Comparative Analysis of Roots from Vicatia thibetica de Boiss and Angelica sinensis Based on Chemical Composition, Antioxidant, Nitrite-Scavenging and Enzyme Inhibition Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of RVT and RAS
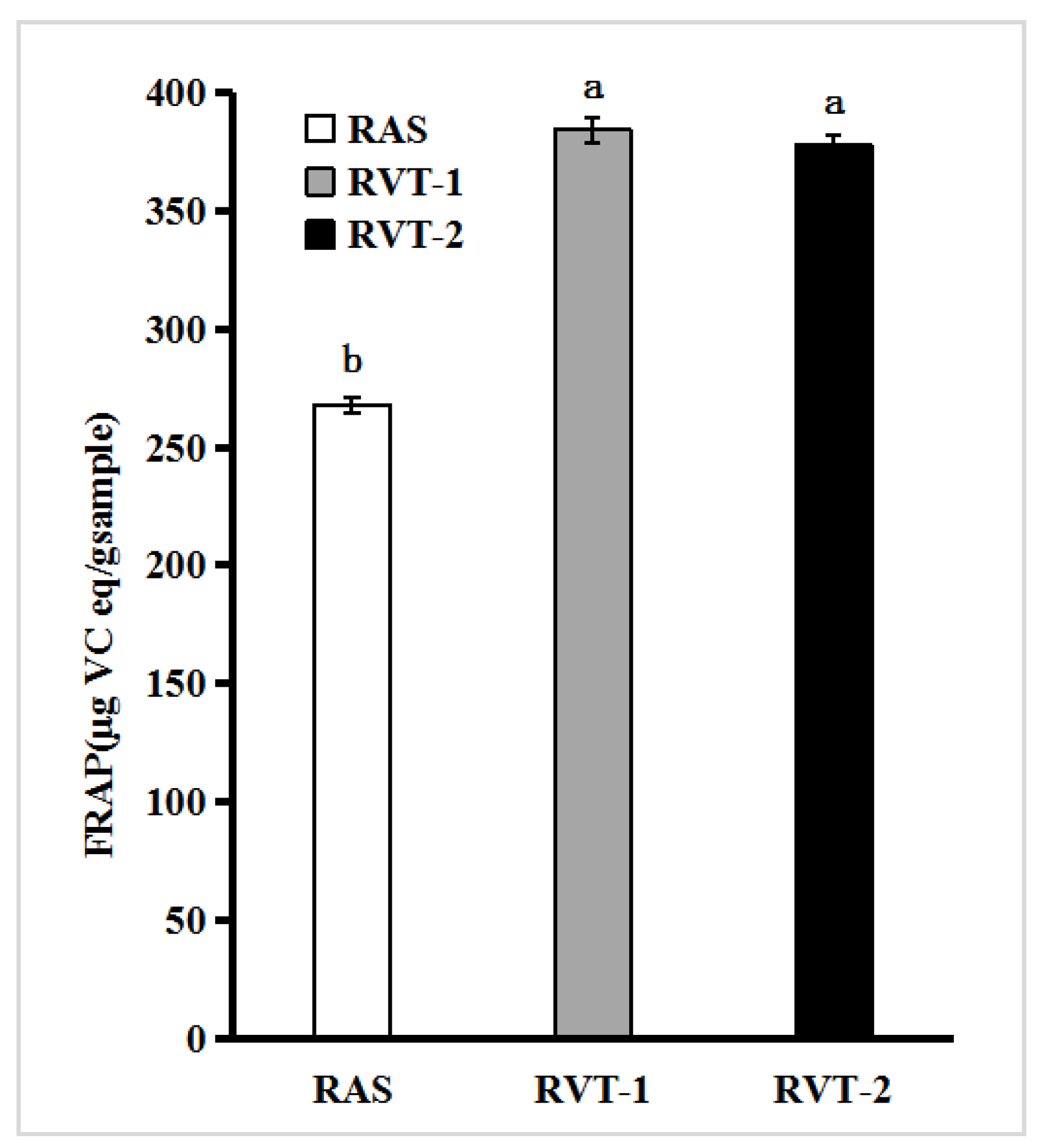
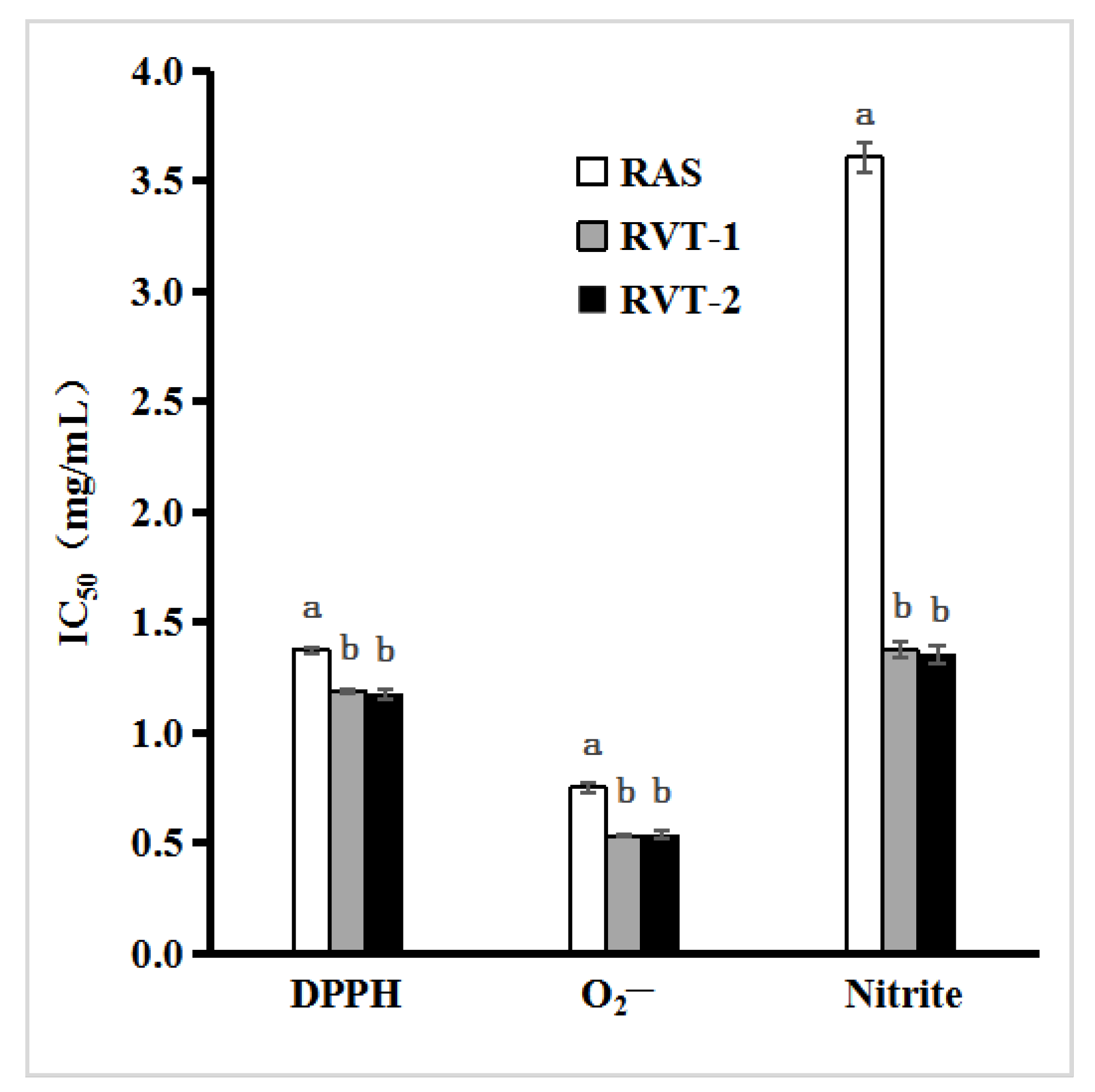
2.2. Antioxidant and Nitrite Scavenging Activities of RVT and RAS
2.3. Tyrosinase Inhibitory Activity of RVT and RAS
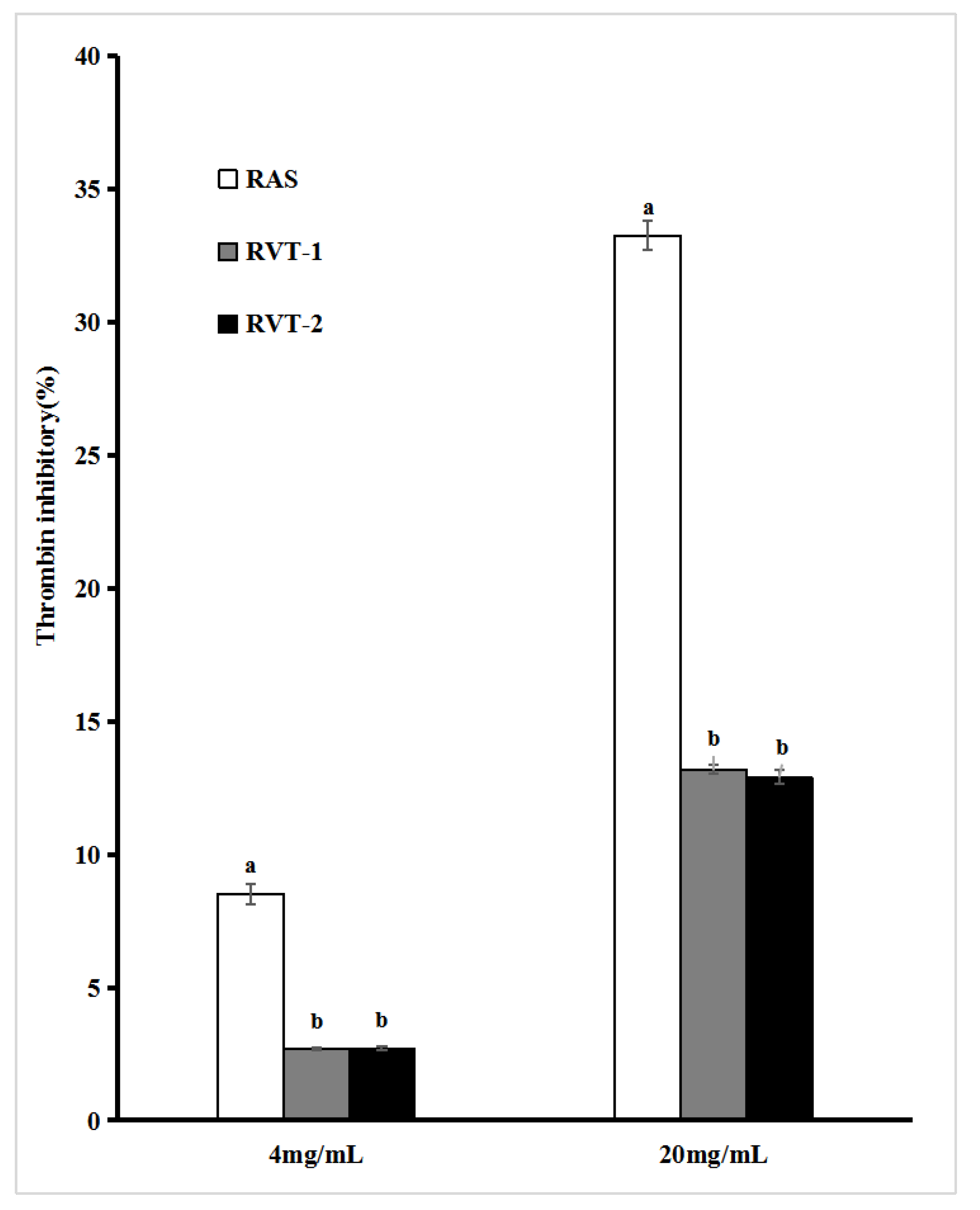
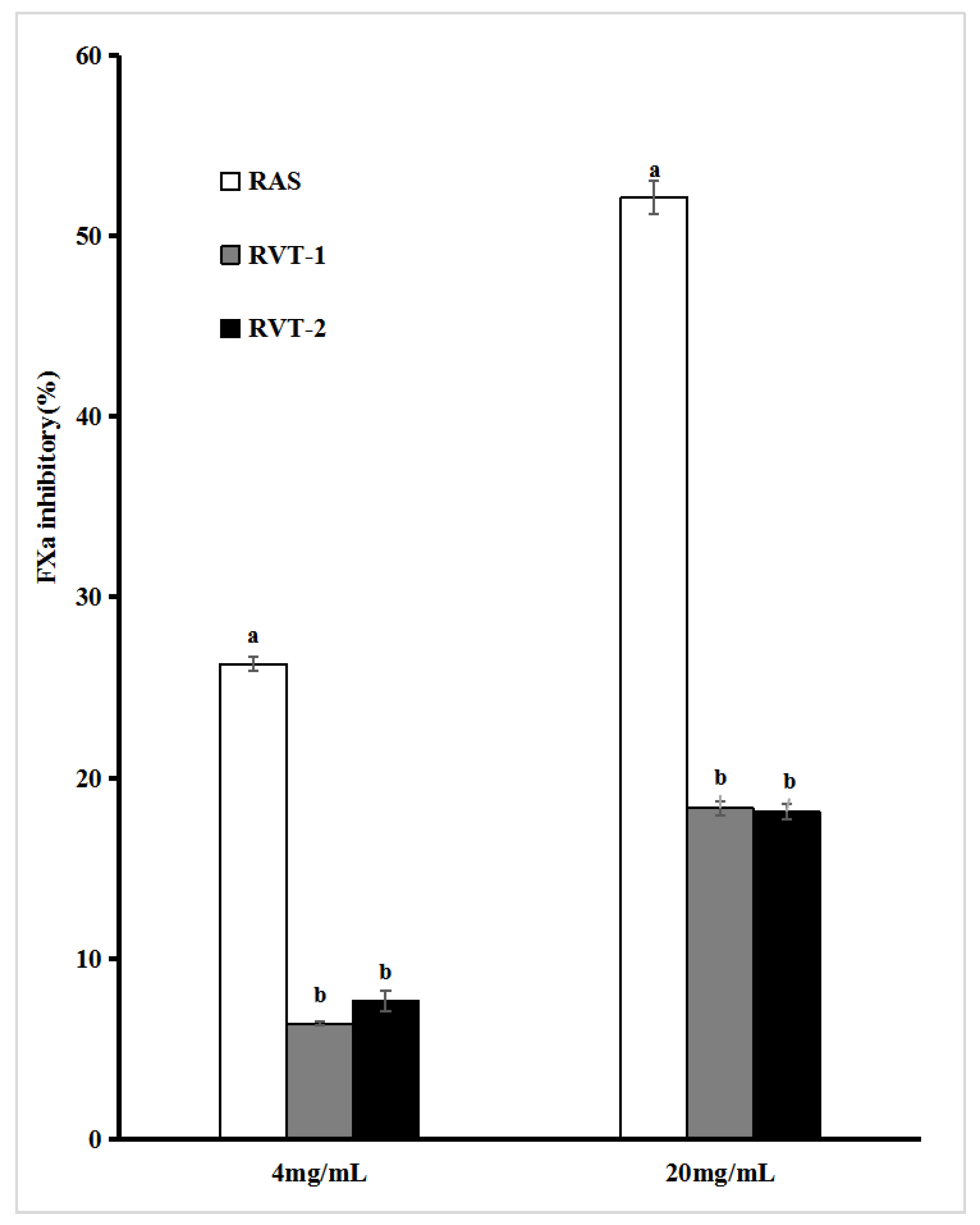
2.4. In Vitro Thrombin and Factor Xa Inhibitory Activities of RVT and RAS
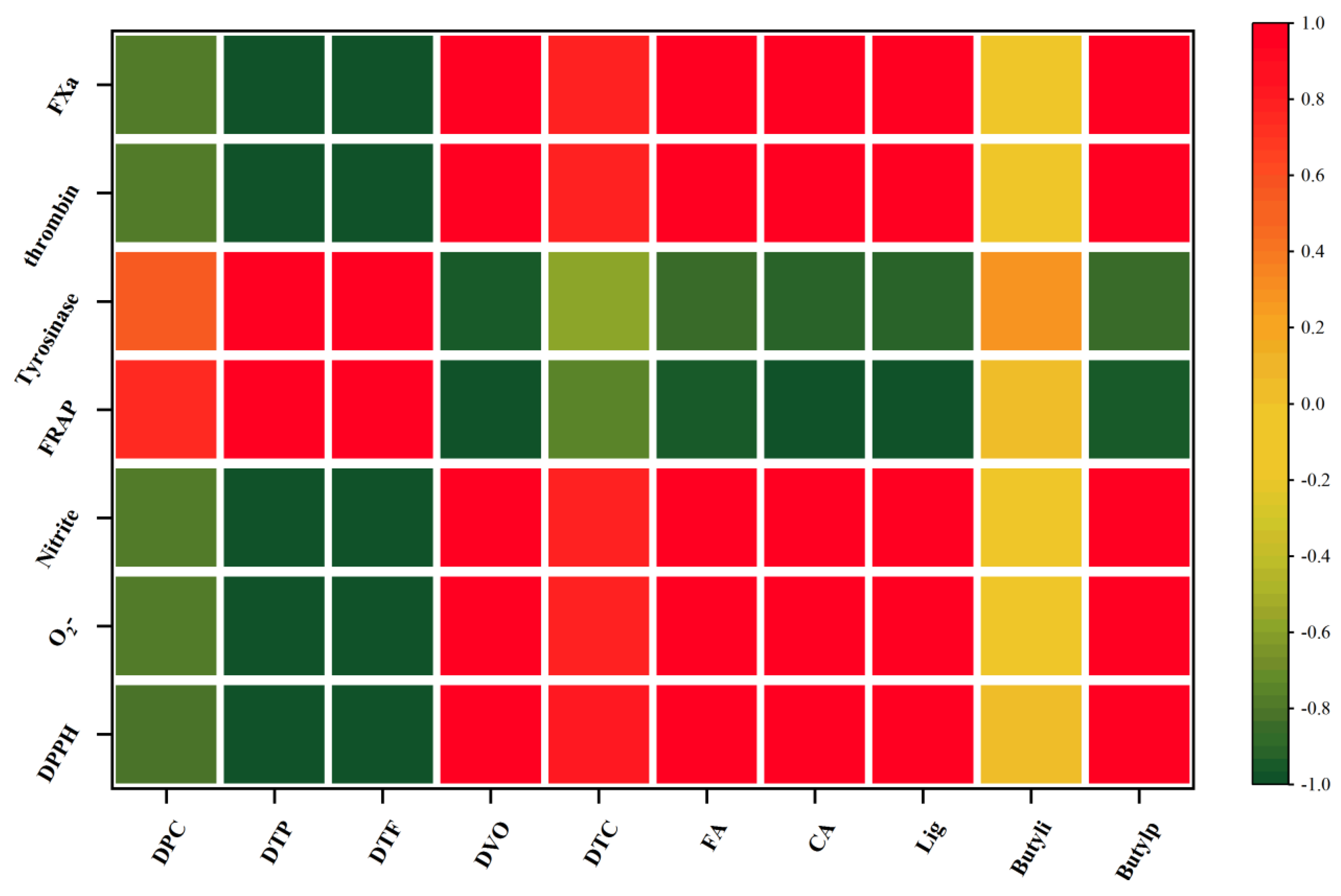
2.5. Correlation Analysis
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Reagents
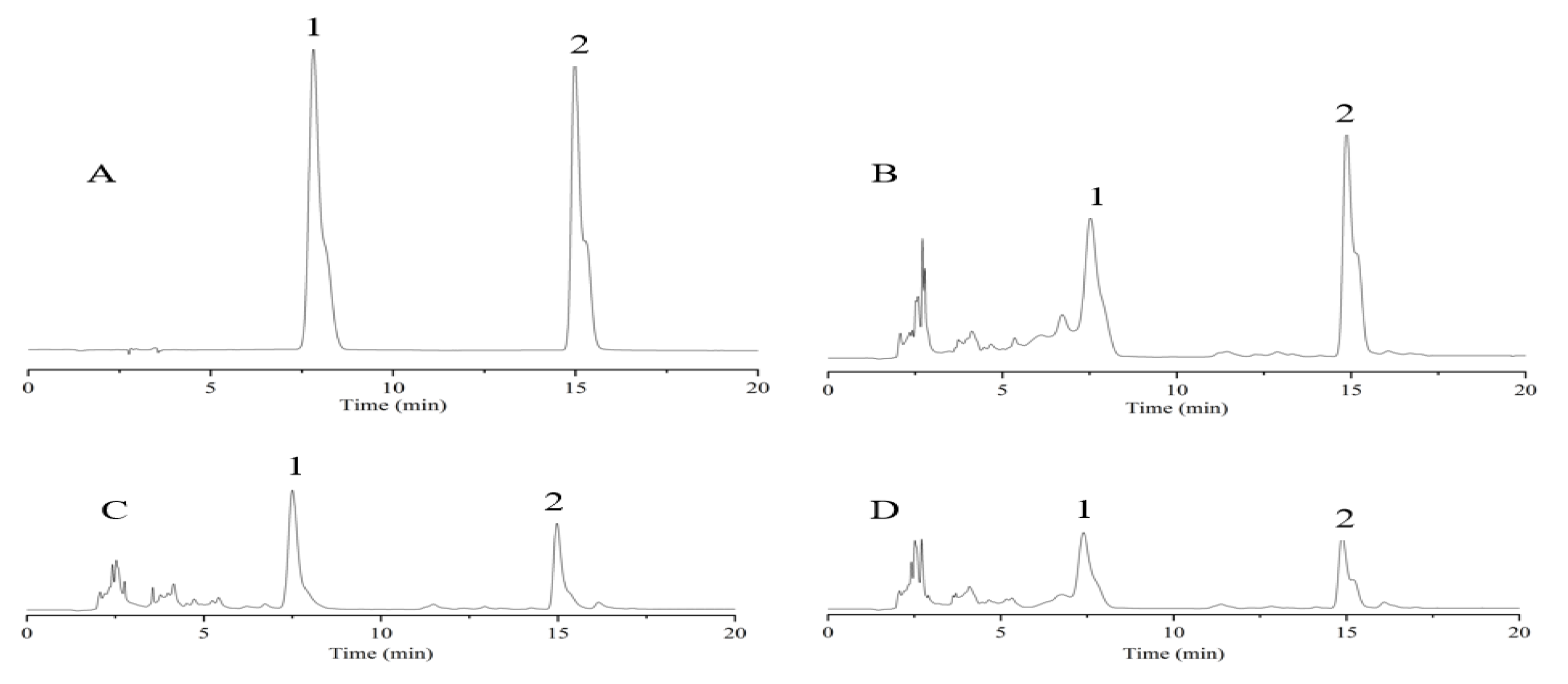
3.3. Phytochemical Analysis
3.4. Determination of Ferulic Acid and Chlorogenic Acid
3.5. Determination of Proteins and Amino Acids
3.6. Essential Oil Analysis
3.7. Antioxidant Activity
3.7.1. FRAP Assay
3.7.2. DPPH Scavenging Assay
3.7.3. O2− Scavenging Assay
3.8. Nitrite Scavenging Activity
3.9. Inhibition of Tyrosinase Activity
3.10. Thrombin and Factor Xa Inhibitory Activities
3.11. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dong, S.T.; Zhang, X.Q.; Hu, Y.; Gong, X.M.; Yang, H.Q. Chemical constituents, quality control and Pharmacology research progress of Xigui. Chin. J. Ethnomed. Ethnopharm. 2018, 27, 40–42. [Google Scholar]
- Cairang, N.J.; Duojie, R.Q.; Wencheng, D.Z.; Meng, X.L. Application of Tibetan celery in Tibetan medicine. Chin. J. Tradit. Chin. Med. Pharm. 2021, 36, 535–537. [Google Scholar]
