Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review
Abstract
1. Introduction
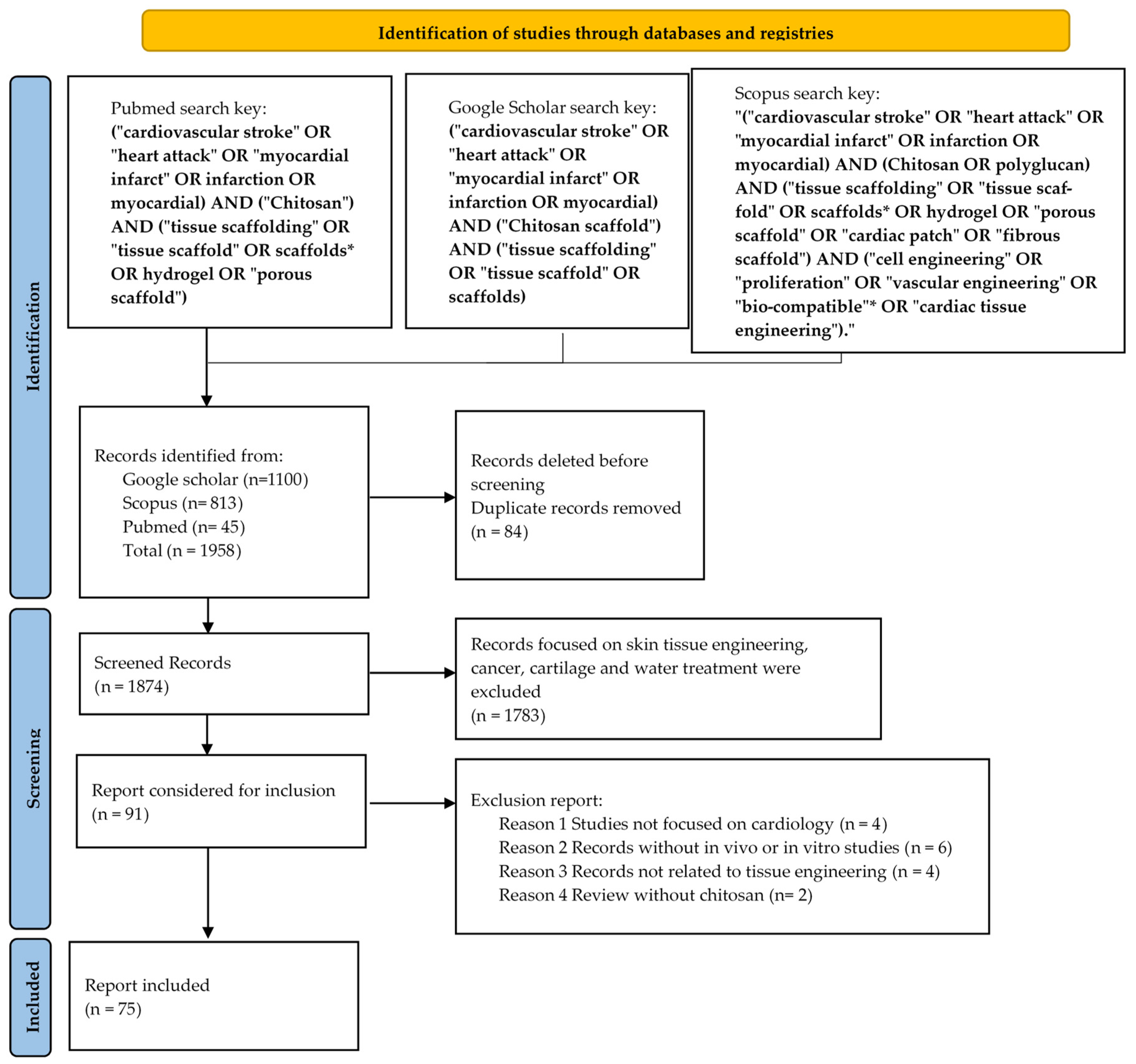
2. Methodology
2.1. Databases and Search Strategies
2.2. Eligibility Criteria
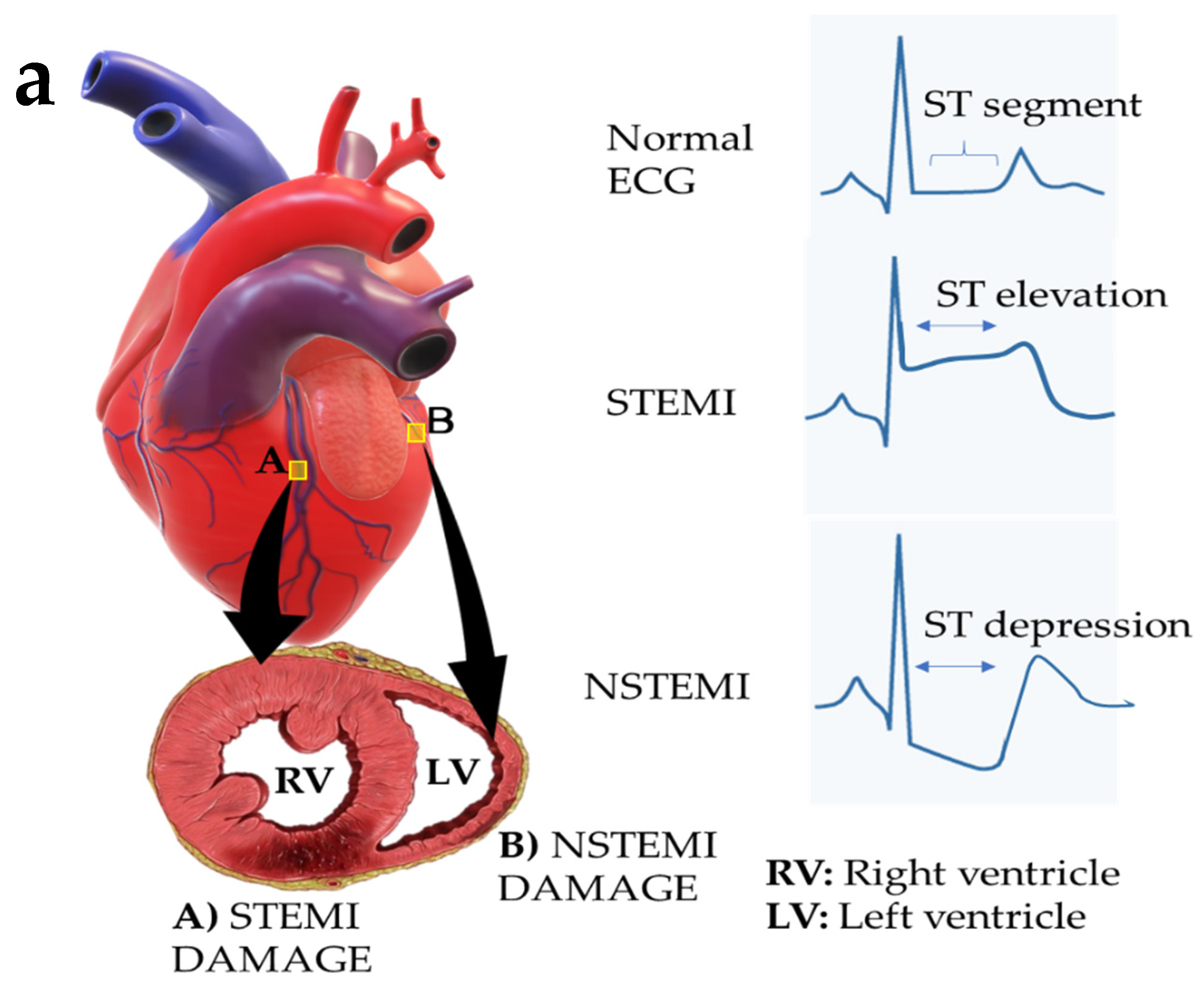
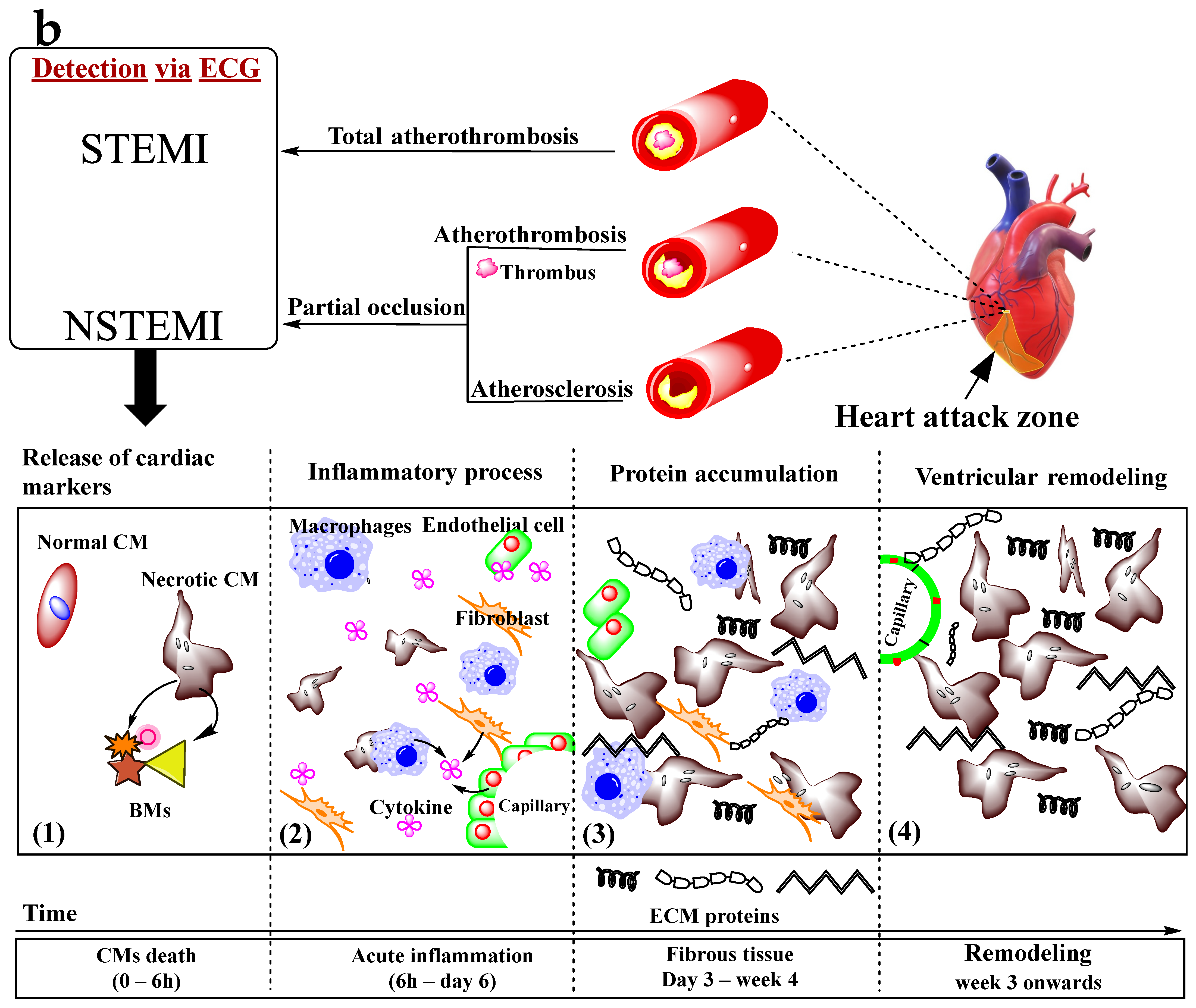
3. Myocardial Infarction
4. Scaffolds for Cardiac Tissue Regeneration
Bioactivity of the Scaffolds in Myocardial Tissue Engineering
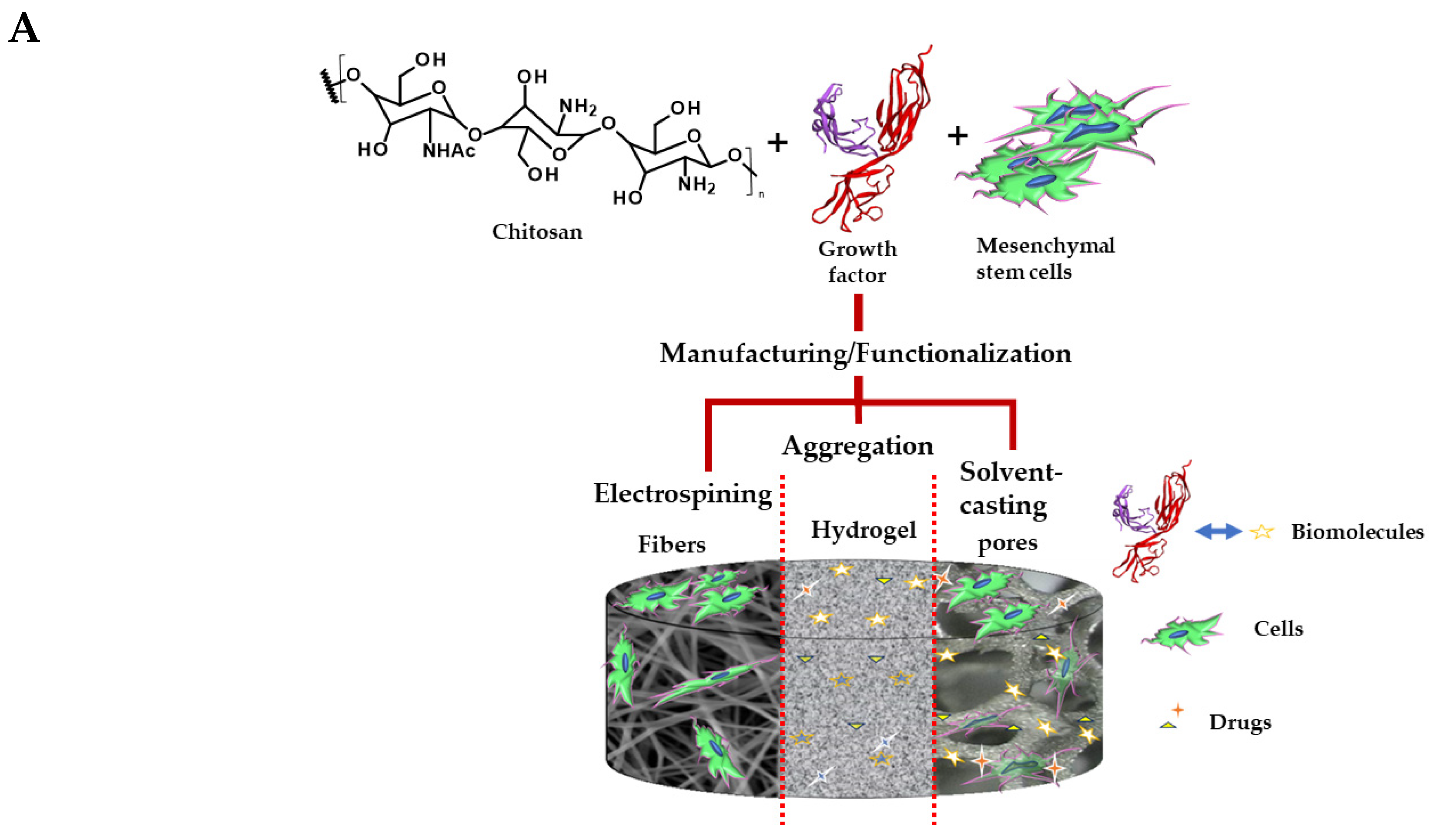
5. Chitosan

5.1. Chitosan’s Role in Cardiovascular Regenerative Therapy
5.1.1. In Vitro Assays of the Chitosan Scaffolds in Cardiovascular Regenerative Therapy
5.1.2. In Vivo Assays of the Chitosan Scaffolds in Cardiovascular Regenerative Therapy
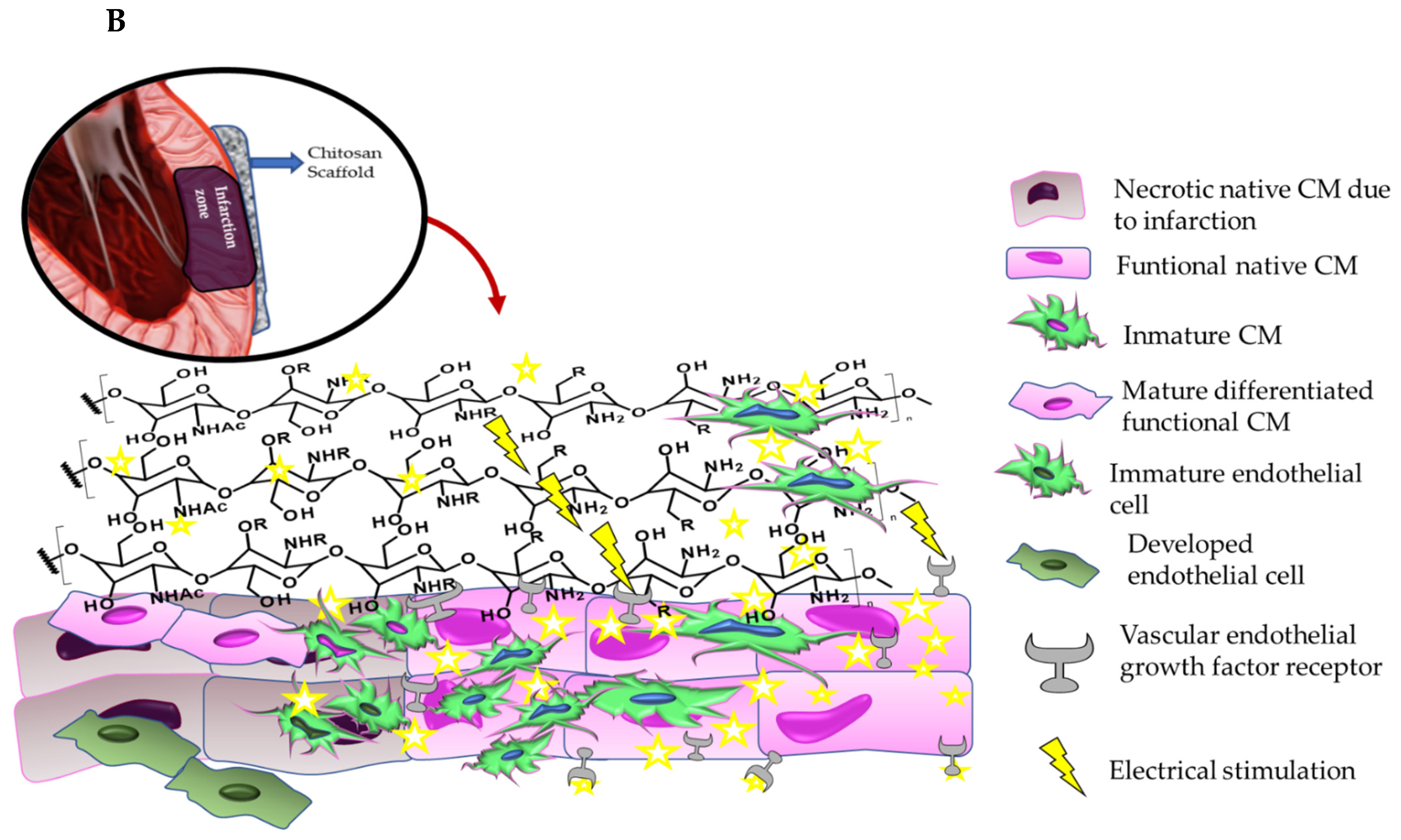
6. Role of the Chitosan Scaffolds in Myocardial Tissue Regeneration: Stimulation of the VEGF Growth Factor Secretion
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 204800401668721. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V. Risk Factors of Acute Myocardial Infarction: A Review. Eurasian J. Med. Investig. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Park, S.; Nguyen, N.B.; Pezhouman, A.; Ardehali, R. Cardiac fibrosis: Potential therapeutic targets. Transl. Res. 2019, 209, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Patel, N.; Kalra, R.; Arora, H.; Bajaj, N.S.; Arora, G.; Arora, P. Incidence and In-Hospital Outcomes of Single-Vessel Coronary Chronic Total Occlusion Treated with Percutaneous Coronary Intervention; Elsevier: Amsterdam, The Netherlands, 2018; Volume 269, ISBN 2059754720. [Google Scholar]
- Patlolla, S.H.; Kanwar, A.; Cheungpasitporn, W.; Doshi, R.P.; Stulak, J.M.; Holmes, D.R.; Bell, M.R.; Singh, M.; Vallabhajosyula, S. Temporal trends, clinical characteristics, and outcomes of emergent coronary artery bypass grafting for acute myocardial infarction in the united states. J. Am. Heart Assoc. 2021, 10, e020517. [Google Scholar] [CrossRef]
- Van Belleghem, S.M.; Mahadik, B.; Snodderly, K.L.; Fisher, J.P. Overview of Tissue Engineering Concepts and Applications, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Sharma, V.; Dash, S.K.; Govarthanan, K.; Gahtori, R.; Negi, N.; Barani, M.; Tomar, R.; Chakraborty, S.; Mathapati, S.; Bishi, D.K.; et al. Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction. Cells 2021, 10, 2538. [Google Scholar] [CrossRef]
- Park, S.-B.; Lih, E.; Park, K.S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Haghighi, P.; Shamloo, A. Fabrication of a novel 3D scaffold for cartilage tissue repair: In-vitro and in-vivo study. Mater. Sci. Eng. C 2021, 128, 112285. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, M.; Kalaee, M.R.; Dehkordi, S.R.; Panahi-Sarmad, M.; Tirgar, M.; Goodarzi, V. Design and characterization of poly(glycerol-sebacate)-co-poly(caprolactone) (PGS-co-PCL) and its nanocomposites as novel biomaterials: The promising candidate for soft tissue engineering. Eur. Polym. J. 2020, 138, 109985. [Google Scholar] [CrossRef]
- Kitsara, M.; Tassis, G.; Papagiannopoulos, A.; Simon, A.; Agbulut, O.; Pispas, S.; Kitsara, M.; Simon, A.; Agbulut, O.; Tassis, G.; et al. Polysaccharide-Protein Multilayers Based on Chitosan-Fibrinogen Assemblies for Cardiac Cell Engineering. Macromol. Biosci. 2022, 22, 2100346. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2018, 8, 1800465. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V.; Singhvi, S. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Lim, Z.Y.J.; Muhamad, N.A.; Liew, F.F. Potential economic value of chitin and its derivatives as major biomaterials of seafood waste, with particular reference to southeast asia. J. Renew. Mater. 2022, 10, 909–938. [Google Scholar] [CrossRef]
- Revkova, V.A.; Grebenik, E.A.; Kalsin, V.A.; Demina, T.S.; Bardakova, K.N.; Shavkuta, B.S.; Melnikov, P.A.; Samoilova, E.M.; Konoplyannikov, M.A.; Efremov, Y.M.; et al. Chitosan-g-oligo(L,L-lactide) Copolymer Hydrogel Potential for Neural Stem Cell Differentiation. Tissue Eng. Part A 2020, 26, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Valencia, C.H.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande-Tovar, C.D. Synthesis and application of scaffolds of chitosan-graphene oxide by the freeze-drying method for tissue regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Ur Rehman, I. Molecular Sciences Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Castro, J.I.; Chaur, M.N.; Humberto, C.; Llano, V.; Eliana, M.; Zapata, V.; Herminsul, J.; Hernandez, M.; Grande-Tovar, C.D.; Castro, J.I.; et al. Biocompatibility Study of Electrospun Nanocomposite Membranes Based on Chitosan/Polyvinyl Alcohol/Oxidized Carbon Nano-Onions. Molecules 2021, 26, 4753. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Rosa, M.; Bomfim, Q.; Henrique De Barros Da Costa Sobrinho, C.; Talissa, D.; Boaz, L.; Da, R.; Lira, S.; Fontes, V.C.; Oliveira Arruda, M.; et al. Characterization, antimicrobial and cytotoxic activity of polymer blends based on chitosan and fish collagen. AMB Express 2022, 12, 102. [Google Scholar] [CrossRef]
- Long, X.; Xu, X.; Sun, D.; Hong, Y.; Wen, C.; Xie, Y.; Yan, B.; Zhang, H.; Ge, Q.; Li, W.; et al. Biomimetic macroporous hydrogel with a triple-network structure for full-thickness skin regeneration. Appl. Mater. Today 2022, 27, 101442. [Google Scholar] [CrossRef]
- Yuan, L.; Fan, W.; Han, L.; Guo, C.; Yan, Z.; Zhu, M.; Mo, X. Evaluation of hydrogels for soft tissue adhesives in vitro and in vivo analyses. Front. Mater. Sci. 2018, 12, 95–104. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Lan, Y.; Zuo, Q.; Li, C.; Zhang, Y.; Guo, R.; Xue, W. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS–PVA hydrogel. J. Tissue Eng. Regen. Med. 2017, 11, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Hu, X.; Ma, L.; You, C.; Zheng, Y.; Sun, H.; Han, C.; Gao, C. Fabrication and characterization of poly(l-lactide-co-glycolide) knitted mesh-reinforced collagen-chitosan hybrid scaffolds for dermal tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 8, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef]
