Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process
Abstract
1. Introduction
2. Results
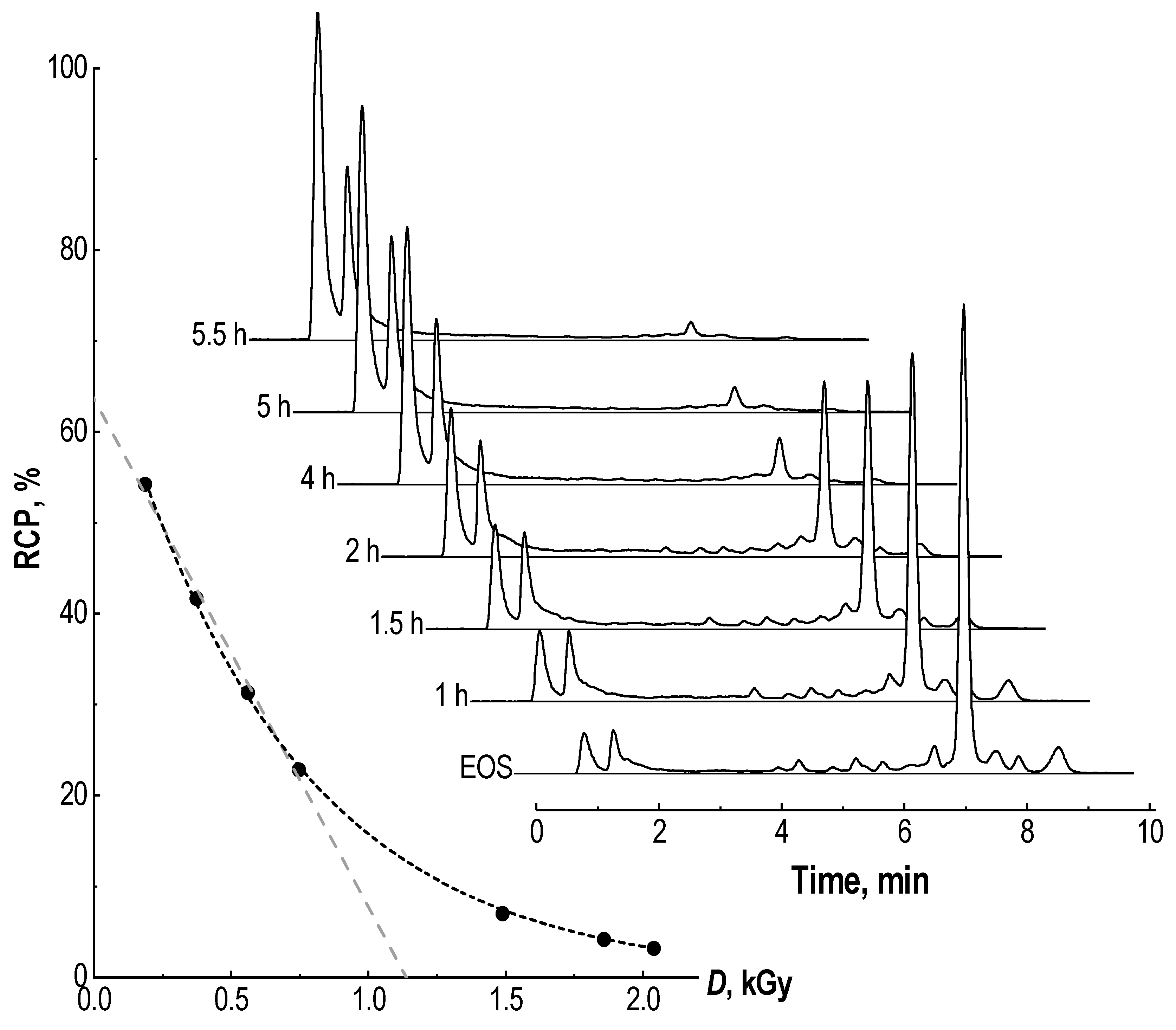
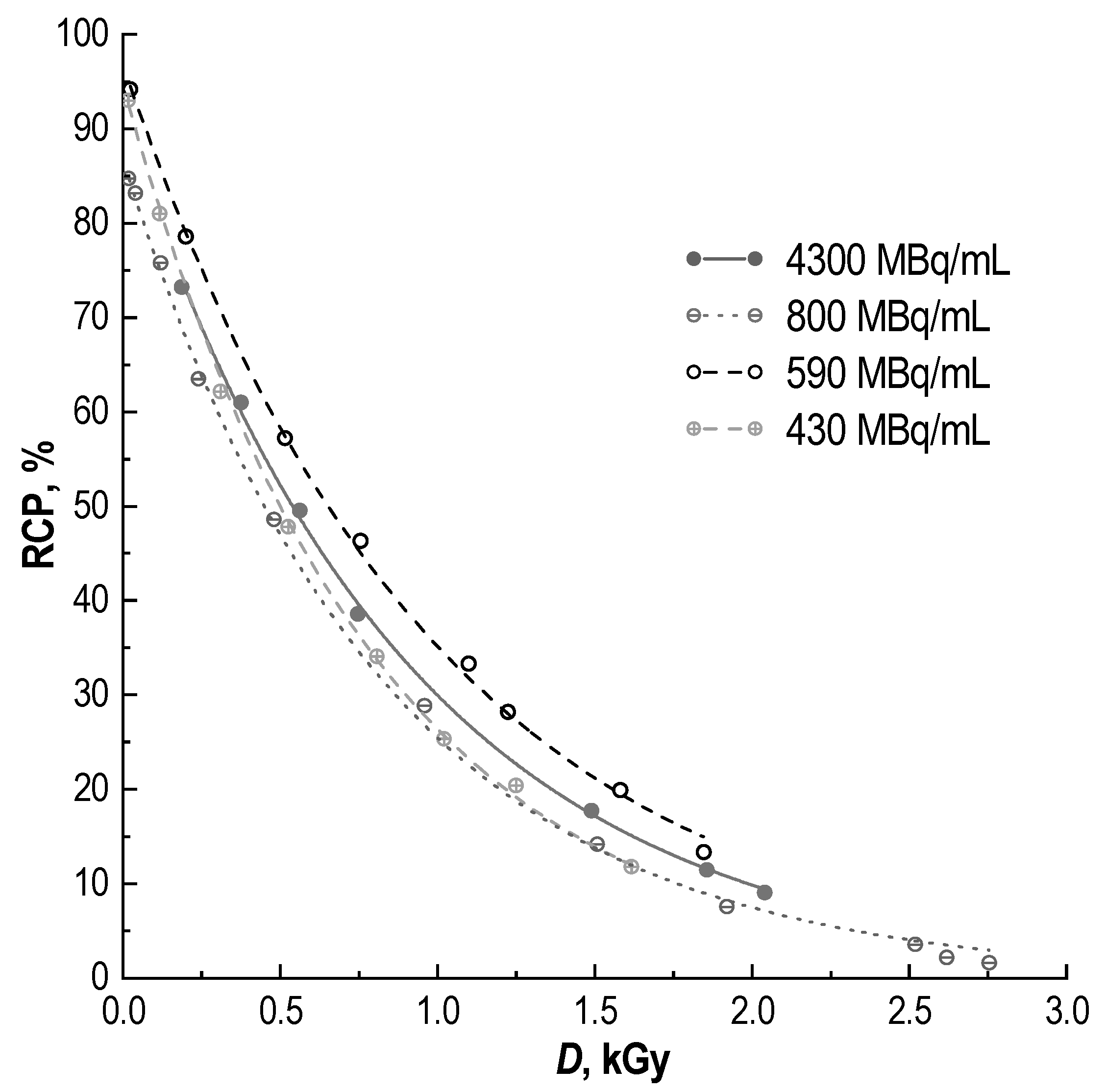
2.1. Radiochemical Purity of [177Lu]Lu-PSMA-617 and Its Radiolysis-Induced Decrease
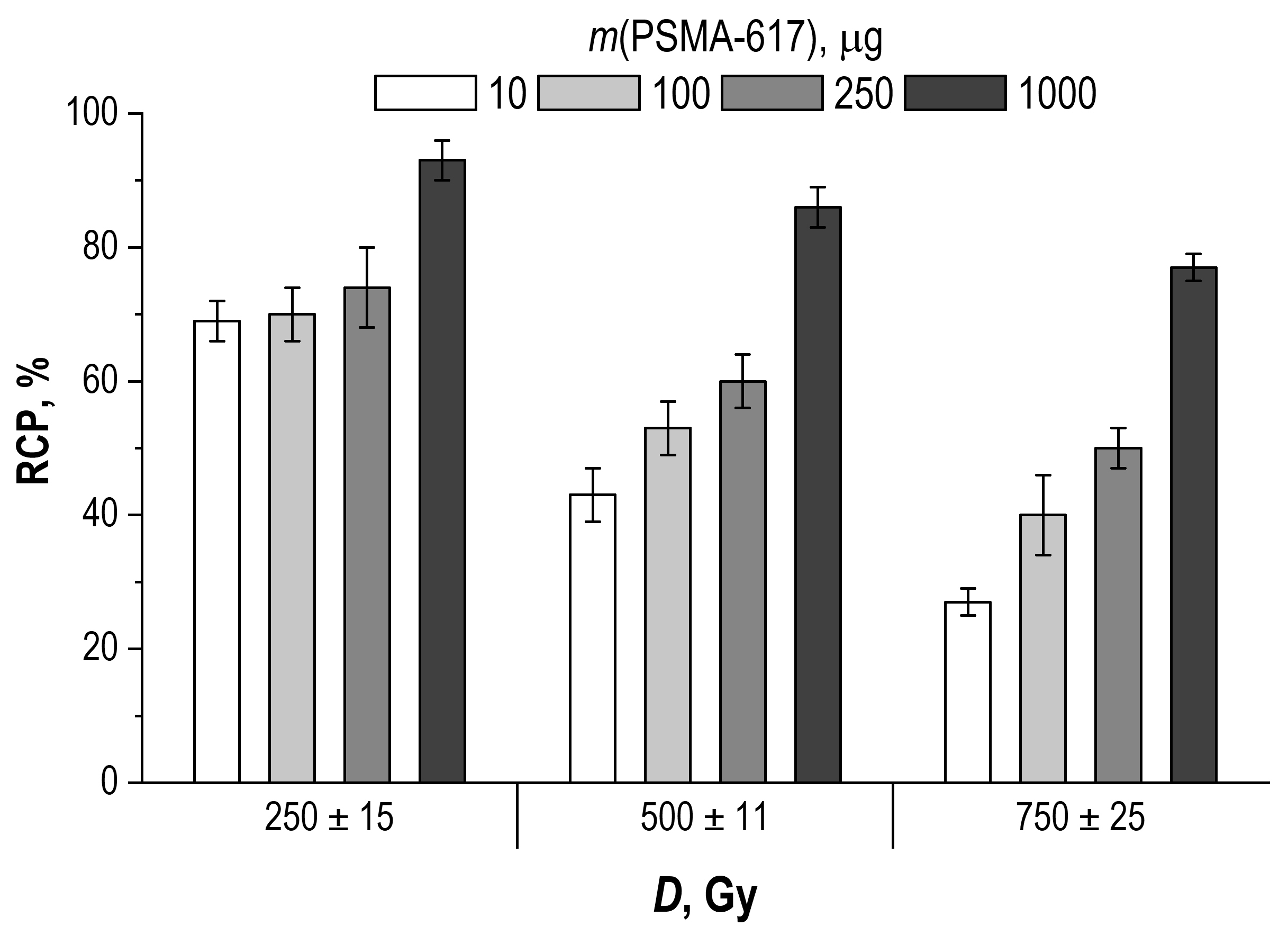
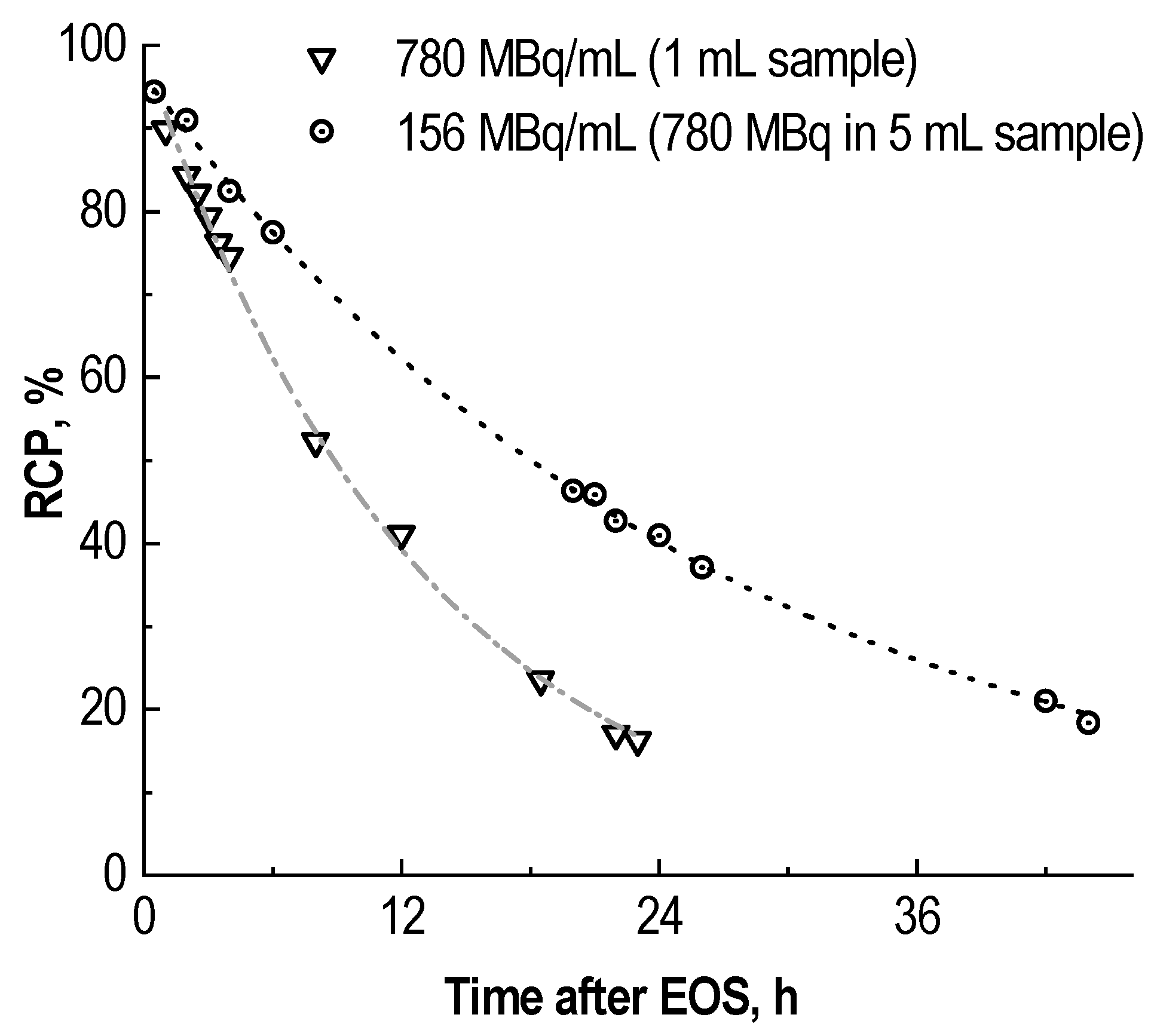
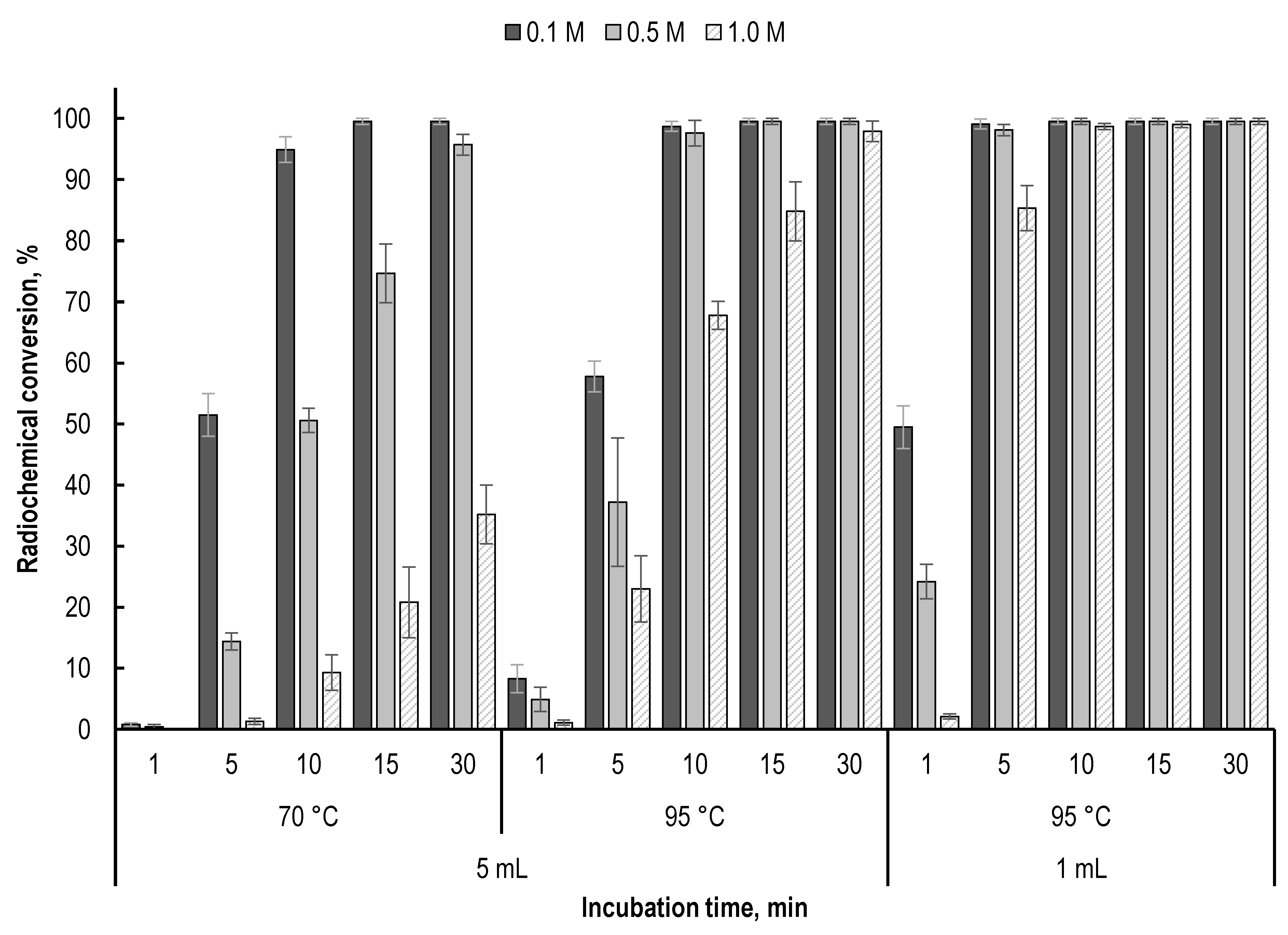
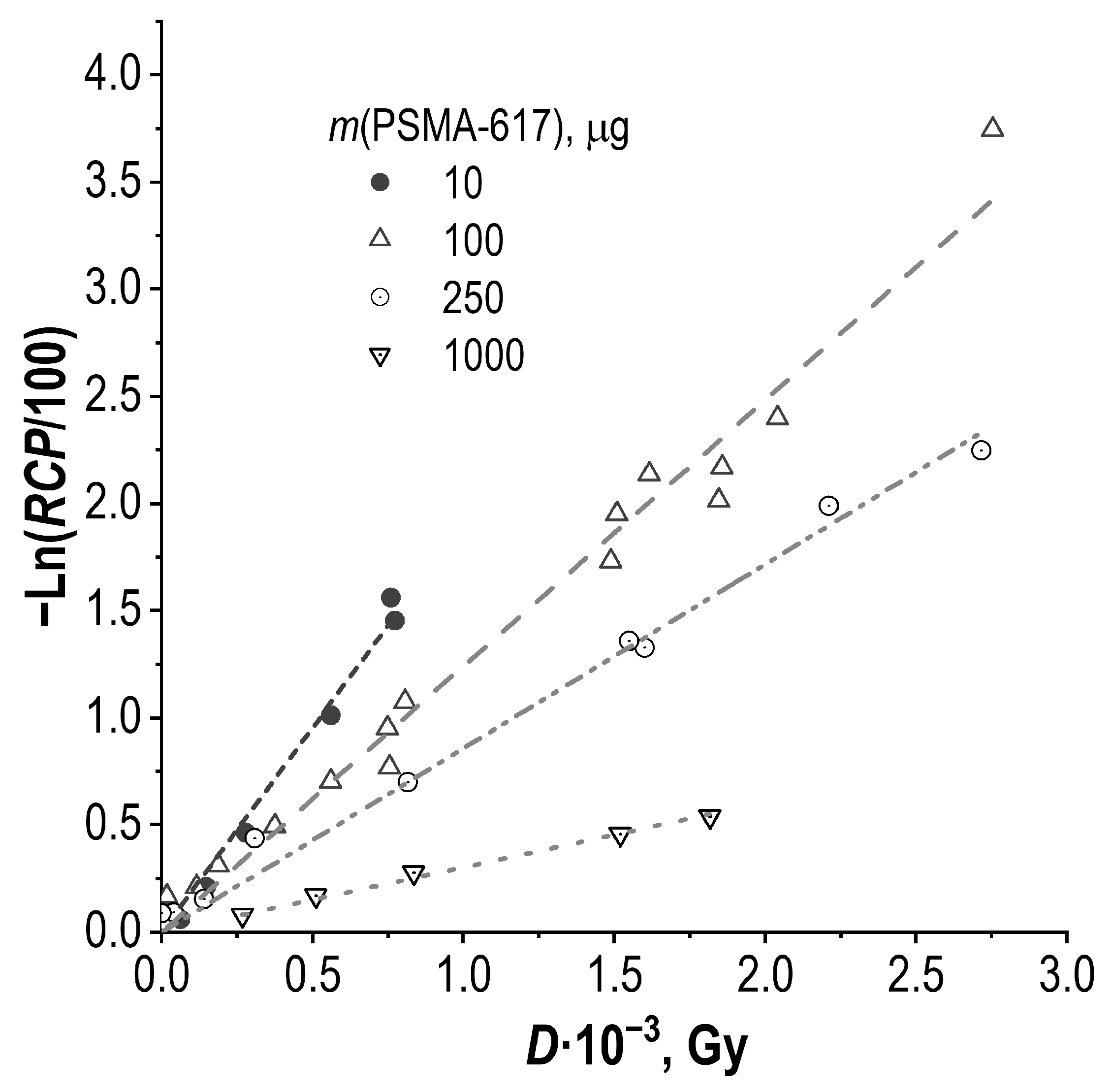
