhani, M.; Nasrabadi, F.Z.; Gouranlou, F. Ultrasound-Promoted Three-Component Reaction of N-Isocyaniminotriphenyl-Phosphorane, (E)-Cinnamic Acids, and Biacetyl. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 20–28. [Google Scholar] [CrossRef]
- Palaska, E.; Sohin, G.; Kalicen, P.; Darlu, N.T.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco 2002, 57, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Coppo, F.T.; Evans, K.A.; Graybill, T.D.; Burton, G. Efficient one-pot preparation of 5-substituted-2-amino-1,3,4-oxadiazoles using resin-bound reagents. Tetrahedron Lett. 2004, 45, 3257–3260. [Google Scholar] [CrossRef]
- Li, J.T.; Meng, X.T.; Zhai, X.L. One-pot synthesis of benzylacetamide from oxime under ultrasound irradiation. Ultrason. Sonochem. 2009, 16, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Y.; Li, H.; Shao, H.W. One-pot three-component Mannich-type reactions using Sulfamic acid catalyst under ultrasound irradiation. Ultrason. Sonochem. 2009, 16, 758–762. [Google Scholar] [CrossRef]
- Rouhani, M.; Ramazani, A.; Joo, S.W. Ultrasonics in isocyanide-based multicomponent reactions: A new, efficient and fast method for the synthesis of fully substituted 1,3,4-oxadiazole derivatives under ultrasound irradiation. Ultrason. Sonochem. 2015, 22, 391–396. [Google Scholar] [CrossRef]
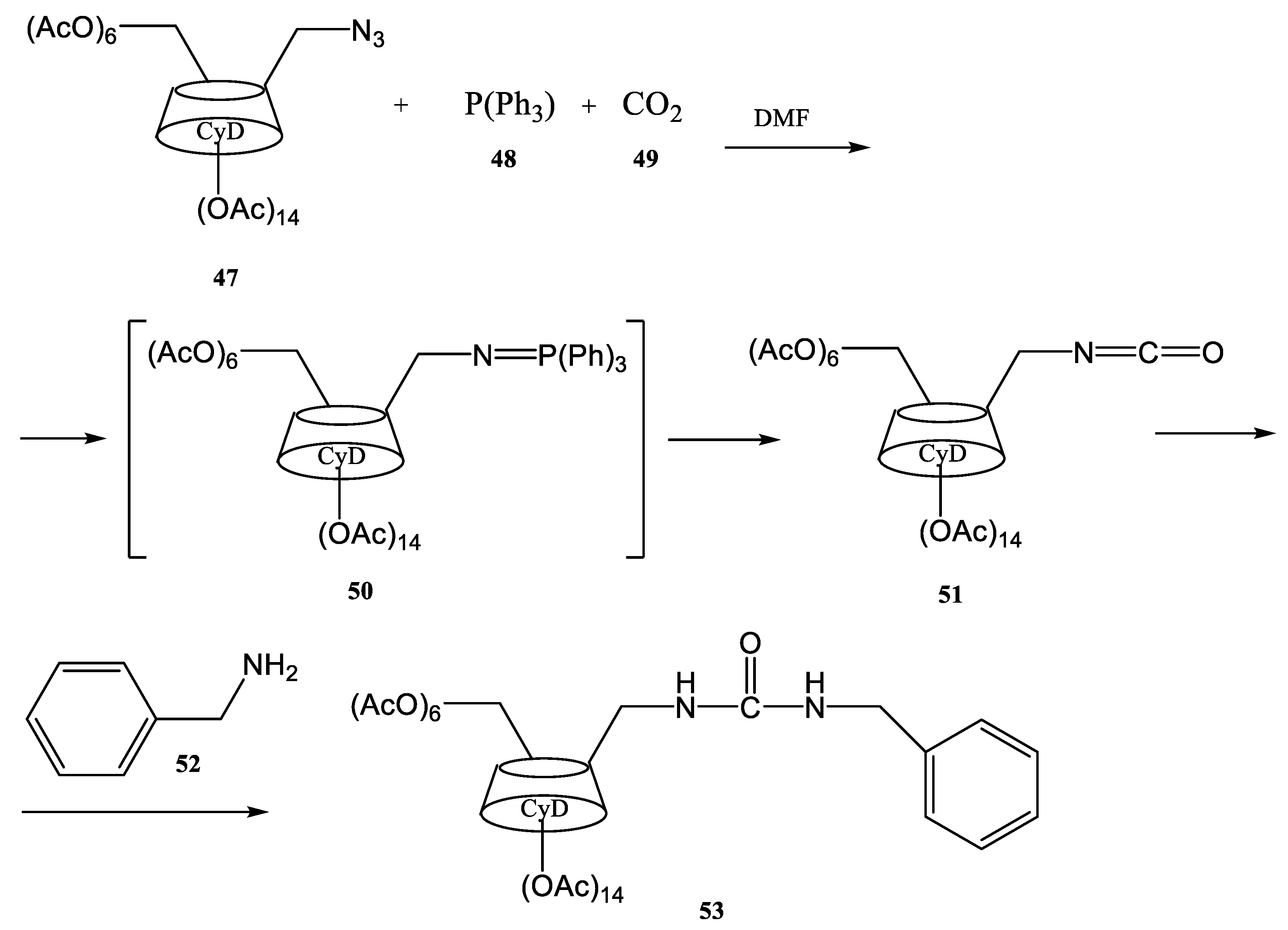
- Scondo, A.; Dumarçay-Charbonnier, F.; Barth, D.; Marsura, A. Ultrasound promoted Staudinger-Aza-Wittig tandem reaction on a monoazido-O-peracetylated-b-cyclodextrin: Effect of ultrasound power. Tetrahedron Lett. 2009, 50, 5582–5584. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef]
- Einhorn, C.; Einhorn, J.; Luche, J.-L. Synthesis 1989, 787; Mason, T. J. Practical Sonochemistry-User’s Guide to Application in Chemistry and Chemical Engineering; Ellis Horwood, Limited: West Sussex, UK, 1991. [Google Scholar]
- Hofmann, J.; Freier, U.; Wecks, M. Ultrasound promoted C-alkylation of benzyl cyanide-effect of reactor and ultrasound parameters. Ultrason. Sonochem. 2003, 10, 271–275. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef]
- Martina, K.; Trotta, F.; Robaldo, B.; Belliardi, N.; Jicsinszky, L.; Cravotto, G. Efficient regioselective functionalizations of cyclodextrins carried out under microwaves or power ultrasound. Tetrahedron Lett. 2007, 48, 9185–9189. [Google Scholar] [CrossRef]
