Facile Transformation from Rofecoxib to a New Near-Infrared Lipid Droplet Fluorescent Probe and Its Investigations on AIE Property, Solvatochromism and Mechanochromism
Abstract
1. Introduction
2. Results and Discussion
2.1. Photophysical Property
2.2. Solvatochromism
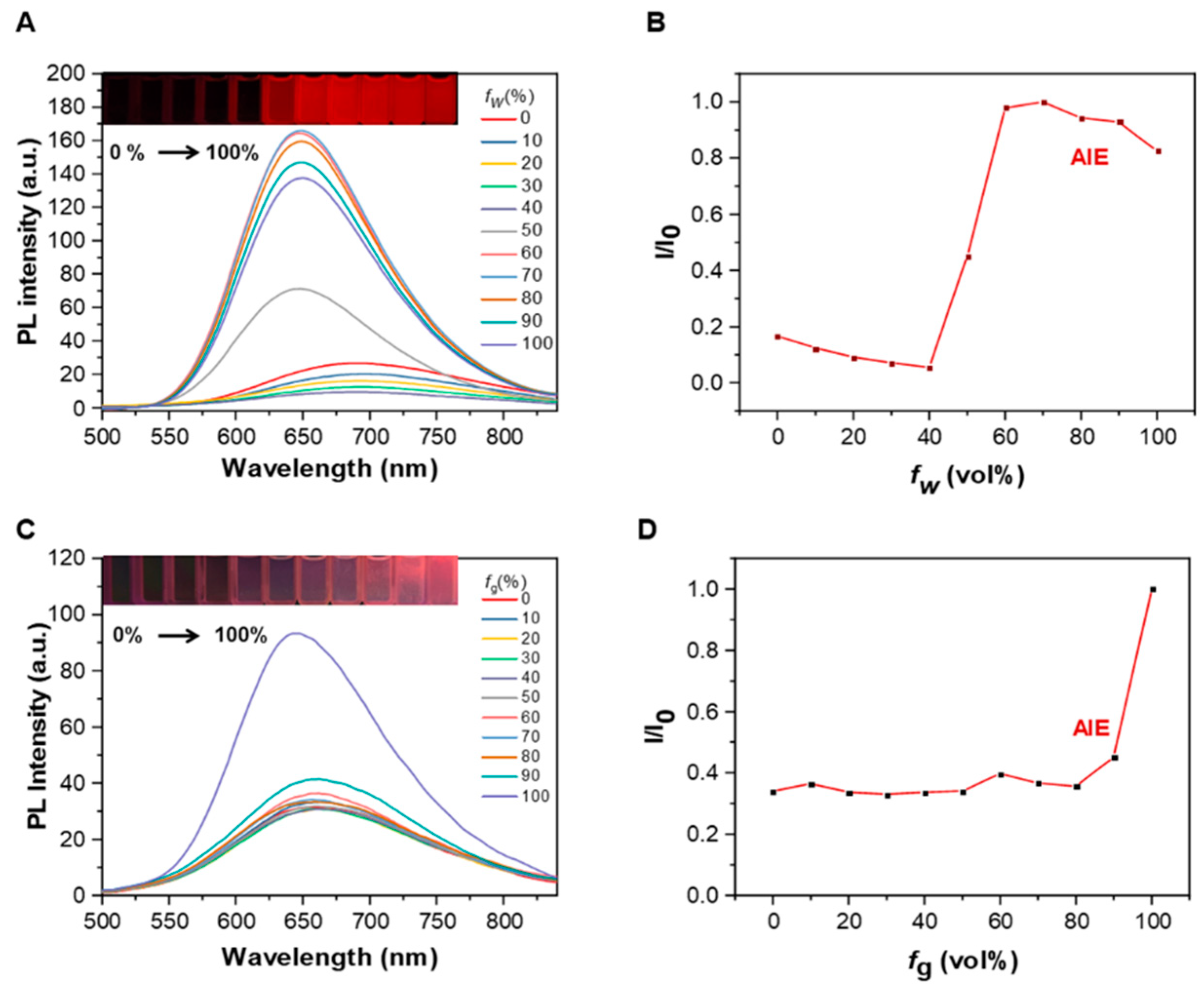
2.3. Aggregation-Induced Emission
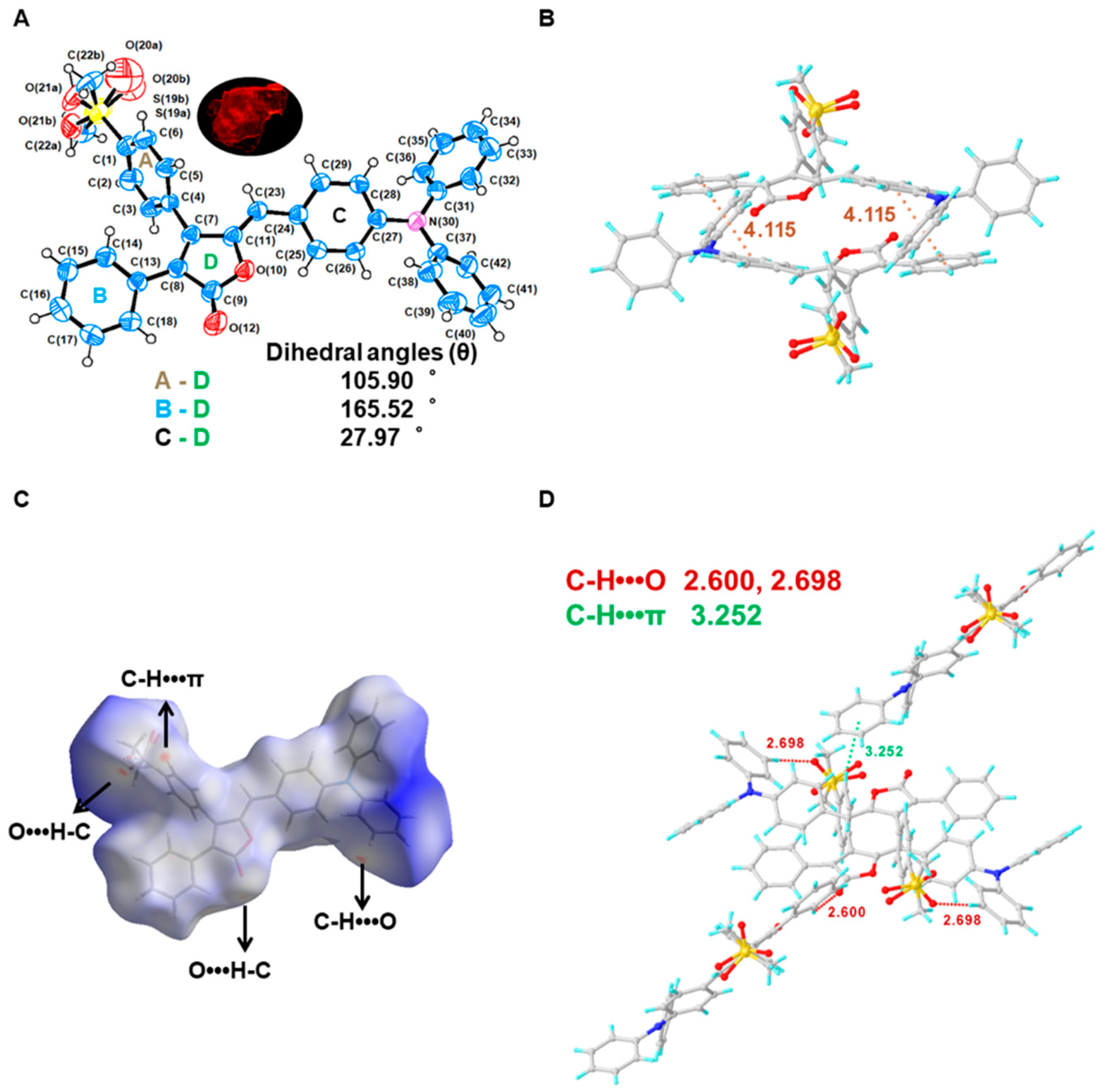