2.2. Effect of Precursor Amount and Sample Dilution
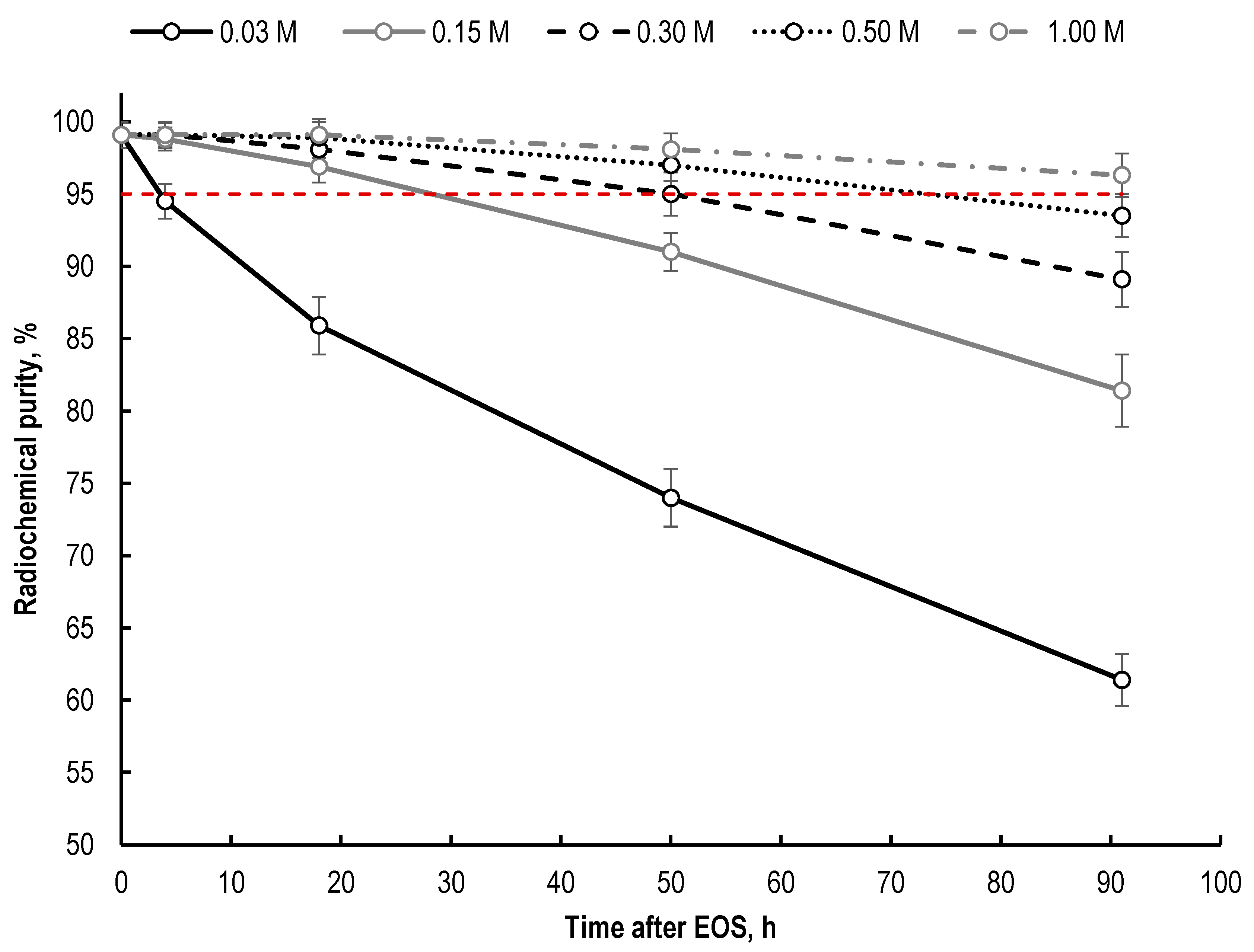
2.3. Effect of Buffering Agent (Sodium Acetate) Concentration
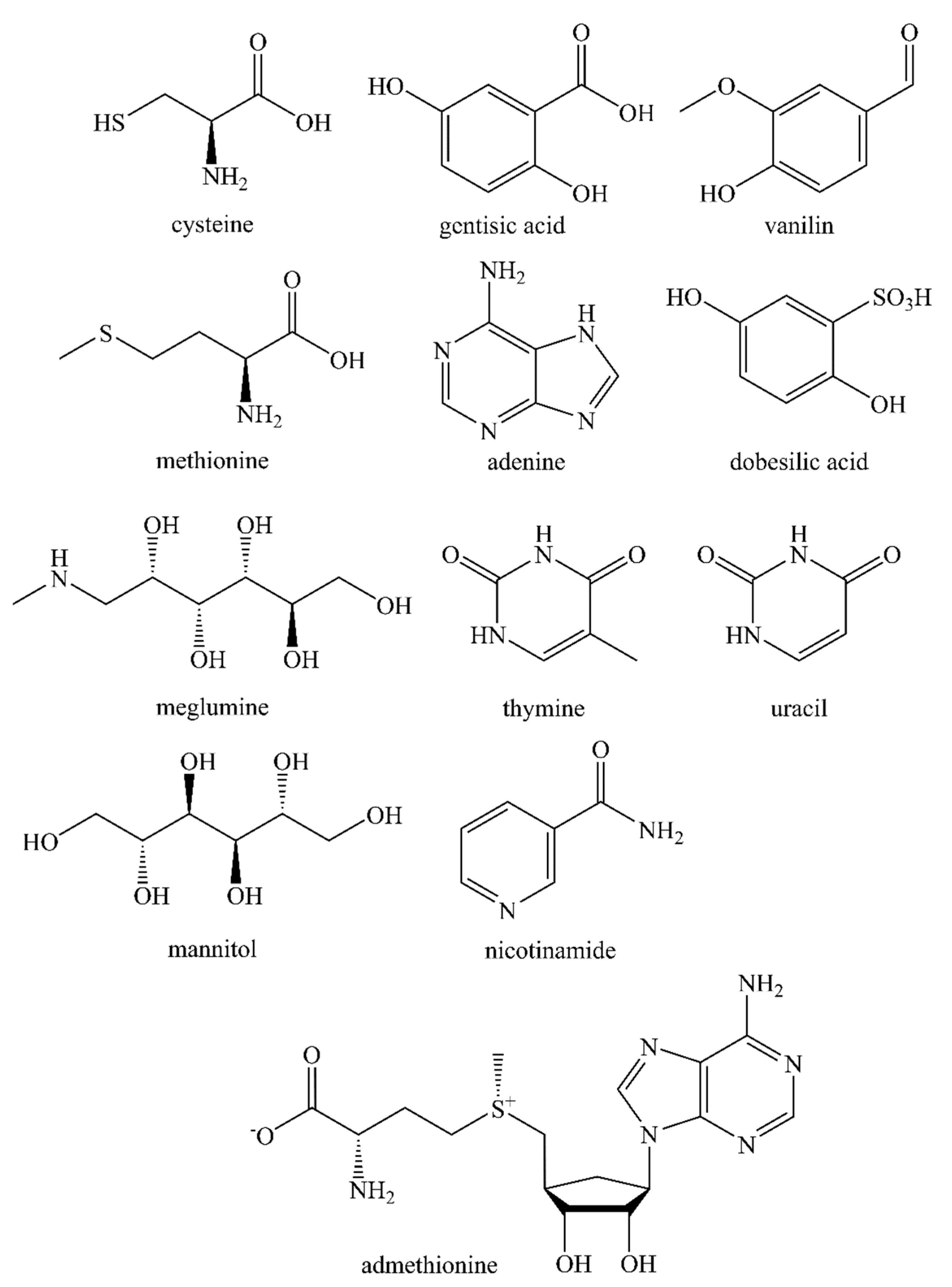
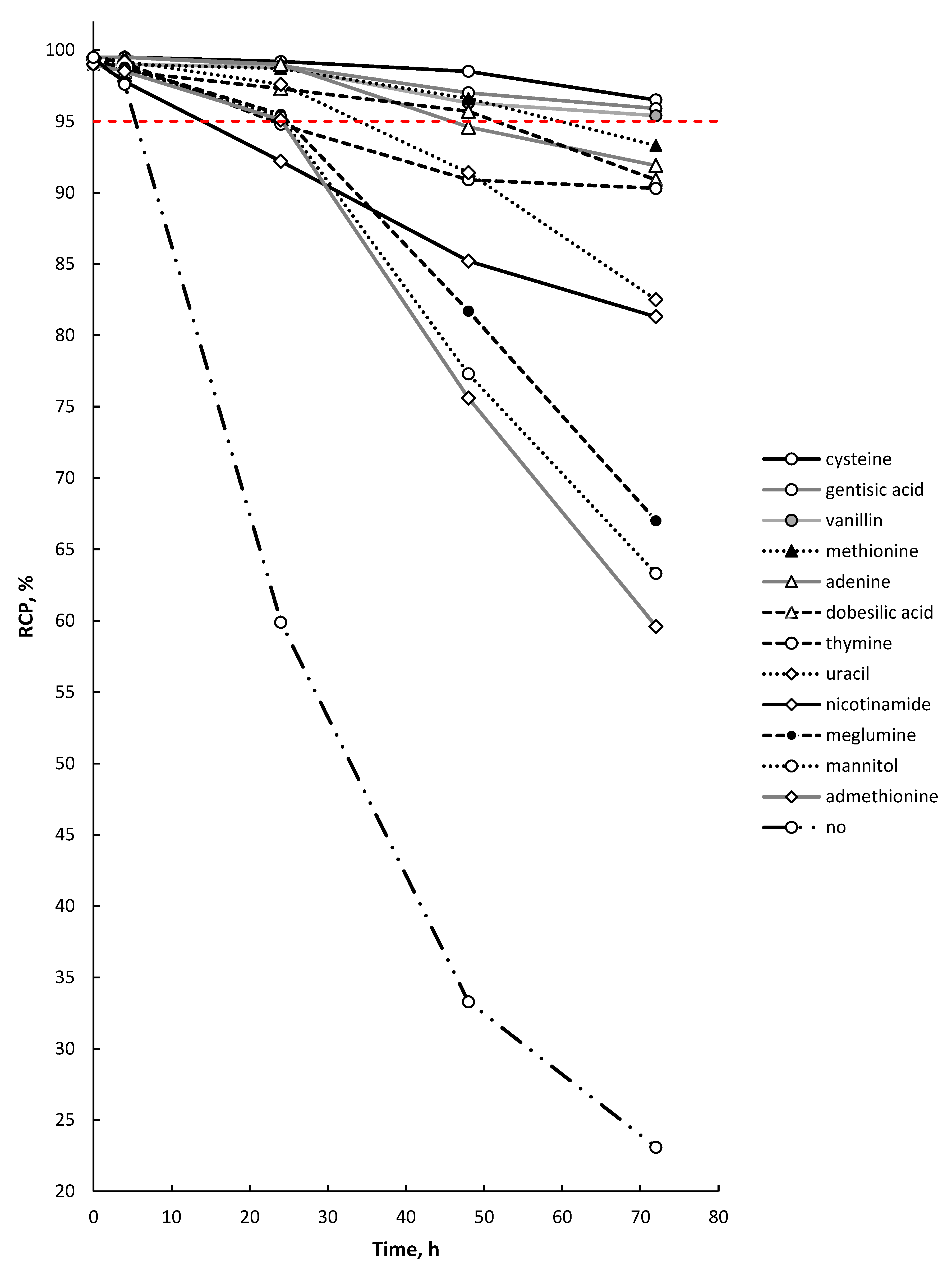
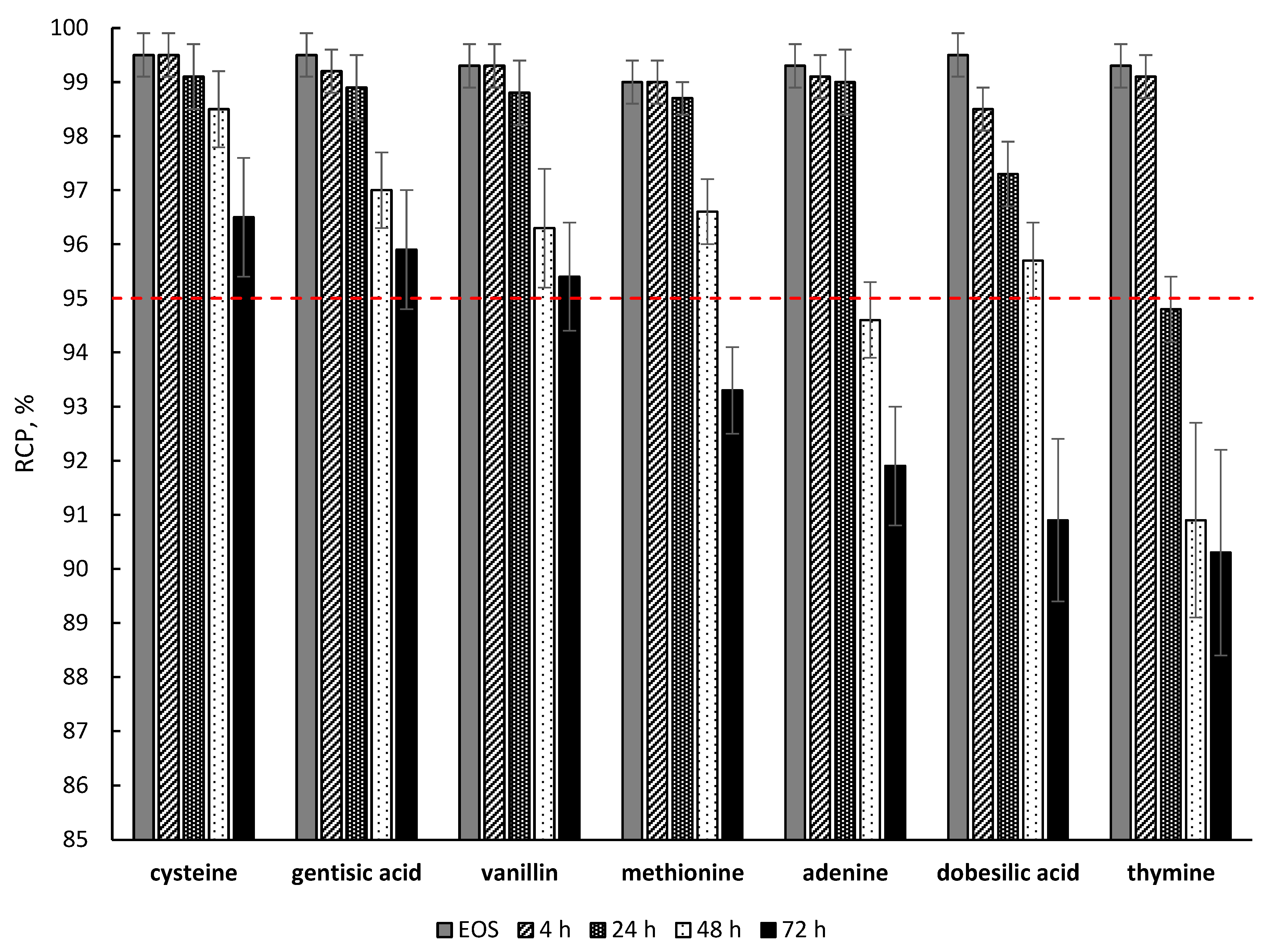
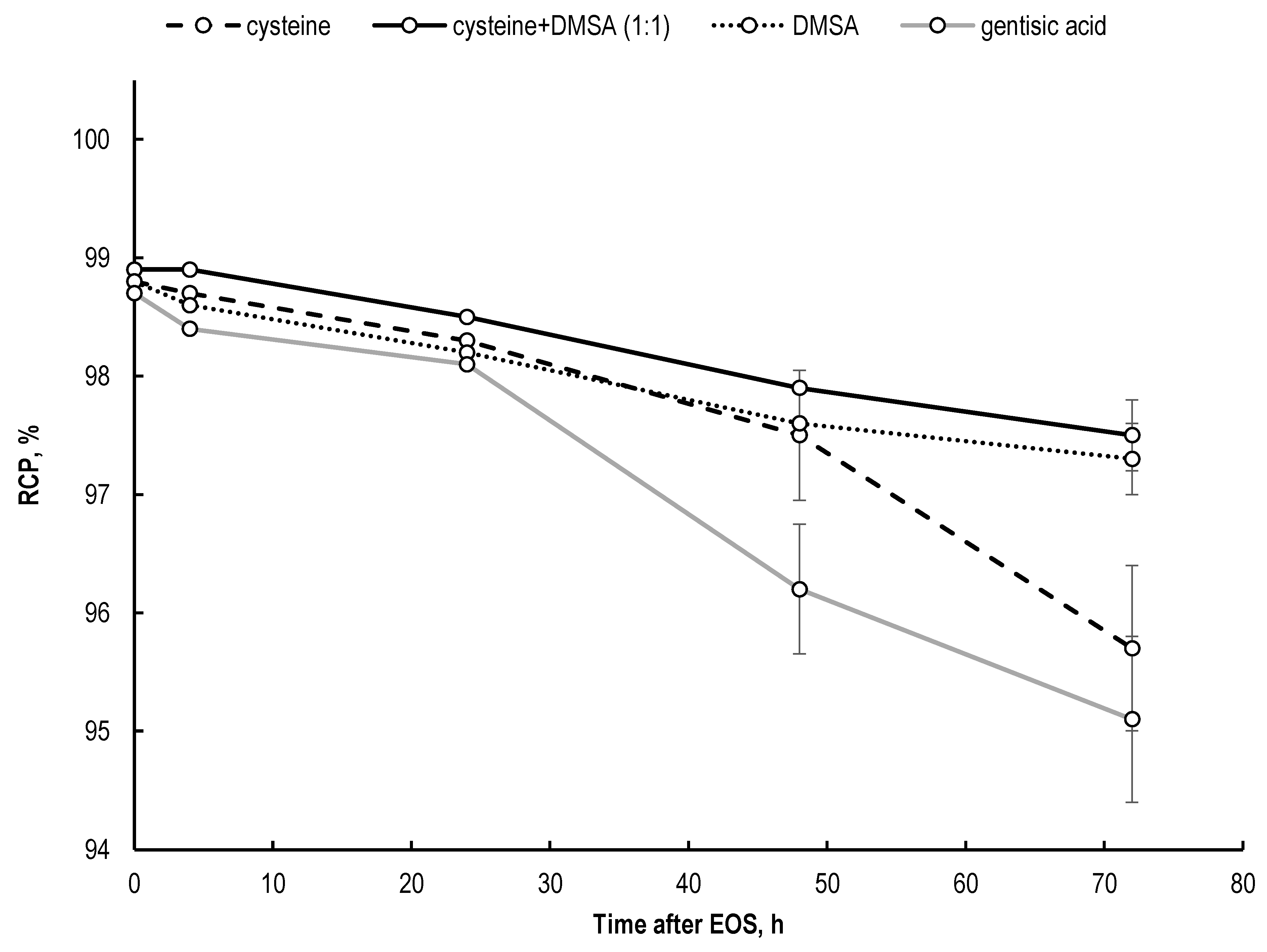
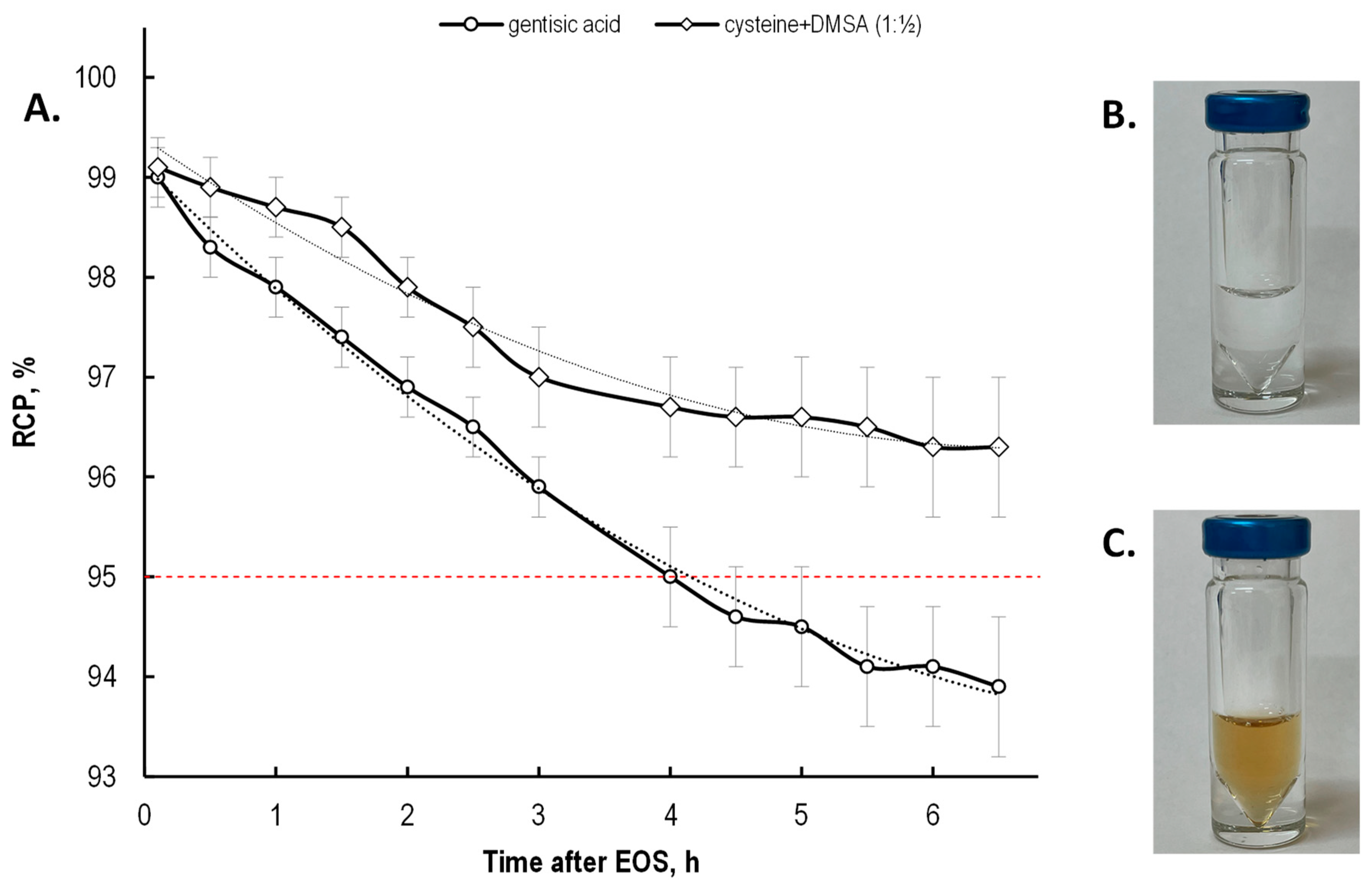
2.4. Effectiveness of Different Quenching Excipients