- Scondo, A.; Dumarcay-Charbonnier, F.; Marsura, A.; Barth, D.J. Supercritical CO2 phosphine imide reaction on peracetylated β-cyclodextrins. Supercrit. Fluids 2009, 48, 41–47. [Google Scholar] [CrossRef]
- Bremmer, D.H. Recent advances in organic synthesis utilizing ultrasound. Ultrasonics Sonochem. 1994, 1, S119–S124. [Google Scholar] [CrossRef]
- Wadsworth, W. Synthetic Applications of Phosphoryl-Stabilized Anions. Wiley Online Library, Org. React. 1977, 25, 73. [Google Scholar]
- Miethchen, R. COST-Workshop—Chemistry under Extreme or Non-classical Conditions; COST-Workshop: Lahnstein, Germany, 1995. [Google Scholar]
- Wadsworth, W.S., Jr.; Emmons, W.D. The Utility of Phosphonate Carbanions in Olefin Synthesis. J. Am. Chem. Soc. 1961, 83, 1733. [Google Scholar] [CrossRef]
- Wadsworth, W.S., Jr.; Emmons, W.D. Ethyl Cyclohexylideneacetate. Org. Synth. Coll. 1973, 5, 547. [Google Scholar]
- Belguendouz, L.; Fremont, L.; Linard, A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997, 53, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Waffo-Teguo, P.; Hawthorne, M.E.; Cuendet, M.; Merillon, J.M.; Kinghorn, A.D.; Pezzuto, J.M.; Mehta, R.G. Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera) cell cultures. Nutr. Cancer 2001, 40, 173–179. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Hamel, E. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia 1989, 45, 209–211. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Sawasdee, K.; Kirtikara, K. Flavonoids and Stilbenoids with COX-1 and COX-2 Inhibitory Activity from Dracaena loureirin, Planta. Med. 2002, 68, 841–843. [Google Scholar]
- Pace-Asciak, C.R.; Rounova, O.; Hahn, S.E.; Diamandis, E.P.; Goldberg, D.M. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin. Chim. Acta. 1996, 246, 163–182. [Google Scholar] [CrossRef]
- Mu, F.; Hamel, E.; Lee, D.J.; Pryor, D.E.; Cushman, M. Synthesis, Anticancer Activity, and Inhibition of Tubulin Polymerization by Conformationally Restricted Analogues of Lavendustin A. J. Med. Chem. 2003, 46, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
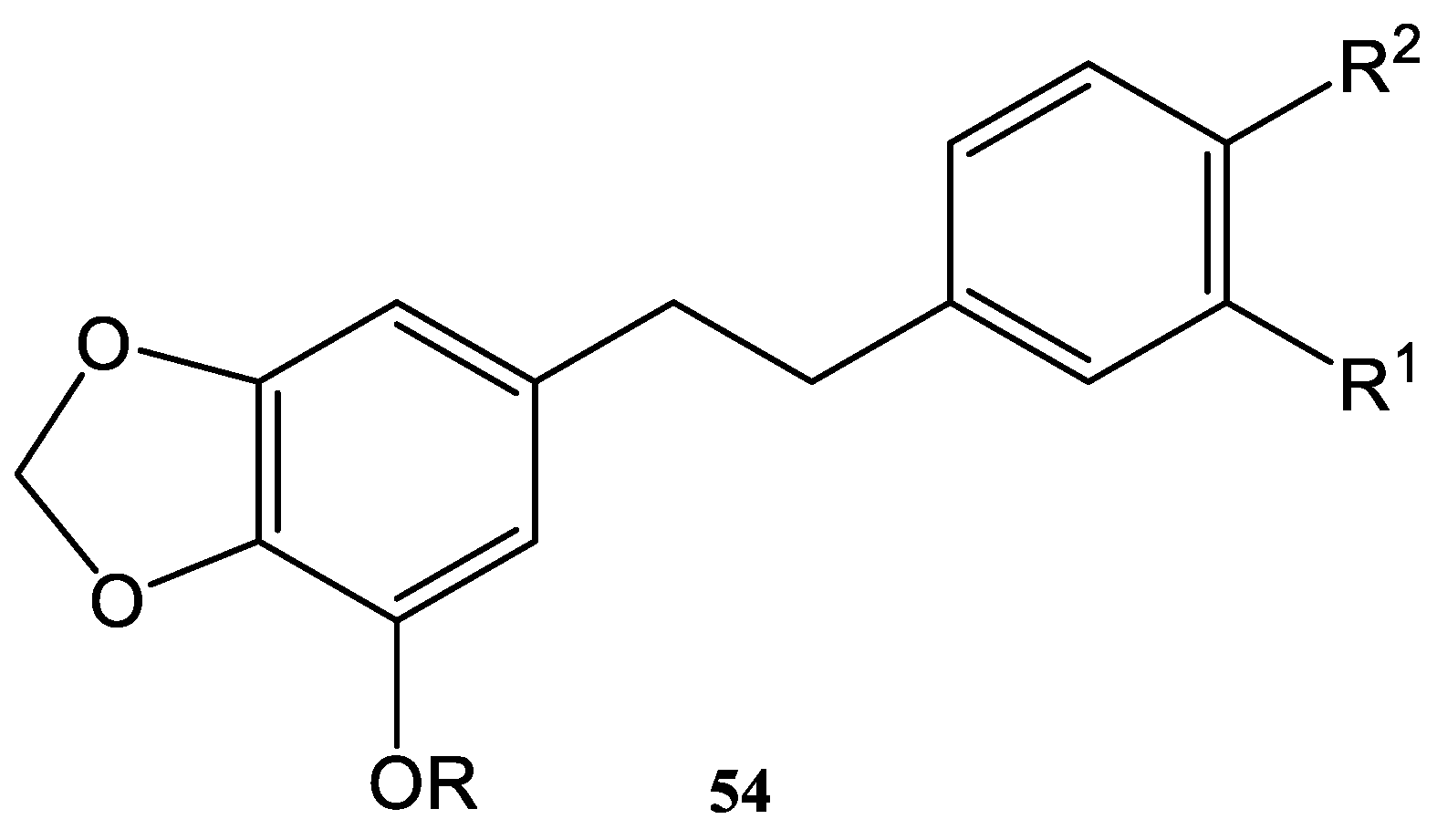
