Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization
Abstract
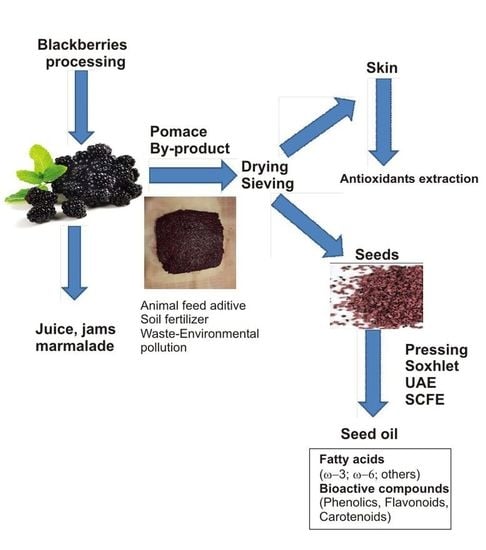
1. Introduction
2. Results and Discussion
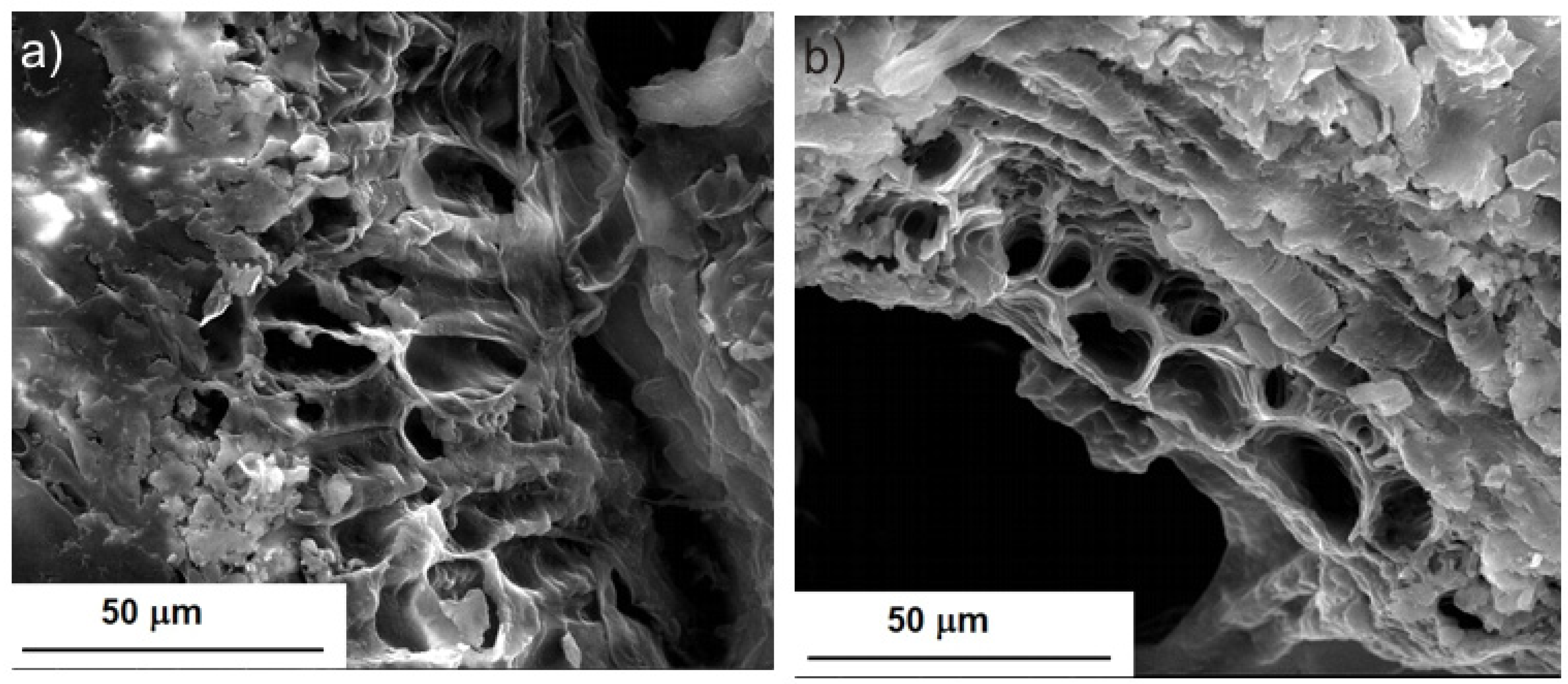
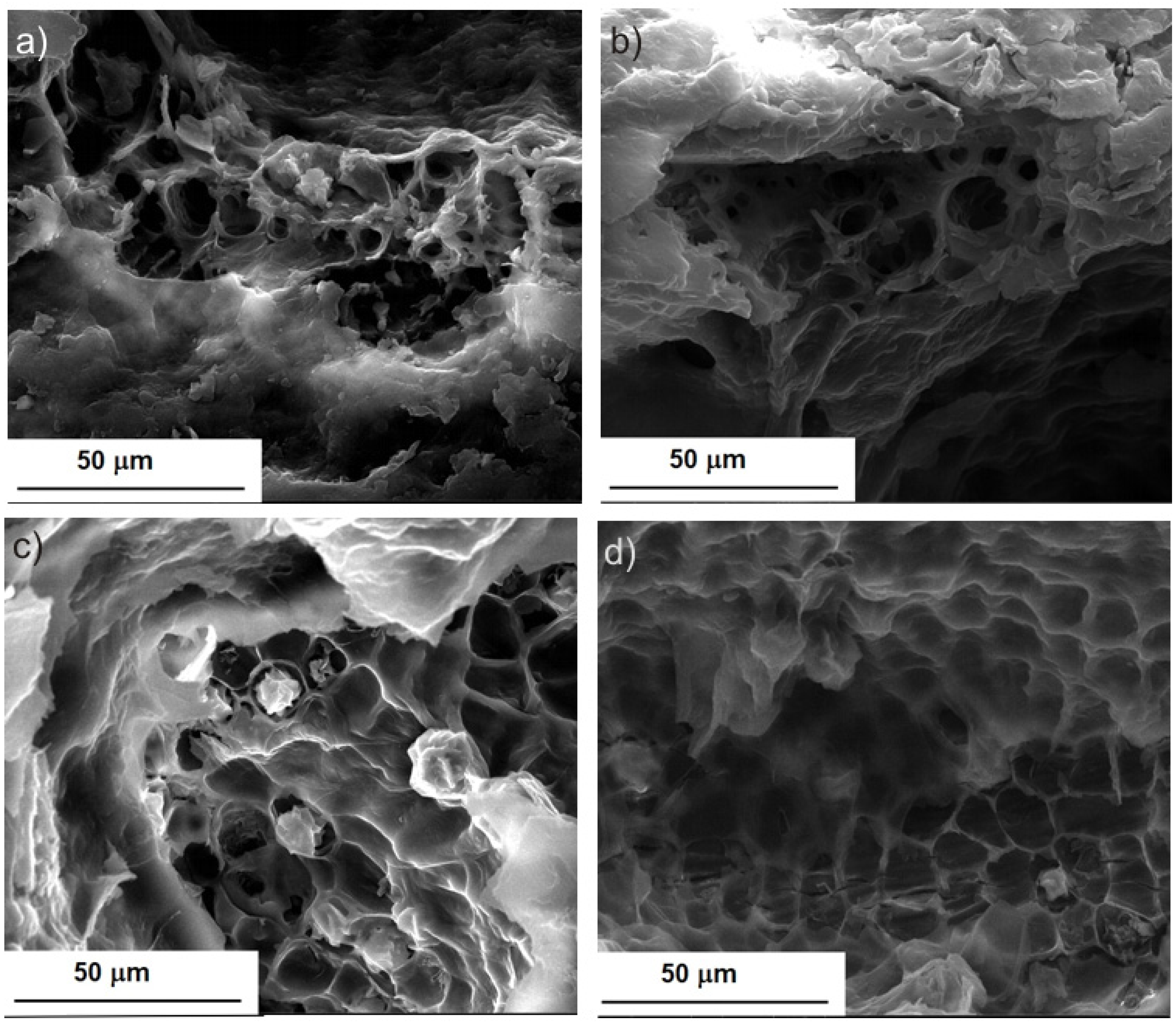
2.1. Morphological Characterization of BB Seeds after UAE
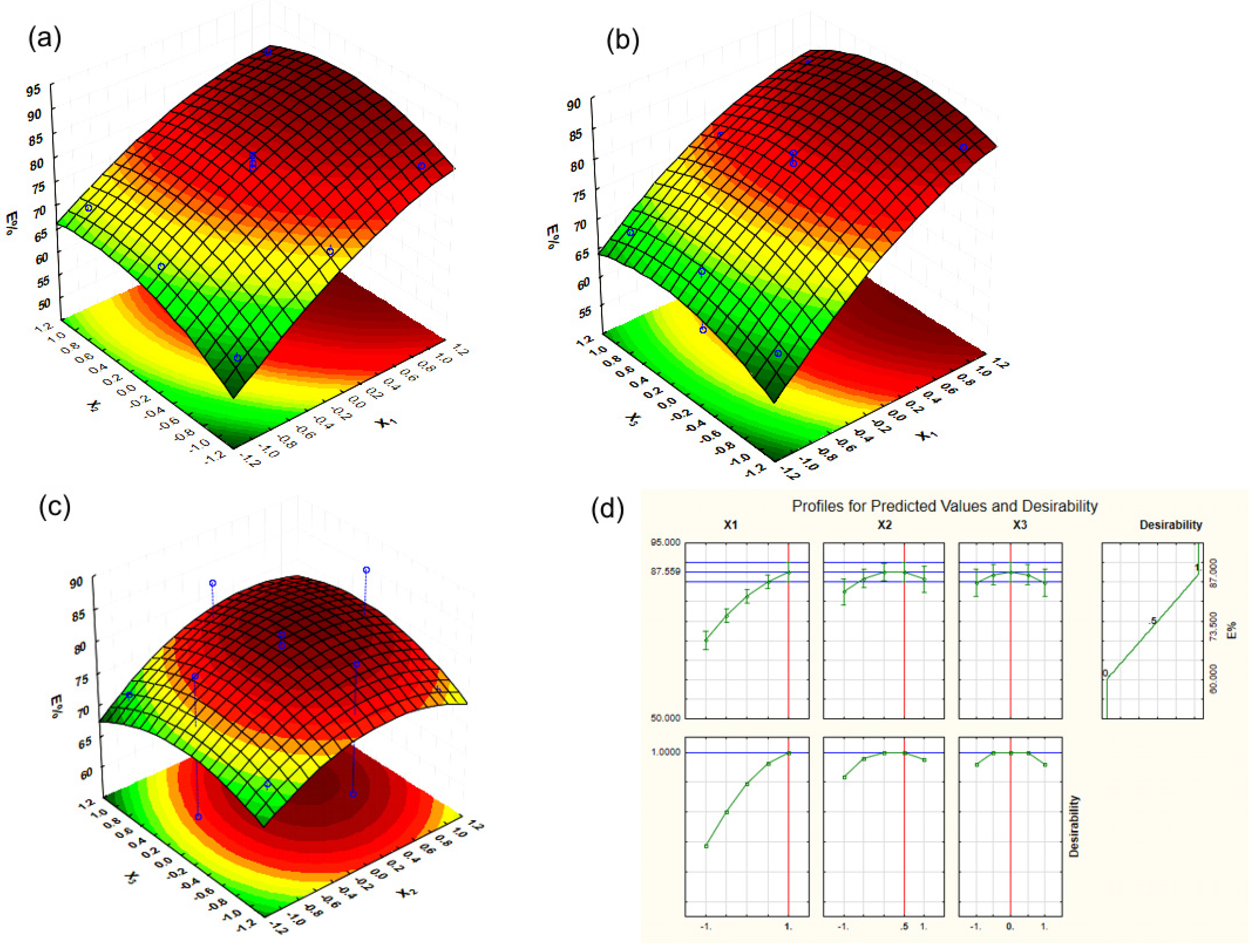
2.2. Extraction Efficiency of UAE for BB Seed Oil Optimized by RSM
2.3. Desirability Optimization for BB Seed Oil
2.4. BB Seed Oil Characterization
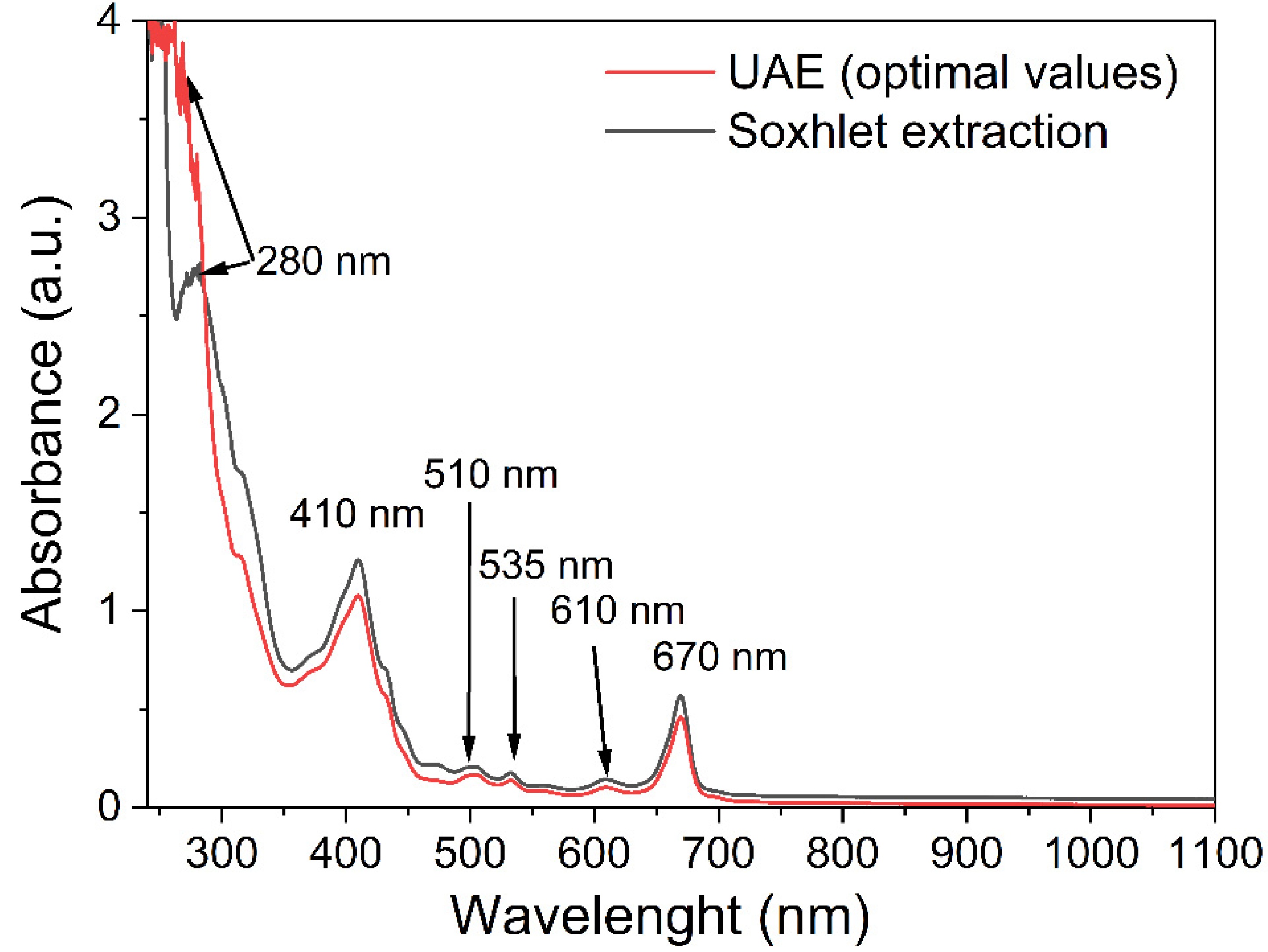
2.5. UV–Vis Spectra of BB Seed Oil
2.6. Total Phenolic Content (TPC) and Anthocyanin Content (TAC) of BB Seed Oil and Defatted Seed Methanolic Extracts and Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Vegetal Material
3.3. Extraction Techniques
3.3.1. Soxhlet Extraction
3.3.2. Ultrasound Assisted Extraction (UAE)
3.4. Experimental Design and Statistical Analysis
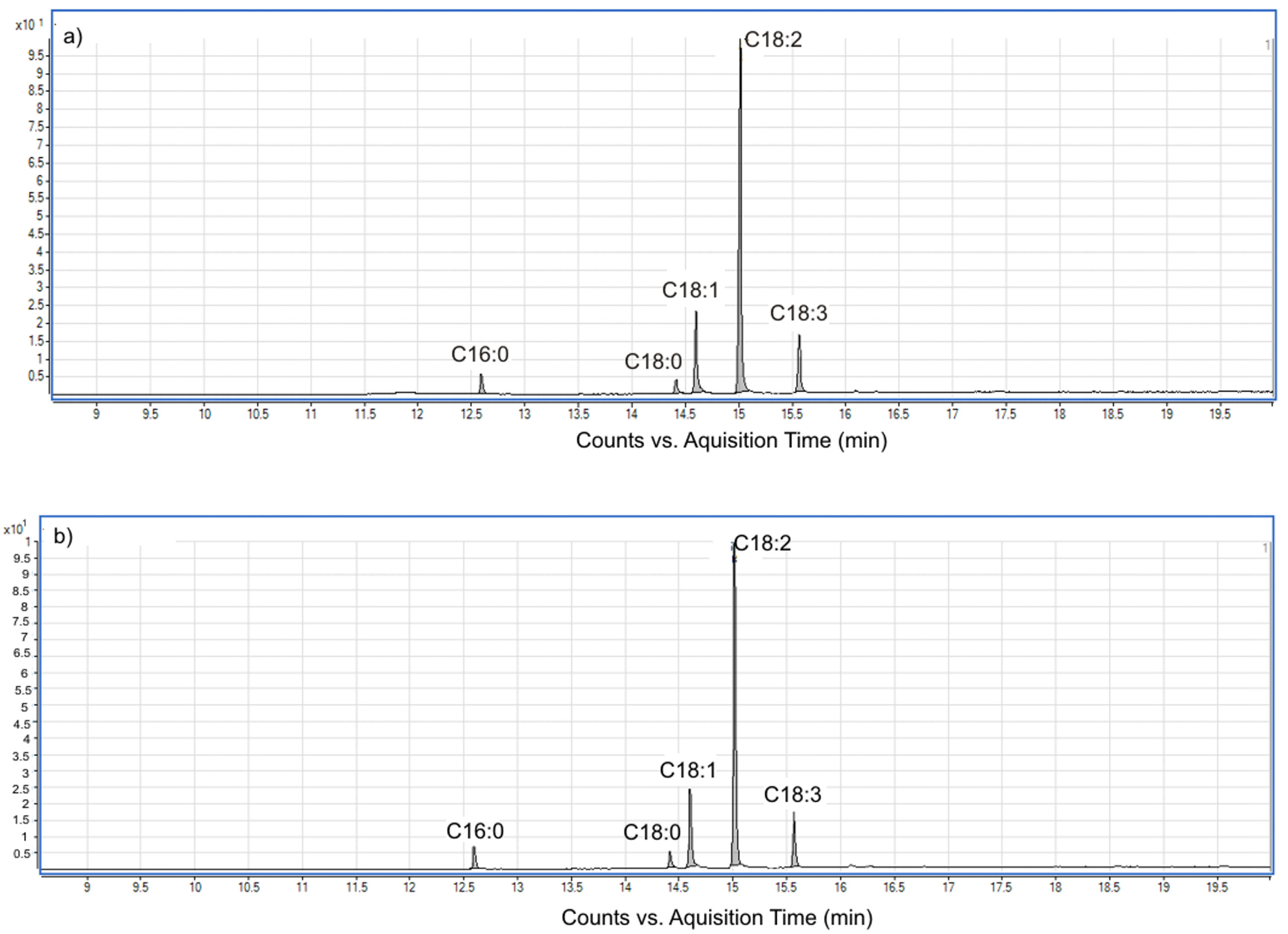
3.5. Analysis of Fatty Acid Composition of BB Seed Oil and Determination of the Oil Quality Indices
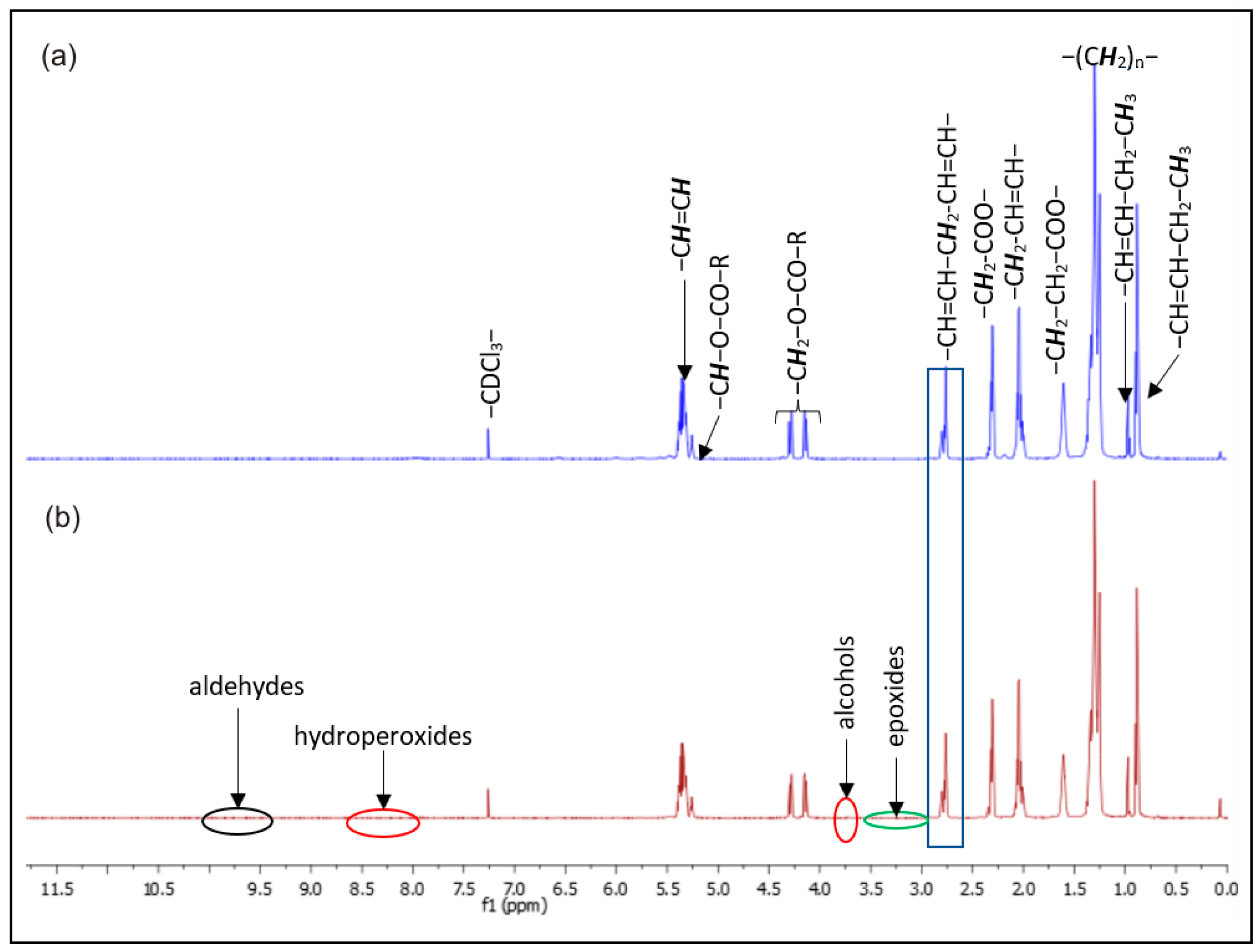
3.6. 1H-NMR Analysis
3.7. UV–Vis Spectra
3.8. Determination of Total Phenolic Content (TPC), Total Anthocyanin Content (TAC), and Antioxidant Capacity of BB Seed Oil and of Defatted BB Seed Methanolic Extract
3.8.1. Sample Preparation
3.8.2. Total Phenolic Content (TPC) and Total Anthocyanin Content (TAC)
3.8.3. Antioxidant Capacity
3.9. BB Seed Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere 2022, 287 Pt 3, 132221. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Kainat, S.; Arshad, M.S.; Khalid, W.; Khalid, M.; Koraqi, H.; Afzal, M.F.; Noreen, S.; Aziz, Z.; Al-Farga, A. Sustainable novel extraction of bioactive compounds from fruits and vegetables waste for functional foods: A review. Int. J. Food Prop. 2022, 25, 2457–2476. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Diaconeasa, Z.M. Introductory Chapter: From Waste to New Resources. In Food Preservation and Waste Exploitation; Socaci, S.A., Farcas, A.C., Aussenac, T., Laguerre, J.-C., Eds.; Intech Open: London, UK, 2020; ISBN 978-1-78985-426-8. [Google Scholar]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energ. Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Qin, S.; Giri, B.S.; Patel, A.K.; Sar, T.; Liu, H.; Chen, H.; Juneja, A.; Kumar, D.; Zhang, Z.; Awasthi, M.K.; et al. Resource recovery and biorefinery potential of apple orchard waste in the circular bioeconomy. Bioresour. Technol. 2021, 321, 124496. [Google Scholar] [CrossRef]