- Bokura, H.; Kobayashi, S. Chitosan decreases total cholesterol in women: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Collins, G.; Yip, D.; Cho, C.H. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnol. Bioeng. 2013, 110, 637–647. [Google Scholar] [CrossRef]
- Chang, T.; Liu, C.; Lu, K.; Wu, Y.; Xu, M.; Yu, Q.; Shen, Z.; Jiang, T.; Zhang, Y. Biomaterials based cardiac patches for the treatment of myocardial infarction. J. Mater. Sci. Technol. 2021, 94, 77–89. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Karimzadeh, F.; Masaeli, E.; Nasr Esfahani, M.-H.M.H.M.-H. Electroconductive Graphene-Containing Polymeric Patch: A Promising Platform for Future Cardiac Repair. ACS Biomater. Sci. Eng. 2020, 6, 4214–4224. [Google Scholar] [CrossRef]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef]
- Kapnisi, M.; Mansfield, C.; Marijon, C.; Guex, A.G.; Perbellini, F.; Bardi, I.; Humphrey, E.J.; Puetzer, J.L.; Mawad, D.; Koutsogeorgis, D.C.; et al. Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction. Adv. Funct. Mater. 2018, 28, 1800618. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Satoh, M.; Fujita, T.; Higo, T.; Sumida, T.; Ko, T.; Yamaguchi, T.; Tobita, T.; Naito, A.T.; Ito, M.; et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat. Commun. 2018, 9, 4435. [Google Scholar] [CrossRef] [PubMed]
- Simon-Yarza, T.; Bataille, I.; Letourneur, D. Cardiovascular Bio-Engineering: Current State of the Art. J. Cardiovasc. Transl. Res. 2017, 10, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Riva, J.J.; Malik, K.M.P.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167–171. [Google Scholar] [PubMed]
- Saleh, M.; Ambrose, J.A. Understanding myocardial infarction [version 1; referees: 2 approved]. F1000Research 2018, 7, F1000 Faculty Rev-1378. [Google Scholar] [CrossRef]
- Uygur, A.; Lee, R.T. Mechanisms of Cardiac Regeneration. Dev. Cell 2016, 36, 362–374. [Google Scholar] [CrossRef]
- Elsayed Azab, A. Acute Myocardial Infarction Risk Factors and Correlation of its Markers with Serum Lipids. J. Appl. Biotechnol. Bioeng. 2017, 3, 00075. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Rankinen, J.; Jneid, H.; Atar, D.; Nikus, K. The Role of ECG in the Diagnosis and Risk Stratification of Acute Coronary Syndromes: An Old but Indispensable Tool. Curr. Cardiol. Rep. 2022, 24, 109–118. [Google Scholar] [CrossRef]
- Vogel, B.; Claessen, B.E.; Arnold, S.V.; Chan, D.; Cohen, D.J.; Giannitsis, E.; Gibson, C.M.; Goto, S.; Katus, H.A.; Kerneis, M.; et al. ST-segment elevation myocardial infarction. Nat. Rev. Dis. Prim. 2019, 5, 39. [Google Scholar] [CrossRef]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Morton, P.G. Acute myocardial infarction. In Critical Care Nursing: A Holistic Approach, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017; pp. 417–443. [Google Scholar] [CrossRef]
- Mehta, A.; Mahtta, D.; Gulati, M.; Sperling, L.S.; Blumenthal, R.S.; Virani, S.S. Cardiovascular Disease Prevention in Focus: Highlights from the 2019 American Heart Association Scientific Sessions. Curr. Atheroscler. Rep. 2020, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.T.; Hsia, J.; Bonaca, M.P. Medical therapy for secondary prevention of atherothrombotic events in peripheral artery disease. Heart Int. 2021, 15, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Michelson, A.D.; Frelinger, A.L.; Bhatt, D.L. Novel aspects of antiplatelet therapy in cardiovascular disease. Res. Pract. Thromb. Haemost. 2018, 2, 439–449. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine-Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Chung, C.M.; Lin, M.S.; Chang, S.T.; Wang, P.C.; Yang, T.Y.; Lin, Y.S. Cardioselective Versus Nonselective β-Blockers After Myocardial Infarction in Adults With Chronic Obstructive Pulmonary Disease. Mayo Clin. Proc. 2022, 97, 531–546. [Google Scholar] [CrossRef]
- Steg, P.G.; Mehta, S.R.; Pollack, C.V.; Bode, C.; Cohen, M.; French, W.J.; Hoekstra, J.; Rao, S.V.; Ruzyllo, W.; Ruiz-Nodar, J.M.; et al. Anticoagulation with otamixaban and ischemic events in non-st-segment elevation acute coronary syndromes: The TAO randomized clinical trial. JAMA-J. Am. Med. Assoc. 2013, 310, 1145–1155. [Google Scholar] [CrossRef]
- Díaz-Munoz, R.; Valle-Caballero, M.J.; Sanchez-Gonzalez, J.; Pizarro, G.; García-Rubira, J.C.; Escalera, N.; Fuster, V.; Fernández-Jiménez, R.; Ibanez, B. Intravenous metoprolol during ongoing STEMI ameliorates markers of ischemic injury: A METOCARD-CNIC trial electrocardiographic study. Basic Res. Cardiol. 2021, 116, 45. [Google Scholar] [CrossRef]
- Zaatari, G.; Fintel, D.J.; Subacius, H.; Germano, J.J.; Shani, J.; Goldberger, J.J. Comparison of Metoprolol Versus Carvedilol After Acute Myocardial Infarction. Am. J. Cardiol. 2021, 147, 1–7. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Voelker, H.; Bhatt, S.P.; Brenner, K.; Casaburi, R.; Come, C.E.; Cooper, J.D.A.; Criner, G.J.; Curtis, J.L.; Han, M.K.; et al. Metoprolol for the prevention of acute exacerbations of COPD. N. Engl. J. Med. 2019, 381, 2304–2314. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Dai, S.-P.; Liu, H.; Xu, Z.-S. Comparison of carvedilol versus metoprolol in patients with acute myocardial infarction A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25855. [Google Scholar] [CrossRef] [PubMed]
- Assimon, M.M.; Brookhart, M.A.; Fine, J.P.; Heiss, G.; Layton, J.B.; Flythe, J.E. A Comparative Study of Carvedilol Versus Metoprolol Initiation and 1-Year Mortality Among Individuals Receiving Maintenance Hemodialysis. Am. J. Kidney Dis. 2018, 72, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Sun Sim, D.; Young Hyun, D.; Ho Jeong, M.; Soo Kim, H.; Chang, K.; Ju Choi, D.; Rok Han, K.; Hoon Ahn, T.; Hwan Bae, J.; Wan Choi, S.; et al. Effect of Low-Dose Nebivolol in Patients with Acute Myocardial Infarction: A Multi-Center Observational Study. Chonnam Med. J. 2020, 56, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Kuwabara, M.; Borghi, C. A critical review of nebivolol and its fixed-dose combinations in the treatment of hypertension. Drugs 2018, 78, 1783–1790. [Google Scholar] [CrossRef]
