A Stable PDLC Film with High Ageing Resistance from an Optimized System Containing Rigid Monomer
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Different LC Contents on Polymer Micromorphology and Mechanical Properties of PDLC Films
2.2. Effect of Different LCs on the Microscopic Morphology of Polymers and the Electro-Optical Properties of PDLC Films
2.3. Effect of Chain Length of Crosslinker on Polymer Micromorphology and Electro-Optical Properties of PDLC Films
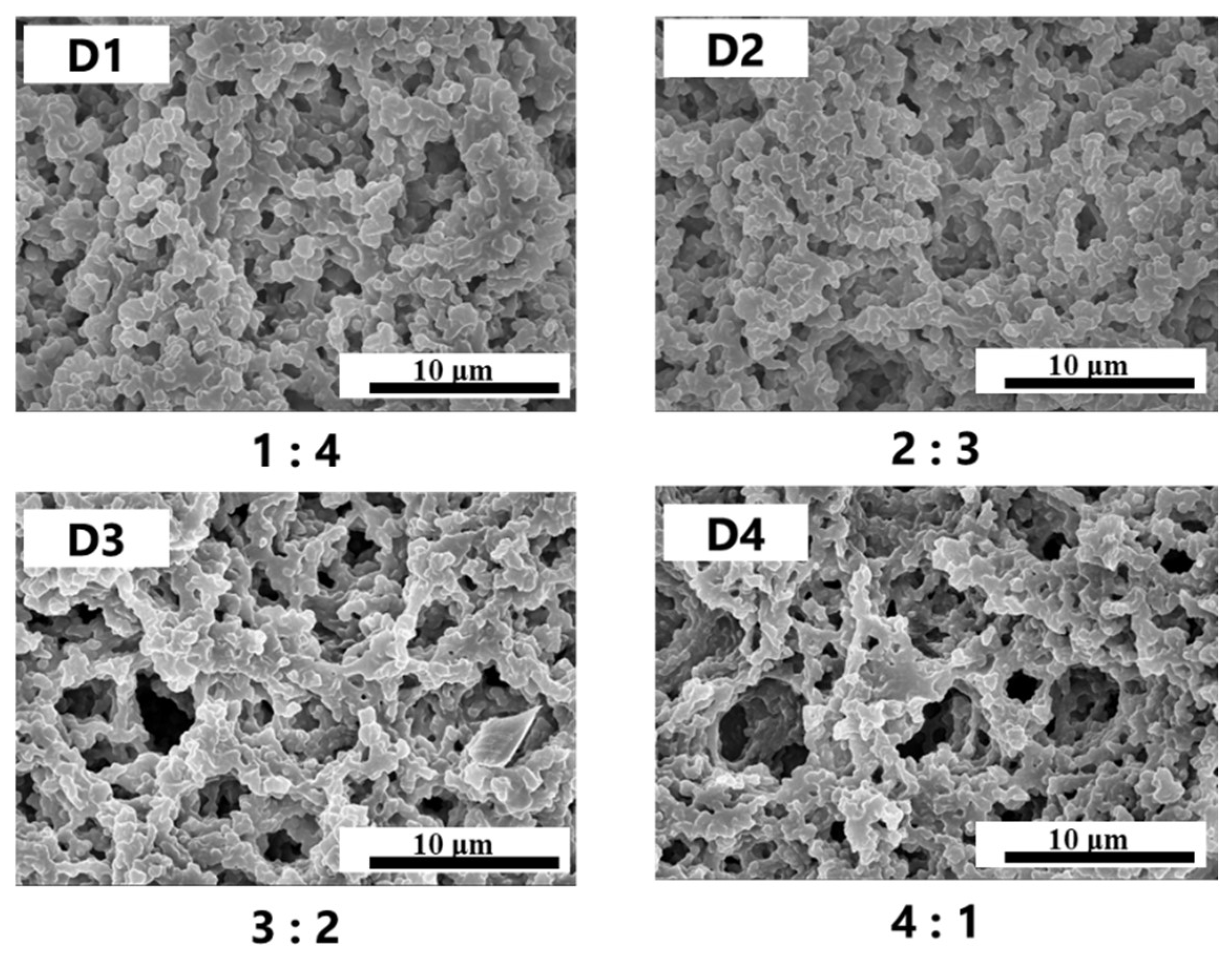
2.4. Effect of Rigid Polymerizable Monomer Content on the Stability of PDLC Films
2.5. Electro-Optical Performance Test of PDLC Films under Different Temperature Conditions
3. Experimental
3.1. Materials
3.2. Preparation of the PDLC Films
3.3. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutierrez-Cuevas, K.G.; Wang, L.; Zheng, Z.-G.; Bisoyi, H.K.; Li, G.; Tan, L.-S.; Vaia, R.A.; Li, Q. Frequency-Driven Self-Organized Helical Superstructures Loaded with Mesogen-Grafted Silica Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 13090–13094. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bisoyi, H.K.; Zheng, Z.; Gutierrez-Cuevas, K.G.; Singh, G.; Kumar, S.; Bunning, T.J.; Li, Q. Stimuli-directed self-organized chiral superstructures for adaptive windows enabled by mesogen-functionalized graphene. Mater. Today 2017, 20, 230–237. [Google Scholar] [CrossRef]
- Li, C.; Chen, M.; Shen, W.; Chen, G.; Zhang, L.; Yang, H. A study on the polymer structures and electro-optical properties of epoxy-mercaptan-based polymer dispersed liquid crystal films. Liq. Cryst. 2019, 46, 1718–1726. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Chen, M.; Guo, S.; Zhang, L.; Li, F.; Guo, S.; Yang, H. A roll-to-roll process for multi-responsive soft-matter composite films containing CsxWO3 nanorods for energy-efficient smart window applications. Nanoscale Horiz. 2017, 2, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Guo, S.; Chen, M.; Li, C.; Wang, Q.; Zou, C.; Zhang, C.; Zhang, L.; Guo, S.; Yang, H. A temperature and electric field-responsive flexible smart film with full broadband optical modulation. Mater. Horiz. 2017, 4, 878–884. [Google Scholar] [CrossRef]
- Ge, D.; Lee, E.; Yang, L.; Cho, Y.; Li, M.; Gianola, D.S.; Yang, S. A Robust Smart Window: Reversibly Switching from High Transparency to Angle-Independent Structural Color Display. Adv. Mater. 2015, 27, 2489–2495. [Google Scholar] [CrossRef]
