Standardization of the Optimum Effects of Indole 3-Butyric Acid (IBA) to Control Root Knot Nematode, Meloidogyne enterolobii, in Guava (Psidium guajava L.)
Abstract
1. Introduction
2. Results
2.1. Effects of Indole 3-Butyric Acid (IBA) on the Morphological Characteristics of M. enterolobii-Infected Lucknow-49 Guava Plants
2.1.1. Plant Height (cm)
2.1.2. Root Length (cm)
2.1.3. Fresh Root Biomass (g)
2.1.4. Dry Root Biomass (g)
2.2. Effects of Indole 3-Butyric Acid (IBA) on the Physiological Characteristics of M. enterolobii-Infected Lucknow-49 Guava Plants
2.2.1. Chlorophyll Index Assessed Using SPAD Meter
2.2.2. Photosynthetic Rate (µmol CO2 m−2 s−1)
2.2.3. Transpiration Rate (µmol H2O m−2 s−1)
2.2.4. Stomatal Conductance (mol H2O m−2 s−1)
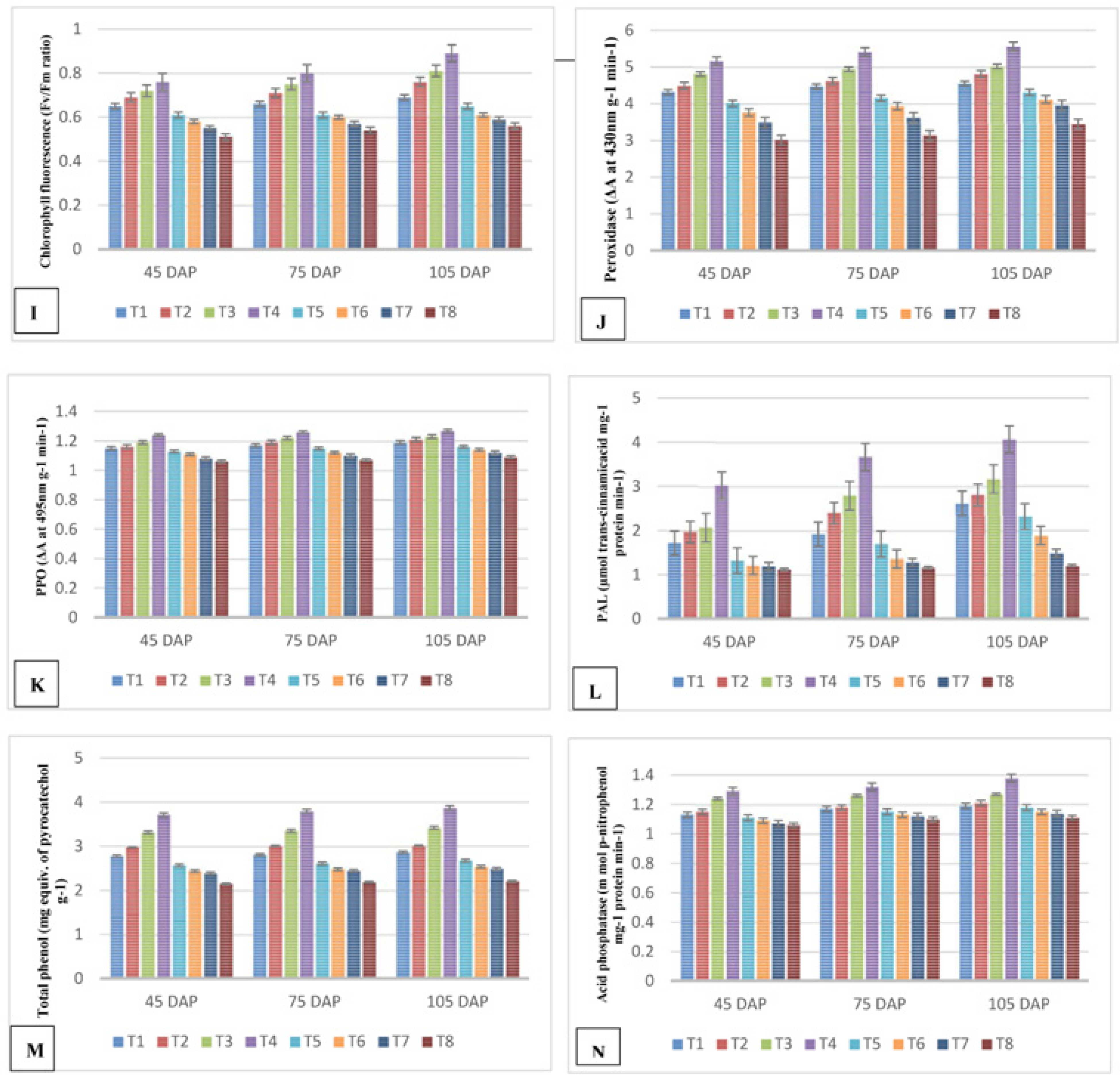
2.2.5. Chlorophyll Fluorescence (Fv/Fm Ratio)
2.3. Effects of Indole 3-Butyric Acid (IBA) on the Biochemical Characteristics of M. enterolobii-Infected Guava Plants (L-49)
2.3.1. Peroxidase Activity (PO)
2.3.2. Polyphenol Oxidase (PPO) Activity
2.3.3. Phenylalanine Ammonia Lyase (PAL) Activity
2.3.4. Total Phenol Content
2.3.5. Acid Phosphatase Activity
2.4. Nematode Population in Soil
2.5. Nematode Population in Root
3. Discussion
3.1. Effects of Indole Butyric Acid on the Plant Height and Root Length of M. enterolobii-Infested Guava
3.2. Effects of Indole Butyric Acid on the Fresh Weight and Dry Root Weight of Guava Infested by M. enterolobii
3.3. Effects of Indole Butyric Acid on the photosynthetic Rate, Transpiration Rate, Stomatal Conductance on M. enteroloii
3.4. Effects of Indole Butyric Acid on Chlorophyll Index in Guava Infested by M. enterolobii
3.5. Effects of Indole Butyric Acid on Peroxidase (PO), Polyphenol Oxidase (PPO), Phenylalanine Ammonia Lyase, Total Phenol Content, and Acid Phosphatase (APS) Activity Levels in Guava Infested by M. enterolobii
4. Materials and Methods
4.1. Experimental Location
Glasshouse Conditions and Planting
4.2. Preparation and Application of Plant Growth Regulator IBA
4.3. Replication Details
4.4. Morphological Characteristics
4.4.1. Plant Height
4.4.2. Root Length
4.4.3. Fresh Root Biomass
4.4.4. Dry Root Biomass
4.5. Physiological Characteristics
4.5.1. Chlorophyll Index
4.5.2. Photosynthetic Rate
4.5.3. Transpiration Rate
4.5.4. Stomatal Conductance