- Pham, D.; Addison, D.; Kayani, W.; Misra, A.; Jneid, H.; Resar, J.; Lakkis, N.; Alam, M. Outcomes of beta blocker use in cocaine-associated chest pain: A meta-analysis. Emerg. Med. J. 2018, 35, 559–563. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, P.; Verma, N.; Vijayvergiya, R.; Singh, A.; Bhalla, A. Propranolol vs. band ligation for primary prophylaxis of variceal hemorrhage in cirrhotic patients with ascites: A randomized controlled trial. Hepatol. Int. 2022, 16, 944–953. [Google Scholar] [CrossRef]
- Zullo, A.R.; Hersey, M.; Lee, Y.; Sharmin, S.; Bosco, E.; Daiello, L.A.; Shah, N.R.; Mor, V.; Boscardin, W.J.; Berard-Collins, C.M.; et al. Outcomes of “diabetes-friendly” vs “diabetes-unfriendly” β-blockers in older nursing home residents with diabetes after acute myocardial infarction. Diabetes Obes. Metab. 2018, 20, 2724–2732. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, D.; Zhang, Q.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Yang, P.; Zhang, W. Rivaroxaban versus Vitamin K Antagonists (warfarin) based on the triple therapy for left ventricular thrombus after ST-Elevation myocardial infarction. Heart Vessel. 2022, 37, 374–384. [Google Scholar] [CrossRef]
- Coleman, C.I.; Kreutz, R.; Sood, N.A.; Bunz, T.J.; Eriksson, D.; Meinecke, A.K.; Baker, W.L. Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Severe Kidney Disease or Undergoing Hemodialysis. Am. J. Med. 2019, 132, 1078–1083. [Google Scholar] [CrossRef]
- Moulson, N.; LaHaye, S.A.; Bertrand, O.F.; MacHaalany, J. Prophylactic warfarin post anterior ST-elevation myocardial infarction: A systematic review and meta-analysis. Cardiovasc. Revascularization Med. 2017, 18, 559–564. [Google Scholar] [CrossRef]
- Cervantes, C.E.; Merino, J.L.; Barrios, V. Edoxaban for the prevention of stroke in patients with atrial fibrillation. Expert Rev. Cardiovasc. Ther. 2019, 17, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lekura, J.; Kalus, J.S. Overview of betrixaban and its role in clinical practice. Am. J. Health-Syst. Pharm. 2018, 75, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.Y.; Mahaffey, K.W. Cangrelor in clinical use. Future Cardiol. 2020, 16, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Rollini, F.; Rivas, A.; Wali, M.; Briceno, M.; Agarwal, M.; Shaikh, Z.; Nawaz, A.; Silva, G.; Been, L.; et al. Platelet Inhibition with Cangrelor and Crushed Ticagrelor in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Results of the CANTIC Study. Circulation 2019, 139, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Rollini, F.; Faz, G.; Rivas, J.R.; Rivas, A.; Agarwal, M.; Briceno, M.; Wali, M.; Nawaz, A.; Silva, G.; et al. Pharmacodynamic effects of vorapaxar in prior myocardial infarction patients treated with potent oral p2y12 receptor inhibitors with and without aspirin: Results of the vora-pratic study. J. Am. Heart Assoc. 2020, 9, e015865. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bliden, K.P.; Chaudhary, R.; Novakovic, M.; Rout, A.; Gurbel, P.A. Vorapaxar in the treatment of cardiovascular diseases. Future Cardiol. 2020, 16, 373–384. [Google Scholar] [CrossRef]
- Misumida, N.; Aoi, S.; Kim, S.M.; Ziada, K.M.; Abdel-Latif, A. Ticagrelor versus clopidogrel in East Asian patients with acute coronary syndrome: Systematic review and meta-analysis. Cardiovasc. Revascularization Med. 2018, 19, 689–694. [Google Scholar] [CrossRef]
- Al-Rubaish, A.M.; Al-Muhanna, F.A.; Alshehri, A.M.; Al-Mansori, M.A.; Alali, R.A.; Khalil, R.M.; Al-Faraidy, K.A.; Cyrus, C.; Sulieman, M.M.; Vatte, C.; et al. Bedside testing of CYP2C19 vs. conventional clopidogrel treatment to guide antiplatelet therapy in ST-segment elevation myocardial infarction patients. Int. J. Cardiol. 2021, 343, 15–20. [Google Scholar] [CrossRef]
- Zeitouni, M.; Nanna, M.G.; Sun, J.L.; Chiswell, K.; Peterson, E.D.; Navar, A.M. Performance of Guideline Recommendations for Prevention of Myocardial Infarction in Young Adults. J. Am. Coll. Cardiol. 2020, 76, 653–664. [Google Scholar] [CrossRef]
- Jain, V.; Al Rifai, M.; Mahtta, D.; Liu, J.; Hussain, A.; Virani, S.S. Highlights from Studies Presented at the Virtual American College of Cardiology Scientific Sessions 2021: Staying Updated with the Latest Advancements in Prevention. Curr. Atheroscler. Rep. 2021, 23, 50. [Google Scholar] [CrossRef]
- Khalil, P.; Kabbach, G. Direct Oral Anticoagulants in Addition to Antiplatelet Therapy for Secondary Prevention after Acute Coronary Syndromes: A Review. Curr. Cardiol. Rep. 2019, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of noninvasive cardiovascular testing, applied clinical nutrition and nutritional supplements in the prevention and treatment of coronary heart disease. Ther. Adv. Cardiovasc. Dis. 2018, 12, 85–108. [Google Scholar] [CrossRef]
- Jackevicius, C.A.; Krumholz, H.M.; Ross, J.S.; Koh, M.; Chong, A.; Austin, P.C.; Stukel, T.A.; Azizi, P.; Ko, D.T. Clinical Outcomes With Beta-Blocker Use in Patients With Recent History of Myocardial Infarction. Can. J. Cardiol. 2020, 36, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, R.F.E. Beta-blockers after myocardial infarction: Are they useful to all patients? And how long should be the beta-blocker therapy? Monaldi Arch. Chest Dis. 2018, 88, 35–36. [Google Scholar] [CrossRef]
- Schaefer, J.K.; Errickson, J.; Li, Y.; Kong, X.; Alexandris-Souphis, T.; Ali, M.A.; Decamillo, D.; Haymart, B.; Kaatz, S.; Kline-Rogers, E.; et al. Adverse Events Associated With the Addition of Aspirin to Direct Oral Anticoagulant Therapy Without a Clear Indication Supplemental content. JAMA Intern. Med. 2021, 181, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Sia, C.H.; Leow, A.S.T.; Tan, B.Y.Q.; Yeo, L.L.L.; Chan, M.Y.Y.; Loh, J.P.Y. Anticoagulation for the treatment of left ventricular thrombus in patients with acute myocardial infarction and renal impairment. Pol. Arch. Intern. Med. 2021, 131, 878–881. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Rosellini, E.; Cristallini, C.; Barbani, N.; Vozzi, G.; Giusti, P. Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 447–453. [Google Scholar] [CrossRef]
