Mechanisms for Catalytic CO Oxidation on SiAun (n = 1–5) Cluster
Abstract
1. Introduction
2. Results and Discussion
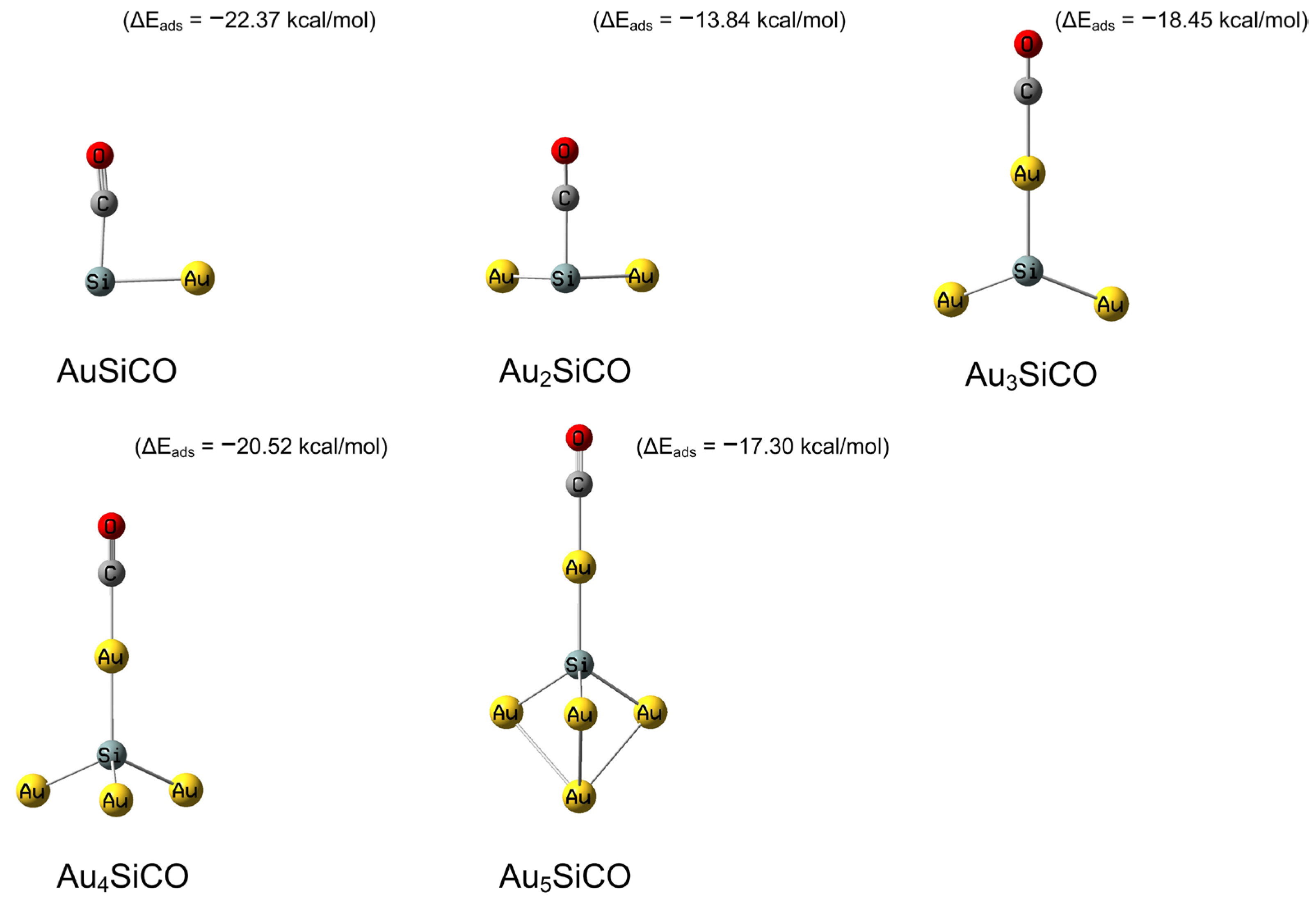
2.1. The Interactions of SiAun (n = 1–5) Cluster with CO and O2
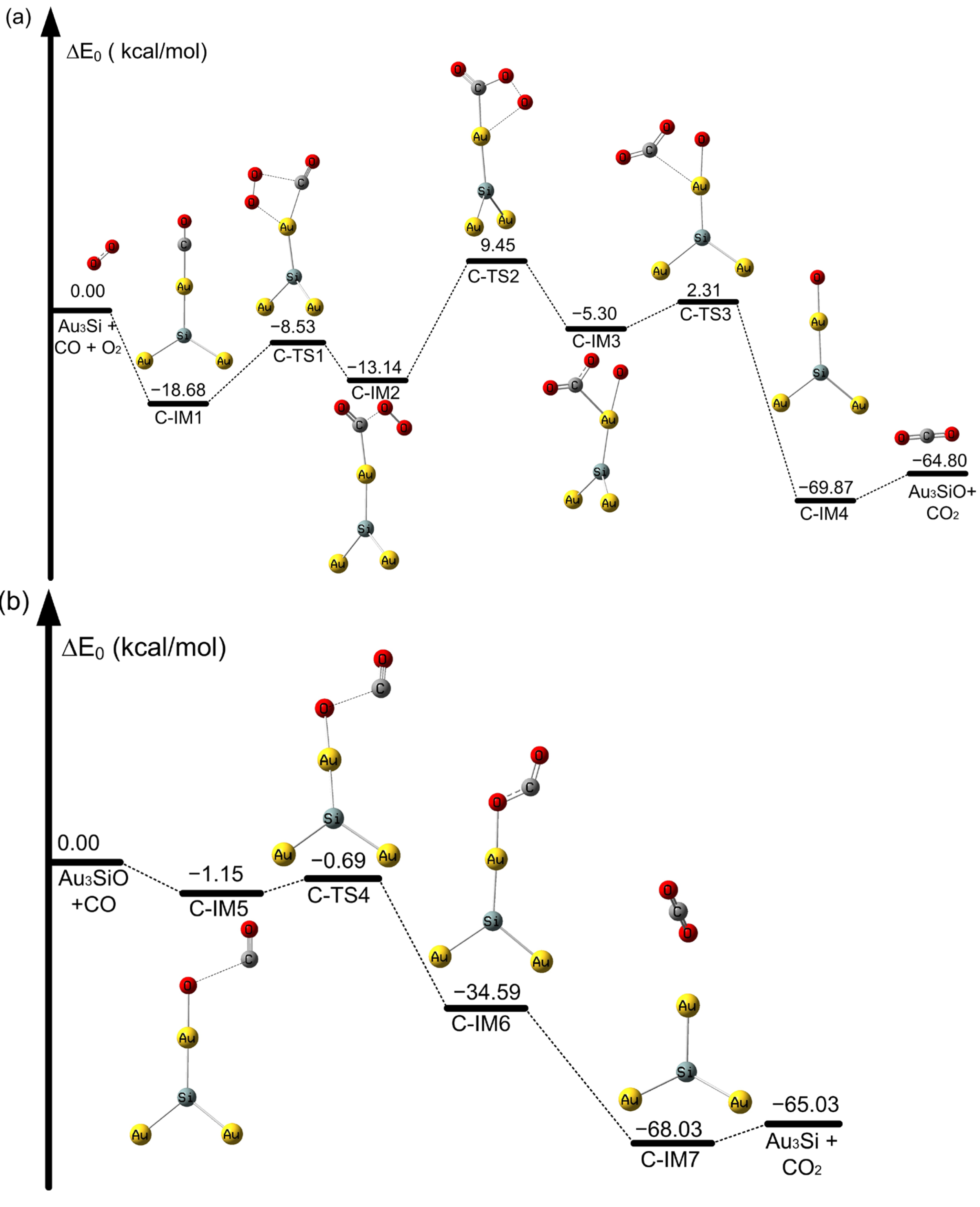
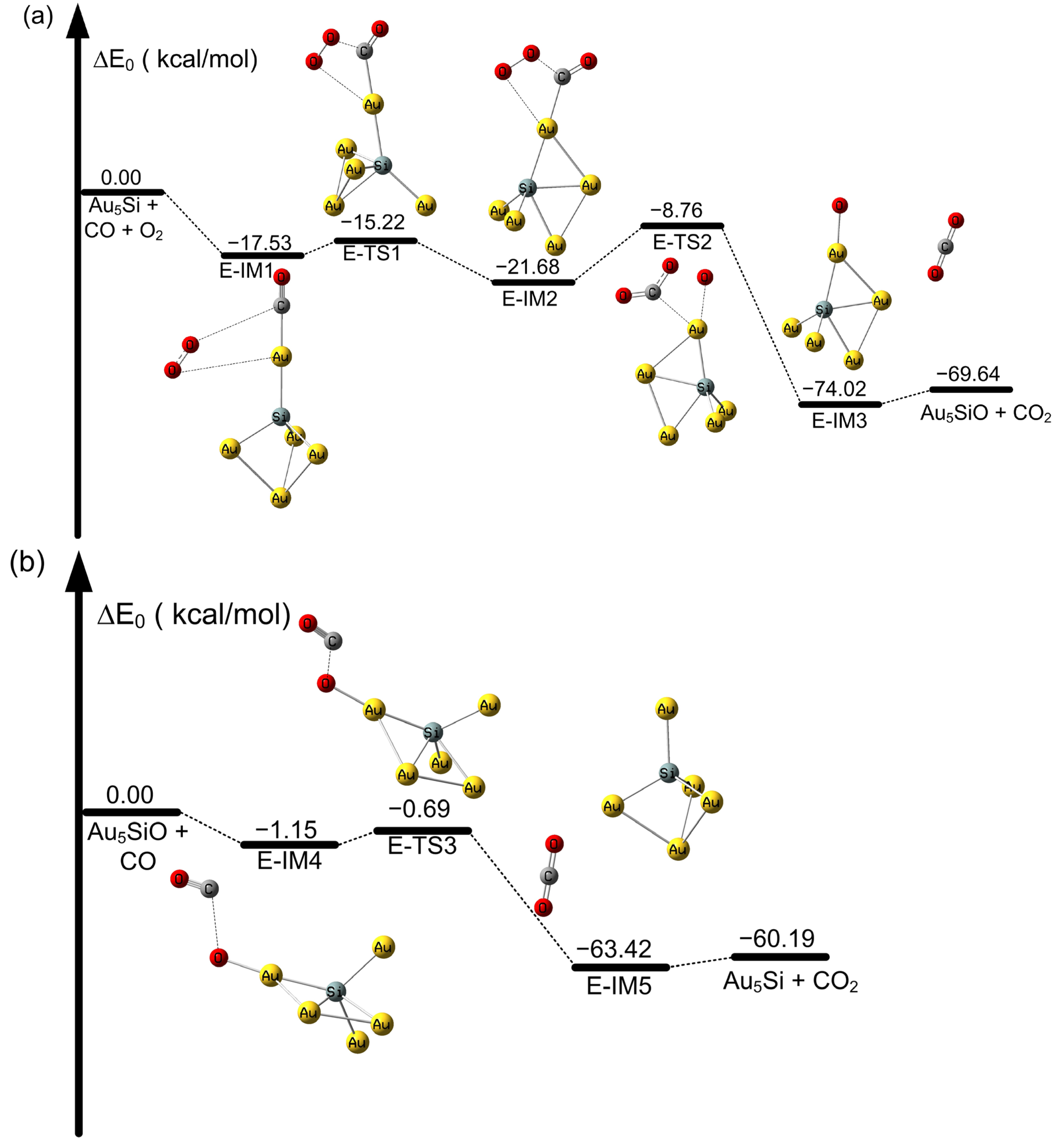
2.2. The Oxidation of CO on SiAun (n = 1–5) Cluster
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Zhang, Z.; Du, P.; Ning, X.; Wang, Y.; Zhang, D.; Liu, J.; Zhang, S.; Lu, X. Embedding Ultrasmall Au Clusters into the Pores of a Covalent Organic Framework for Enhanced Photostability and Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 6082–6089. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Y. Evolution of catalytic activity driven by structural fusion of icosahedral gold cluster cores. Chin. J. Catal. 2021, 42, 245–250. [Google Scholar] [CrossRef]
- Niu, W.; Du, Z.; Zhang, C.; Xu, D.; Li, J.; Sun, M.; Wu, L.; Yao, H.; Zhao, L.; Gao, X. Broken electron transfer pathway in enzyme: Gold clusters inhibiting TrxR1/Trx via cell studies and theory simulations. Chin. Chem. Lett. 2022, 33, 3488–3491. [Google Scholar] [CrossRef]
- Pembere, A.M.S.; Cui, C.; Wu, H.; Luo, Z. Small gold clusters catalyzing oxidant-free dehydrogenation of glycerol initiated by methene hydrogen atom transfer. Chin. Chem. Lett. 2019, 30, 1000–1004. [Google Scholar] [CrossRef]
- King, A.K.; Brar, A.; Findlater, M. A tertiary phosphine oxide ligand-based recyclable system for the Suzuki–Miyaura and Negishi reactions: Evidence for pseudo-homogeneous catalysis. Catal. Sci. Technol. 2023, 13, 301–304. [Google Scholar] [CrossRef]
- Witzel, S.; Hashmi, A.S.K.; Xie, J. Light in Gold Catalysis. Chem. Rev. 2021, 121, 8868–8925. [Google Scholar] [CrossRef]
- Hendrich, C.M.; Sekine, K.; Koshikawa, T.; Tanaka, K.; Hashmi, A.S.K. Homogeneous and Heterogeneous Gold Catalysis for Materials Science. Chem. Rev. 2021, 121, 9113–9163. [Google Scholar] [CrossRef]
- Ye, L.-W.; Zhu, X.-Q.; Sahani, R.L.; Xu, Y.; Qian, P.-C.; Liu, R.-S. Nitrene Transfer and Carbene Transfer in Gold Catalysis. Chem. Rev. 2021, 121, 9039–9112. [Google Scholar] [CrossRef]
- Rezvani, A.; Abdel-Mageed, A.M.; Ishida, T.; Murayama, T.; Parlinska-Wojtan, M.; Behm, R.J. CO2 Reduction to Methanol on Au/CeO2 Catalysts: Mechanistic Insights from Activation/Deactivation and SSITKA Measurements. ACS Catal. 2020, 10, 3580–3594. [Google Scholar] [CrossRef]