4.5.5. Chlorophyll Fluorescence (Fv/Fm Ratio)
4.6. Statistical Analysis
4.7. Biochemical Parameters
4.7.1. Estimation of Total Phenols
4.7.2. Assay of Peroxidase
4.7.3. Assay of Polyphenol Oxidase
4.7.4. Assay of Acid Phosphatase
4.7.5. Assay of Phenylalanine Ammonia Lyase
4.8. Nematode Population
4.8.1. Soil Nematode Population
4.8.2. Nematode Population in Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakasone, H.Y.; Paull, R.E. Tropical Fruits; Cab International: Wallingford, UK, 1998. [Google Scholar]
- Prakash, D.P.; Narayanaswamy, P.; Sondur, S.N. Analysis of molecular diversity in guava using RAPD markers. J. Hortic. Sci. Biotechnol. 2002, 77, 287–293. [Google Scholar] [CrossRef]
- NHB. Data base. In Yearly; National Horticultural Board, Department of Agriculture and Co-operation, Government of India: Gurugram, India, 2017. [Google Scholar]
- Morton, J.F. Guava. In Fruits of Warm Climates; J.F. Morton: Miami, FL, USA, 1987; pp. 356–363. [Google Scholar]
- Ashokkumar, N.; Poornima, K. Occurrence and distribution of root knot nematode, Meloidogyne enterolobii in guava (Psidium guajava L.) in Tamil Nadu. J. Pharmacogn. Phytochem. 2019, 8, 1922–1924. [Google Scholar]
- Ashokkumar, N.; Poornima, K.; Kalaiarasan, P. Embryogenesis, Penetration and Post penetration Development of Meloidogyne enterolobii in guava (Psidium guajava L.). Ann. Plant Prot. Sci. 2019, 27, 140–145. [Google Scholar] [CrossRef]
- Ashokkumar, N.; Poornima, K.; Kalaiarasan, P.; Jeyakumar, P.; Uma, D.; Kavino, M.; Kothai, S. Induction of defence-related proteins by selected plant growth regulators and biocontrol agents against guava root knot nematode, Meloidogyne enterolobii. J. Nematol. 2021, 53, e2021–e2081. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.A.; Shah, K.; Ahmed, I.; Basit, A.; Sajid, M.; Bano, A.S.; Ara, G.; Shahid, U. Influence of indole butyric acid (IBA) concentrations on air layerage in guava (Psidium guajava L.) cv. Sufeda. Pure Appl. Biol. 2019, 8, 355–362. [Google Scholar] [CrossRef]
- Sukhjit, K. Evaluation of different doses of Indole-3-bic acid (IBA) on the rooting survival and vegetative growth performance of hardwood cuttings of Flordaguard peach (Prunus persica L.Batch). J. Appl. Nutr. Sci. 2017, 9, 173–180. [Google Scholar]