- Khandelwal, H.; Schenning, A.P.H.J.; Debije, M.G. Infrared Regulating Smart Window Based on Organic Materials. Adv. Energy Mater. 2017, 7, 1602209. [Google Scholar] [CrossRef]
- Park, S.; Hong, J.W. Polymer dispersed liquid crystal film for variable-transparency glazing. Thin Solid Film. 2009, 517, 3183–3186. [Google Scholar] [CrossRef]
- Saeed, M.H.; Zhang, S.; Cao, Y.; Zhou, L.; Hu, J.; Muhammad, I.; Xiao, J.; Zhang, L.; Yang, H. Recent Advances in The Polymer Dispersed Liquid Crystal Composite and Its Applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef]
- Doane, J.W.; Golemme, A.; West, J.L.; Whitehead, J.B.; Wu, B.G. Polymer Dispersed Liquid Crystals for Display Application. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1988, 165, 511–532. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Chen, M.; Jiang, T.; Zhang, L.; Yang, Z.; Yang, H. Research of Liquid-Crystal Materials for a High-Performance FFS-TFT Display. Molecules 2023, 28, 754. [Google Scholar] [CrossRef] [PubMed]
- Drzaic, P.S.; Vaz, N.A.; Montgomery, J.G.P.; Efron, U. Electrical properties of polymer-dispersed liquid crystal films. In Liquid Crystal Materials, Devices, and Applications; Society of Photo Optical: Bellingham, WA, USA, 1992; pp. 64–79. [Google Scholar]
- Shi, Z.; Shao, L.; Zhang, Y.; Guan, Y.; Wang, F.; Deng, F.; Liu, Y.; Wang, Y. Fabrication of polymer-dispersed liquid crystals with low driving voltage based on the thiol-ene click reaction. Polym. Int. 2017, 66, 1094–1098. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Yu, Y.; Zhao, Y.; Guo, Z.; Yao, R.; Gao, J.; Zhang, Y.; Wang, D. Electro-Optical Characteristics of Polymer Dispersed Liquid Crystal Doped with MgO Nanoparticles. Molecules 2022, 27, 7265. [Google Scholar] [CrossRef]
- Zhang, W.; Nan, Y.; Wu, Z.; Shen, Y.; Luo, D. Photothermal-Driven Liquid Crystal Elastomers: Materials, Alignment and Applications. Molecules 2022, 27, 4330. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, H.; Jia, D.; Liu, T. Recent Development of Tunable Optical Devices Based on Liquid. Molecules 2022, 27, 8025. [Google Scholar] [CrossRef]
- Feng, Y.-Q.; Lv, M.-L.; Yang, M.; Ma, W.-X.; Zhang, G.; Yu, Y.-Z.; Wu, Y.-Q.; Li, H.-B.; Liu, D.-Z.; Yang, Y.-S. Application of New Energy Thermochromic Composite Thermosensitive Materials of Smart Windows in Recent Years. Molecules 2022, 27, 1638. [Google Scholar] [CrossRef]