- Lee, R.J.; Hinson, A.; Bauernschmitt, R.; Matschke, K.; Fang, Q.; Mann, D.L.; Dowling, R.; Schiller, N.; Sabbah, H.N. The feasibility and safety of Algisyl-LVRTM as a method of left ventricular augmentation in patients with dilated cardiomyopathy: Initial first in man clinical results. Int. J. Cardiol. 2015, 199, 18–24. [Google Scholar] [CrossRef]
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook. From ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, T.; Yu, Y.; Shi, K.; Bei, Z.; Qian, Y.; Qian, Z. An injectable conductive hydrogel restores electrical transmission at myocardial infarct site to preserve cardiac function and enhance repair. Bioact. Mater. 2023, 20, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Goyal, A.K. Biomaterial-based scaffolds-current status and future directions. Expert Opin. Drug Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; AnilKumar, S.; Roman, B.; Tasnim, N.; Joddar, B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl. Res. 2019, 211, 64–83. [Google Scholar] [CrossRef]
- Qian, B.; Yang, Q.; Wang, M.; Huang, S.; Jiang, C.; Shi, H.; Long, Q.; Zhou, M.; Zhao, Q.; Ye, X. Encapsulation of lyophilized platelet-rich fibrin in alginate-hyaluronic acid hydrogel as a novel vascularized substitution for myocardial infarction. Bioact. Mater. 2022, 7, 401–411. [Google Scholar] [CrossRef]
- Peng, L.; Li, M.; Zhao, K.; Ma, C.; Tang, H.; Li, Y. Evaluation of an Injectable Hydrogel Based on Hyaluronic Acid–Chitosan/β-Glycerophosphate-Loaded Mesenchymal Stem Cells in Enhancing the Therapeutic Efficacy of Myocardial Infarction. Macromol. Biosci. 2022, 22, 2100286. [Google Scholar] [CrossRef]
- Zhan, J.; Liao, X.; Fan, X.; Zhang, J.; Li, H.; Cai, Y.; Qiu, X. An injectable and conductive TEMPOL/polypyrrole integrated peptide co-assembly hydrogel promotes functional maturation of cardiomyocytes for myocardial infarction repair. Compos. Part B Eng. 2022, 236, 109794. [Google Scholar] [CrossRef]
- Chandika, P.; Heo, S.Y.; Kim, T.H.; Oh, G.W.; Kim, G.H.; Kim, M.S.; Jung, W.K. Recent advances in biological macromolecule based tissue-engineered composite scaffolds for cardiac tissue regeneration applications. Int. J. Biol. Macromol. 2020, 164, 2329–2357. [Google Scholar] [CrossRef]
- Guan, G.; Huo, D.; Li, Y.; Zhao, X.; Li, Y.; Qin, Z.; Sun, D.; Yang, G.; Yang, M.; Tan, J.; et al. Engineering hiPSC-CM and hiPSC-EC laden 3D nanofibrous splenic hydrogel for improving cardiac function through revascularization and remuscularization in infarcted heart. Bioact. Mater. 2021, 6, 4415–4429. [Google Scholar] [CrossRef]
- Ai, J.; Farzin, A.; Zamiri, S.; Hadjighassem, M.; Ebrahimi-Barough, S.; Ai, A.; Mohandesnezhad, S.; Karampour, A.; Sagharjoghi Farahani, M.; Goodarzi, A. Repair of injured spinal cord using platelet-rich plasma- and endometrial stem cells-loaded chitosan scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1002–1011. [Google Scholar] [CrossRef]
- Meyers, K.; Lee, B.P.; Rajachar, R.M. Electroactive polymeric composites to mimic the electromechanical properties of myocardium in cardiac tissue repair. Gels 2021, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Liu, J.; Zhang, J.; Shi, J.; Jin, Z.; Li, Y.; Ding, X.; Zhu, X.; Yuan, C.; Xiu, B.; et al. Conductive single-wall carbon nanotubes/extracellular matrix hybrid hydrogels promote the lineage-specific development of seeding cells for tissue repair through reconstructing an integrin-dependent niche. J. Nanobiotechnol. 2021, 19, 252. [Google Scholar] [CrossRef] [PubMed]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Hassanabad, A.F.; Heydari, B.; Mikami, Y.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Wagner, K.T.; Tarraf, S.A.; et al. Acellular bioscaffolds redirect cardiac fibroblasts and promote functional tissue repair in rodents and humans with myocardial injury. Sci. Rep. 2020, 10, 9459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Feng, Y.; Xue, L.; Cui, M.; Zhang, Q.; Xu, F.; Peng, N.; Jiang, Z.; Gao, D.; Zhang, X. Anisotropic conductive reduced graphene oxide/silk matrices promote post-infarction myocardial function by restoring electrical integrity. Acta Biomater. 2022, 139, 190–203. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Wang, M.; Gupta, R.C.; Rastogi, S.; Ilsar, I.; Sabbah, M.S.; Kohli, S.; Helgerson, S.; Lee, R.J. Augmentation of left ventricular wall thickness with alginate hydrogel implants improves left ventricular function and prevents progressive remodeling in dogs with chronic heart failure. JACC Heart Fail. 2013, 1, 252–258. [Google Scholar] [CrossRef]
- Lee, L.C.; Klepach, D.; Hinson, A.; Lee, R.; Guccione, J. Algisyl-LVR Reduces Left Ventricular Wall Stress and Improves Function in the Failing Human Heart. J. Card. Fail. 2012, 18, S57–S58. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef]
- Sarkar, D.; Zhao, W.; Schaefer, S.; Ankrum, J.A.; Teo, G.S.L.; Pereira, M.N.; Ferreira, L.; Karp, J.M. Overview of Tissue Engineering Concepts and Applications. In Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1122–1137. [Google Scholar] [CrossRef]
- Smagul, S.; Kim, Y.; Smagulova, A.; Raziyeva, K.; Nurkesh, A.; Saparov, A. Biomaterials loaded with growth factors/cytokines and stem cells for cardiac tissue regeneration. Int. J. Mol. Sci. 2020, 21, 5952. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Karimzadeh, F. A conductive film of chitosan-polycaprolcatone-polypyrrole with potential in heart patch application. Polym. Test. 2019, 75, 254–261. [Google Scholar] [CrossRef]
- De Alvarenga, G.; Hryniewicz, B.M.; Jasper, I.; Silva, R.J.; Klobukoski, V.; Costa, F.S.; Cervantes, T.N.M.; Amaral, C.D.B.; Schneider, J.T.; Bach-Toledo, L.; et al. Recent trends of micro and nanostructured conducting polymers in health and environmental applications. J. Electroanal. Chem. 2020, 879, 114754. [Google Scholar] [CrossRef]