- Costa, J.M.; Ampese, L.C.; Ziero, H.D.D.; Sganzerla, W.G.; Forster-Carneiro, T. Apple pomace biorefinery: Integrated approaches for the production of bioenergy, biochemicals, and value-added products—An updated review. J. Environ. Chem. Eng. 2022, 10, 108358. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Kim, I.J.; Jeong, D.; Kim, S.R. Upstream processes of citrus fruit waste biorefinery for complete valorization. Bioresour. Technol. 2022, 362, 127776. [Google Scholar] [CrossRef] [PubMed]
- Metzner Ungureanu, C.-R.; Lupitu, A.I.; Moisa, C.; Rivis, A.; Copolovici, L.O.; Poiana, M.-A. Investigation on High-Value Bioactive Compounds and Antioxidant Properties of Blackberries and Their Fractions Obtained by Home-Scale Juice Processing. Sustainability 2020, 12, 5681. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. A biorefinery for efficient processing and utilization of spent pulp of Colombian Andes Berry (Rubus glaucus Benth.): Experimental, techno-economic and environmental assessment. Bioresour. Technol. 2017, 223, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Magama, P.; Chiyanzu, I.; Mulopo, J. A systematic review of sustainable fruit and vegetable waste recycling alternatives and possibilities for anaerobic biorefinery. Bioresour. Technol. Rep. 2022, 18, 101031. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Brodowska, A.J. Raspberry pomace—Composition, properties and application. Eur. J. Biol. Res. 2017, 7, 86–96. [Google Scholar]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 8, 1215–1225. [Google Scholar] [CrossRef]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food. Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Sirohi, R.; Ahluwalia, V.; Zhou, Y.; Sindhu, R.; Binod, P.; Singhnia, R.R.; Patel, A.K.; Juneja, A.; et al. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour. Technol. 2021, 332, 125181. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kavaliauskaitė, A.; Tamkutė, L.; Pukalskienė, M.; Syrpas, M.; Venskutonis, P.R. Zero waste biorefining of lingonberry (Vaccinium vitisidaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020, 322, 126767. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus Fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Freitas Chim, J. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Ledwożyw-Smoleń, I.; Augustynowicz, J.; Wyżgolik, G.; Kruczek, M.; Kaszycki, P. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 2015, 13, 1313–1325. [Google Scholar] [CrossRef]