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Lutetium-177
4.3. Synthesis of [177Lu]Lu-PSMA-617 Preparations
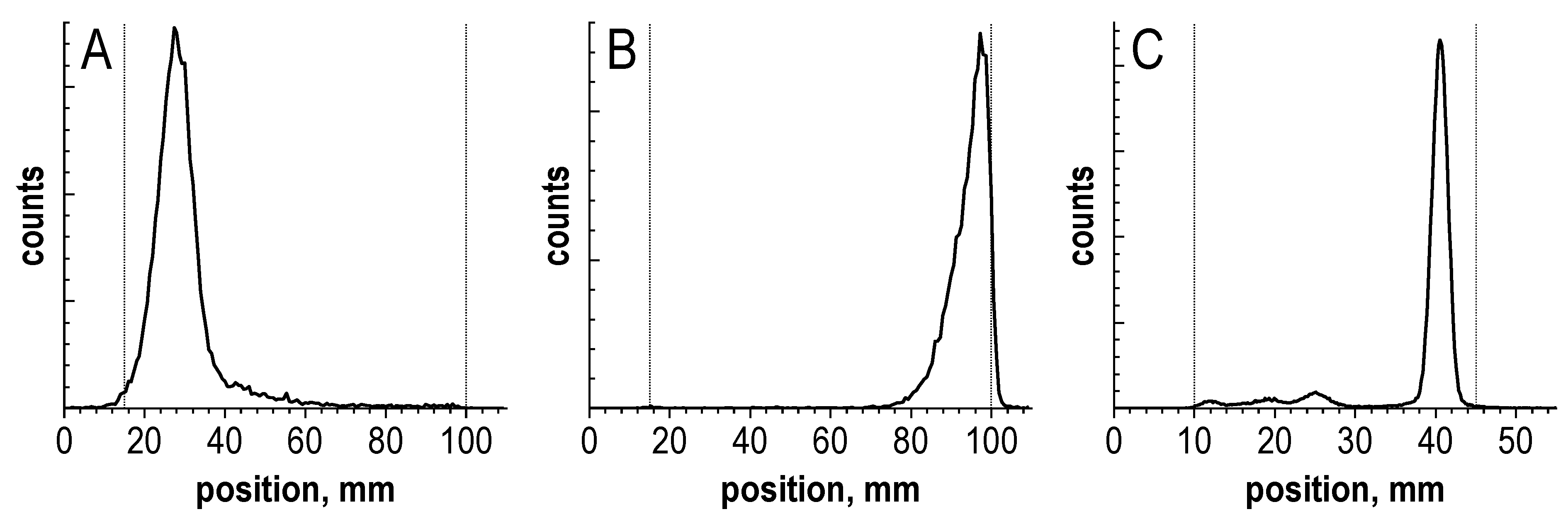
4.4. TLC and HPLC Analysis
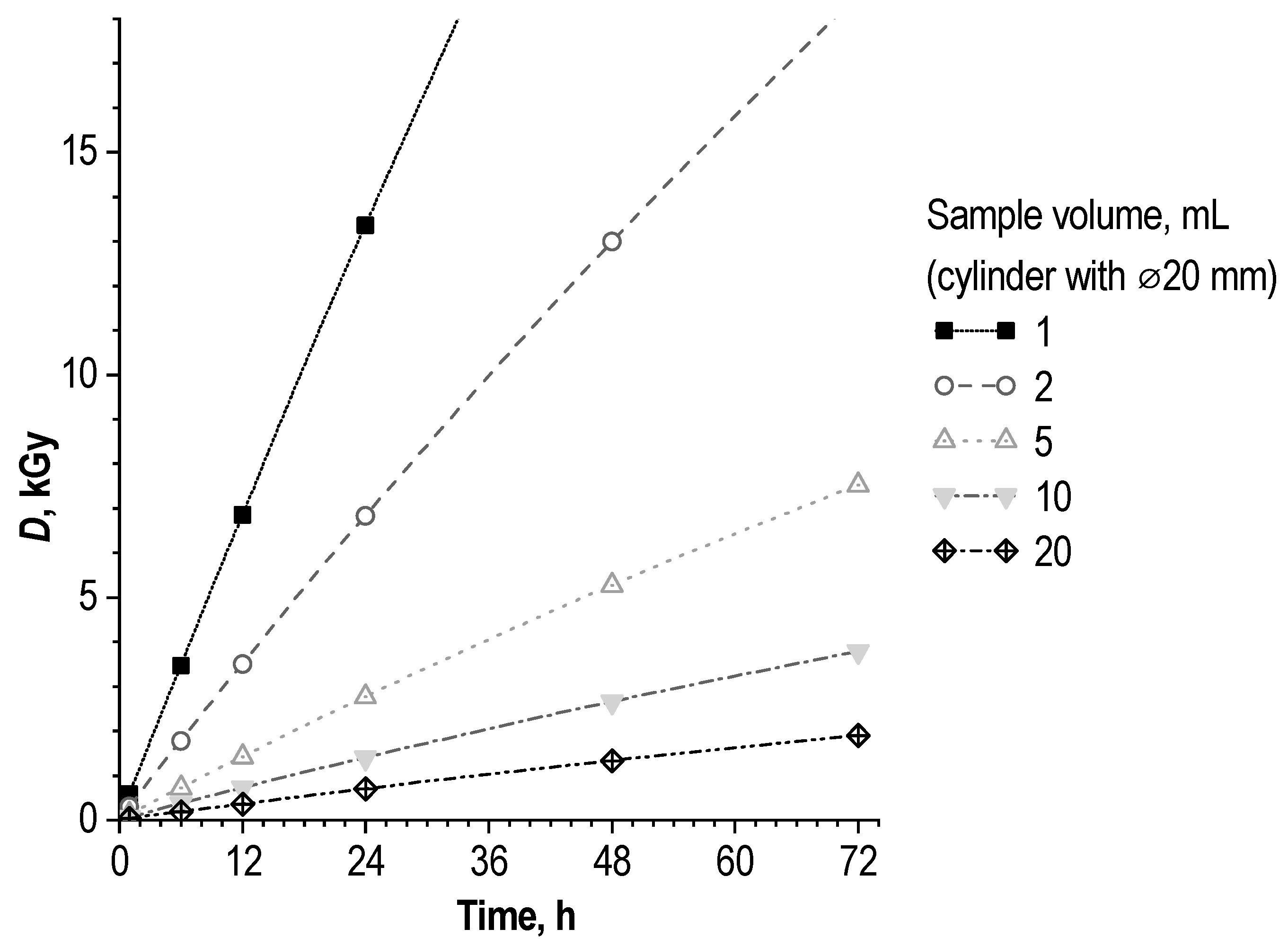
4.5. Absorbed Dose Estimation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Pini, C.; Gelardi, F.; Sollini, M. Present and Future of Target Therapies and Theranostics: Refining Traditions and Exploring New Frontiers—Highlights from Annals of Nuclear Medicine 2021. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3613–3621. [Google Scholar] [CrossRef]
- Te Beek, E.T.; Burggraaf, J.; Teunissen, J.J.M.; Vriens, D. Clinical Pharmacology of Radiotheranostics in Oncology. Clin. Pharmacol. Ther. 2023, 113, 260–274. [Google Scholar] [CrossRef]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in Oncology: Current Challenges and Emerging Opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef]
- Radiotherapy Market—Growth Drivers & Opportunities|MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/radiotherapy-monitoring-devices-market-567.html (accessed on 10 January 2023).
- Radiotherapy Market Size, Growth Trends & Forecast 2030. Available online: https://www.strategicmarketresearch.com/market-report/radiotherapy-market (accessed on 10 January 2023).