- Yi, L.; Liang, Y.; Wu, H.; Yuan, D. The analysis of Radix Angelicae sinensis (Danggui). J. Chromatogr. A 2009, 1216, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-a review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef]
- Tian, J.L.; Guo, M.X.; Liu, G.M.; Shen, L.; Liu, X.B. Effects of Xigui drug serum on inflammatory factors in lipopolysaccharide–stimulated murine macrophages RAW264.7. J. Pharm. Res. 2018, 37, 497–499. [Google Scholar]
- Liu, X.Q.; Ding, J.C.; Li, X. Immunomodulation of Polysaccharides from Xigui in Exercise–induced Fatigue Mice. Sci. Tech. Food Ind. 2021, 42, 317–321, 327. [Google Scholar]
- Marques Jucá, M.; Sales Cysne Filho, F.M.; De Almeida, J.C.; Da Silva Mesquita, D.; Rodrigues de Moraes Barriga, J.; Ferreira Dias, K.C.; Matias Barbosa, T.; Costa Vasconcelos, L.; Kalyne Almeida Moreira Leal, L.; Eduardo Ribeiro, J.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2018, 34, 692–705. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohyd. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef]
- Zou, Y.F.; Li, C.Y.; Fu, Y.P.; Jiang, Q.X.; Peng, X.; Li, L.X.; Song, X.; Zhao, X.H.; Li, Y.P.; Chen, X.F. The comparison of preliminary structure and intestinal anti-inflammatory and anti-oxidative activities of polysaccharides from different root parts of Angelica sinensis (Oliv.) Diels. J. Ethnopharmacol. 2022, 295, 115446. [Google Scholar] [CrossRef]
