Rhombohedral Boron Monosulfide as a p-Type Semiconductor
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure of Synthesized r-BS
2.2. Optical Properties of r-BS
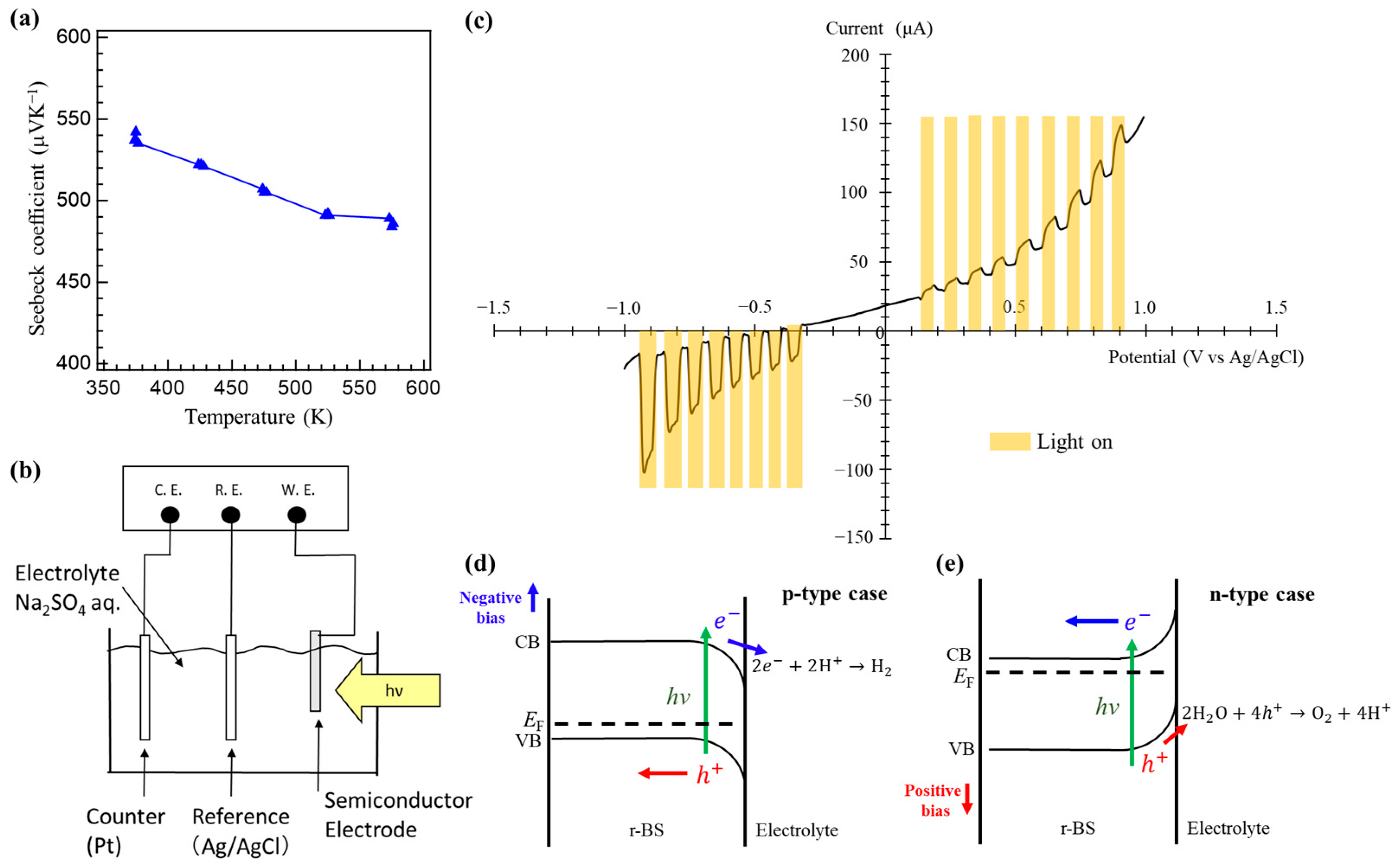
2.3. p-Type Property of Synthesized r-BS
3. Materials and Methods
3.1. Starting Material
3.2. Synthesis of r-BS
3.3. X-ray Diffraction (XRD)
3.4. Fourire Transform Infrared Absorption Spectroscopy (FT-IR)
3.5. Ultraviolet-Visible Adsorption Spectroscopy (UV-vis)
3.6. Seebeck Coefficient Measurement
3.7. Photoelectrochemical Measurements
3.8. Thermogravimetry Analysis (TGA)
3.9. ESR
3.10. DFT Calculation and Simulation of Vibrational Spectrum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Osada, M.; Sasaki, T. Two-dimensional dielectric nanosheets: Novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 2012, 24, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Matsuda, I. Chemically modified borophene. In 2D Boron: Boraphene, Borophene, Boronene; Matsuda, I., Wu, K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 89–119. [Google Scholar] [CrossRef]
- Kondo, T. Recent progress in boron nanomaterials. Sci. Technol. Adv. Mater. 2017, 18, 780–804. [Google Scholar] [CrossRef]
- Zhang, Z.; Penev, E.S.; Yakobson, B.I.; Boron, T.-D. Two-dimensional boron: Structures, properties and applications. Chem. Soc. Rev. 2017, 46, 6746–6763. [Google Scholar] [CrossRef]
- Penev, E.S.; Bhowmick, S.; Sadrzadeh, A.; Yakobson, B.I. Polymorphism of two-dimensional boron. Nano Lett. 2012, 12, 2441–2445. [Google Scholar] [CrossRef]
- Wu, X.; Dai, J.; Zhao, Y.; Zhuo, Z.; Yang, J.; Zeng, X.C.; Boron, T.-D. Two-dimensional boron Monolayer sheets. ACS Nano 2012, 6, 7443–7453. [Google Scholar] [CrossRef]
- Boustani, I. New quasi-planar surfaces of bare boron. Surf. Sci. 1997, 370, 355–363. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, F.; Bell, J.; Bilic, A.; Du, A.; Boron, T.-D. Two-Dimensional Boron Hydride sheets: High stability, massless dirac fermions, and excellent mechanical properties. Angew. Chem. 2016, 128, 10448–10451. [Google Scholar] [CrossRef]
- Fan, D.; Lu, S.; Chen, C.; Jiang, M.; Li, X.; Hu, X. Versatile two-dimensional boron monosulfide polymorphs with tunable bandgaps and superconducting properties. Appl. Phys. Lett. 2020, 117, 013103. [Google Scholar] [CrossRef]
- Arnold, F.M.; Seifert, G.; Kunstmann, J. Thermodynamic stability of borophene, B2O3 and other B1−xOx sheets thermodynamic stability of borophene, B2O3 and other B1−xOx sheets. J. Phys. Commun. 2020, 4, 031001. [Google Scholar] [CrossRef]
- Şahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R.T.; Ciraci, S. Monolayer honeycomb structures of Group-IV elements and III-V binary compounds: First-principles calculations. Phys. Rev. B 2009, 80, 155453. [Google Scholar] [CrossRef]
- Oganov, A.R.; Solozhenko, V.L. Boron: A hunt for superhard polymorphs. J. Superhard Mater. 2009, 31, 285–291. [Google Scholar] [CrossRef]