- Zhang, W.-G.; Zhao, R.; Ren, J.; Ren, L.-X.; Lin, J.-G.; Liu, D.-L.; Wu, Y.-L.; Yao, X.-S. Synthesis and Anti-proliferative in-vitro Activity of Two Natural Dihydrostilbenes and their Analogues. Arch. Pharm. Chem. Life Sci. 2007, 340, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wigerinck, P.; Aerschot, A.; Claes, P.; Balzarini, J.; De Clercq, E.; Herdewijn, P. 3′-(1,2,3-Triazol-1-yl)-2′,3′-dideoxythymidine and 3′-(1,2,3-triazol-1-yl)-2′,3′-dideoxyuridine. J. Heterocycl. Chem. 1989, 26, 1635–1642. [Google Scholar] [CrossRef]
- Peters, D.; Pautet, F.; ElFakih, H.; Fillion, H.; Luche, J.-L. Competition between isomerization and addition in the Sonication of Vinyl Sulfones in the presence of bromotrichloromethane. J. Prakt. Chem. 1995, 337, 363–367. [Google Scholar] [CrossRef]
- Miller, A.O.; Peters, D.; Zur, C.; Frank, M.; Miethchen, R. Organofluorine compounds and fluorinating agents. Part 17: Sonochemical-forced preparation of perfluoroalkanals and their use for non-conventional acetalations of carbohydrates. J. Fluor. Chem. 1997, 82, 33–38. [Google Scholar] [CrossRef]