- Milivojević, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and antioxidant properties of cultivated and wild fragaria and rubus berries. J. Food Qual. 2011, 34, 1–79. [Google Scholar] [CrossRef]
- Van de Velde, F.; Grace, M.H.; Esposito, D.; Pirovani, M.E.; Lila, M.A. Quantitative comparison of phytochemical profile, antioxidant, and anti-inflammatory properties of blackberry fruits adapted to Argentina. J. Food Compos. Anal. 2016, 47, 82–89. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Zorenc, Z.; Koron, D.; Senica, M. Fruit Quality Characteristics and Biochemical Composition of Fully Ripe Blackberries Harvested at Different Times. Foods 2021, 10, 1581. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Go’sciniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Papetti, A.; Dabić Zagorac, D.Č.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.J.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Kraujalis, P.; Kraujalienè, V.; Kazernaviĉiūtè, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluids 2017, 122, 99–108. [Google Scholar] [CrossRef]
- Kitrytė, V.; Narkevičiūtė, A.; Tamkutė, L.; Syrpas, M.; Pukalskienė, M.; Venskutonis, P.R. Consecutive high-pressure and enzyme assisted fractionation of blackberry (Rubus fruticosus L.) pomace into functional ingredients: Process optimization and product characterization. Food Chem. 2020, 312, 126072. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.K.; Breil, C.; Vian, M.A.; Chemat, F.; Venskutonis, P.R. Biorefining of Bilberry (Vaccinium myrtillus L.) Pomace Using Microwave, Hydro-Diffusion and Gravity, Ultrasound Assisted and Bead Milling extraction. ACS Sustain. Chem. Eng. 2018, 6, 4185–4193. [Google Scholar] [CrossRef]
- Basegmez, H.I.O.; Povilaitis, D.; Kitrytè, V.; Kraujalienè, V.; Šulniūtè, V.; Alasalvar, C.; Venskutonis, P.R. Biorefining of blackcurrant pomace into high value functional ingredients using supercritical CO2, pressurized liquid and enzyme assisted extractions. J. Supercrit. Fluids 2017, 124, 10–19. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrné, L.; Alminger, M. Supercritical Fluid Extraction of Berry Seeds: Chemical Composition and Antioxidant Activity. J. Food Qual. 2018, 2018, 6046074. [Google Scholar] [CrossRef]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; De Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO₂ Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Arturo-Perdomo, D.; Mora, J.P.J.; Ibáñez, E.; Cifuentes, A.; Hurtado-Benavides, A.; Montero, L. Extraction and Characterization of the Polar Lipid Fraction of Blackberry and Passion Fruit Seeds Oils Using Supercritical Fluid Extraction. Food Anal. Methods 2021, 14, 2026–2037. [Google Scholar] [CrossRef]
- Correa, M.S.; Fetzer, D.L.; Hamerski, F.; Corazza, M.L.; Scheer, A.P.; Hoffmann Ribani, R. Pressurized extraction of high-quality blackberry (Rubus spp. Xavante cultivar) seed oils. J. Supercrit. Fluids 2021, 169, 105101. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L.; Huang, Q.; Wang, J.; Lin, Q.; Liu, M.; Lee, W.Y.; Song, H. Ultrasonic-Assisted Extraction of Raspberry Seed Oil and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. PLoS ONE 2016, 11, e0153457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanaga, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Cui, E.-h.; Fang, L.; Wu, W.-l.; Li, W.-l. Optimization of Ultrasonic-Assisted Extraction of Blackberry Seed Oil by Response Surface Methodology. Food Sci. 2012, 33, 26–30. (In Chinese) [Google Scholar]
- Khadhraoui, B.; Fabiano-Tixier, A.S.; Petitcolas, E.; Robinet, P.; Imbert, R.; El Maâtaoui, M.; Chemat, F. Microscopic imaging as a tool to target spatial and temporal extraction of bioactive compounds through ultrasound intensification. Ultrason. Sonochem. 2019, 53, 214–225. [Google Scholar] [CrossRef]
- Van Hoed, V.; Barbouche, I.; De Clercq, N.; Dewettinck, K.; Slah, M.; Leber, E.; Verhé, R. Influence of filtering of cold pressed berry seed oils on their antioxidant profile and quality characteristics. Food Chem. 2011, 127, 1848–1855. [Google Scholar] [CrossRef]
- Dimić, E.; Vujasinović, V.; Radočaj, O.; Pastor, O. Characteristics of blackberry and raspberry seeds and oils. Acta Period. Technol. 2012, 43, 1–9. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease—7 dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Bessa, R.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Krížová, L.; Hasonová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed]
- Chira, N.; Todaşcǎ, C.; Nicolescu, A.; Pǎunescu, G.; Roşca, S. Determination of the Technical Quality Indices of Vegetable Oils by Modern Physical Techniques. UPB Sci. Bull. Ser. B 2009, 71, 3–12. [Google Scholar]