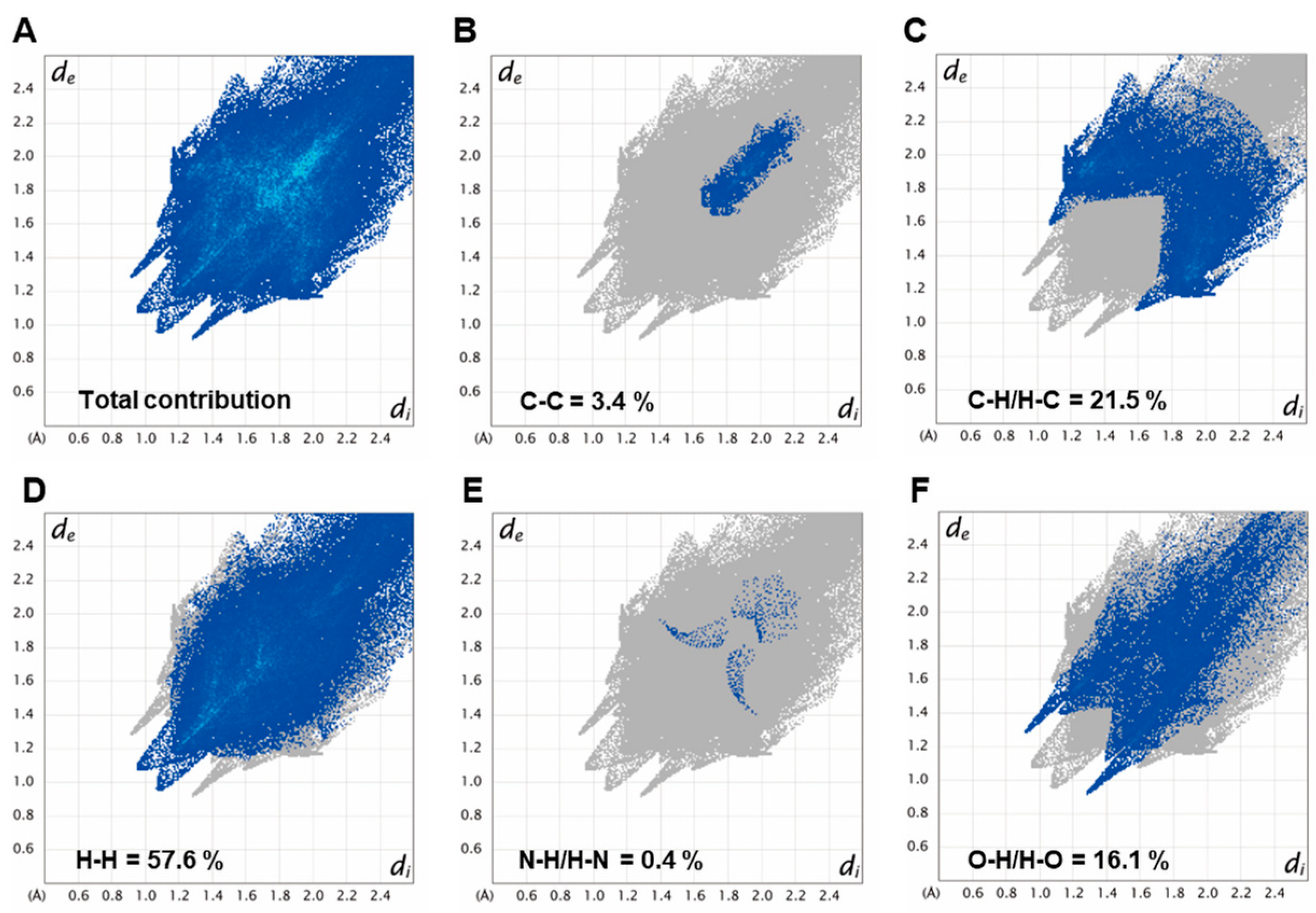
2.4. Single Crystal X-ray Analysis
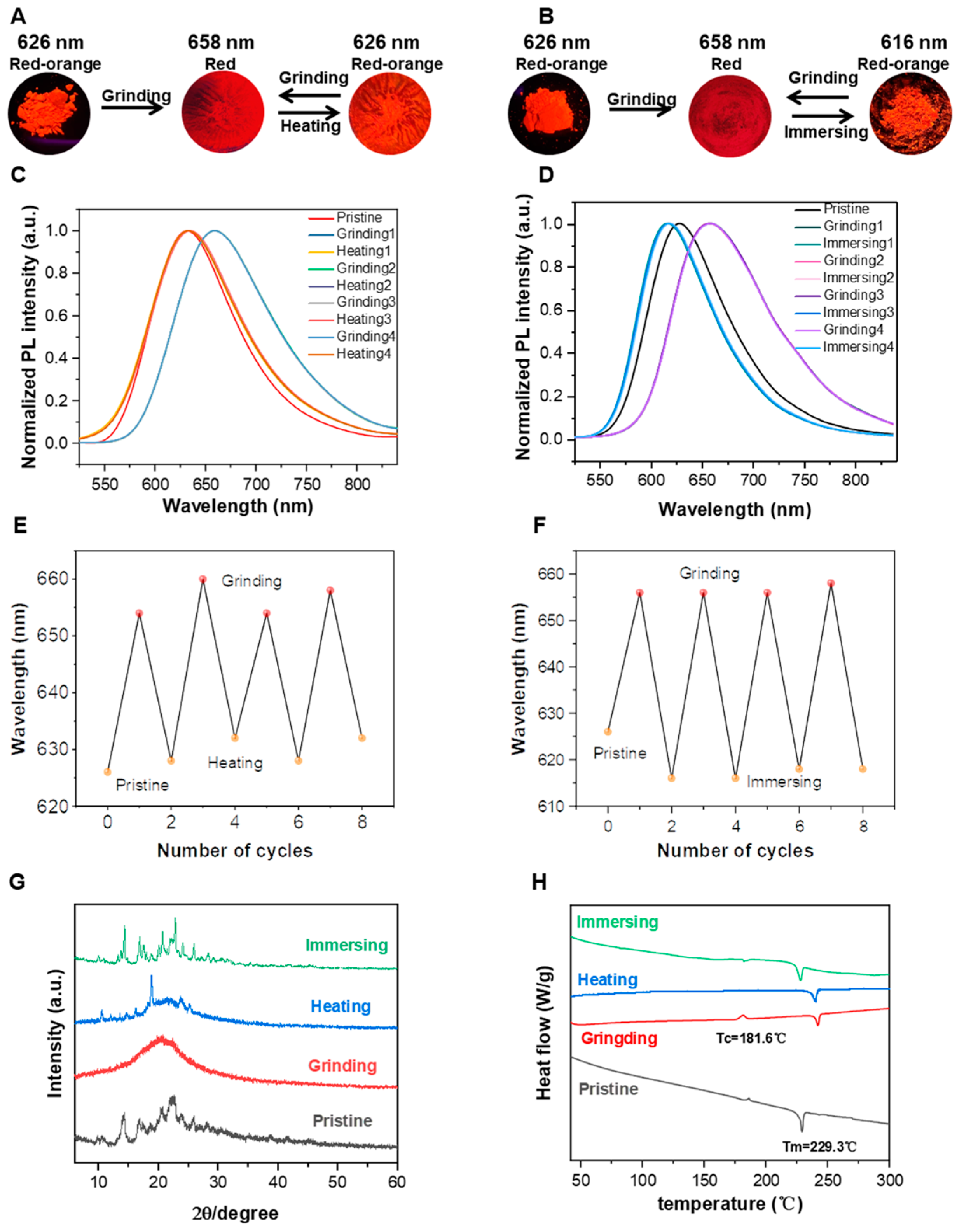
2.5. Mechanochromic Properties