- Zhao, B.; Kang, Q.; Peng, Y. Effect of Angelica sinensis root extract on cancer prevention in different stages of an AOM / DSS mouse model. Int. J. Mol. Sci. 2017, 18, 1750. [Google Scholar] [CrossRef]
- Matsuo, Y.; Yamaguchi, E.; Hakamata, R.; Ootomo, K.; Takatori, K.; Fukaya, H.; Mimaki, Y. Benzofuran and coumarin derivatives from the root of Angelica dahurica and their PPAR-γ ligand-binding activity. Phytochemistry 2020, 173, 112301. [Google Scholar] [CrossRef] [PubMed]
- Committee, C.P. 2020 Edition of Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020; p. 319. [Google Scholar]
- Choi, J.H.; Park, J.K.; Kim, K.M. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 1, 1–9. [Google Scholar]
- Zheng, R.L.; Zhang, H. Effects of ferulic acid on fertile and asthenozo-ospermic infertile human sperm motility, viability, lipid peroxidation, and cyclic nucleotides. Free Radic. Biol. Med. 1997, 22, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, Z.; Meade, J.A. Pharmacologic overview of chlorogenic acid and its metabolites in chronic pain and inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S. Antibacterial activity and mecha-nism of action of chlorogenic acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Martínez Sanz, J.M.; Norte Navarro, A.; Salinas García, E.; Sospedra López, I. An overview on essential amino acids and branched Chain amino acids. In Nutrition and Enhanced Sports Performance, 2nd ed.; Bagchi, D., Nair, S., Sen, C.