- Fricke, C.; Dahiya, A.; Reid, W.B.; Schoenebeck, F. Gold-Catalyzed C–H Functionalization with Aryl Germanes. ACS Catal. 2019, 9, 9231–9236. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, P.; Zhang, Z.; Zhang, L.; Yang, G.; Han, B. Efficient Hydrogenation of CO2 to Methanol over Supported Subnanometer Gold Catalysts at Low Temperature. ChemCatChem 2017, 9, 3691–3696. [Google Scholar] [CrossRef]
- Wang, J.; Tan, H.; Yu, S.; Zhou, K. Morphological Effects of Gold Clusters on the Reactivity of Ceria Surface Oxygen. ACS Catal. 2015, 5, 2873–2881. [Google Scholar] [CrossRef]
- Chen, S.; Luo, L.; Jiang, Z.; Huang, W. Size-Dependent Reaction Pathways of Low-Temperature CO Oxidation on Au/CeO2 Catalysts. ACS Catal. 2015, 5, 1653–1662. [Google Scholar] [CrossRef]
- Wu, X.; Senapati, L.; Nayak, S.K.; Selloni, A.; Hajaligol, M. A density functional study of carbon monoxide adsorption on small cationic, neutral, and anionic gold clusters. J. Chem. Phys. 2002, 117, 4010–4015. [Google Scholar] [CrossRef]
- Fang, H.-C.; Li, Z.H.; Fan, K.-N. CO oxidation catalyzed by a single gold atom: Benchmark calculations and the performance of DFT methods. Phys. Chem. Chem. Phys. 2011, 13, 13358–13369. [Google Scholar] [CrossRef]
- Heiz, U.; Sanchez, A.; Abbet, S.; Schneider, W.D. The reactivity of gold and platinum metals in their cluster phase. Eur. Phys. J. D 1999, 9, 35–39. [Google Scholar] [CrossRef]
- Bond, G.C. Gold: A relatively new catalyst. Catal. Today 2002, 72, 5–9. [Google Scholar] [CrossRef]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef]
- Pyykkö, P. Theoretical Chemistry of Gold. Angew. Chem. Int. Ed. 2004, 43, 4412–4456. [Google Scholar] [CrossRef]
- Chen, S.; Abdel-Mageed, A.M.; Hauble, A.; Ishida, T.; Murayama, T.; Parlinska-Wojtan, M.; Behm, R.J. Performance of Au/ZnO catalysts in CO2 reduction to methanol: Varying the Au loading / Au particle size. Appl. Catal. A 2021, 624, 118318. [Google Scholar] [CrossRef]
- Cai, R.; Ellis, P.R.; Yin, J.; Liu, J.; Brown, C.M.; Griffin, R.; Chang, G.; Yang, D.; Ren, J.; Cooke, K.; et al. Performance of Preformed Au/Cu Nanoclusters Deposited on MgO Powders in the Catalytic Reduction of 4-Nitrophenol in Solution. Small 2018, 14, 1703734. [Google Scholar] [CrossRef]
- Niihori, Y.; Hashimoto, S.; Koyama, Y.; Hossain, S.; Kurashige, W.; Negishi, Y. Dynamic Behavior of Thiolate-Protected Gold–Silver 38-Atom Alloy Clusters in Solution. J. Phys. Chem. C 2019, 123, 13324–13329. [Google Scholar] [CrossRef]