- Herrero Álvarez, N.; Bauer, D.; Hernández-Gil, J.; Lewis, J.S. Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem 2021, 16, 2909–2941. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S. A Review of Advances in the Last Decade on Targeted Cancer Therapy Using 177Lu: Focusing on 177Lu Produced by the Direct Neutron Activation Route. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 443. [Google Scholar]
- Hofland, J.; Brabander, T.; Verburg, F.A.; Feelders, R.A.; de Herder, W.W. Peptide Receptor Radionuclide Therapy. J. Clin. Endocrinol. Metab. 2022, 107, 3199–3208. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM Procedure Guidelines for Radionuclide Therapy with 177Lu-Labelled PSMA-Ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hofman, M.S. Prostate-Specific Membrane Antigen Theranostics: Therapy with Lutetium-177. Curr. Opin. Urol. 2018, 28, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA Radionuclide Therapy for Men with Prostate Cancer: A Review of the Current Literature and Discussion of Practical Aspects of Therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Vimalnath, K.V.; Chakravarty, R.; Sarma, H.D.; Dash, A. Multidose Formulation of Ready-to-Use 177Lu-PSMA-617 in a Centralized Radiopharmacy Set-Up. Appl. Radiat. Isot. 2018, 139, 91–97. [Google Scholar] [CrossRef]
- De Zanger, R.M.S.; Chan, H.S.; Breeman, W.A.P.; de Blois, E. Maintaining Radiochemical Purity of [177Lu]Lu-DOTA-PSMA-617 for PRRT by Reducing Radiolysis. J. Radioanal. Nucl. Chem. 2019, 321, 285–291. [Google Scholar] [CrossRef]
- Serdons, K.; Verbruggen, A.; Bormans, G. The Presence of Ethanol in Radiopharmaceutical Injections. J. Nucl. Med. 2008, 49, 2071. [Google Scholar] [CrossRef]
- Evans, E.A. Control of Self-Irradiation Decomposition of Tritium-Labelled Compounds at High Specific Activity. Nature 1966, 209, 169–171. [Google Scholar] [CrossRef]
- Evans, E.A. Stability of a Radioactive Drug—Tetrasodium 2-Methyl-1,4-Naphthaquinol Diphosphate Labelled with Tritium. Nature 1966, 209, 196–197. [Google Scholar] [CrossRef]
- Miller, G.G.; Raleigh, J.A. Action of Some Hydroxyl Radical Scavengers on Radiation-Induced Haemolysis. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 2009, 43, 411–419. [Google Scholar] [CrossRef]
- Kim, S.; Han, E. Changes in the Radiochemical Purity of [18F]FDG Radiopharmaceutical According to the Amount of Ethanol Added. Int. J. Radiat. Res. 2020, 18, 593–598. [Google Scholar] [CrossRef]
- Mu, L.; Hesselmann, R.; Oezdemir, U.; Bertschi, L.; Blanc, A.; Dragic, M.; Löffler, D.; Smuda, C.; Johayem, A.; Schibli, R. Identification, Characterization and Suppression of Side-Products Formed during the Synthesis of High Dose 68Ga-DOTA-TATE. Appl. Radiat. Isot. 2013, 76, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Walters, L.R.; Martin, K.J.; Jacobson, M.S.; Hung, J.C.; Mosman, E.A. Stability Evaluation of 18f-FDG at High Radioactive Concentrations. J. Nucl. Med. Technol. 2012, 40, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.S.; Dankwart, H.R.; Mahoney, D.W. Radiolysis of 2-[18F]Fluoro-2-Deoxy-d-Glucose ([18F]FDG) and the Role of Ethanol and Radioactive Concentration. Appl. Radiat. Isot. 2009, 67, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Meisenheimer, M.; Kürpig, S.; Essler, M.; Eppard, E. Ethanol Effects on 68Ga-Radiolabelling Efficacy and Radiolysis in Automated Synthesis Utilizing NaCl Post-Processing. EJNMMI Radiopharm. Chem. 2019, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ellars, C.E.; Edwards, D.S. Ascorbic Acid: Useful as a Buffer Agent and Radiolytic Stabilizer for Metalloradiopharmaceuticals. Bioconjug. Chem. 2003, 14, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Edwards, D.S. Stabilization of 90Y-Labeled DOTA-Biomolecule Conjugates Using Gentisic Acid and Ascorbic Acid. Bioconjug. Chem. 2001, 12, 554–558. [Google Scholar] [CrossRef]
- Kumar, K.S.A.; Mathur, A. A Convenient Total Synthesis of PSMA-617: A Prostate Specific Membrane Antigen (PSMA) Ligand for Prostate Cancer Endotherapeutic Applications. Eur. J. Med. Chem. Rep. 2022, 6, 100084. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lohar, S.; Dash, A.; Sarma, H.D.; Samuel, G.; Korde, A. Single Vial Kit Formulation of DOTATATE for Preparation of 177Lu-Labeled Therapeutic Radiopharmaceutical at Hospital Radiopharmacy. J. Label. Compd. Radiopharm. 2015, 58, 166–172. [Google Scholar] [CrossRef]
- Nanabala, R.; Sasikumar, A.; Joy, A.; Pillai, M. Preparation of [177Lu]PSMA-617 Using Carrier Added (CA) 177Lu for Radionuclide Therapy of Prostate Cancer. J. Nucl. Med. Radiat. Ther. 2016, 7, 306. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Golubitskii, G.B.; Budko, E.V.; Basova, E.M.; Kostarnoi, A.V.; Ivanov, V.M. Stability of Ascorbic Acid in Aqueous and Aqueous-Organic Solutions for Quantitative Determination. J. Anal. Chem. 2007, 62, 742–747. [Google Scholar] [CrossRef]
- Herbig, A.L.; Renard, C.M.G.C. Factors That Impact the Stability of Vitamin C at Intermediate Temperatures in a Food Matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Washino, K. Radiation Protection Agents Suitable for Use with Radiopharmaceuticals Comprising Reducible Active Ingredients. EP0832654B1, 15 January 2003. [Google Scholar]
- Scott, P.J.H.; Kilbourn, M.R. Determination of Residual Kryptofix 2.2.2 Levels in [18F]-Labeled Radiopharmaceuticals for Human Use. Appl. Radiat. Isot. 2007, 65, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Tönnesmann, R.; Hierlmeier, I.; Maus, S.; Rosar, F.; Ruf, J.; Holland, J.P.; Ezziddin, S.; Bartholomä, M.D. Identification, Characterization, and Suppression of Side Products Formed during the Synthesis of [177Lu]Lu-PSMA-617. J. Med. Chem. 2021, 64, 4960–4971. [Google Scholar] [CrossRef] [PubMed]
- Boas, C.A.W.V.; Dias, L.A.P.; Matsuda, M.M.N.; de Araújo, E.B. Stability in the Production and Transport of 177Lu Labelled PSMA. Braz. J. Radiat. Sci. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary Experience with Dosimetry, Response and Patient Reported Outcome after 177 Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer. Oncotarget 2016, 8, 3581–3590. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga-and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Guleria, M.; Amirdhanayagam, J.; Sarma, H.D.; Rallapeta, R.P.; Krishnamohan, V.S.; Nimmagadda, A.; Ravi, P.; Patri, S.; Kalawat, T.; Das, T. Preparation of 177Lu-PSMA-617 in Hospital Radiopharmacy: Convenient Formulation of a Clinical Dose Using a Single-Vial Freeze-Dried PSMA-617 Kit Developed In-House. Biomed Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Das, T.; Banerjee, S.; Shinto, A.; Kamaleshwaran, K.K.; Sarma, H.D. Preparation of Therapeutic Dose of 177Lu-DOTA-TATE Using a Novel Single Vial Freeze-Dried Kit: A Comparison with ‘In-Situ’ Preparation at Hospital Radiopharmacy. Curr. Radiopharm. 2014, 7, 12–19. [Google Scholar] [CrossRef]
- Mathur, A.; Prashant, V.; Sakhare, N.; Chakraborty, S.; Vimalnath, K.V.; Mohan, R.K.; Arjun, C.; Karkhanis, B.; Seshan, R.; Basu, S.; et al. Bulk Scale Formulation of Therapeutic Doses of Clinical Grade Ready-to-Use 177Lu-DOTA-TATE: The Intricate Radiochemistry Aspects. Cancer Biother. Radiopharm. 2017, 32, 266–273. [Google Scholar] [CrossRef]
- Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.C.; Kopka, K.; Pietzsch, H.J.; Petrik, M.; Mamat, C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting Psma—A Proof of Concept Study. Cancers 2021, 13, 1974. [Google Scholar] [CrossRef]
- Ghosh, A.; Woolum, K.; Kothandaraman, S.; Tweedle, M.F.; Kumar, K. Stability Evaluation and Stabilization of a Gastrin-Releasing Peptide Receptor (GRPR) Targeting Imaging Pharmaceutical. Molecules 2019, 24, 2878. [Google Scholar] [CrossRef]
- Trindade, V.; Balter, H. Oxidant and Antioxidant Effects of Gentisic Acid in a 177 Lu-Labelled Methionine-Containing Minigastrin Analogue. Curr. Radiopharm. 2019, 13, 107–119. [Google Scholar] [CrossRef]
- Di Iorio, V.; Boschi, S.; Cuni, C.; Monti, M.; Severi, S.; Paganelli, G.; Masini, C. Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials. Molecules 2022, 27, 4143. [Google Scholar] [CrossRef]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. Antioxidant Activity and Free Radical Scavenging Reactions of Gentisic Acid: In-Vitro and Pulse Radiolysis Studies. Free Radic. Res. 2012, 46, 11–20. [Google Scholar] [CrossRef]
- Lutathera|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera (accessed on 17 January 2023).
- Pluvicto|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pluvicto (accessed on 17 January 2023).