- Stapleton, L.; Zhu, Y.; Woo, Y.-P.J.; Appel, E. Engineered biomaterials for heart disease. Curr. Opin. Biotechnol. 2020, 66, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Efraim, Y.; Sarig, H.; Cohen Anavy, N.; Sarig, U.; de Berardinis, E.; Chaw, S.Y.; Krishnamoorthi, M.; Kalifa, J.; Bogireddi, H.; Duc, T.V.; et al. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater. 2017, 50, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Soleimani, M.; Imani, M.; Sahebjam, M.; Ghiaseddin, A.; Nassiri, S.M.; Majd Ardakani, J.; Tajik Rostami, M.; Jalali, A.; Mousanassab, B.; et al. Regenerating Heart Using a Novel Compound and Human Wharton Jelly Mesenchymal Stem Cells. Arch. Med. Res. 2017, 48, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, S.; Soleimani, M.; Tafti, S.H.A.; Aghdam, R.M.; Hojati, Z. Improvement of Heart Function After Transplantation of Encapsulated Stem Cells Induced with miR-1/Myocd in Myocardial Infarction Model of Rat. Cell Transplant. 2021, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, L.; Feng, L.-F.; Yue, Z.-W.; Zhao, N.-H.; Li, Z.; He, Z.-X. IGF-1C domain-modified hydrogel enhanced the efficacy of stem cells in the treatment of AMI. Stem Cell Res. Ther. 2020, 11, 136. [Google Scholar] [CrossRef]
- Song, X.; Mei, J.; Ye, G.; Wang, L.; Ananth, A.; Yu, L.; Qiu, X. In situ pPy-modification of chitosan porous membrane from mussel shell as a cardiac patch to repair myocardial infarction. Appl. Mater. Today 2019, 15, 87–99. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Akagi, T.; Akashi, M. Vascularized cardiac tissue construction with orientation by layer-by-layer method and 3D printer. Sci. Rep. 2020, 10, 5484. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Extracellular Matrix in Heart Disease: Focus on Circulating Collagen Type I and III Derived Peptides as Biomarkers of Myocardial Fibrosis and Their Potential in the Prognosis of Heart Failure: A Concise Review. Metabolites 2022, 12, 297. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Tovar, C.D.G.; Hernandez, J.H.M. The role of chitosan and graphene oxide in bioactive and antibacterial properties of acrylic bone cements. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M. A combination of sugar esters and chitosan to promote in vivo wound care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Melgarejo-Ramírez, Y.; Sánchez-Sánchez, R.; García-Carvajal, Z.; García-López, J.; Gutiérrez-Gómez, C.; Luna-Barcenas, G.; Ibarra, C.; Velasquillo, C. Biocompatibility of Human Chondrocytes Grown on a Chitosan/Polyvnyl/Epichlorohydrin Based Hydrogel for Application in Tissue Engineering. Int. J. Morphol. 2014, 32, 1347–1356. [Google Scholar] [CrossRef]
- Jayakumar, R.; Selvamurugan, N.; Nair, S.V.; Tokura, S.; Tamura, H. Preparative methods of phosphorylated chitin and chitosan-An overview. Int. J. Biol. Macromol. 2008, 43, 221–225. [Google Scholar] [CrossRef]
- Kosowska, K.; Domalik-Pyzik, P.; Krok-Borkowicz, M.; Chłopek, J. Synthesis and characterization of chitosan/reduced graphene oxide hybrid composites. Materials 2019, 12, 2077. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Sha, D.; Liu, Y.; Xu, J.; Shi, K.; Yu, C.; Ji, X. Injectable and antibacterial ε-poly(L-lysine)-modified poly(vinyl alcohol)/chitosan/AgNPs hydrogels as wound healing dressings. Polymer 2021, 212, 123155. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Valencia Zapata, M.E.; Restrepo, Y.J.; Mina Hernandez, J.H.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/Polyvinyl Alcohol/Tea Tree Essential Oil Composite Films for Biomedical Applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Zhao, M.; Fan, C.; Fast, V.G.; Valarmathi, M.T.; Zhu, W.; Zhang, J. N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovasc. Res. 2020, 116, 671–685. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, X. Cardiomyocyte Induction and Regeneration for Myocardial Infarction Treatment: Cell Sources and Administration Strategies. Adv. Healthc. Mater. 2020, 9, 2001175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, S.; Wang, S.; Li, W.; Liu, J. Electroactive nanoparticles loaded Silk protein/Chitosan macromolecular injectable hydrogel to improve therapeutic efficacy of mesenchymal stem cells in functional recovery after ischemic myocardial infarction. Res. Sq. 2021; manuscript in preparation. [Google Scholar] [CrossRef]
- Si, R.; Gao, C.; Guo, R.; Lin, C.; Li, J.; Guo, W. Human mesenchymal stem cells encapsulated-coacervated photoluminescent nanodots layered bioactive chitosan/collagen hydrogel matrices to indorse cardiac healing after acute myocardial infarction. J. Photochem. Photobiol. B Biol. 2020, 206, 111789. [Google Scholar] [CrossRef]
- Mousavi, A.; Mashayekhan, S.; Baheiraei, N.; Pourjavadi, A. Biohybrid oxidized alginate/myocardial extracellular matrix injectable hydrogels with improved electromechanical properties for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 692–708. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 045003. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Akagi, T.; Shima, F.; Akashi, M. Fabrication of Orientation-Controlled 3D Tissues Using a Layer-by-Layer Technique and 3D Printed a Thermoresponsive Gel Frame. Tissue Eng. Part C Methods 2017, 23, 357–366. [Google Scholar] [CrossRef]
- Esmaeili Pourfarhangi, K.; Mashayekhan, S.; Asl, S.G.; Hajebrahimi, Z. Construction of scaffolds composed of acellular cardiac extracellular matrix for myocardial tissue engineering. Biologicals 2018, 53, 10–18. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, D.; Wang, Z.; Zhang, Z.; Xia, Y.; Xue, H.; Liu, Y. Preparation of an Electrically Conductive Graphene Oxide/Chitosan Scaffold for Cardiac Tissue Engineering. Appl. Biochem. Biotechnol. 2019, 188, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart. Sci. Rep. 2018, 8, 15069. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Hong, S.; Fang, Y.H.; Song, M.; Son, K.H.; Son, H.S.; Kim, S.K.; Sun, K.; Park, Y. Differential regeneration of myocardial infarction depending on the progression of disease and the composition of biomimetic hydrogel. J. Biosci. Bioeng. 2014, 118, 461–468. [Google Scholar] [CrossRef]