- Chen, B.; Guo, H.; Liu, C.; Shang, L.; Ye, X.; Chen, L.; Feng, C.; Hayashi, K. Molecularly imprinted sol-gel/Au@Ag core-shell nano-urchin localized surface plasmon resonance sensor designed in reflection mode for detection of organic acid vapors. Biosens. Bioelectron. 2020, 169, 112639. [Google Scholar] [CrossRef]
- Lu, Z.; Piernavieja-Hermida, M.; Turner, C.H.; Wu, Z.; Lei, Y. Effects of TiO2 in Low Temperature Propylene Epoxidation Using Gold Catalysts. J. Phys. Chem. C 2018, 122, 1688–1698. [Google Scholar] [CrossRef]
- de Boed, E.J.J.; de Rijk, J.W.; de Jongh, P.E.; Donoeva, B. Steering the Selectivity in Gold–Titanium-Catalyzed Propene Oxidation by Controlling the Surface Acidity. J. Phys. Chem. C 2021, 125, 16557–16568. [Google Scholar] [CrossRef]
- Kapil, N.; Weissenberger, T.; Cardinale, F.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Precisely Engineered Supported Gold Clusters as a Stable Catalyst for Propylene Epoxidation. Angew. Chem. Int. Ed. 2021, 60, 18185–18193. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, G.; Mao, J.; Wang, Y.; Yang, H.; Guo, X. The Effect of Gold Nanoparticles on the Catalytic Activity of NiTiO3 for Hydrodeoxygenation of Guaiacol. Catalysts 2021, 11, 994. [Google Scholar] [CrossRef]
- Neal, R.D.; Inoue, Y.; Hughes, R.A.; Neretina, S. Catalytic Reduction of 4-Nitrophenol by Gold Catalysts: The Influence of Borohydride Concentration on the Induction Time. J. Phys. Chem. C 2019, 123, 12894–12901. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, Y. Green Synthesis and Catalytic Activity of Gold Nanoparticles/Graphene Oxide Nanocomposites Prepared By Tannic Acid. J. Nanosci. Nanotechnol. 2018, 18, 2536–2546. [Google Scholar] [CrossRef]
- Tathe, A.G.; Urvashi; Yadav, A.K.; Chintawar, C.C.; Patil, N.T. Gold-Catalyzed 1,2-Aminoarylation of Alkenes with External Amines. ACS Catal. 2021, 11, 4576–4582. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Toste, F.D. Modern Gold Catalyzed Synthesis; John Wiley & Sons: Weinheim, Germany, 2012. [Google Scholar]
- Chintawar, C.C.; Yadav, A.K.; Patil, N.T. Gold-Catalyzed 1,2-Diarylation of Alkenes. Angew. Chem. Int. Ed. 2020, 59, 11808–11813. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Niu, L.; Zong, X.; An, L.; Qu, D.; Wang, X.; Sun, Z. Ambient photothermal catalytic CO oxidation over a carbon-supported palladium catalyst. Appl. Catal. B 2022, 313, 121439. [Google Scholar] [CrossRef]
- Chizallet, C. Achievements and Expectations in the Field of Computational Heterogeneous Catalysis in an Innovation Context. Top. Catal. 2022, 65, 69–81. [Google Scholar] [CrossRef]