- Sasaki, T.; Takizawa, H.; Uheda, K.; Endo, T. High pressure synthesis of binary B-S compounds. Phys. Status Solidi (B) 2001, 223, 29–33. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, D.; Sonvane, Y.; Ahuja, R.; Boron Monochalcogenide, T.-D. Monolayer for thermoelectric material. Sustain. Energy Fuels. 2020, 4, 2363–2369. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, D.; Sonvane, Y.; Ahuja, R. Metal-functionalized 2D boron sulfide monolayer material enhancing hydrogen storage capacities. J. Appl. Phys. 2020, 127, 184305. [Google Scholar] [CrossRef]
- Kusaka, H.; Ishibiki, R.; Toyoda, M.; Fujita, T.; Tokunaga, T.; Yamamoto, A.; Miyakawa, M.; Matsushita, K.; Miyazaki, K.; Li, L.; et al. Crystalline boron monosulfide nanosheets with tunable bandgaps. J. Mater. Chem. A 2021, 9, 24631–24640. [Google Scholar] [CrossRef]
- Mori, T. Thermoelectric and magnetic properties of rare earth borides: Boron cluster and layered compounds. J. Solid State Chem. 2019, 275, 70–82. [Google Scholar] [CrossRef]
- Lu, S.; Shen, P.; Zhang, H.; Liu, G.; Guo, B.; Cai, Y.; Chen, H.; Xu, F.; Zheng, T.; Xu, F.; et al. Towards n-type conductivity in hexagonal boron nitride. Nat. Commun. 2022, 13, 3109. [Google Scholar] [CrossRef]
- Cherednichenko, K.A.; Kruglov, I.A.; Oganov, A.R.; Le Godec, Y.; Mezouar, M.; Solozhenko, V.L. Boron monosulfide: Equation of state and pressure-induced phase transition. J. Appl. Phys. 2018, 123, 135903. [Google Scholar] [CrossRef]
- Cherednichenko, K.A.; Sokolov, P.S.; Kalinko, A.; Le Godec, Y.; Polian, A.; Itié, J.P.; Solozhenko, V.L. Optical phonon modes in rhombohedral boron monosulfide under high pressure. J. Appl. Phys. 2015, 117, 185904. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Yang, M.; Yu, J.; Li, W.; Li, X.; Feng, S. Experimental realization and computational investigations of B2S2as a new 2D material with potential applications. ACS Appl. Mater. Interfaces. 2022, 14, 32330–32340. [Google Scholar] [CrossRef] [PubMed]
- Fano, U. Effects of configuration interaction on intensities and phase shifts. Phys. Rev. 1961, 124, 1866–1878. [Google Scholar] [CrossRef]
- Kuzmenko, A.B.; Benfatto, L.; Cappelluti, E.; Crassee, I.; van der Marel, D.; Blake, P.; Novoselov, K.S.; Geim, A.K. Gate tunable infrared phonon anomalies in bilayer graphene. Phys. Rev. Lett. 2009, 103, 116804. [Google Scholar] [CrossRef]
- Cappelluti, E.; Benfatto, L.; Kuzmenko, A.B. Phonon switching and combined Fano-Rice effect in optical spectra of bilayer graphene. Phys. Rev. B 2010, 82, 041402(R). [Google Scholar] [CrossRef]
- Cappelluti, E.; Benfatto, L.; Manzardo, M.; Kuzmenko, A.B. Charged-phonon theory and Fano effect in the optical spectroscopy of bilayer graphene. Phys. Rev. B 2012, 86, 115439. [Google Scholar] [CrossRef]
- Humlíček, J. Ellipsometric study of Fano resonance in heavily doped p-type Si and SiGe alloys. Thin Solid Films 1998, 313–314, 656–660. [Google Scholar] [CrossRef]