- Hager, C.; Miethchen, R.; Reinke, H. Organofluorine compounds and fluorinating agents, Part 26 New reversed nucleosides - perfluoroalkyl substituted 1,2,3-triazoles linked to D-galactose and D-altroses. J. Fluor. Chem. 2000, 104, 135–142. [Google Scholar] [CrossRef]
- Bohm, R.; Karow, C. Reaction of 3-nitro-and 3-bromo-3-nitroacrylates with sodium azide. Pharmazie 1981, 36, 243. [Google Scholar]
- Dehne, H. 1,2,3-Triazoles, in Houben-Weyl, 8th ed.; Thieme-Verlag: Stuttgart, Germany, 1994; Volume 308. [Google Scholar]
- Contreras, A.; Sanchez-Perez, R.M.; Alonso, G. Halomethyl-1,2,3-triazole derivatives: A new type of alkylating agent active in mouse transplantable tumors. Cancer Chemother. Pharmacol. 1978, 1, 243–247. [Google Scholar] [CrossRef]
- Fakih, H.E.; Pautet, F.; Fillion, H.; Luche, J.-L. Sonochemical Wittig-Horner synthesis of vinyl sulfones from 4-nitro acetophenone as a model for enolisable ketones. Tetrahedron Lett. 1992, 33, 4909–4910. [Google Scholar] [CrossRef]
- Pletcher, D.; Green, R.A.; Brown, R.C.D. Flow Electrolysis Cells for the Synthetic Organic Chemistry Laboratory. Chem. Rev. 2018, 118, 4573–4591. [Google Scholar] [CrossRef]
- Schlosser, M. Top. Stereochem.; Eliel, E.L., Allinger, N.L., Eds.; Wiley: New York, NY, USA, 1970; Volume 5. [Google Scholar]