- Camposeco, R.; Torres, A.E.; Zanella, R. Influence of the Preparation Method of Au, Pd, Pt, and Rh/TiO2 Nanostructures and Their Catalytic Activity on the CO Oxidation at Low Temperature. Top. Catal. 2022, 65, 798–816. [Google Scholar] [CrossRef]
- Samadi, P.; Binczarski, M.J.; Maniukiewicz, W.; Pawlaczyk, A.; Rogowski, J.; Szubiakiewicz, E.; Szynkowska-Jozwik, M.I.; Witonska, I.A. Zn Modification of Pd/TiO2/Ti Catalyst for CO Oxidation. Materials 2023, 16, 1216. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhou, J.; Li, D.; Ao, Z. Humidity tuning CO oxidation on Ti decorated V2CO2 monolayer (MXene) catalyst: A density functional calculation study. Appl. Surf. Sci. 2023, 616, 156497. [Google Scholar] [CrossRef]
- Chen, W.; Xu, J.; Huang, F.; Zhao, C.; Guan, Y.; Fang, Y.; Hu, J.; Yang, W.; Luo, Z.; Guo, Y. CO Oxidation over CuOx/TiO2 Catalyst: The Importance of Oxygen Vacancies and Cu+ Species. Appl. Surf. Sci. 2023, 618, 156539. [Google Scholar] [CrossRef]
- Yoshida, T.; Murayama, T.; Sakaguchi, N.; Okumura, M.; Ishida, T.; Haruta, M. Carbon Monoxide Oxidation by Polyoxometalate-Supported Gold Nanoparticulate Catalysts: Activity, Stability, and Temperature- Dependent Activation Properties. Angew. Chem. Int. Ed. 2018, 57, 1523–1527. [Google Scholar] [CrossRef]
- Shao, B.; Zhao, W.; Miao, S.; Huang, J.; Wang, L.; Li, G.; Shen, W. Facet-dependent anchoring of gold nanoparticles on TiO2 for CO oxidation. Chin. J. Catal. 2019, 40, 1534–1539. [Google Scholar] [CrossRef]
- Soni, Y.; Erumpukuthical Ashok Kumar, A.; Nayak, C.; Deepak, F.L.; Vinod, C.P. A Convenient Route for Au@Ti–SiO2 Nanocatalyst Synthesis and Its Application for Room Temperature CO Oxidation. J. Phys. Chem. C 2017, 121, 4946–4957. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-Temperature Oxidation of CO over Gold Supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Buratto, S.K.; Bowers, M.T.; Metiu, H.; Tong, X.; Benz, L.; Kemper, P.; Chretien, S. Aun and Agn (n = 1−8) Nanocluster Catlysts: Gas-Phase Reativity to Deposited Structures. Chem. Phys. Solid Surf. 2007, 12, 151–199. [Google Scholar]
- Li, H.; Li, L.; Pedersen, A.; Gao, Y.; Khetrapal, N.; Jónsson, H.; Zeng, X.C. Magic-Number Gold Nanoclusters with Diameters from 1 to 3.5 nm: Relative Stability and Catalytic Activity for CO Oxidation. Nano Lett. 2015, 15, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Jena, N.K.; Chandrakumar, K.R.S.; Ghosh, S.K. DNA Base–Gold Nanocluster Complex as a Potential Catalyzing Agent: An Attractive Route for CO Oxidation Process. J. Phys. Chem. C 2012, 116, 17063–17069. [Google Scholar] [CrossRef]