- Ivanova, M.; Hanganu, A.; Dumitriu, R.; Tociu, M.; Ivanov, G.; Stavarache, C.; Popescu, L.; Ghendov-Mosanu, A.; Sturza, R.; Deleanu, C.; et al. Saponification Value of Fats and Oils as Determined from 1H-NMR Data: The Case of Dairy Fats. Foods 2022, 11, 1466. [Google Scholar] [CrossRef]
- Pegg, R.B. Measurement of Primary Lipid Oxidation Products. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; Protocol D2.1.4–D2.1.6. [Google Scholar]
- Hanganu, A.; Chira, N.-A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Matei, P.L.; Busuioc, C.; Ionescu, N.; Stoica-Guzun, A.; Chira, N.-A. Cnicus benedictus Oil as a Raw Material for Biodiesel: Extraction Optimization and Biodiesel Yield. Sustainability 2021, 13, 13193. [Google Scholar] [CrossRef]
- Stroescu, M.; Stoica-Guzun, A.; Ghergu, S.; Chira, N.-A.; Jipa, I. Optimization of fatty acids extraction from Portulaca oleracea seed using response surface methodology. Ind. Crops Prod. 2016, 43, 405–411. [Google Scholar] [CrossRef]
- Isopencu, G.; Stroescu, M.; Brosteanu, A.; Chira, N.; Pârvulescu, O.C.; Busuioc, C.; Stoica-Guzun, A. Optimization of ultrasound and microwave assisted oil extraction from sea buckthorn seeds by response surface methodology. J. Food Proc. Eng. 2018, 42, e12947. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Knothe, G.; Kenar, J.A. Determination of the fatty acid profile by 1H-NMR Spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- Ampem, G.; Le Gresley, A.; Grootveld, M.; Naughton, D.P. Nuclear Magnetic Resonance Spectroscopic Analysis of the Evolution of Peroxidation Products Arising from Culinary Oils Exposed to Thermal Oxidation: An Investigation Employing 1H and 1H-1H COSY and TOCSY Techniques. Foods 2022, 11, 1864. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Shen, D.; Han, T.; Wu, W.; Lyu, L.; Li, W. Composition and Antioxidant Activity of Anthocyanins and Non-Anthocyanin Flavonoids in Blackberry from Different Growth Stages. Foods 2022, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Machate, D.J.; Candido, C.J.; Inada, A.C.; Franco, B.C.; Carvalho, I.R.A.; de Oliveira, L.C.S.; Cortes, M.R.; Caires, A.R.L.; da Silva, R.H.; Hiane, P.A.; et al. Fatty acid profile and physicochemical, optical and thermal characteristics of Campomanesia adamantium (Cambess.) O. Berg seed oil. Food Sci. Technol. 2020, 40, 538–544. [Google Scholar] [CrossRef]