- Simonian, A.W.; Sproul, A.B.; Shi, Z.; Gauja, E. Observation of Fano resonance in heavily doped p-type silicon at room temperature. Phys. Rev. B 1995, 52, 5672–5674. [Google Scholar] [CrossRef]
- Perdew, J.P. Density functional theory and the band gap problem. Int. J. Quant. Chem. 1985, 28, 497–523. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yang, W.; Burke, K.; Yang, Z.; Gross, E.K.; Scheffler, M.; Scuseria, G.E.; Henderson, T.M.; Zhang, I.Y.; Ruzsinszky, A.; et al. Understanding band gaps of solids in generalized Kohn–Sham theory. Proc. Natl. Acad. Sci. USA 2017, 114, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Matsuzaki, K.; Yamaguchi, A.; Miyauchi, M. Visible-Light-Active Photoelectrochemical Z-Scheme System Based on Top 5 Clarke-Number Elements. ACS Appl. Energy Mater. 2018, 1, 5954. [Google Scholar] [CrossRef]
- Nukui, Y.; Srinivasan, N.; Shoji, S.; Atarashi, D.; Sakai, E.; Miyauchi, M. Vertically aligned hexagonal WO3 nanotree electrode for photoelectrochemical water oxidation. Chem. Phys. Lett. 2015, 635, 306–311. [Google Scholar] [CrossRef]
- Yamaoka, S.; Akaishi, M.; Kanda, H.; Osawa, T.; Taniguchi, T.; Sei, H.; Fukunaga, O. Development of belt type high pressure apparatus for material synthesis at 8 GPa. J. High Press. Inst. Jpn. 1992, 30, 249–258. [Google Scholar]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Hartwigsen, C.; Goedecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641–3662. [Google Scholar] [CrossRef]
- Rappe, A.M.; Rabe, K.M.; Kaxiras, E.; Joannopoulos, J.D. Optimized pseudopotentials. Phys. Rev. B 1990, 41, 1227. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, N.; Miyazaki, K.; Toyoda, M.; Takeyasu, K.; Tsujii, N.; Kusaka, H.; Yamamoto, A.; Saito, S.; Miyakawa, M.; Taniguchi, T.; et al. Rhombohedral Boron Monosulfide as a p-Type Semiconductor. Molecules 2023, 28, 1896. https://doi.org/10.3390/molecules28041896
Watanabe N, Miyazaki K, Toyoda M, Takeyasu K, Tsujii N, Kusaka H, Yamamoto A, Saito S, Miyakawa M, Taniguchi T, et al. Rhombohedral Boron Monosulfide as a p-Type Semiconductor. Molecules. 2023; 28(4):1896. https://doi.org/10.3390/molecules28041896
Chicago/Turabian StyleWatanabe, Norinobu, Keisuke Miyazaki, Masayuki Toyoda, Kotaro Takeyasu, Naohito Tsujii, Haruki Kusaka, Akiyasu Yamamoto, Susumu Saito, Masashi Miyakawa, Takashi Taniguchi, and et al. 2023. "Rhombohedral Boron Monosulfide as a p-Type Semiconductor" Molecules 28, no. 4: 1896. https://doi.org/10.3390/molecules28041896
APA StyleWatanabe, N., Miyazaki, K., Toyoda, M., Takeyasu, K., Tsujii, N., Kusaka, H., Yamamoto, A., Saito, S., Miyakawa, M., Taniguchi, T., Aizawa, T., Mori, T., Miyauchi, M., & Kondo, T. (2023). Rhombohedral Boron Monosulfide as a p-Type Semiconductor. Molecules, 28(4), 1896. https://doi.org/10.3390/molecules28041896