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 509–519. [Google Scholar]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. A sensitive HPLC-FLD method combined with multivariate analysis for the determination of amino acids in L-citrulline rich vegetables. J. Food Drug Anal. 2019, 27, 717. [Google Scholar] [CrossRef]
- Wu, X.R. Amino Acid; Beijing Agricultural University Press: Beijing, China, 1988. [Google Scholar]
- Yao, W.L.; Zhang, L.; Hua, Y.L.; Ji, P.; Li, P.L.; Li, J.X.; Zhong, L.J.; Zhao, H.F.; Wei, Y.M. The investigation of anti-inflammatory activity of volatile oil of Angelica sinensis by plasma metabolomics approach. Int. Immunopharmacol. 2015, 29, 269–277. [Google Scholar] [CrossRef]
- Yeh, J.C.; Garrard, I.J.; Chantal Cho, C.W.; Annie Bligh, S.W.; Lu, G.H.; Fan, T.P.; Fisher, D. Bioactivity-guided fractionation of the volatile oil of Angelica sinensis radix designed to preserve the synergistic effects of the mixture followed by identification of the active principles. J. Chromatogra. A 2012, 1236, 132–138. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.B.; Tian, D.Q.; Fang, X.P.; Yu, Y.M.; Zhu, H.Q. Antioxidant properties and color parameters of herbal teas in China. Ind. Crops Prod. 2016, 87, 198–209. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.Y.; Wang, Z.Z.; Wang, C.N.; Wang, E.L.; Zhang, Y.; Liu, J.B. Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Bryan, N.S.; Alexander, D.D.; Coughlin, J.R.; Milkowski, A.L.; Boffetta, P. Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food Chem. Toxicol. 2012, 50, 3646–3665. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidantactivity. J. Agric. Food Chem. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 5, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med.Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L. The extraction process and effect of tyrosinase activity from aqueous in matrimony vine. J. Wuhan Instit. Tech. 2011, 33, 30–32. [Google Scholar]
- Kim, D.; Park, J.; Kim, J. Flavonoids asmushroom tyrosinase inhibitors: A fluorescence quenching study. J. Agric. Food Chem. 2006, 54, 935–941. [Google Scholar] [CrossRef]
- Sheng, F.; Wang, D.B. Effect of tyrosinase activity from polysaccharide in angelica. J. Kunming Higher Normal College 2005, 27, 61–62. [Google Scholar]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326. [Google Scholar] [CrossRef]
- Nunez-Navarro, N.E.; Santana, F.M.; Parra, L.P.; Zacconi, F.C. Surfing the blood coagulation cascade: Insight into the vital factor Xa. Curr. Med. Chem. 2019, 26, 3175. [Google Scholar] [CrossRef]
- Eller, T.; Busse, J.; Dittrich, M.; Flieder, T.; Alban, S.; Knabbe, C.; Birschmann, I. Dabigatran rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin. Chem. Lab. Med. 2014, 52, 835. [Google Scholar] [CrossRef]
- Tomkowski, W.Z.; Davidson, B.L. Thromboprophylaxis by rivaroxaban, aspirin, both, or placebo after hospitalization for medical illness. Thromb. Res. 2019, 180, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.R.; Wang, J.; Yu, D.K.; Chen, Y.S.; He, Y.; Wang, C.Y. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-yenous shunt thrombosis in vivo in rats. Yakugaku. Zasshi. 