- Pelouch, V.; Dixon, I.M.C.; Golfman, L.; Beamish, R.E.; Dhalla, N.S. Role of extracellular matrix proteins in heart function. Mol. Cell. Biochem. 1993, 129, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.T.; Humphrey, J.D. Denaturation of collagen via heating: An irreversible rate process. Annu. Rev. Biomed. Eng. 2002, 4, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Joanne, P.; Kitsara, M.; Boitard, S.E.; Naemetalla, H.; Vanneaux, V.; Pernot, M.; Larghero, J.; Forest, P.; Chen, Y.; Menasché, P.; et al. Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials 2016, 80, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Guo, Y.; Qu, Y.; Yu, J.; Song, L.; Chen, S.; Qin, Z.; Gong, J.; Zhan, H.; Gao, Y.; Zhang, J. A chitosan-vitamin C based injectable hydrogel improves cell survival under oxidative stress. Int. J. Biol. Macromol. 2022, 202, 102–111. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Selvamani, S.; Prasanna, M.; Muthukumaran, A. A novel biocompatible chitosan–Selenium nanoparticles (SeNPs) film with electrical conductivity for cardiac tissue engineering application. Mater. Sci. Eng. C 2018, 92, 151–160. [Google Scholar] [CrossRef]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.H.; et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef]
- Ke, X.; Li, M.; Wang, X.; Liang, J.; Wang, X.; Wu, S.; Long, M.; Hu, C. An injectable chitosan/dextran/β-glycerophosphate hydrogel as cell delivery carrier for therapy of myocardial infarction. Carbohydr. Polym. 2020, 229, 115516. [Google Scholar] [CrossRef]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, P.; Qiao, C.; Wu, T.; Sun, X.; Wen, M.; Zhang, W. Chitosan Hydrogel Enhances the Therapeutic Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells for Myocardial Infarction by Alleviating Vascular Endothelial Cell Pyroptosis. J. Cardiovasc. Pharmacol. 2020, 75, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Cacopardo, L.; Marques Costa, J.; Fedak, P.W.M.; Vasanthan, V.; Hassanabad, A.F.; Pattar, S.; Niklewski, P.; Wagner, K. Promoting Cardiac Regeneration and Repair Using Acellular Biomaterials. Front. Bioeng. Biotechnol. 2020, 8, 291. [Google Scholar] [CrossRef]
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642. [Google Scholar] [CrossRef] [PubMed]
- Blan, N.R.; Birla, R.K. Design and fabrication of heart muscle using scaffold-based tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 86, 195–208. [Google Scholar] [CrossRef]
- Ozawa, T.; Mickle, D.A.G.; Weisel, R.D.; Koyama, N.; Wong, H.; Ozawa, S.; Li, R.-K. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J. Thorac. Cardiovasc. Surg. 2002, 124, 1157–1164. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S. V In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Pok, S.; Benavides, O.M.; Hallal, P.; Jacot, J.G. Use of myocardial matrix in a chitosan-based full-thickness heart patch. Tissue Eng. Part A 2014, 20, 1877–1887. [Google Scholar] [CrossRef]
- Pok, S.; Stupin, I.V.; Tsao, C.; Pautler, R.G.; Gao, Y.; Nieto, R.M.; Tao, Z.-W.; Fraser, C.D.; Annapragada, A.V.; Jacot, J.G. Full-Thickness Heart Repair with an Engineered Multilayered Myocardial Patch in Rat Model. Adv. Healthc. Mater. 2017, 6, 228–237. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Musial-Wysocka, A.; Kot, M.; Sulkowski, M.; Majka, M. Regenerative potential of the product “cardiocell” derived from the wharton’s jelly mesenchymal stem cells for treating hindlimb ischemia. Int. J. Mol. Sci. 2019, 20, 4632. [Google Scholar] [CrossRef]
- He, S.; Wu, J.; Li, S.-H.; Wang, L.; Sun, Y.; Xie, J.; Ramnath, D.; Weisel, R.D.; Yau, T.M.; Sung, H.-W.; et al. The conductive function of biopolymer corrects myocardial scar conduction blockage and resynchronizes contraction to prevent heart failure. Biomaterials 2020, 258, 120285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhang, R. Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol. 2017, 103, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Al Rez, M.F.; Binobaid, A.; Alghosen, A.; Mirza, E.H.; Alam, J.; Fouad, H.; Hashem, M.; Alsalman, H.; Almalak, H.M.; Mahmood, A.; et al. Tubular poly(ε-caprolactone)/chitosan nanofibrous scaffold prepared by electrospinning for vascular tissue engineering applications. J. Biomater. Tissue Eng. 2017, 7, 427–436. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.; Deng, B.; Liu, X.; Wang, L.; Zhu, Q.-L. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp. Ther. Med. 2017, 13, 588–594. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Zhuang, J.; Zhu, P. Fabrication of engineered nanoparticles on biological macromolecular (PEGylated chitosan) composite for bio-active hydrogel system in cardiac repair applications. Int. J. Biol. Macromol. 2018, 117, 553–558. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Nirwan, V.P.; Munnier, E.; Chourpa, I.; Fahmi, A.; Kabashin, A.V. Toward multifunctional hybrid platforms for tissue engineering based on chitosan(PEO) nanofibers functionalized by bare laser-synthesized Au and Si nanoparticles. RSC Adv. 2017, 7, 31759–31766. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef]
- Gao, L.; Yi, M.; Xing, M.; Li, H.; Zhou, Y.; Xu, Q.; Zhang, Z.; Wen, Z.; Chang, J. In situ activated mesenchymal stem cell (MSCs) by bioactive for myocardial infarction treatment. J. Mater. Chem. B 2021, 8, 7713–7722. [Google Scholar] [CrossRef] [PubMed]
- Giacca, M. Cardiac Regeneration After Myocardial Infarction: An Approachable Goal. Curr. Cardiol. Rep. 2020, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, P.; Nazeri, N.; Derakhshan, M.A.M.A.M.A.; Ghanbari, H. Preparation and characterization of polyurethane/chitosan/CNT nanofibrous scaffold for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Lin, S.; Mequanint, K. Preparation and characterization of electrospun rGO-poly(ester amide) conductive scaffolds. Mater. Sci. Eng. C 2019, 98, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Sadeghi, D.; Solouk, A.; Bagheri, S.; Ghalei, S.; Mirzadeh, H. Chitosan/gum tragacanth/PVA hybrid nanofibrous scaffold for tissue engineering applications. Bioinspired Biomim. Nanobiomater. 2020, 9, 16–23. [Google Scholar] [CrossRef]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.-Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.-W.; Li, R.-K. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef]