- Chen, J.; Linder, K.E.; Cagnolini, A.; Metcalfe, E.; Raju, N.; Tweedle, M.F.; Swenson, R.E. Synthesis, Stabilization and Formulation of [177Lu]Lu-AMBA, a Systemic Radiotherapeutic Agent for Gastrin Releasing Peptide Receptor Positive Tumors. Appl. Radiat. Isot. 2008, 66, 497–505. [Google Scholar] [CrossRef]
- De Blois, E.; Sze Chan, H.; Konijnenberg, M.; de Zanger, R.; Breeman, A.P.W. Effectiveness of Quenchers to Reduce Radiolysis of 111In- or 177Lu-Labelled Methionine-Containing Regulatory Peptides. Maintaining Radiochemical Purity as Measured by HPLC. Curr. Top. Med. Chem. 2013, 12, 2677–2685. [Google Scholar] [CrossRef]
- De Blois, E.; Chan, H.S.; de Zanger, R.; Konijnenberg, M.; Breeman, W.A.P. Application of Single-Vial Ready-for-Use Formulation of 111In- or 177Lu-Labelled Somatostatin Analogs. Appl. Radiat. Isot. 2014, 85, 28–33. [Google Scholar] [CrossRef]
- De Blois, E.; de Zanger, R.M.S.; Chan, H.S.; Konijnenberg, M.; Breeman, W.A.P. Radiochemical and Analytical Aspects of Inter-Institutional Quality Control Measurements on Radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Maus, S.; de Blois, E.; Ament, S.J.; Schreckenberger, M.; Breeman, W.A.P. Aspects on Radiolabeling of 177Lu-DOTA-TATE: After C18 Purification Re-Addition of Ascorbic Acid Is Required to Maintain Radiochemical Purity. Int. J. Diagn. Imaging 2014, 1, 5. [Google Scholar] [CrossRef]
- Larenkov, A.A.; Mitrofanov, Y.A.; Rakhimov, M.G. Features and Practical Aspects of Radiochemical Purity Determination of Receptor-Specific Lu-177 Radiopharmaceuticals as Exemplified by [177Lu]Lu–PSMA-617. Bull. Sci. Cent. Expert Eval. Med. Prod. Regul. Res. Med. Eval. 2022, 12, 455–467. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, S.D. Bifunctional Chelators for Therapeutic Lanthanide Radiopharmaceuticals. Bioconjug. Chem. 2001, 12, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.P. Practical Aspects of Labeling DTPA-and DOTA-Peptides with 90Y, 111In, 177Lu, 68Ga for Peptide-Receptor Scintigraphy and Peptide-Receptor Radionuclide Therapy in Preclinical and Clinical Applications. Univ. New Mex. Health Sci. Center Coll. Pharm. 2012, 16, 1–34. [Google Scholar]
- Breeman, A.P.W.; Sze Chan, H.; de Zanger, M.S.R.; Konijnenberg, K.M.; de Blois, E. Overview of Development and Formulation of 177 Lu-DOTA-TATE for PRRT. Curr. Radiopharm. 2015, 9, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Maruk, A.Y.; Larenkov, A.A. Determination of Ionic 68Ga Impurity in Radiopharmaceuticals: Major Revision of Radio-HPLC Methods. J. Radioanal. Nucl. Chem. 2020, 323, 189–195. [Google Scholar] [CrossRef]
- Larenkov, A.A.; Maruk, A.Y.; Kodina, G.E. Intricacies of the Determination of the Radiochemical Purity of 68Ga Preparations: Possibility of Sorption of Ionic 68Ga Species on Reversed-Phase Columns. Radiochemistry 2018, 60, 625–633. [Google Scholar] [CrossRef]
- Mitrofanov, Y.A.; Bubenshchikov, V.B.; Belousov, A.V.; Lunev, A.A.; Larenkov, A.A. Evaluation of the Applicability of External X-Ray Irradiation to Simulate the Autoradiolysis Processes in Therapeutic Radiopharmaceuticals (Exemplified by [153Sm]Sm-PSMA-617 and [177Lu]Lu-PSMA-617). High Energy Chem. 2023, 57, 36–45. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The Antioxidant Action of N-Acetylcysteine: Its Reaction with Hydrogen Peroxide, Hydroxyl Radical, Superoxide, and Hypochlorous Acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Allaveisi, F.; Hashemi, B.; Mortazavi, S.M.J. Radioprotective Effect of N-Acetyl-L-Cysteine Free Radical Scavenger on Compressive Mechanical Properties of the Gamma Sterilized Cortical Bone of Bovine Femur. Cell Tissue Bank. 2014, 16, 97–108. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, H.J.; Cho, W.Y.; Yeon, S.J.; Lee, C.H. In Vitro Antioxidant Actions of Sulfur-Containing Amino Acids. Arab. J. Chem. 2020, 13, 1678–1684. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, M.; Song, M.; Shi, C.; Zhang, P.; Xu, D.; You, L.; Zhuang, R.; Su, X.; Liu, T.; et al. Synthesis and Evaluation of 99mTc-Labeled Dimeric Folic Acid for FR-Targeting. Molecules 2016, 21, 817. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, S.; Iwama, T. Determination of a Small Quantity of Cystine in the Presence of a Large Amount of Cysteine. Biosci. Biotechnol. Biochem. 1999, 63, 1503–1505. [Google Scholar] [CrossRef]
- Dewey, D.L.; Beecher, J. Interconversion of Cystine and Cysteine Induced by X-Rays. Nature 1965, 206, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Markakis, P.; Tappel, A.L. Products of γ-Irradiation of Cysteine and Cystine. J. Am. Chem. Soc. 1960, 82, 1613–1617. [Google Scholar] [CrossRef]
- Anbar, M.; Neta, P. A Compilation of Specific Bimolecular Rate Constants for the Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals with Inorganic and Organic Compounds in Aqueous Solution. Int. J. Appl. Radiat. Isot. 1967, 18, 493–523. [Google Scholar] [CrossRef]
- Plyku, D.; Ghaly, M.; Li, Y.; Brown, J.L.; O’Reilly, S.; Khamwan, K.; Goodkind, A.B.; Sexton-Stallone, B.; Cao, X.; Zurakowski, D.; et al. Renal 99mTc-DMSA Pharmacokinetics in Pediatric Patients. EJNMMI Phys. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Hernández-Valdés, D.; Blanco-González, A.; García-Fleitas, A.; Rodríguez-Riera, Z.; Meola, G.; Alberto, R.; Jáuregui-Haza, U. Insight into the Structure and Stability of Tc and Re DMSA Complexes: A Computational Study. J. Mol. Graph. Model. 2017, 71, 167–175. [Google Scholar] [CrossRef]
- Tarkington, M.A.; Fildes, R.D.; Levin, K.; Ziessman, H.; Harkness, B.; Gibbons, M.D. High Resolution Single Photon Emission Computerized Tomography (SPECT) 99mTechnetium-Dimercapto-Succinic Acid Renal Imaging: A State of the Art Technique. J. Urol. 1990, 144, 598–600. [Google Scholar] [CrossRef]
- Parvex, P.; Rozen, R.; Dziarmaga, A.; Goodyer, P. Studies of Urinary Cystine Precipitation in Vitro: Ontogeny of Cystine Nephrolithiasis and Identification of Meso-2,3-Dimercaptosuccinic Acid as a Potential Therapy for Cystinuria. Mol. Genet. Metab. 2003, 80, 419–425. [Google Scholar] [CrossRef]