2009, 129, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, K.; Ponczek Michał, B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of Vitamin K-Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules 2020, 25, 1465. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, J.; Zhang, M.; Li, C.X.; Li, Y.Y.; Qiu, L.; Hou, Y.H.; Liu, Y.Q.; Jin, X.J. Material Basis and Molecular Mechanism of Angelica Sinensis Radix in activating blood: Based on computer-aided drug design. China J. Chin. Mater. Medica 2022, 47, 1942–1954. [Google Scholar]
- Wang, T.; Huang, H.; Zhang, Y. Role of Effective Composition on Antioxidant, Anti-Inflammatory, Sedative-Hypnotic Capacities of 6 Common Edible Lilium Varieties. J. Food Sci. 2015, 80, 857–868. [Google Scholar] [CrossRef]
- Tian, S.Y.; Hao, C.C.; Xu, G.K.; Yang, J.J.; Sun, R.G. Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J. Food. Drug. Anal. 2017, 25, 766–775. [Google Scholar] [CrossRef]
- Dinh, C.D.; Nguyen, T.N.Y.; Tri, D.L.; Khanh, Q.N.H.; Nguyen, D.C.; Bach, L.G. Extraction conditions of Polyphenol, Flavonoid compounds with Antioxidant activity from Veronia amygdalina Del. Leaves: Modeling and optimization of the process using the response surface methodology RSM. Mate. Today: Proc. 2019, 18, 4004–4010. [Google Scholar] [CrossRef]
- GB 5009.124-2016; National Food Safety Standards Determination of Amino Acids in Foods. National Health and Family Planning Commission of PRC, CFDA: Beijing China, 2016.