- Mori, Y.; Miyahara, F.; Tsutsumi, Y.; Kondo, R. Effects of combinational treatment with ethephon and indole-3-butyric acid on adventitious rooting of Pinus thunbergii cuttings. Plant Growth Regul. 2011, 63, 271–278. [Google Scholar] [CrossRef]
- Bielenin, M. Rooting and gas exchange of conifer cuttings treated with indolebutyric acid. J. Fruit Ornam. Plant Res. 2003, 11, 99–106. [Google Scholar]
- Arora, R.; Singh, N. Growth regulators for yield and quality enhancement in litchi (Litchi chinensis L.)—A review. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2152–2159. [Google Scholar] [CrossRef]
- Pospisilova, J. Interaction of cytokinins and abscisic acid during regulation of stomatal opening in bean leaves. Photosynthetica 2003, 41, 49–56. [Google Scholar] [CrossRef]
- Perez, L.P.; Montesinos, Y.P.; Olmedo, J.G.; Sánchez, R.R.; Montenegro, O.N.; Rodriguez, R.B.; Ribalta, O.H.; Escriba, R.; Daniels, D.; Gómez-Kosky, R. Effects of different culture conditions (photoautotrophic, photomixotrophic) and the auxin indole-butyric acid on the in vitro acclimatization of papaya (Carica papaya L. var. Red Maradol) plants using zeolite as support. Afr. J. Biotechnol. 2015, 14, 2622–2635. [Google Scholar]
- El-Shraiy, A.M.; Hegazi, A.M. Effect of acetylsalicylic acid, indole-3-bytric acid and gibberellic acid on plant growth and yield of Pea (Pisum sativum L.). Aust. J. Basic Appl. Sci. 2009, 3, 3514–3523. [Google Scholar]
- Porfirio, S.; Calado, M.L.; Noceda, C.; Cabrita, M.J.; da Silva, M.G.; Azadi, P.; Peixe, A. Tracking biochemical changes during adventitious root formation in olive (Olea europaea L.). Sci. Hortic. 2016, 204, 41–53. [Google Scholar] [CrossRef]
- Aslmoshtaghi, E.; Shahsavar, A.R. Peroxidase, polyphenol oxidase and protein changes in olives during adventitious root formation. Trakia J. Sci. 2016, 14, 176–182. [Google Scholar] [CrossRef]
- Ashokkumar, N.; Poornima, K.; Kalaiarasan, P.; Kavino, M. Screening and histological characterization of guava (Psidium guajava L.) cultivars against root knot nematode, Meloidogyne enterolobii. Pest Manag. Hortic. Ecosyst. 2019, 25, 84–92. [Google Scholar] [CrossRef]
- Malick, C.P.; Singh, M.B. Phenolics. In Plant Enzymology and Histoenzymology; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Rad, A.M.; Ghourchian, H.; Moosavi-Movahedi, A.A.; Hong, J.; Nazari, K. Spectrophotometric assay for horseradish peroxidase activity based on pyrocatechol-aniline coupling hydrogen donor. Anal. Biochem. 2007, 362, 38–43. [Google Scholar]