- Yan, P.; Shu, S.; Shi, X.; Li, J. Promotion effect of Au single-atom support graphene for CO oxidation. Chin. Chem. Lett. 2022, 33, 4822–4827. [Google Scholar] [CrossRef]
- Centeno, M.A.; Ramírez Reina, T.; Ivanova, S.; Laguna, O.H.; Odriozola, J.A. Au/CeO2 Catalysts: Structure and CO Oxidation Activity. Catalysts 2016, 6, 158. [Google Scholar] [CrossRef]
- Johnson, G.E.; Reilly, N.M.; Tyo, E.C.; Castleman, A.W., Jr. Gas-Phase Reactivity of Gold Oxide Cluster Cations with CO. J. Phys. Chem. C 2008, 112, 9730–9736. [Google Scholar] [CrossRef]
- Bürgel, C.; Reilly, N.M.; Johnson, G.E.; Mitrić, R.; Kimble, M.L.; Castleman, A.W., Jr.; Bonačić-Koutecký, V. Influence of Charge State on the Mechanism of CO Oxidation on Gold Clusters. J. Am. Chem. Soc. 2008, 130, 1694–1698. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, N.; Pei, Y.; Chen, Z.; Zeng, X.C. Catalytic Activities of Subnanometer Gold Clusters (Au16–Au18, Au20, and Au27–Au35) for CO Oxidation. ACS Nano 2011, 5, 7818–7829. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-M.; Pal, R.; Huang, W.; Zeng, X.C.; Wang, L.-S. Tuning the electronic properties of the golden buckyball by endohedral doping: M@Au16− (M = Ag,Zn,In). J. Chem. Phys. 2009, 130, 051101. [Google Scholar] [CrossRef] [PubMed]
- Zorriasatein, S.; Joshi, K.; Kanhere, D.G. Electronic and structural investigations of gold clusters doped with copper: Aun−1Cu− (n = 13–19). J. Chem. Phys. 2008, 128, 184314. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-M.; Bai, J.; Lechtken, A.; Huang, W.; Schooss, D.; Kappes, M.M.; Zeng, X.C.; Wang, L.-S. Magnetic doping of the golden cage cluster M@Au16− (M = Fe,Co,Ni). Phys. Rev. B 2009, 79, 033413. [Google Scholar] [CrossRef]
- García-Cruz, R.; Gonzalez-Torres, J.; Montoya-Moreno, A.; Domínguez-Soria, V.; Luna-García, H.; Poulain, E.; Arellano, J.S.; Olvera-Neria, O. The π back-donation influence in CO oxidation on small and oxidized Au–Ag clusters. Chem. Phys. 2022, 556, 111462. [Google Scholar] [CrossRef]
- Jena, N.K.; Chandrakumar, K.R.S.; Ghosh, S.K. Beyond the Gold–Hydrogen Analogy: Doping Gold Cluster with H-atom–O2 Activation and Reduction of the Reaction Barrier for CO Oxidation. J. Phys. Chem. Lett. 2011, 2, 1476–1480. [Google Scholar] [CrossRef]
- Cao, Y.; Peng, Y.; Cheng, D.; Chen, L.; Wang, M.; Shang, C.; Zheng, L.; Ma, D.; Liu, Z.-P.; He, L. Room-Temperature CO Oxidative Coupling for Oxamide Production over Interfacial Au/ZnO Catalysts. ACS Catal. 2023, 13, 735–743. [Google Scholar] [CrossRef]