- Abedi, A.; Bakhshandeh, B.; Babaie, A.; Mohammadnejad, J.; Vahdat, S.; Mombeiny, R.; Moosavi, S.R.; Amini, J.; Tayebi, L. Concurrent application of conductive biopolymeric chitosan/ polyvinyl alcohol/ MWCNTs nanofibers, intracellular signaling manipulating molecules and electrical stimulation for more effective cardiac tissue engineering. Mater. Chem. Phys. 2021, 258, 123842. [Google Scholar] [CrossRef]
- Jafarkhani, M.; Salehi, Z.; Mashayekhan, S.; Kowsari-Esfahan, R.; Orive, G.; Dolatshahi-Pirouz, A.; Bonakdar, S.; Shokrgozar, M.A. Induced cell migration based on a bio-active hydrogel sheet combined with a perfused microfluidic system. Biomed. Mater. 2020, 14, 045010. [Google Scholar] [CrossRef]
- Torabi, H.; Mehdikhani, M.; Varshosaz, J.; Shafiee, F. An innovative approach to fabricate a thermosensitive melatonin-loaded conductive pluronic/chitosan hydrogel for myocardial tissue engineering. J. Appl. Polym. Sci. 2021, 138, app50327. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Zhang, L.; Gong, W.; Sun, W. Biomimetic design and fabrication of scaffolds integrating oriented micro-pores with branched channel networks for myocardial tissue engineering. Biofabrication 2019, 11, 035004. [Google Scholar] [CrossRef]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Joz Majidi, H.; Babaei, A.; Kazemi-Pasarvi, S.; Arab-Bafrani, Z.; Amiri, M. Tuning polylactic acid scaffolds for tissue engineering purposes by incorporating graphene oxide-chitosan nano-hybrids. Polym. Adv. Technol. 2021, 32, 1654–1666. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Cieślar-Pobuda, A.; Ejneby, M.S.; Sammalisto, L.; Gelmi, A.; de Muinck, E.; Brask, J.; Łos, M.J.; Rafat, M. Rational Design of a Conductive Collagen Heart Patch. Macromol. Biosci. 2017, 17, 1600446. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.D.; Caridade, S.G.; Sousa, M.P.; Azevedo, S.; Kandur, M.Y.; Öner, E.T.; Alves, N.M.; Mano, J.F. Adhesive free-standing multilayer films containing sulfated levan for biomedical applications. Acta Biomater. 2018, 69, 183–195. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in cardiomyocytes and heart diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Wu, Q.; Bao, F.; Yang, H.; Qiu, X.; Chang, J. Chitosan/Calcium Silicate Cardiac Patch Stimulates Cardiomyocyte Activity and Myocardial Performance after Infarction by Synergistic Effect of Bioactive Ions and Aligned Nanostructure. ACS Appl. Mater. Interfaces 2019, 11, 1449–1468. [Google Scholar] [CrossRef]
| Treatment | Drug | Function | Featured Findings | Precautions | Ref. |
|---|---|---|---|---|---|
| β-blockers | Metoprolol® | Blocks β-1 receptors of cardiomyocytes, reducing blood pressure and muscle contractility | Decreases ischemic damage in people with STEMI. Increases the odds of surviving a second heart attack | Hospitalization in patients with obstructive problems; pulmonary severe or moderate | [51,52,53] |
| Carvedilol® | Blocks β-1, β-2 and α1 receptors on cells in the body | Helps in the ventricular remodeling processes. Promotes diastolic ventricular filling and decreases ischemic heart disease | Population on hemodialysis could experience cardiovascular complications in the first year of medication | [54,55] | |
| Nabivolol® | β-1 receptor antagonist with vasodilation properties | Well tolerated in elderly patients with heart failure. Decreases the probability of heart failure due to its vasodilator effect | It could cause headaches, diarrhea, nausea, bradycardia and other mild side effects | [56,57] | |
| Propanolol® | Non-specific inhibitor of β-adrenergic receptors | Reduces mortality in post-infarction patients and regulates cardiac arrhythmias | Contraindicated in some patients with grade ≥2 cirrhosis due to a possible increase in chronic kidney damage | [58,59] | |
| Bisoprolol® | Non-specific inhibitor of β receptors. Helps in arterial diminution and vasodilation | It could increase the risk of a heart attack, heart failure and hospitalization vs. β-selective drugs | Hospitalization in type 2 diabetics due to hyperglycemia | [49,60] | |
| Anticoagulants | Rivaroxaban® | Decreases thrombotic events by inhibiting serine protease coagulating factor Xa | Helps in the treatment of the left ventricular thrombi after an ischemic episode | Does not significantly reduce the risk of systemic ischemia or embolism in patients with stage 5 renal failure | [61,62] |
| Warfarin® | Decreases the probability of death, heart attack and systemic embolism by inhibiting coagulation factor II | Helps reduce the likelihood of recurrent heart attacks in people with chronic heart disease | Possible increase in bleeding due to its anticoagulant action | [63] | |
| Edoxaban® | Reduces the risks of ischemia and systemic embolisms through the inhibition of coagulating factor Xa | Prevents ischemia in patients with atrial fibrillation | Adverse liver events in a population with liver problems and atrial fibrillation | [64] | |
| Betrixaban® | Clotting factor inhibitor Xa | Applied in patients with venous thromboembolism | Risk of bleeding | [65] | |
| Antiplatelet | Cangrelor® | ATP analog P2Y12 receptor antagonist that regulates the platelet segregation | Decreases the probability of a second heart attack. Clinically used to reduce perioperative complications associated with percutaneous coronary interventions | Risk of bleeding | [66,67] |
| Voraxapar® | Inhibits protease receptor 1, decreasing thrombin synthesis and therefore hindering platelet synthesis | It is administered with P2Y12 inhibitor drugs for the biosynthetic decrease in platelets in post-infarction patients | Increases the risk of bleeding | [68,69] | |
| Clopidrogel® | P2Y12 receptor antagonist that regulates the platelet segregation | The use of this drug decreases the probability of heart failure and sudden death in long-term patients | Increased risk of bleeding in isolated cases | [70,71] |
| Composition | Disadvantages | Advantages | Type of Assay | Implantation Site | Animal Model | Cell Type | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan/porcine decellularized extracellular matrix | Slow cell proliferation within the scaffold of stem cells seeded in the hydrogel | Improves the ejection fractions in rats with acute myocardial infarction and stimulates tissue re-epithelization | In vivo/ In vitro | Left ventricle | Male Wistar rats | - | [106] |