- Mayer, A.M.; Harel, E.; Ben-Shaul, R. Assay of catechol oxidase-a critical comparison of methods. Phytochemistry 1966, 5, 783–789. [Google Scholar] [CrossRef]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium Carbonum. Physiol. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Schindler, A. A simple substitute for a Baermann funnel. Plant Dis. Report. 1961, 45, 747. [Google Scholar]
- McBeth, C.W.; Taylor, A.L.; Smith, A.L. Note on staining nematodes in root tissues. Proc. Helminthol. Soc. Wash. 1941, 8, 186–201. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natarajan, A.; Selvam, D.; Palaniappan, K.; Subbaiah Balamurali, A.; Perumal, C.; Durai, R.; Sadasivam, S.; Asokan, A.; Sivalingam, R.; Subiramaniyan, A. Standardization of the Optimum Effects of Indole 3-Butyric Acid (IBA) to Control Root Knot Nematode, Meloidogyne enterolobii, in Guava (Psidium guajava L.). Molecules 2023, 28, 1839. https://doi.org/10.3390/molecules28041839
Natarajan A, Selvam D, Palaniappan K, Subbaiah Balamurali A, Perumal C, Durai R, Sadasivam S, Asokan A, Sivalingam R, Subiramaniyan A. Standardization of the Optimum Effects of Indole 3-Butyric Acid (IBA) to Control Root Knot Nematode, Meloidogyne enterolobii, in Guava (Psidium guajava L.). Molecules. 2023; 28(4):1839. https://doi.org/10.3390/molecules28041839
Chicago/Turabian StyleNatarajan, Ashokkumar, Dharani Selvam, Kalaiarasan Palaniappan, Akshaya Subbaiah Balamurali, Chandrasekaran Perumal, Rameshkumar Durai, Shakila Sadasivam, Akino Asokan, Ramadass Sivalingam, and Ashok Subiramaniyan. 2023. "Standardization of the Optimum Effects of Indole 3-Butyric Acid (IBA) to Control Root Knot Nematode, Meloidogyne enterolobii, in Guava (Psidium guajava L.)" Molecules 28, no. 4: 1839. https://doi.org/10.3390/molecules28041839
APA StyleNatarajan, A., Selvam, D., Palaniappan, K., Subbaiah Balamurali, A., Perumal, C., Durai, R., Sadasivam, S., Asokan, A., Sivalingam, R., & Subiramaniyan, A. (2023). Standardization of the Optimum Effects of Indole 3-Butyric Acid (IBA) to Control Root Knot Nematode, Meloidogyne enterolobii, in Guava (Psidium guajava L.). Molecules, 28(4), 1839. https://doi.org/10.3390/molecules28041839