- Barrow, R.F.; Gissane, W.J.M.; Travis, D.N. Electronic Spectra of some Gaseous Diatomic Compounds of Gold. Nature 1964, 201, 603–604. [Google Scholar] [CrossRef]
- Pal, R.; Wang, L.-M.; Huang, W.; Wang, L.-S.; Zeng, X.C. Structural Evolution of Doped Gold Clusters: MAux− (M = Si, Ge, Sn; x = 5−8). J. Am. Chem. Soc. 2009, 131, 3396–3404. [Google Scholar] [CrossRef]
- Li, X.; Kiran, B.; Wang, L.-S. Gold as Hydrogen. An Experimental and Theoretical Study of the Structures and Bonding in Disilicon Gold Clusters Si2Aun- and Si2Aun (n = 2 and 4) and Comparisons to Si2H2 and Si2H4. J. Phys. Chem. A 2005, 109, 4366–4374. [Google Scholar] [CrossRef]
- Kiran, B.; Li, X.; Zhai, H.-J.; Cui, L.-F.; Wang, L.-S. [SiAu4]: Aurosilane. Angew. Chem. Int. Ed. 2004, 43, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Li, Y.-C. Au@Sin: Growth behavior, stability and electronic structure. Phys. Lett. A 2010, 374, 2736–2742. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Catalysis with Transition Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape Dependence and Stability. J. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Behafarid, F.; Croy, J.R.; Ono, L.K.; Li, L.; Yang, J.C.; Frenkel, A.I.; Cuenya, B.R. Shape-Dependent Catalytic Properties of Pt Nanoparticles. J. Am. Chem. Soc. 2010, 132, 15714–15719. [Google Scholar] [CrossRef]
- Li, Y.-F.; Mao, A.-J.; Li, Y.; Kuang, X.-Y. Density functional study on size-dependent structures, stabilities, electronic and magnetic properties of AunM (M = Al and Si, n = 1–9) clusters: Comparison with pure gold clusters. J. Mol. Model. 2012, 18, 3061–3072. [Google Scholar] [CrossRef]
- Deka, A.; Deka, R.C. Structural and electronic properties of stable Aun (n = 2–13) clusters: A density functional study. J. Mol. Struct. THEOCHEM 2008, 870, 83–93. [Google Scholar] [CrossRef]
- Liu, J.-X.; Filot, I.A.W.; Su, Y.; Zijlstra, B.; Hensen, E.J.M. Optimum Particle Size for Gold-Catalyzed CO Oxidation. J. Phys. Chem. C 2018, 122, 8327–8340. [Google Scholar] [CrossRef]
- Schwerdtfeger, P.; Lein, M.; Krawczyk, R.P.; Jacob, C.R. The adsorption of CO on charged and neutral Au and Au2: A comparison between wave-function based and density functional theory. J. Chem. Phys. 2008, 128, 124302. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Pan, L.-L.; Dai, B.; Kiran, B.; Li, J.; Wang, L.-S. Chemisorption-induced Structural Changes and Transition from Chemisorption to Physisorption in Au6(CO)n− (n = 4−9). J. Phys. Chem. C 2008, 112, 11920–11928. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Kiran, B.; Dai, B.; Li, J.; Wang, L.-S. Unique CO Chemisorption Properties of Gold Hexamer: Au6(CO)n- (n = 0−3). J. Am. Chem. Soc. 2005, 127, 12098–12106. [Google Scholar] [CrossRef]
