A Benzothiadiazole-Based Self-Assembled Cage for Cadmium Detection
Abstract
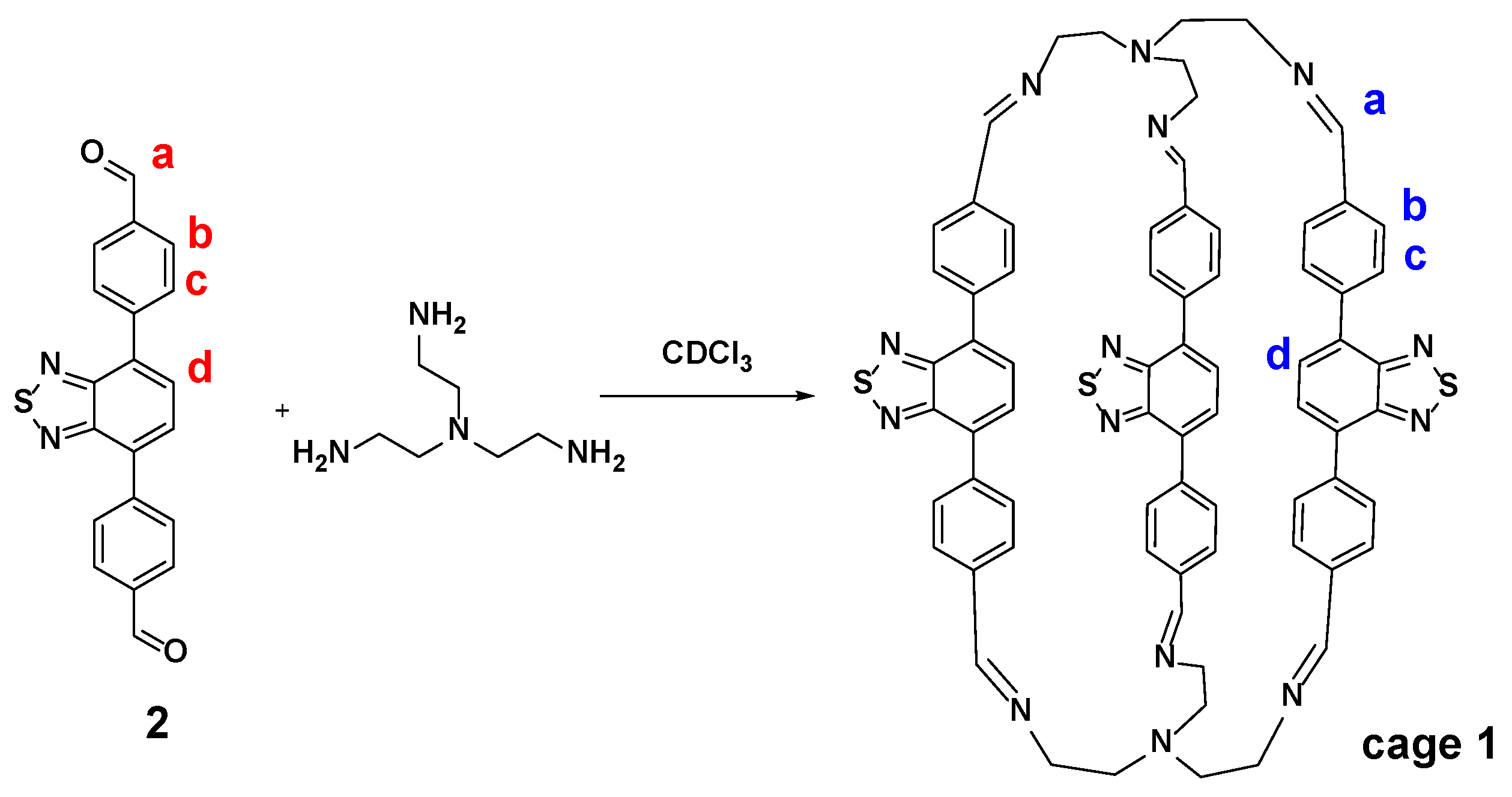
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Typical Procedure for the Synthesis of Cage 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Durot, S.; Taesch, J.; Heitz, V. Multiporphyrinic Cages: Architectures and Functions. Chem. Rev. 2014, 114, 8542–8578. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M. Porous Shape-Persistent Organic Cage Compounds of Different Size, Geometry, and Function. Acc. Chem. Res. 2018, 51, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Montá-Gonzále, G.; Sancenón, F.; Martínez-Máñez, R.; Martí-Centelles, V. Purely Covalent Molecular Cages and Containers for Guest Encapsulation. Chem. Rev. 2022, 122, 13636–13708. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Zhang, G.; Mastalerz, M. Organic Cage Compounds—From Shape-Persistency to Function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Stoddart, J.F. Review Emergent Behavior in Nanoconfined Molecular Containers. Chem 2021, 7, 919–947. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, C.F. Synthesis and Structure of a TriptyceneBased Nanosized Molecular Cage. J. Org. Chem. 2007, 72, 9339–9341. [Google Scholar] [CrossRef]
- Dietrich, B.; Lehn, J.M.; Sauvage, J.P. Les Cryptates. Tetrahedron Lett. 1969, 10, 2889–2892. [Google Scholar] [CrossRef]
- Little, M.A.; Cooper, A.I. The Chemistry of Porous Organic Molecular Materials. Adv. Funct. Mater. 2020, 30, 1909842. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Zhang, Z.Y.; Wang, Y.; Jia, X.; Li, C. One-pot and Shape-controlled Synthesis of Organic Cages. Angew. Chem. Int. Ed. 2021, 60, 17904–17909. [Google Scholar] [CrossRef]
- Schneider, M.W.; Oppel, I.M.; Mastalerz, M. ExoFunctionalized Shape-Persistent 2 + 3 Cage Compounds: Influence of Molecular Rigidity on Formation and Permanent Porosity. Chem. Eur. J. 2012, 18, 4156–4160. [Google Scholar] [CrossRef]
- Ma, J.X.; Li, J.; Chen, Y.F.; Ning, R.; Ao, Y.F.; Liu, J.M.; Sun, J.; Wang, D.X.; Wang, Q.Q. Cage Based Crystalline Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 3843–3848. [Google Scholar] [CrossRef]
- Fang, S.; Wang, M.; Wu, Y.; Guo, Q.H.; Li, E.; Li, H.; Huang, F. Cagearenes: Synthesis, characterization, and application for programmed vapour release. Chem. Sci. 2022, 13, 6254–6261. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.M.; Rebek, J. Molecules in Confined Spaces: Reactivities and Possibilities in Cavitands. Chem 2020, 6, 1265–1274. [Google Scholar] [CrossRef]
- Warmuth, R. Reactions Inside Carcerands. In Molecular Encapsulation; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 227–268. [Google Scholar]
- Pappalardo, A.; Puglisi, R.; Sfrazzetto, G.T. Catalysis inside Supramolecular Capsules: Recent Developments. Catalysts 2019, 9, 630. [Google Scholar] [CrossRef]
- Zhang, Q.; Catti, L.; Tiefenbacher, K. Catalysis inside the Hexameric Resorcinarene Capsule. Acc. Chem. Res. 2018, 51, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Ao, Y.F.; Wang, D.X.; Wang, Q.Q. Exploiting anion–π interactions for efficient and selective catalysis with chiral molecular cages. Angew. Chem. Int. Ed. 2021, 60, 20650–20655. [Google Scholar] [CrossRef]