- Li, X.C. Improved Pyrogallol Autoxidation Method: A Reliable and Cheap Superoxide-scavenging Assay Suitable for All Antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Menezesde Sá, A.Á.; DosSantos, E.W.P.; Santana, M.H.S.; Santos, A.J.; De Araujo, G.R.S.; Santana, D.G.; Arguelho, M.L.P.M. Evaluation of the incorporation of essential oils in microemulsions as a promising formulation in the inhibition of tyrosinase. Ind. Crops. Prod. 2020, 154, 112654. [Google Scholar]
- Jablonka, W.; Kotsyfakis, M.; Mizurini, D.M.; Monteiro, R.Q.; Lukszo, J.; Drake, S.K.; Ribeiro, J.M.C.; Andersen, J.F. Identification and mechanistic analysis of a novel tick-derived inhibitor of thrombin. PLoS ONE 2015, 10, e0133991. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Chen, X.T.; Zhao, P.; Qu, Z.; Ma, D.; Zhao, C.C.; Gao, W.Y. Effect of stir-frying time during Angelica Sinensis Radix processing with wine on physicochemical, structure properties and bioactivities of polysaccharides. Process. Biochem. 2019, 81, 188–196. [Google Scholar] [CrossRef]


| Samples | DPC (%) | DTC (mg OS/gsample) | DTP (mg GA/gsample) | DTF (mg RU/gsample) | CA (mg/g) | FA (mg/g) | Protein (%) | DVO (%) |
|---|---|---|---|---|---|---|---|---|
| RVT-1 | 20.44 ± 0.45 bB | 5.98 ± 0.03 bB | 8.59 ± 0.02 aA | 6.53 ± 0.05 aA | 0.46 ± 0.01 bB | 0.59 ± 0.01 bB | 19.59 ± 0.22 aA | 0.37 ± 0.03 bB |
| RVT-2 | 21.83 ± 0.36 aA | 5.52 ± 0.13 cC | 7.80 ± 0.15 bB | 5.77 ± 0.15 bB | 0.42 ± 0.01 cC | 0.39 ± 0.02 cC | 19.00 ± 0.43 aA | 0.33 ± 0.04 bB |
| RAS | 19.53 ± 0.16 cC | 6.29 ± 0.07 aA | 2.29 ± 0.19 cC | 0.54 ± 0.01 cC | 0.95 ± 0.01 aA | 1.20 ± 0.01 aA | 10.52 ± 0.21 bB | 1.53 ± 0.05 aA |
| Amino Acid | Amino Acid Content (%) | ||
|---|---|---|---|
| RVT-1 | RVT-2 | RAS | |
| Asp | 1.96 | 2.13 | 1.15 |
| Thr | 0.63 | 0.66 | 0.54 |
| Ser | 0.74 | 0.72 | 0.43 |
| Glu | 3.10 | 3.12 | 2.06 |
| Gly | 0.76 | 0.79 | 0.64 |
| Ala | 0.76 | 0.78 | 0.67 |
| Cys | 0.06 | 0.04 | 0.04 |
| Val | 0.97 | 1.01 | 0.67 |
| Met | 0.29 | 0.29 | 0.15 |
| Ile | 0.72 | 0.70 | 0.55 |
| Leu | 1.35 | 1.34 | 0.92 |
| Tyr | — | 0.38 | 0.33 |
| Phe | 1.24 | 1.25 | 0.47 |
| His | 0.45 | 0.50 | 0.27 |
| Lys | 7.49 | 1.14 | 1.02 |
| Arg | 2.62 | 3.16 | 4.89 |
| Pro | 0.41 | 0.43 | 0.23 |
| Total content | 23.56 | 18.44 | 15.02 |
| No | Compounds | MF | MW | RAS | RVT-1 | RVT-2 | |||
|---|---|---|---|---|---|---|---|---|---|
| RT (min) | A% | RT (min) | A% | RT (min) | A% | ||||
| 1 | Butyraldehyde | C4H8O | 72.11 | 5.454 | 3.82 | — | — | 5.460 | 2.01 |
| 2 | Acetal | C6H14O2 | 118.17 | 5.868 | 0.13 | — | — | 5.874 | 3.51 |
| 3 | 1R-α-Pinene | C10H16 | 136.23 | 8.108 | 1.11 | — | — | — | — |
| 4 | α-Pinene | C10H16 | 136.23 | 8.279 | 0.72 | — | — | — | — |
| 5 | 2,6-dimethyl-2,4,6-Octatriene | C10H16 | 136.23 | — | — | 9.527 | 0.16 | 9.522 | 8.89 |
| 6 | β-Pinene | C10H16 | 136.23 | 11.792 | 0.27 | — | — | — | — |
| 7 | β-Caryophyllene | C15H24 | 204.35 | — | — | 11.920 | 2.03 | 12.009 | 0.11 |
| 8 | β-Myrcene | C10H16 | 136.23 | 12.127 | 0.15 | — | — | ||
| 9 | (+)-Ledene | C15H24 | 204.35 | — | — | 16.407 | 0.31 | — | — |
| 10 | β-selinene | C15H24 | 204.35 | — | — | 17.388 | 0.24 | 17.376 | 0.09 |
| 11 | α-Farnesene | C15H24 | 204.35 | — | — | 19.775 | 0.14 | — | — |
| 12 | 3-ethyl-1,5,5-trimethyl cyclohexene | C10H16 | 136.23 | 21.382 | 1.34 | — | — | — | — |
| 12 | 6-n-butyl-1,4-cycloheptadiene | C11H18 | 204.35 | — | — | 21.394 | 0.61 | 21.394 | 0.19 |
| 13 | Amyl ketone | C11H22O | 170.29 | — | — | 21.875 | 0.43 | — | — |
| 14 | D-limonene | C10H16 | 136.23 | 24.378 | 5.27 | — | — | — | — |
| 15 | p-isopropyltoluene | C10H14 | 136.23 | 27.331 | 0.24 | — | — | 27.339 | 4.65 |