- Phala, N.S.; Klatt, G.; Steen, E.v. A DFT study of hydrogen and carbon monoxide chemisorption onto small gold clusters. Chem. Phys. Lett. 2004, 395, 33–37. [Google Scholar] [CrossRef]
- Tielens, F.; Gracia, L.; Polo, V.; Andrés, J. A Theoretical Study on the Electronic Structure of Au−XO(0,−1,+1) (X = C, N, and O) Complexes: Effect of an External Electric Field. J. Phys. Chem. A 2007, 111, 13255–13263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, Q. Reactions of Gold Atoms and Small Clusters with CO: Infrared Spectroscopic and Theoretical Characterization of AunCO (n = 1−5) and Aun(CO)2 (n = 1, 2) in Solid Argon. J. Phys. Chem. A 2005, 109, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Abbet, S.; Heiz, U.; Schneider, W.D.; Häkkinen, H.; Barnett, R.N.; Landman, U. When Gold Is Not Noble: Nanoscale Gold Catalysts. J. Phys. Chem. A 1999, 103, 9573–9578. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, N.; Bulusu, S.; Zeng, X.C. Effective CO Oxidation on Endohedral Gold-Cage Nanoclusters. J. Phys. Chem. C 2008, 112, 8234–8238. [Google Scholar] [CrossRef]
- Manzoor, D.; Krishnamurty, S.; Pal, S. Endohedrally doped gold nanocages: Efficient catalysts for O2 activation and CO oxidation. Phys. Chem. Chem. Phys. 2016, 18, 7068–7074. [Google Scholar] [CrossRef]
- Manzoor, D.; Krishnamurty, S.; Pal, S. Effect of Silicon Doping on the Reactivity and Catalytic Activity of Gold Clusters. J. Phys. Chem. C 2014, 118, 7501–7507. [Google Scholar] [CrossRef]
- Gurtu, S.; Rai, S.; Ehara, M.; Priyakumar, U.D. Ability of density functional theory methods to accurately model the reaction energy pathways of the oxidation of CO on gold cluster: A benchmark study. Theor. Chem. Acc. 2016, 135, 93. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef]
- An, H.; Kwon, S.; Ha, H.; Kim, H.Y.; Lee, H.M. Reactive Structural Motifs of Au Nanoclusters for Oxygen Activation and Subsequent CO Oxidation. J. Phys. Chem. C 2016, 120, 9292–9298. [Google Scholar] [CrossRef]
- Hou, M.; Mei, Q.; Han, B. Solvent effects on geometrical structures and electronic properties of metal Au, Ag, and Cu nanoparticles of different sizes. J. Colloid Interface Sci. 2015, 449, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, D.; Pal, S. Hydrogen Atom Chemisorbed Gold Clusters as Highly Active Catalysts for Oxygen Activation and CO Oxidation. J. Phys. Chem. C 2014, 118, 30057–30062. [Google Scholar] [CrossRef]