- Galan, A.; Ballester, P. Stabilization of Reactive Species by Supramolecular Encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. [Google Scholar] [CrossRef]
- Ballester, P. Anion Binding in Covalent and Self-Assembled Molecular Capsules. Chem. Soc. Rev. 2010, 39, 3810–3830. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, W.; Chen, C.H.; Flood, A.H. Chloride capture using a C-H hydrogen-bonding cage. Science 2019, 365, 159–161. [Google Scholar] [PubMed]
- MacDowell, D.; Nelson, J. Facile synthesis of a new family of cage molecules. Tetrahedron Lett. 1988, 29, 385–386. [Google Scholar] [CrossRef]
- Cao, N.; Wang, Y.; Zheng, X.; Jiao, T.; Li, H. Controllable Self-Assembly of Pills and Cages via Imine Condensation for Silver Cation Detection. Org. Lett. 2018, 20, 7447–7450. [Google Scholar] [CrossRef]
- Fang, S.; Sun, W.; Lin, C.; Huang, F.; Li, H. Self-Assembled Cage for In Situ Detecting Silver Cation in Water. Inorg. Chem. 2022, 62, 1776–1780. [Google Scholar] [CrossRef]
- Ding, Y.; Alimi, L.O.; Moosa, B.; Maaliki, C.; Jacquemin, J.; Huang, F.; Khashab, N.M. Selective adsorptive separation of cyclohexane over benzene using thienothiophene cages. Chem. Sci. 2021, 12, 5315–5318. [Google Scholar] [CrossRef]
- Moosa, B.A.; Alimi, L.O.; Shkurenko, A.; Fakim, A.; Bhatt, P.M.; Zhang, G.; Eddaoudi, M.; Khashab, N.M. A polymorphic azobenzene cage for energy efficient and highly selective p-Xylene separation. Angew. Chem. Int. Ed. 2020, 59, 21367–21371. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Herber, R.F.M.; Alessio, L. Cadmium in the Human Environment; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Shi, C.T.; Huang, Z.Y.; Wu, A.B.; Hu, Y.X.; Wang, N.C.; Zhang, Y.; Shu, W.M.; Yu, W.C. Recent progress in cadmium fluorescent and colorimetric probes. RSC Adv. 2021, 11, 29632–29660. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Goshisht, M.K.; Patra, G.K.; Tripathi, N. Fluorescent Schiff base sensors as a versatile tool for metal ion detection: Strategies, mechanistic insights, and applications. Mater. Adv. 2022, 3, 2612–2669. [Google Scholar] [CrossRef]
- Chaney, R.L.; Ryan, J.A.; Li, Y.M.; Brown, S.L. Cadmium in Soils and Plants; McLaughlin, M.J., Singh, B., Eds.; Kluwer: Boston, MA, USA, 1999. [Google Scholar]
- Goyer, R.A.; Liu, J.; Waalkes, M.P. Cadmium and cancer of prostate and testis. BioMetals 2004, 17, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.B.; Williams, D.J.; Moore, M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.; Yoon, J. Fluorescent and colorimetric chemosensors for detection of nucleotides, FAD and NADH: Highlighted research during 2004–2010. Chem. Soc. Rev. 2011, 40, 2222–2235. [Google Scholar] [CrossRef]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, J.S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, K.; Iniya, M.; Jeyanthi, D.; Siva, A.; Chellappa, D. A new multifunctional benzimidazole tagged coumarin as ratiometric fluorophore for the detection of Cd2+/F− ions and imaging in live cells. Spectrochim. Acta Part A 2018, 205, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.B.; Li, S.Y.; Lu, R.M.; Liu, G.; Pu, S.Z. A multi-functional hydrazinobenzothiazole-based diarylethene derivative: Highly efficient discrimination cadmium ion from zinc ion and near-infrared absorption detection of hydroxide ion. Dye. Pigment. 