- Taha, F.S.; Helmy, H.E.; El-Nockrashy, A.S. Changes in cottonseed oil when used for frying vegetable products containing chlorophyll. J. Am. Oil Chem. Soc. 1988, 65, 267–271. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Nguyen, M.T.; Nguyen, V.T.; Le, V.M.; Trieu, L.H.; Le, X.T.; Khang, T.V.; Giang, N.T.L.; Thach, N.Q.; Hung, T.T. The effects of different extraction conditions on the polyphenol, flavonoids components and antioxidant activity of Polyscias fruticosa roots. IOP Conf. Ser. Mat. Sci. Eng. 2020, 736, 022067. [Google Scholar] [CrossRef]
- Luo, Y.; Yuan, F.; Li, Y.; Wang, J.; Gao, B.; Yu, L. Triacylglycerol and Fatty Acid Compositions of Blackberry, Red Raspberry, Black Raspberry, Blueberry and Cranberry Seed Oils by Ultra-Performance Convergence Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Foods 2021, 10, 2530. [Google Scholar] [CrossRef]
- Jazic, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace. Int. J. Food Sci. Technol. 2018, 54, 194–201. [Google Scholar] [CrossRef]
- Sariburun, E.; Şhanin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis ‘Starkrimson’ fruit peel. Food Chem. 2019, 297, 124993. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.-K.; Chan, E.-S.; Lee, Y.-Y. Physicochemical and antioxidative properties of ultrasound-assisted extraction of mahua (Madhuca longifolia) seed oil in comparison with conventional Soxhlet and mechanical extractions. Ultrason. Sonochem. 2023, 92, 106280. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.M.; Trifan, A.; Gaspar-Pintilescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.; Wrolstad, R. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Stoica Guzun, A.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Akter, J.; Hossain, A.; Takara, K.; Islam, Z.; Hou, D.-X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp.): Isolation of active compounds. Comp. Biochem. Physiol. C 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef]
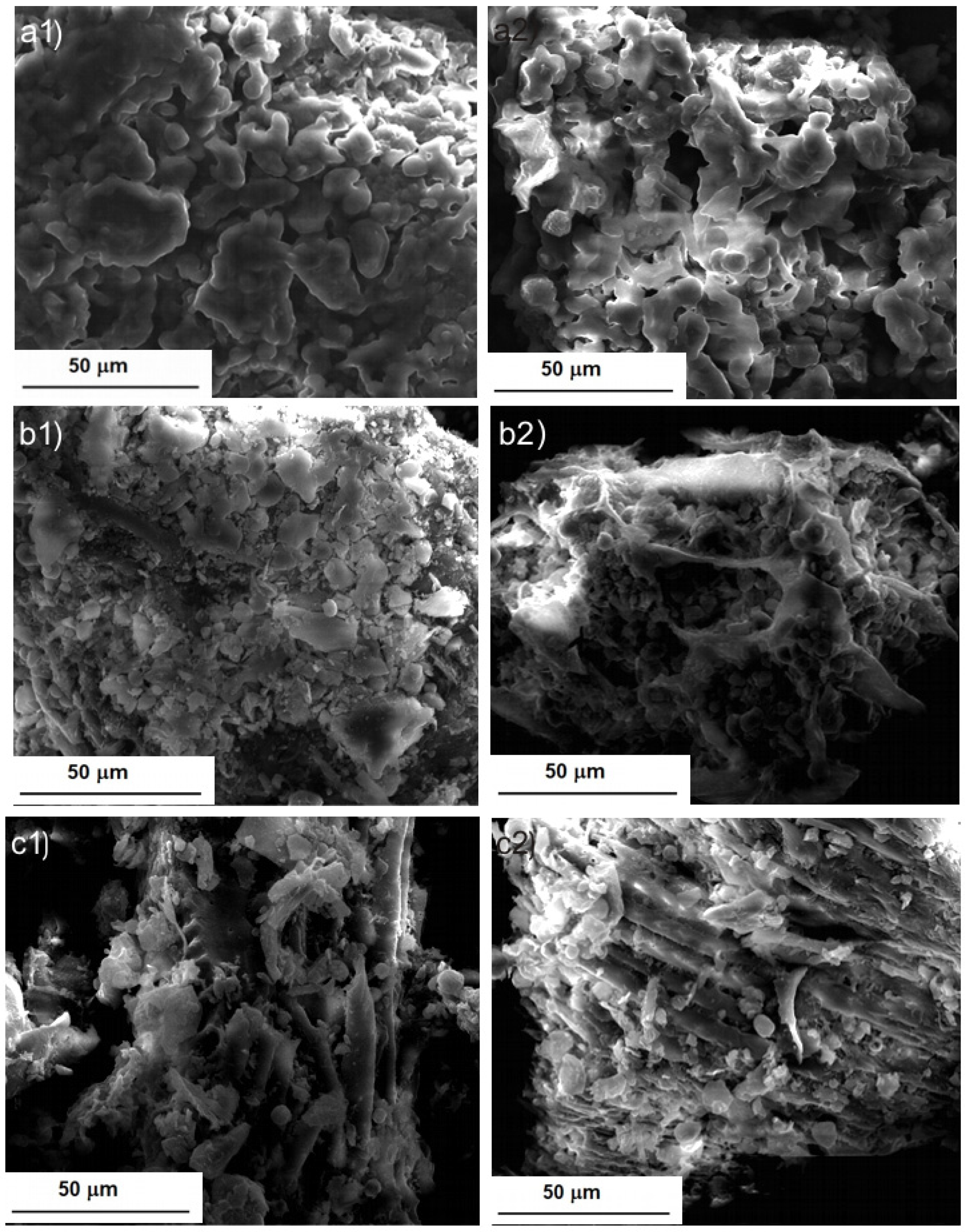

| Run | X1 | X2 | X3 | Eexp (%) | Epredicted (%) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 60 ± 0.17 | 61.25 |
| 2 | −1 | 0 | −1 | 65 ± 0.25 | 64.62 |
| 3 | −1 | 0 | 1 | 68 ± 0.13 | 67.62 |
| 4 | −1 | 1 | 0 | 70 ± 0.09 | 69.50 |
| 5 | 0 | −1 | −1 | 73 ± 0.45 | 72.12 |
| 6 | 0 | −1 | 1 | 72 ± 0.34 | 71.12 |
| 7 | 0 | 0 | 0 | 81 ± 0.25 | 80.74 |
| 8 | 0 | 1 | −1 | 75 ± 0.16 | 75.87 |
| 9 | 0 | 1 | 1 | 78 ± 0.34 | 78.87 |