| Chitosan/collagen | Cellular limitation due to low pore connectivity | It would help to up-regulate MicroRNA-1/Myocardin in internally seeded stem cells and reduce the infarcted zone | In vivo/ In vitro | Left ventricle | Male Sprague–Dawley rats | Mesenchymal stem cell | [108] |
| Chitosan/PEG/TiO2 nanoparticles | Its rapid biodegradation affects the results of the cell viability studies | This hydrogel presented high biocompatibility, encapsulation and short-term cell binding protein segregation | In vitro | - | - | Neonatal rats cardiomyocytes | [162] |
| Chitosan/silk protein | Activated the hypertrophy process in native cardiomyocytes | Could improve the cellular cardiomyoplasty processes and the hemodynamics of the infarcted heart | In vitro/ in vivo | - | Adult male Sprague–Dawley rats | Bone marrow-derived mesenchymal stem cells | [129] |
| Chitosan/graphene oxide | This material could generate apoptosis in some stem cells | Helps improve cell anchoring and electrical conductivity in the infarcted zone | In vitro | - | - | Cardiomyocytes derived from an engineered human embryonic stem cell line | [163] |
| Chitosan/PPi | Made it difficult to visualize the cells in the cell staining techniques such as hematoxylin and eosin (H&E). The study showed that there was no regenerative capacity of the material. | Improved cell anchoring and electrical conductivity in the infarcted zone, orientation and ordering of the regenerated tissue | In vivo/in vitro | Left ventricle | Female Sprague–Dawley rats | CMs from 1-day-old neonatal Sprague–Dawley rats | [171] |
| Chitosan/PPi | Its application failed to regenerate the infarcted tissue significantly | Used to stimulate the fibrous area of infarction electrically, synchronizing the infarcted tissue and improving the hemodynamics of the heart | In vitro/ In vivo | Left ventricle | Female Sprague-Dawley rats | Fibrotic tissue matrix | [159] |
| Chitosan/polyvinyl alcohol/MWCNTs | Several factors are required for the scaffold to develop functional heart tissue | Scaffold is capable of propagating electrical impulses. Helps induce stem cell differentiation into the cardiomyocytes | In vitro | - | - | Unrestricted somatic stem cells | [172] |
| Chitosan/Polyvinyl alcohol/CNTs | Low levels in the genes that express the differentiation to cardiomyocytes | The scaffold is capable of conducting electricity and with good cell viability values (viability > 80%) | In vitro | - | - | Undifferentiated mesenchymal stem cells from thighbone marrow of young male rats | [84] |
| Polyurethane/chitosan/CNTs | No in vitro/in vivo assay information found | Scaffold can conduct electricity and has good cell viability values in the H9C2 cells | - | - | - | - | [168] |
| Chitosan/decellularized tissue of the bovine heart | The biocompatibility of the scaffold against cardiomyocytes is not known. | Formation of blood vessels inside the scaffold with potential application in the regeneration of cardiac tissue | In vitro | - | - | Human umbilical vein endothelial cell | [173] |
| Chitosan/placental insulin-like growth factor 1 C-domain peptide | Rapid biodegradation in vivo that did not allow for cell maturation | Can improve the hemodynamics of the heart by removing the fibrous tissue in the area of infarction | In vivo/in vitro | Left ventricle | C57BL/6 transgenic mice | Human placenta-derived mesenchymal stem cells/Neonatal rat cardiomyocytes | [109] |
| Chitosan/Cardiomyocytes derived from pluripotent human stem cells/decellularized porcine extracellular matrix | Its application could decrease the capacity of cell maturation | Using this hydrogel improved the concentration of Ca2+ and the proteins necessary for the maturation of the stem cells. In addition, it is an isoproterenol-releasing drug. | In vitro | - | - | Human-induced pluripotent stem cell-derived cardiomyocytes | [145] |
| Chitosan/graphene quantum dots/collagen | The hydrogel does not have the necessary capabilities to be injected | The hydrogel could increase the endothelial cells and reduce the infarcted zone in rats, improving the hemodynamics of the treated heart. | In vitro/ In vivo | Left ventricle | Male Fischer rats | Human coronary artery endothelial cells/Human mesenchymal stem cells | [130] |
| Chitosan/ bone marrow-derived mesenchymal stem cells | The hydrogel does not have the necessary capabilities to be injected into the myocardium | Its superficial application can improve the regeneration of the infarcted tissue with anti-inflammatory effects | In vivo | Left ventricle | Transgenic FVB-Fluc/GFP mice | Bone marrow-derived Mesenchymal stem cell | [148] |
| Chitosan/ Dextran/ β-glycerophosphate | It was not possible to measure the regenerative capacity of the hydrogel | In the biomodels, the scaffold demonstrated the stimulation of the differentiation capacity of the mesenchymal stem cells from the umbilical cord to immature cardiac cells | In vitro | - | - | Umbilical cord mesenchymal stem cells | [146] |
| Chitosan/Au nanoparticles/Polyglycerol sebacate | In vitro tests showed a cytotoxicity of 10% | Its composition can allow for the injection of stem cells into the infarcted tissue | In vitro | - | - | H9C2 cells | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleño Acosta, B.; Advincula, R.C.; Grande-Tovar, C.D. Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review. Molecules 2023, 28, 1920. https://doi.org/10.3390/molecules28041920
Beleño Acosta B, Advincula RC, Grande-Tovar CD. Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review. Molecules. 2023; 28(4):1920. https://doi.org/10.3390/molecules28041920
Chicago/Turabian StyleBeleño Acosta, Bryan, Rigoberto C. Advincula, and Carlos David Grande-Tovar. 2023. "Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review" Molecules 28, no. 4: 1920. https://doi.org/10.3390/molecules28041920
APA StyleBeleño Acosta, B., Advincula, R. C., & Grande-Tovar, C. D. (2023). Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review. Molecules, 28(4), 1920. https://doi.org/10.3390/molecules28041920