2017, 146, 305–315. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, R.J.; Fan, C.B.; Liu, G.; Pu, S.Z. A highly selective fluorescent sensor for Cd2+ based on a new diarylethene with a 1,8-naphthyridine unit. Dyes Pigment. 2017, 139, 208–217. [Google Scholar] [CrossRef]
- Li, S.; Liu, K.; Feng, X.-C.; Li, Z.-X.; Zhang, Z.Y.; Wang, B.; Li, M.; Bai, Y.-L.; Cui, L.; Li, C. Synthesis and macrocyclization-induced emission enhancement of benzothiadiazole-based macrocycle. Nat. Commun. 2022, 13, 2850. [Google Scholar] [CrossRef]
- Ding, M.H.; Tang, L.L.; Liao, J.; Ou, G.C.; Zeng, F. High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene. Chin. Chem. Lett. 2021, 5, 1665–1668. [Google Scholar] [CrossRef]
- Zeng, F.; Liao, J.; Ding, M.H.; Ou, G.C. Self-assembled macrocycle that binds polycyclic aromatic hydrocarbons. Dye. Pigment. 2021, 192, 109430. [Google Scholar] [CrossRef]
- Zeng, F.; Cheng, L.; Ou, G.C.; Tang, L.L.; Ding, M.H. Pyromellitic Diimide-Extended Pillar[6]arene: Synthesis, Structure, and Its Complexation with Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2022, 87, 3863–3867. [Google Scholar] [CrossRef]
- Zeng, F.; Cheng, L.; Zhang, W.J.; Tang, L.L.; Wang, X.F. Phenanthrene[2]arene: Synthesis and application as nonporous adaptive crystals in the separation of benzene from cyclohexane. Org. Chem. Front. 2022, 9, 3307–3311. [Google Scholar] [CrossRef]
- Zeng, F.; Xiao, X.S.; Gong, S.F.; Yuan, L.; Tang, L.L. An electron-deficient supramolecular macrocyclic host for the selective separation of aromatics and cyclic aliphatics. Org. Chem. Front. 2022, 9, 4829–4833. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Peng, B.; Chang, Y.; Li, Y.; Zhong, C. A thiadiazole-based covalent triazine framework nanosheet for highly selective and sensitive primary aromatic amine detection among various amines. J. Mater. Chem. A 2020, 8, 16542–16550. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-C.; Tan, Y.-Z.; Yu, H.; Bao, W.-H.; Tang, L.-L.; Zeng, F. A Benzothiadiazole-Based Self-Assembled Cage for Cadmium Detection. Molecules 2023, 28, 1841. https://doi.org/10.3390/molecules28041841
Wang Z-C, Tan Y-Z, Yu H, Bao W-H, Tang L-L, Zeng F. A Benzothiadiazole-Based Self-Assembled Cage for Cadmium Detection. Molecules. 2023; 28(4):1841. https://doi.org/10.3390/molecules28041841
Chicago/Turabian StyleWang, Zong-Cheng, Ying-Zi Tan, Hui Yu, Wen-Hu Bao, Lin-Li Tang, and Fei Zeng. 2023. "A Benzothiadiazole-Based Self-Assembled Cage for Cadmium Detection" Molecules 28, no. 4: 1841. https://doi.org/10.3390/molecules28041841
APA StyleWang, Z.-C., Tan, Y.-Z., Yu, H., Bao, W.-H., Tang, L.-L., & Zeng, F. (2023). A Benzothiadiazole-Based Self-Assembled Cage for Cadmium Detection. Molecules, 28(4), 1841. https://doi.org/10.3390/molecules28041841