- Busbee, J.D.; Yuhl, A.T.; Natarajan, L.V.; Tongdilia, V.P.; Bunning, T.J.; Vaia, R.A.; Braun, P.V. SiO2Nanoparticle Sequestration via Reactive Functionalization in Holographic Polymer-Dispersed Liquid Crystals. Adv. Mater. 2009, 21, 3659–3662. [Google Scholar] [CrossRef]
- Yaroshchuk, O.V.; Dolgov, L.O.; Kiselev, A.D. Electro-optics and structural peculiarities of liquid crystal–nanoparticle-polymer composites. Phys. Rev. E 2005, 72, 051715. [Google Scholar] [CrossRef]
- Guo, S.-M.; Liang, X.; Zhang, C.-H.; Chen, M.; Shen, C.; Zhang, L.-Y.; Yuan, X.; He, B.-F.; Yang, H. Preparation of a Thermally Light-Transmittance-Controllable Film from a Coexistent System of Polymer-Dispersed and Polymer-Stabilized Liquid Crystals. ACS Appl. Mater. Interfaces 2017, 9, 2942–2947. [Google Scholar] [CrossRef]
- Zhong, T.; Mandle, R.J.; Goodby, J.W.; Zhang, C.; Zhang, L. Thiol-ene reaction based polymer dispersed liquid crystal composite films with low driving voltage and high contrast ratio. Liq. Cryst. 2019, 47, 2171–2183. [Google Scholar] [CrossRef]
- Jayoti, D.; Khushboo; Malik, P.; Singh, A. Effect of polymer concentration on morphology, dielectric and optical properties in a polymer-dispersed ferroelectric liquid crystal. Liq. Cryst. 2016, 43, 623–631. [Google Scholar] [CrossRef]
- Saeed, M.H.; Gao, Y.; Zhou, L.; Zhong, T.; Zhang, S.; Li, C.; Zhang, L.; Yang, H. Effects of multifunctional acrylates and thiols on the morphology and electro-optical properties of polymer-dispersed liquid crystal films. Liq. Cryst. 2021, 48, 1457–1466. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L. Eco-friendly PDLC composites based on chitosan and cholesteryl acetate. J. Mol. Liq. 2021, 321, 114466. [Google Scholar] [CrossRef]
- Montgomery, G.P.; Vaz, N.A. Light-scattering analysis of the temperature-dependent transmittance of a polymer-dispersed liquid-crystal film in its isotropic phase. Phys. Rev. A 1989, 40, 6580–6591. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cao, H.; Mao, Q.; Song, P.; Yang, H. Effects of monomer structure on the morphology of polymer networks and the electro-optical properties of polymer-dispersed liquid crystal films. Liq. Cryst. 2012, 39, 419–424. [Google Scholar] [CrossRef]