| 10 | 1 | −1 | 0 | 82 ± 0.53 | 82.50 |
| 11 | 1 | 0 | −1 | 85 ± 0.17 | 85.37 |
| 12 | 1 | 0 | 1 | 84 ± 0.40 | 84.37 |
| 13 | 1 | 1 | 0 | 87 ± 0.32 | 85.75 |
| 14 | 0 | 0 | 0 | 79 ± 0.19 | 80.74 |
| 15 | 0 | 0 | 0 | 82.7 ± 0.25 | 80.74 |
| 16 | 0 | 0 | 0 | 81 ± 0.16 | 80.74 |
| 17 | 0 | 0 | 0 | 80 ± 0.34 | 80.74 |
| Term | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 907.3533 | 9 | 108.8170 | 47.67729 | 0.000019 a |
| X1 | 703.1250 | 1 | 703.1250 | 372.4179 | 0.000042 a |
| X2 | 66.1250 | 1 | 66.1250 | 35.0238 | 0.004083 a |
| X3 | 2.0000 | 1 | 2.0000 | 1.0593 | 0.361533 |
| X12 | 26.2106 | 1 | 26.2106 | 13.8827 | 0.020368 a |
| X22 | 51.4317 | 1 | 51.4317 | 27.2414 | 0.006430 a |
| X32 | 31.7264 | 1 | 31.7264 | 16.8042 | 0.014862 a |
| X1X2 | 6.2500 | 1 | 6.2500 | 3.3104 | 0.142969 |
| X1X3 | 4.0000 | 1 | 4.0000 | 2.1186 | 0.219218 |
| X2X3 | 4.0000 | 1 | 4.0000 | 2.1186 | 0.219218 |
| Lack of Fit | 7.2500 | 3 | 2.4167 | 1.2800 | 0.394875 |
| Pure error | 7.5520 | 4 | 1.8880 | ||
| Total SS | 922.1553 | 16 | |||
| R2 = 0.983 | Adj R2 = 0.963 | CV = 1.77% |
| No. | Fatty Acid | Lipid Number | Fatty Acid Profile of BB Seed Oil (Molar) * | |
|---|---|---|---|---|
| Soxhlet Extraction (n-hexane, 8 h) | UAE (13.77 W/cm2 UI, 45 °C, 15 min) (Optimum Values) | |||
| 1 | Palmitic | C16:0 | 4.20 ± 0.16 | 4.75 ± 0.18 |
| 2 | Stearic | C18:0 | 3.14 ± 0.02 | 3.55 ± 0.09 |
| 3 | Oleic | C18:1 | 16.52 ± 0.57 | 17.21 ± 0.63 |
| 4 | Linoleic | C18:2 | 64.86 ± 0.96 | 63.53 ± 0.78 |
| 5 | α-Linolenic | C18:3 | 11.28 ± 0.34 | 10.97 ± 0.36 |
| ∑ SAFA | 7.34 | 8.3 | ||
| ∑MUFA | 16.52 | 17.21 | ||
| ∑PUFA | 76.14 | 74.5 | ||
| n-6/n-3 | 5.73 | 5.79 | ||
| Total fat (g/100 g dried seeds) | 14.56 ± 0.53 | |||
| Oil health indices ** | ||||
| AI | 0.04 | 0.04 | ||
| TI | 0.10 | 0.11 | ||
| P/S ratio | 10.37 | 8.98 | ||
| H/H ratio | 29.51 | 25.83 | ||
| HPI | 29.51 | 25.83 | ||
| DI | 81.38 | 80.74 | ||
| Oil technical quality indices | ||||
| IV (g I2/100 g oil) ** | 157.6 ± 0.8 | 157.8 ± 0.9 | ||
| SV (mg KOH/g oil) ** | 191.5 ± 0.5 | 191.6 ± 0.9 | ||
| PV (meq. active oxygen/kg of oil) | 4.23 ± 0.34 | 4.31 ± 0.34 | ||
| No. | δ (ppm) | Proton | Compound |
|---|---|---|---|
| 1 | 0.85 | –CH2–CH2–CH2–CH3 | all acids except linolenic acid |
| 2 | 0.85 | –CH=CH–CH2-CH3 | linolenic acid |
| 3 | 1.24 | –(CH2)n- | all fatty acids |
| 4 | 1.64 | –CH2–CH2–COO– | all fatty acids |