| 16 | g-Terpinene | C10H16 | 136.23 | 28.141 | 0.11 | — | — | — | — |
| 17 | 3,7-dimethyl-1,3,6-Octatriene | C10H16 | 136.23 | 28.476 | 0.26 | — | — | 28.482 | 0.25 |
| 18 | 3,7-dimethyl-1,3,7-Octatriene | C10H16 | 136.23 | 28.939 | 4.29 | — | — | — | — |
| 19 | 1,4-cyclohexadiene-1,2-dicarboxylic anhydride | C8H6O3 | 150.13 | 35.728 | 0.14 | — | — | 35.758 | 1.03 |
| 20 | 4-ethyl-methyl ester Benzoic acid | C9H10O2 | 150.17 | 39.436 | 0.07 | — | — | — | — |
| 21 | 2,6-dimethyl-2,4,6-Octatriene | C10H16 | 136.23 | 46.073 | 0.42 | — | — | — | — |
| 22 | Guaiene | C15H24 | 204.35 | 50.061 | 0.51 | — | — | — | — |
| 23 | α-caryophyllene | C15H24 | 204.35 | — | — | — | — | 51.559 | 1.31 |
| 24 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.17 | 52.667 | 1.94 | — | — | 52.685 | 0.72 |
| 25 | β-Farnesene | C15H24 | 204.35 | 53.300 | 0.45 | — | — | — | — |
| 26 | 2,4,5-trimethyl-Benzaldehyde | C10H12O | 148.20 | 53.818 | 0.57 | — | — | — | — |
| 27 | β-Chamigrene | C15H24 | 204.35 | 54.366 | 0.19 | — | — | — | — |
| 28 | β-Bisabolene | C15H24 | 204.35 | 55.115 | 0.66 | 55.036 | 0.18 | — | — |
| 29 | (+)-β-Cedrene | C15H24 | 204.35 | 56.826 | 1.13 | — | — | — | — |
| 30 | (+)-α-Longipinene | C15H24 | 204.35 | 57.301 | 0.57 | — | — | — | — |
| 31 | γ-Elemene | C15H24 | 204.35 | 59.133 | 0.86 | — | — | 59.127 | 0.86 |
| 32 | Senkyunolide A | C12H16O2 | 192.25 | 62.720 | 0.11 | — | — | — | — |
| 33 | Heptacosane | C27H56 | 380.73 | 64.839 | 1.09 | — | — | — | — |
| 34 | 4-Isopropylbenzaldehyde | C10H12O | 148.20 | 69.369 | 3.23 | — | — | — | — |
| 36 | 3-Butylidenephthalide | C12H12O2 | 188.22 | 70.818 | 9.79 | 70.819 | 14.19 | 70.813 | 5.63 |
| 37 | Z-Ligustilide | C12H14O2 | 190.24 | 73.047 | 26.92 | 73.071 | 2.76 | 73.607 | 0.79 |
| 38 | E-Ligustilide | C12H14O2 | 190.24 | 73.260 | 3.95 | 73.259 | 0.15 | — | — |
| 39 | 3-N-Butylphthalide | C12H14O2 | 190.24 | 75.435 | 14.68 | 75.437 | 55.22 | 75.421 | 56.96 |
| 40 | Diisobutyl phthalate | C16H22O4 | 278.38 | 75.707 | 2.27 | — | — | — | — |
| 41 | Pentadecanoic acid | C15H30O2 | 242.40 | — | — | 71.451 | 0.41 | — | — |
| 42 | Margaric acid | C17H34O2 | 270.45 | — | — | 76.204 | 0.29 | 76.189 | 0.23 |
| 43 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 77.571 | 0.48 | 77.576 | 4.21 | 77.556 | 1.63 |
| 44 | oleic acid | C18H34O2 | 282.46 | — | — | 79.061 | 2.26 | 79.032 | 1.65 |
| 45 | Linoleic acid | C18H32O2 | 280.45 | 79.884 | 2.67 | 79.835 | 8.13 | 79.984 | 3.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Chen, Y.; Guo, F. Comparative Analysis of Roots from Vicatia thibetica de Boiss and Angelica sinensis Based on Chemical Composition, Antioxidant, Nitrite-Scavenging and Enzyme Inhibition Activities. Molecules 2023, 28, 1942. https://doi.org/10.3390/molecules28041942
Tang W, Chen Y, Guo F. Comparative Analysis of Roots from Vicatia thibetica de Boiss and Angelica sinensis Based on Chemical Composition, Antioxidant, Nitrite-Scavenging and Enzyme Inhibition Activities. Molecules. 2023; 28(4):1942. https://doi.org/10.3390/molecules28041942
Chicago/Turabian StyleTang, Wenwen, Yuan Chen, and Fengxia Guo. 2023. "Comparative Analysis of Roots from Vicatia thibetica de Boiss and Angelica sinensis Based on Chemical Composition, Antioxidant, Nitrite-Scavenging and Enzyme Inhibition Activities" Molecules 28, no. 4: 1942. https://doi.org/10.3390/molecules28041942
APA StyleTang, W., Chen, Y., & Guo, F. (2023). Comparative Analysis of Roots from Vicatia thibetica de Boiss and Angelica sinensis Based on Chemical Composition, Antioxidant, Nitrite-Scavenging and Enzyme Inhibition Activities. Molecules, 28(4), 1942. https://doi.org/10.3390/molecules28041942