- Vedejs, E.; Snoble, K.A.J. Direct observation of oxaphosphetanes from typical Wittig reactions. J. Am. Chem. Soc. 1973, 95, 5778–5780. [Google Scholar] [CrossRef]
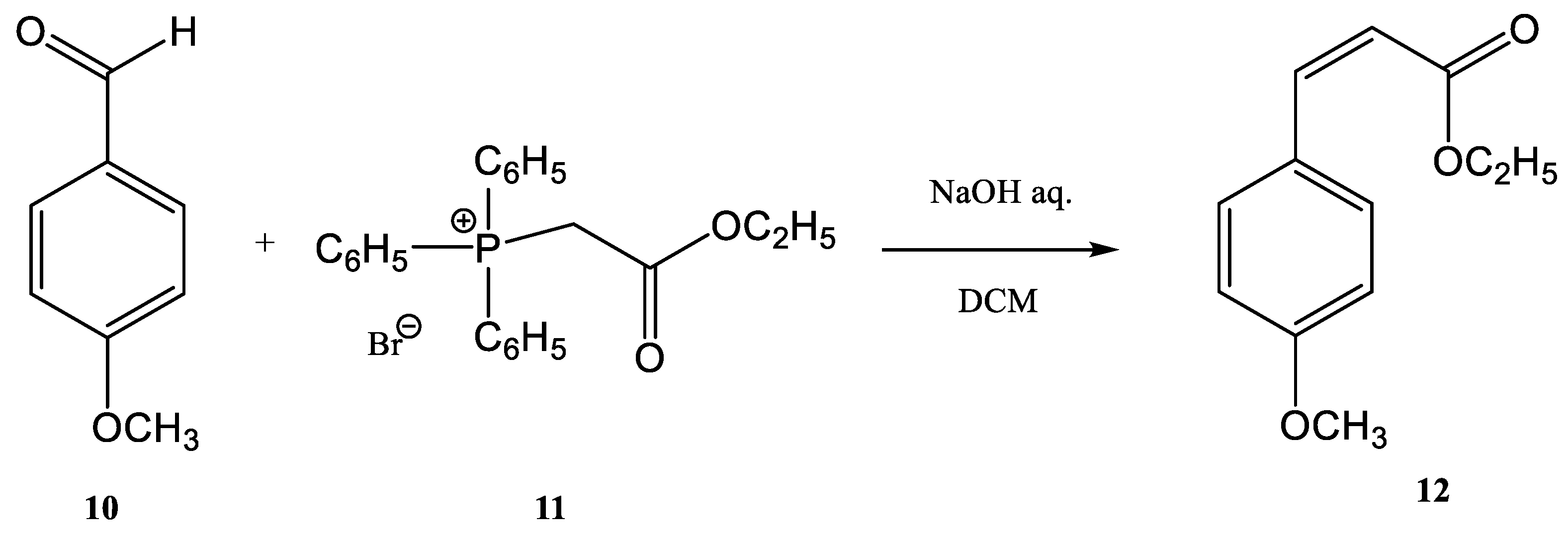
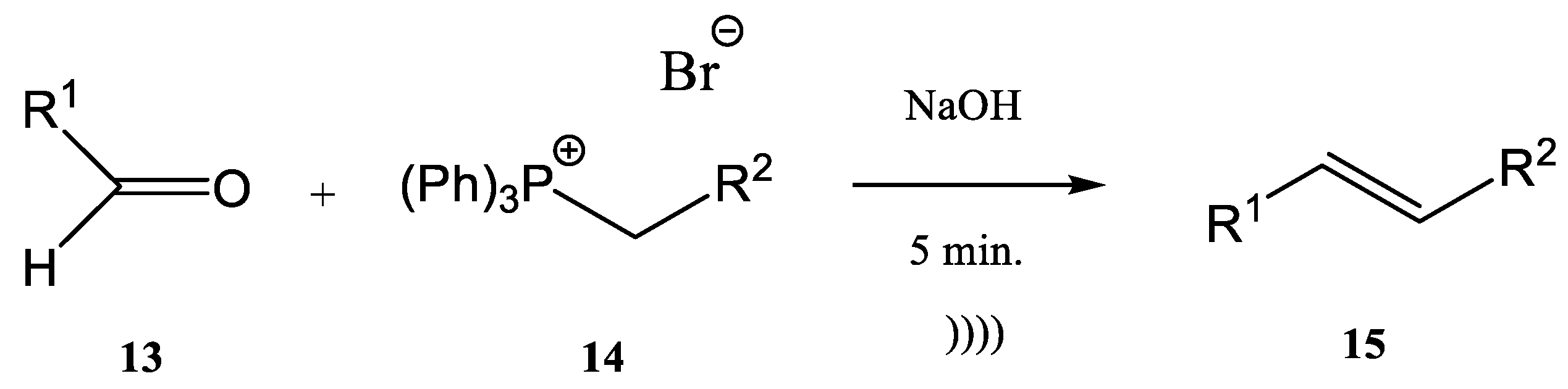
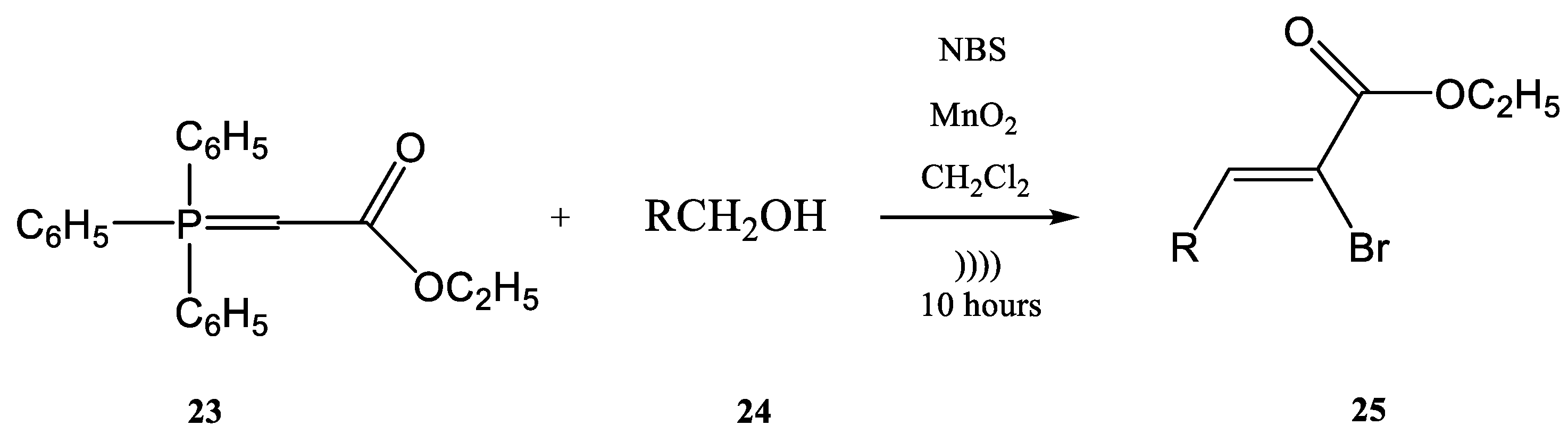

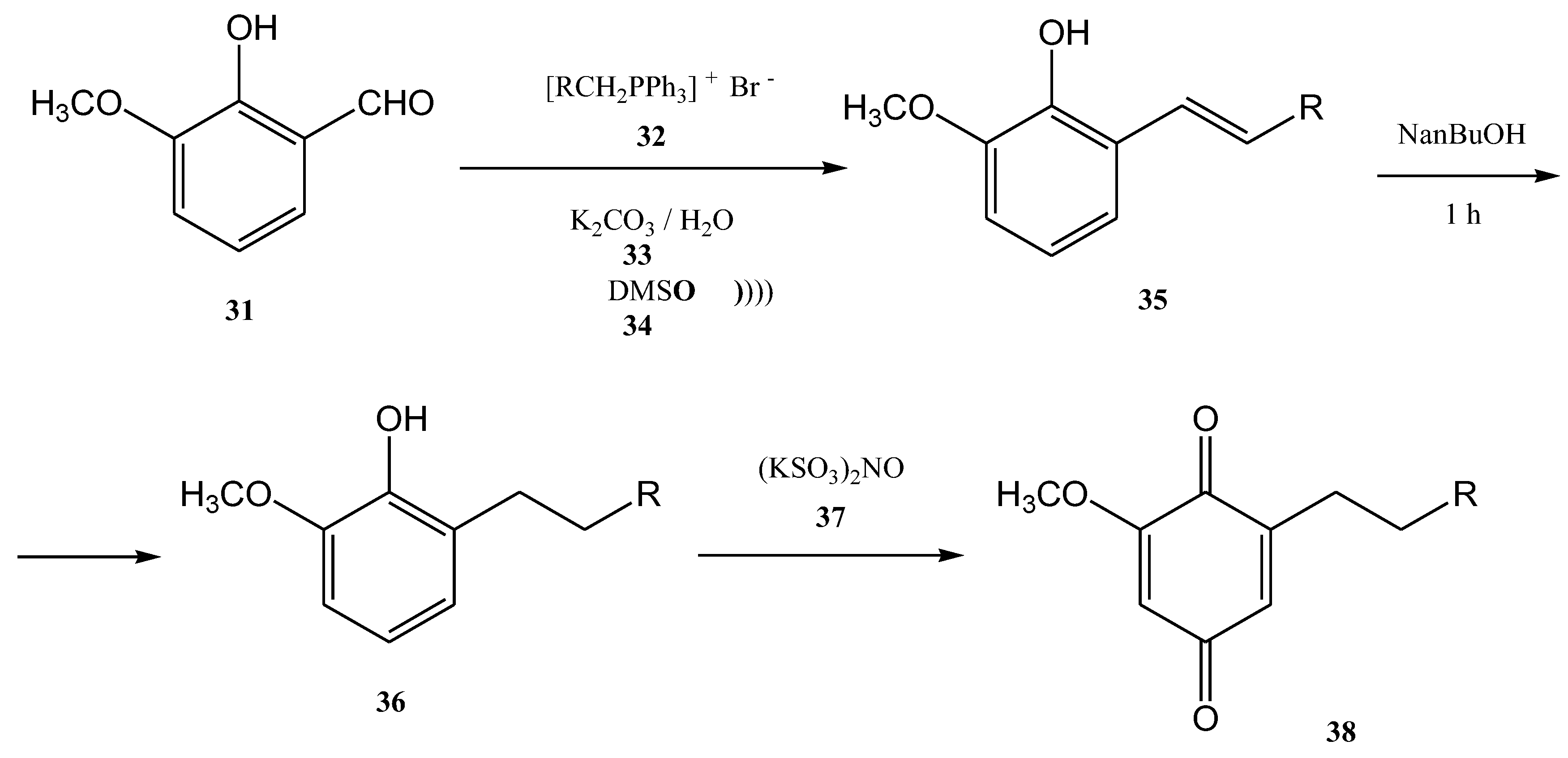


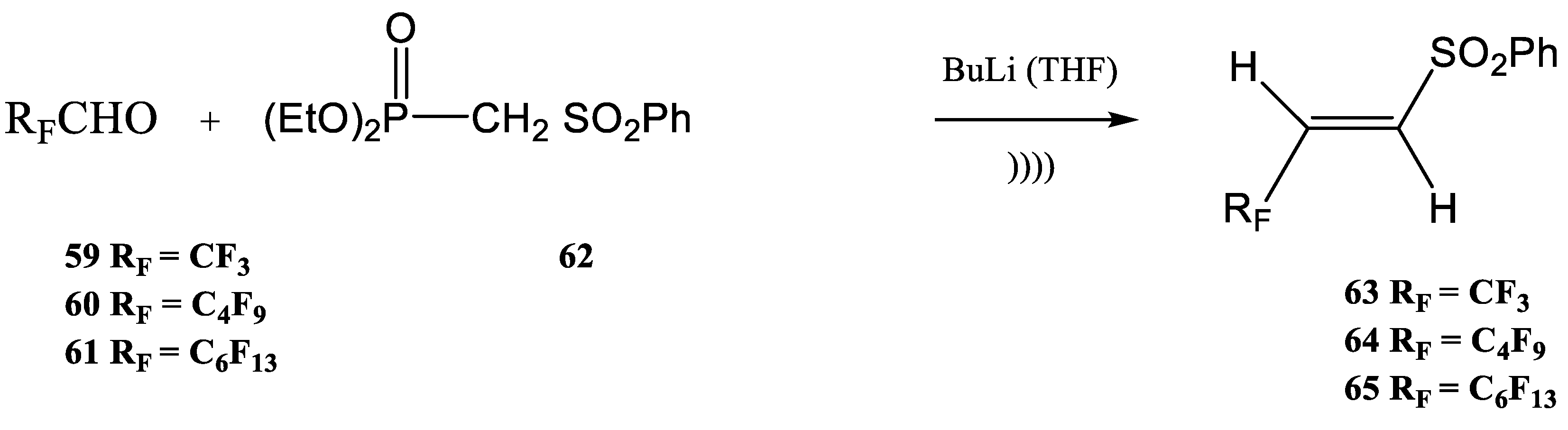


| R | Product | Yield (%) | Z:E Ratio |
|---|---|---|---|
 | 25a | 85 | 94:6 |
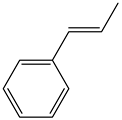 | 25b | 81 | 95:5 |
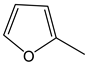 | 25c | 91 | 89:11 |
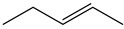 | 25d | 90 | 100:0 |
 | 25e | 86 | 92:8 |
 | 25f | 87 | 76:24 |
 | 25g | 21 | 90:10 |
| Dihydrostilbenes | R | R1 | R2 |
|---|---|---|---|
| 54a | CH3 | OH | H |
| 54b | H | OH | H |
| 54c | CH3 | NH2 | H |
| 54d | H | NH2 | H |
| 54e | CH3 | H | OH |
| 54f | H | H | OH |
| 54g | CH3 | H | F |
| 54h | H | H | F |
| 54i | H | H | OCH3 |
| 54j | H | H | N(CH3)2 |
| 54k | CH3 | OCH3 | OH |
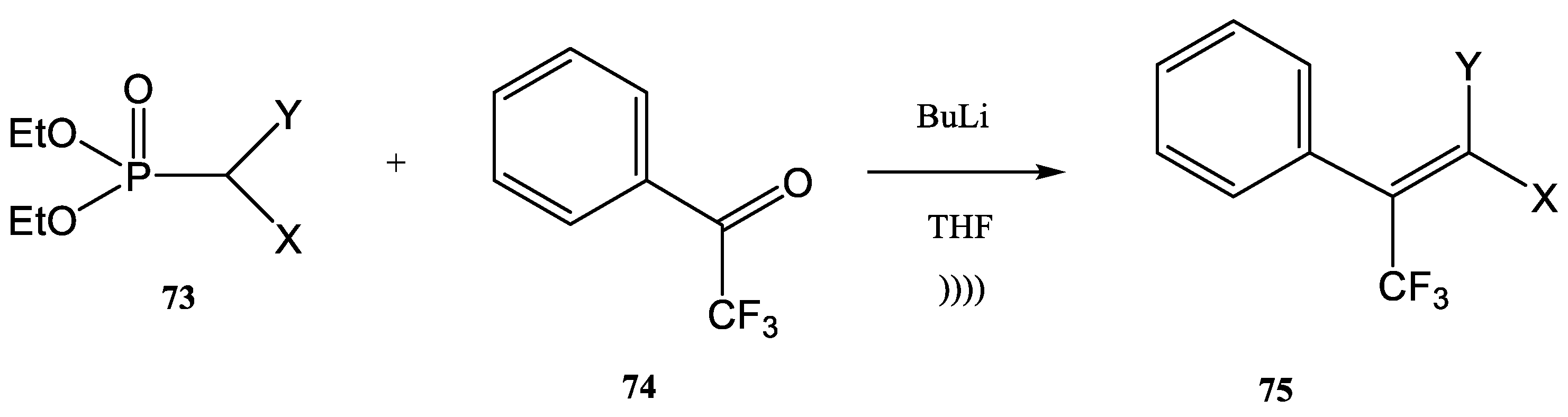
| Base | Conditions | Ratio 72:71 | Ratio E/Z | Yield (%) |
|---|---|---|---|---|