- Zhang, J.; Dolg, M. Global optimization of clusters of rigid molecules using the artificial bee colony algorithm. Phys. Chem. Chem. Phys. 2016, 18, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dolg, M. ABCluster: The artificial bee colony algorithm for cluster global optimization. Phys. Chem. Chem. Phys. 2015, 17, 24173–24181. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Figgen, D.; Rauhut, G.; Dolg, M.; Stoll, H. Energy-consistent pseudopotentials for group 11 and 12 atoms: Adjustment to multi-configuration Dirac–Hartree–Fock data. Chem. Phys. 2005, 311, 227–244. [Google Scholar] [CrossRef]
- Peterson, K.A.; Puzzarini, C. Systematically convergent basis sets for transition metals. II. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor. Chem. Acc. 2005, 114, 283–296. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Staroverov, V.N. Chapter 24—Progress in the development of exchange-correlation functionals. In Theory and Applications of Computational Chemistry; Dykstra, C.E., Frenking, G., Kim, K.S., Scuseria, G.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 669–724. [Google Scholar]
- Hratchian, H.P.; Schlegel, H.B. Using Hessian Updating To Increase the Efficiency of a Hessian Based Predictor-Corrector Reaction Path Following Method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a Hessian based predictor–corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Stability analysis for solutions of the closed shell Kohn–Sham equation. J. Chem. Phys. 1996, 104, 9047–9052. [Google Scholar] [CrossRef]
- Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XVIII. Constraints and stability in Hartree–Fock theory. J. Chem. Phys. 1977, 66, 3045–3050. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ren, D. Mechanisms for Catalytic CO Oxidation on SiAun (n = 1–5) Cluster. Molecules 2023, 28, 1917. https://doi.org/10.3390/molecules28041917
Zhang Y, Ren D. Mechanisms for Catalytic CO Oxidation on SiAun (n = 1–5) Cluster. Molecules. 2023; 28(4):1917. https://doi.org/10.3390/molecules28041917
Chicago/Turabian StyleZhang, Yang, and Dasen Ren. 2023. "Mechanisms for Catalytic CO Oxidation on SiAun (n = 1–5) Cluster" Molecules 28, no. 4: 1917. https://doi.org/10.3390/molecules28041917
APA StyleZhang, Y., & Ren, D. (2023). Mechanisms for Catalytic CO Oxidation on SiAun (n = 1–5) Cluster. Molecules, 28(4), 1917. https://doi.org/10.3390/molecules28041917