| 5 | 2.02 | –CH2–CH=CH– | allylic acids (from all unsaturated fatty acids) |
| 6 | 2.26 | –CH2–COO– | all fatty acids |
| 7 | 2.76 | –CH = CH–CH2–CH=CH– | bis-allylic protons (from linolenic and linoleic acid) |
| 8 | 3.60 | –COO–CH3 | methyl ester moiety |
| 9 | 4.19 | –CH2OCOR | H in the sn-1/3 position of the glycerol backbone |
| 10 | 5.20 | –CHOCOR | H in the sn-2 position of the glycerol backbone |
| 11 | 5.29 | –CH=CH- | all unsaturated fatty acids |
| Methanolic Extract | TPC | TAC | TEAC | AE | |
|---|---|---|---|---|---|
| mg GAE/100 g dw | mg CAE/100 g dw | mg C3GE/100 g dw | (μg/g Extract) | % | |
| BB seed oil (Soxhlet extraction) | 3.19 ± 0.17 | 2.90 ± 0.15 | 0.41 ± 0.02 | 72.91 ± 0.35 | 33.11 |
| BB seed oil (UAE) | 3.34 ± 0.19 | 3.48 ± 0.20 | 0.46 ± 0.02 | 81.73 ± 0.6 | 28.51 |
| Defatted BB seed residue (Soxhlet extraction) | 2984.71 ± 88.28 | 2712.69 ± 80.23 | 37.21 ± 1.19 | 266.71 ± 7.27 | 89.07 |
| Defatted BB seed residue (UAE) | 3471.12 ± 73.73 | 3155.04 ± 66.98 | 40.86 ± 2.51 | 267.50 ± 9.25 | 89.37 |
| Independent Variables | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| UI (W/cm2) | X1 | 5.06 | 9.64 | 13.77 |
| Extraction temperature (°C) | X2 | 30 | 40 | 50 |
| Extraction time (min) | X3 | 10 | 15 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, P.L.; Deleanu, I.; Brezoiu, A.M.; Chira, N.A.; Busuioc, C.; Isopencu, G.; Cîlțea-Udrescu, M.; Alexandrescu, E.; Stoica-Guzun, A. Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization. Molecules 2023, 28, 2486. https://doi.org/10.3390/molecules28062486
Matei PL, Deleanu I, Brezoiu AM, Chira NA, Busuioc C, Isopencu G, Cîlțea-Udrescu M, Alexandrescu E, Stoica-Guzun A. Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization. Molecules. 2023; 28(6):2486. https://doi.org/10.3390/molecules28062486
Chicago/Turabian StyleMatei, Petronela L., Iuliana Deleanu, Ana M. Brezoiu, Nicoleta A. Chira, Cristina Busuioc, Gabriela Isopencu, Mihaela Cîlțea-Udrescu, Elvira Alexandrescu, and Anicuta Stoica-Guzun. 2023. "Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization" Molecules 28, no. 6: 2486. https://doi.org/10.3390/molecules28062486
APA StyleMatei, P. L., Deleanu, I., Brezoiu, A. M., Chira, N. A., Busuioc, C., Isopencu, G., Cîlțea-Udrescu, M., Alexandrescu, E., & Stoica-Guzun, A. (2023). Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization. Molecules, 28(6), 2486. https://doi.org/10.3390/molecules28062486