| BuLi | Stirring | 95:5 | 75:25 | 33 |
| BuLi | Ultrasound | 85:15 | 60:40 | 48 |
| NaH | Ultrasound | 60:40 | 80:20 | 66 |
| X | Y | Yield % | Z:E |
|---|---|---|---|
| SO2CH3 | H | 54 | 61:39 |
| SO2C6H5 | H | 63 | 43:57 |
| OCH3 | CN | 50 | 58:42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilia, G.; Simulescu, V.; Plesu, N.; Chiriac, V.; Merghes, P. Wittig and Wittig–Horner Reactions under Sonication Conditions. Molecules 2023, 28, 1958. https://doi.org/10.3390/molecules28041958
Ilia G, Simulescu V, Plesu N, Chiriac V, Merghes P. Wittig and Wittig–Horner Reactions under Sonication Conditions. Molecules. 2023; 28(4):1958. https://doi.org/10.3390/molecules28041958
Chicago/Turabian StyleIlia, Gheorghe, Vasile Simulescu, Nicoleta Plesu, Vlad Chiriac, and Petru Merghes. 2023. "Wittig and Wittig–Horner Reactions under Sonication Conditions" Molecules 28, no. 4: 1958. https://doi.org/10.3390/molecules28041958
APA StyleIlia, G., Simulescu, V., Plesu, N., Chiriac, V., & Merghes, P. (2023). Wittig and Wittig–Horner Reactions under Sonication Conditions. Molecules, 28(4), 1958. https://doi.org/10.3390/molecules28041958