- Kockler, K.B.; Haehnel, A.P.; Fleischhaker, F.; Schneider-Baumann, M.; Misske, A.M.; Barner-Kowollik, C. No Apparent Correlation ofkpwith Steric Hindrance for Branched Acrylates. Macromol. Chem. Phys. 2015, 216, 1573–1582. [Google Scholar] [CrossRef]










| Samples | Bis-EMA15 | PEGDA | HPMA | LMA | LC | ||
|---|---|---|---|---|---|---|---|
| Group A | |||||||
| A1 | 2.4 | PEGDA 600 | 3.6 | 14.4 | 9.6 | LC-1717 | 70.0 |
| A2 | 2.8 | 4.2 | 16.8 | 11.2 | 65.0 | ||
| A3 | 3.2 | 4.8 | 19.2 | 12.8 | 60.0 | ||
| A4 | 3.6 | 5.4 | 21.6 | 14.4 | 55.0 | ||
| A5 | 4.0 | 6.0 | 24.0 | 16.0 | 50.0 | ||
| Group B | |||||||
| B1 | 4.0 | PEGDA 600 | 6.0 | 24.0 | 16.0 | LC-1717 | 50.0 |
| B2 | 4.0 | 6.0 | 24.0 | 16.0 | LC-6011 | 50.0 | |
| B3 | 4.0 | 6.0 | 24.0 | 16.0 | LC-6015 | 50.0 | |
| Group C | |||||||
| C1 | 4.0 | PEGDA 200 | 6.0 | 24.0 | 16.0 | LC-6011 | 50.0 |
| C2 | 4.0 | PEGDA 400 | 6.0 | 24.0 | 16.0 | 50.0 | |
| C3 | 4.0 | PEGDA 600 | 6.0 | 24.0 | 16.0 | 50.0 | |
| C4 | 4.0 | PEGDA 700 | 6.0 | 24.0 | 16.0 | 50.0 | |
| C5 | 4.0 | PEGDA1000 | 6.0 | 24.0 | 16.0 | 50.0 | |
| Group D | |||||||
| D1 | 2.0 | PEGDA 700 | 8.0 | 24.0 | 16.0 | LC-6011 | 50.0 |
| D2 | 4.0 | 6.0 | 24.0 | 16.0 | 50.0 | ||
| D3 | 6.0 | 4.0 | 24.0 | 16.0 | 50.0 | ||
| D4 | 8.0 | 2.0 | 24.0 | 16.0 | 50.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, X.; Xu, J.; Hu, W.; Yu, M.; Zhang, L.; Jiang, Y.; Yang, H. A Stable PDLC Film with High Ageing Resistance from an Optimized System Containing Rigid Monomer. Molecules 2023, 28, 1887. https://doi.org/10.3390/molecules28041887
Chen H, Wang X, Xu J, Hu W, Yu M, Zhang L, Jiang Y, Yang H. A Stable PDLC Film with High Ageing Resistance from an Optimized System Containing Rigid Monomer. Molecules. 2023; 28(4):1887. https://doi.org/10.3390/molecules28041887
Chicago/Turabian StyleChen, Hongren, Xiao Wang, Jianjun Xu, Wei Hu, Meina Yu, Lanying Zhang, Yong Jiang, and Huai Yang. 2023. "A Stable PDLC Film with High Ageing Resistance from an Optimized System Containing Rigid Monomer" Molecules 28, no. 4: 1887. https://doi.org/10.3390/molecules28041887
APA StyleChen, H., Wang, X., Xu, J., Hu, W., Yu, M., Zhang, L., Jiang, Y., & Yang, H. (2023). A Stable PDLC Film with High Ageing Resistance from an Optimized System Containing Rigid Monomer. Molecules, 28(4), 1887. https://doi.org/10.3390/molecules28041887