- Louise, F.Ø.; Katja, V.P.; Søren, K.L.; Susanne, S.O.; Helene, U.J.; Kim, H.A.; Pedersen, K.V.; Lildal, S.K. The Challenges of Cystinuria in the Twenty-First Century—A Mini Review. J. Rare Dis. Res. Treat 2016, 1, 41–45. [Google Scholar]
- Maiorino, R.M.; Bruce, D.C.; Aposhian, H.V. Determination and Metabolism of Dithiol Chelating Agents: VI. Isolation and Identification of the Mixed Disulfides of Meso-2,3-Dimercaptosuccinic Acid with l-Cysteine in Human Urine. Toxicol. Appl. Pharmacol. 1989, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Polt, R.; Li, Y.; Fernando, Q.; Rivera, M. A Synthetic Method for Unsymmetrical Disulfides of Cysteine: The Bis-Cysteine Disulfide of Meso-2,3-Dimercaptosuccinic Acid. Tetrahedron Lett. 1992, 33, 2961–2964. [Google Scholar] [CrossRef]
- Hosokawa, S.; Shukuya, K.; Sogabe, K.; Ejima, Y.; Morinishi, T.; Hirakawa, E.; Ohsaki, H.; Shimosawa, T.; Tokuhara, Y. Novel Absorbance Peak of Gentisic Acid Following the Oxidation Reaction. PLoS ONE 2020, 15, e0232263. [Google Scholar] [CrossRef]
- Lee, B.; Lee, M. Decomposition of 2,4,6-Trinitrotoluene (TNT) by Gamma Irradiation. Environ. Sci. Technol. 2005, 39, 9278–9285. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, A.; Yablonskiy, M.; Petrov, V.; Mitrofanov, A. DFT Prediction of Radiolytic Stability of Conformationally Flexible Ligands. Energies 2022, 16, 257. [Google Scholar] [CrossRef]
- Stabin, M.G.; Konijnenberg, M.W. Re-Evaluation of Absorbed Fractions for Photons and Electrons in Spheres of Various Sizes. J. Nucl. Med. 2000, 41, 149–160. [Google Scholar]
- Ruigrok, E.A.M.; Tamborino, G.; de Blois, E.; Roobol, S.J.; Verkaik, N.; De Saint-Hubert, M.; Konijnenberg, M.W.; van Weerden, W.M.; de Jong, M.; Nonnekens, J. In Vitro Dose Effect Relationships of Actinium-225- and Lutetium-177-Labeled PSMA-I&T. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3627–3638. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Madden, K.P.; Mezyk, S.P. Critical Review of Aqueous Solution Reaction Rate Constants for Hydrogen Atoms. J. Phys. Chem. Ref. Data 2011, 40, 023103. [Google Scholar] [CrossRef]
- Mahal, H.S.; Badheka, L.P.; Mukherjee, T. Radical Scavenging Properties of a Flavouring Agent-Vanillin. Res. Chem. Intermed. 2001, 27, 595–604. [Google Scholar] [CrossRef]
- Lungu, V.; Chiper, D.; Niculae, D. Radiolabelling of Meso-2,3-DMSA with 177Lu. Preliminary Results Regarding the Stability and Biospecificity of 177Lu–DMSA. J. Label. Compd. Radiopharm. 2007, 50, 565–566. [Google Scholar] [CrossRef]
- Allison, J.; Amako, K.; Apostolakis, J.; Arce, P.; Asai, M.; Aso, T.; Bagli, E.; Bagulya, A.; Banerjee, S.; Barrand, G.; et al. Recent Developments in Geant4. In Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment; Elsevier: Amsterdam, The Netherlands, 2016; Volume 835, pp. 186–225. [Google Scholar] [CrossRef]
- Khusnulina, A. Researching the Possibility to Use Fricke Dosimeter for Measurement of Absorbed Dose Generated by Pulse Electron Beam. IOP Conf. Ser. Mater. Sci. Eng. 2014, 66, 012032. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 1–337. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Wayner, D.D.M.; Burton, G.W.; Ingold, K.U.; Barclay, L.R.C.; Locke, S.J. The Relative Contributions of Vitamin E, Urate, Ascorbate and Proteins to the Total Peroxyl Radical-Trapping Antioxidant Activity of Human Blood Plasma. Biochim. Biophys. Acta Gen. Subj. 1987, 924, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational Strategies for Predicting Free Radical Scavengers’ Protection against Oxidative Stress: Where Are We and What Might Follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef]
| # | TLC Plates | Solvent | Rf of 177Lu Species | ||
|---|---|---|---|---|---|
| Unbound [177Lu]Lu3+ | [177Lu]Lu-PSMA-617 | [177Lu]Lu-PSMA-617 Degradation Products | |||
| 1 | iTLC-SG glass microfiber chromatography paper impregnated with a silica gel (Agilent, Santa Clara, CA, USA) | 0.05 M H3Citraq. | 0.95 ± 0.05 | 0.15 ± 0.05 | 0.2–1.0 |
| 2 | NH3-Ethanol-H2O (1:5:10) | 0–0.05 | 0.95 ± 0.05 | 0.95 ± 0.05 | |
| 3 | TLC Silica gel 60 sheets with aluminum support (5553, Merck, Darmstadt, Germany) | MeCN-H2O (1:1) | 0–0.05 | 0.85 ± 0.05 | 0.15–0.75 |
| 4 | TLC Cellulose F sheets with plastic support (5565, Merck, Darmstadt, Germany) | 0–0.05 | 0.95 ± 0.05 | 0.95 ± 0.05 | |
| # | HPLC Column | Gradient Profile, Flow Rate, Solvents | Rt of 177Lu Species | |||
|---|---|---|---|---|---|---|
| Unbound [177Lu]Lu3+ | [177Lu]Lu-PSMA-617 | [177Lu]Lu-PSMA-617 Degradation Products | [177Lu]Lu-PSMA-617 Cyclisation Products | |||
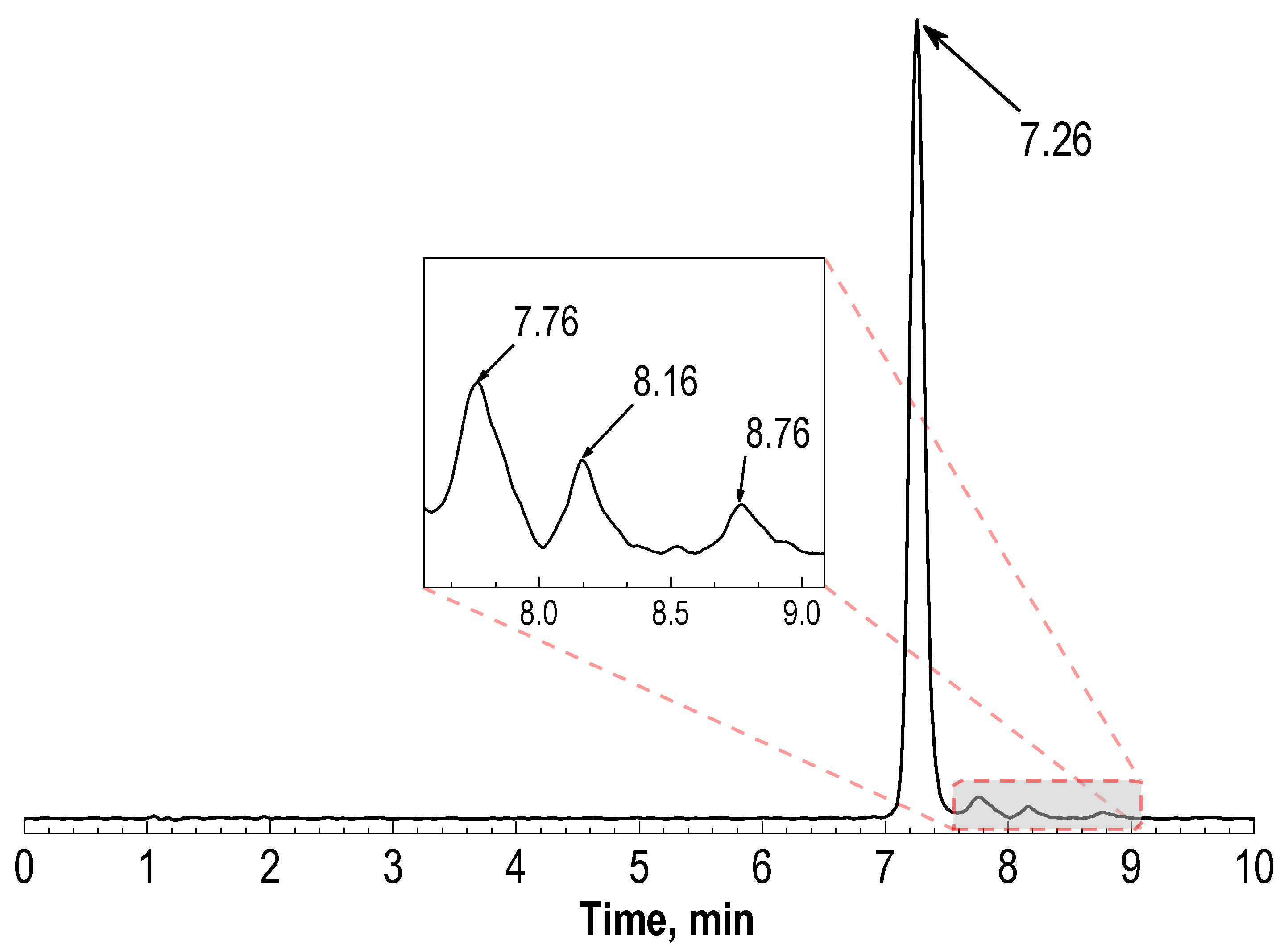
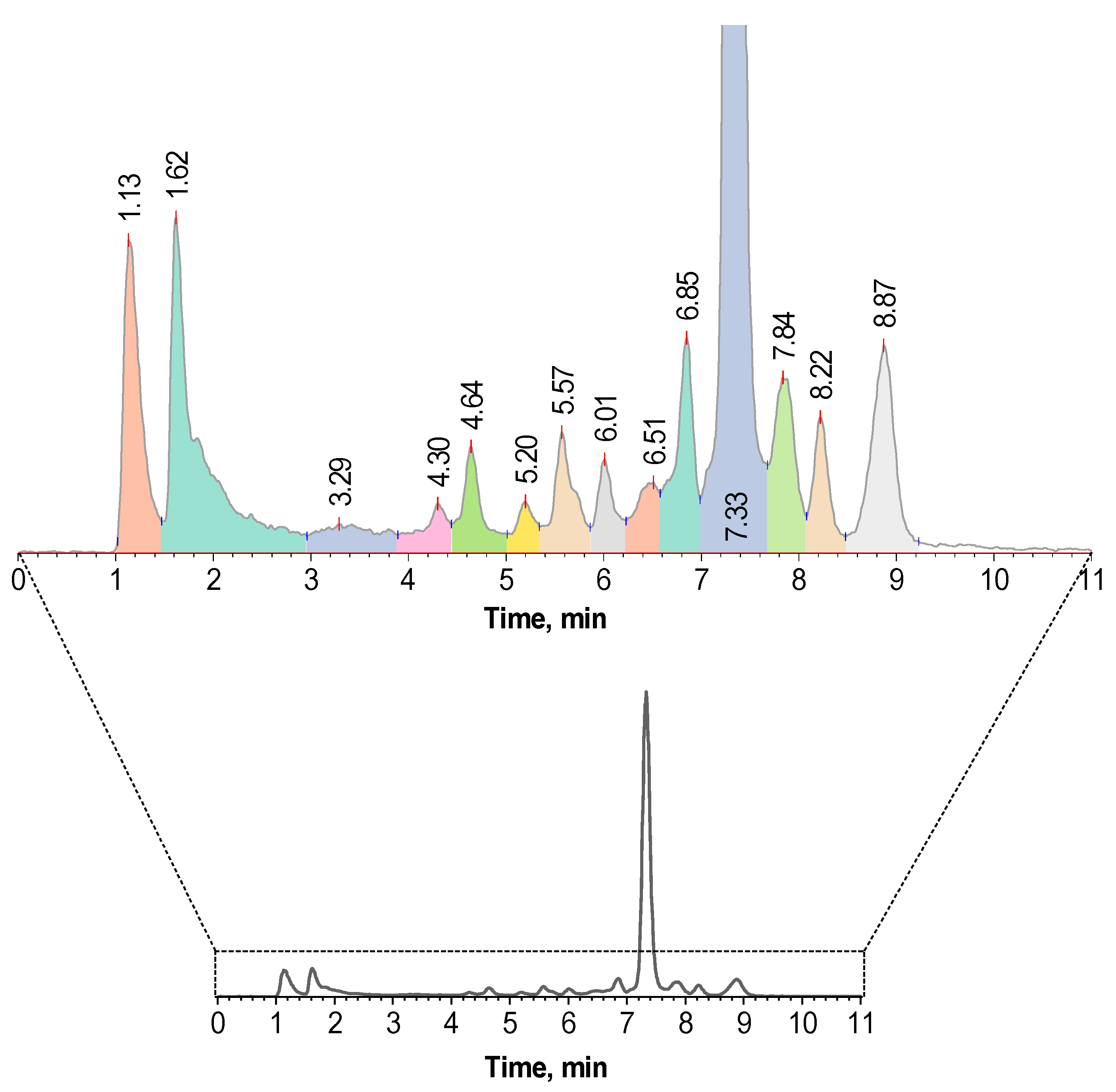
| 1 | Phenomenex® Luna 150 × ⌀3 mm, 5 μm, 100 Å | 0–5–15–20 min = 17–25–25–17%B 0.75 mL/min, B—0.1% TFA in acetonitrile | 1.11 ± 0.05 | 7.26 ± 0.04 | 1.4–7.0 | 7.6–9.3 |
| 2 | Phenomenex® Jupiter 250 × ⌀4.6 mm, 5 μm, 300 Å | 0–1–25–27–29–32 min = 5–5–50–95–95–5%B, 1 mL/min, B—0.1% TFA in acetonitrile [37] | 3.64 ± 0.08 | 16.90 ± 0.10 | 4.0–16.7 | 17.2–18.8 |
| 3 | Phenomenex® Jupiter 250 × ⌀4.6 mm, 5 μm, 300 Å | 0–20–25–25.01–30 min = 15–100–100–15–15% B, 1 mL/min, B—methanol [17] | 3.54 ± 0.08 | 13.61 ± 0.72 | 3.7–13.2 | 14.2–15.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larenkov, A.; Mitrofanov, I.; Pavlenko, E.; Rakhimov, M. Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process. Molecules 2023, 28, 1884. https://doi.org/10.3390/molecules28041884
Larenkov A, Mitrofanov I, Pavlenko E, Rakhimov M. Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process. Molecules. 2023; 28(4):1884. https://doi.org/10.3390/molecules28041884
Chicago/Turabian StyleLarenkov, Anton, Iurii Mitrofanov, Ekaterina Pavlenko, and Marat Rakhimov. 2023. "Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process" Molecules 28, no. 4: 1884. https://doi.org/10.3390/molecules28041884
APA StyleLarenkov, A., Mitrofanov, I., Pavlenko, E., & Rakhimov, M. (2023). Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process. Molecules, 28(4), 1884. https://doi.org/10.3390/molecules28041884