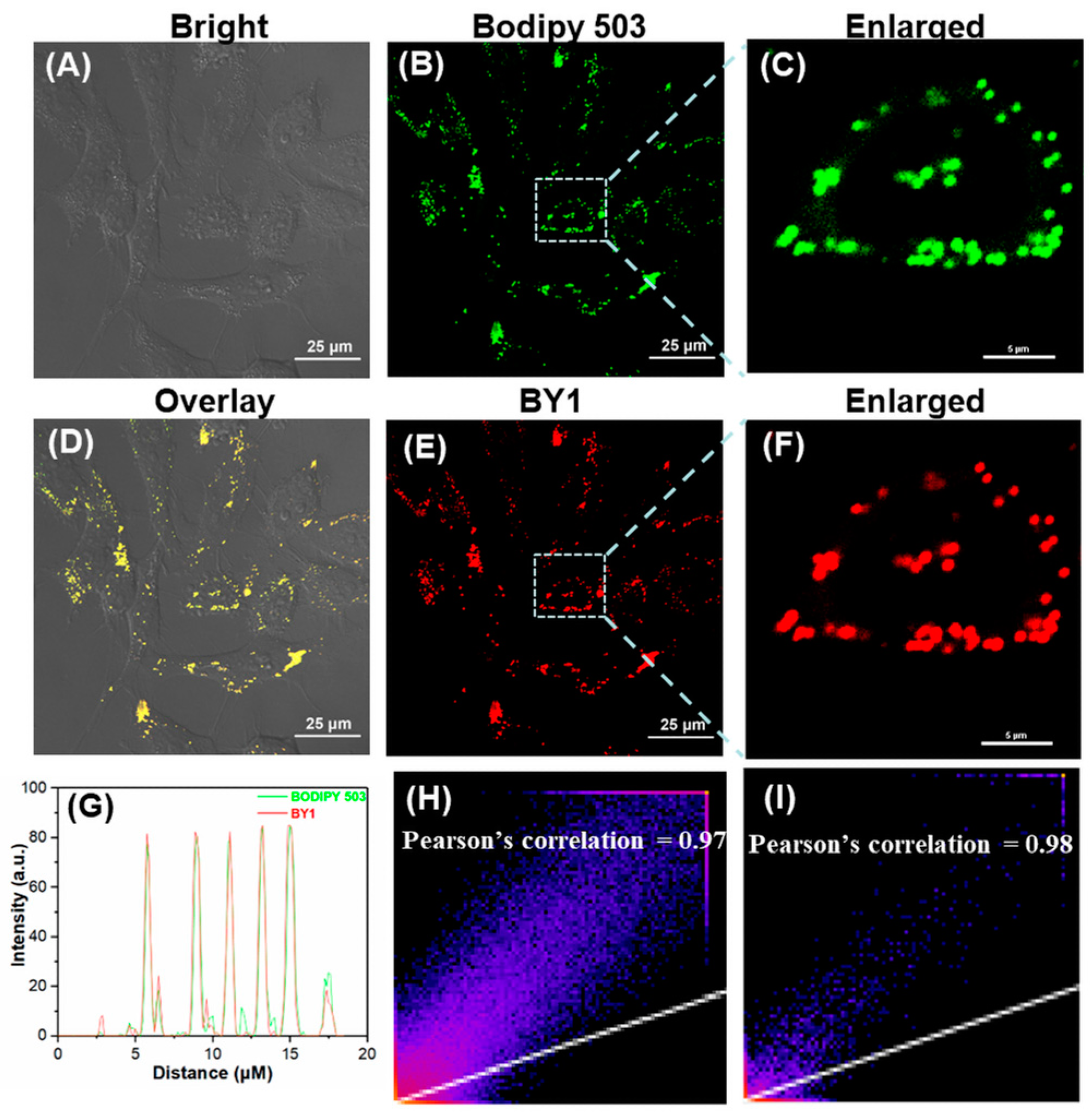
2.6. Lipid Droplet Imaging
3. Materials and Methods
3.1. General Information and Materials
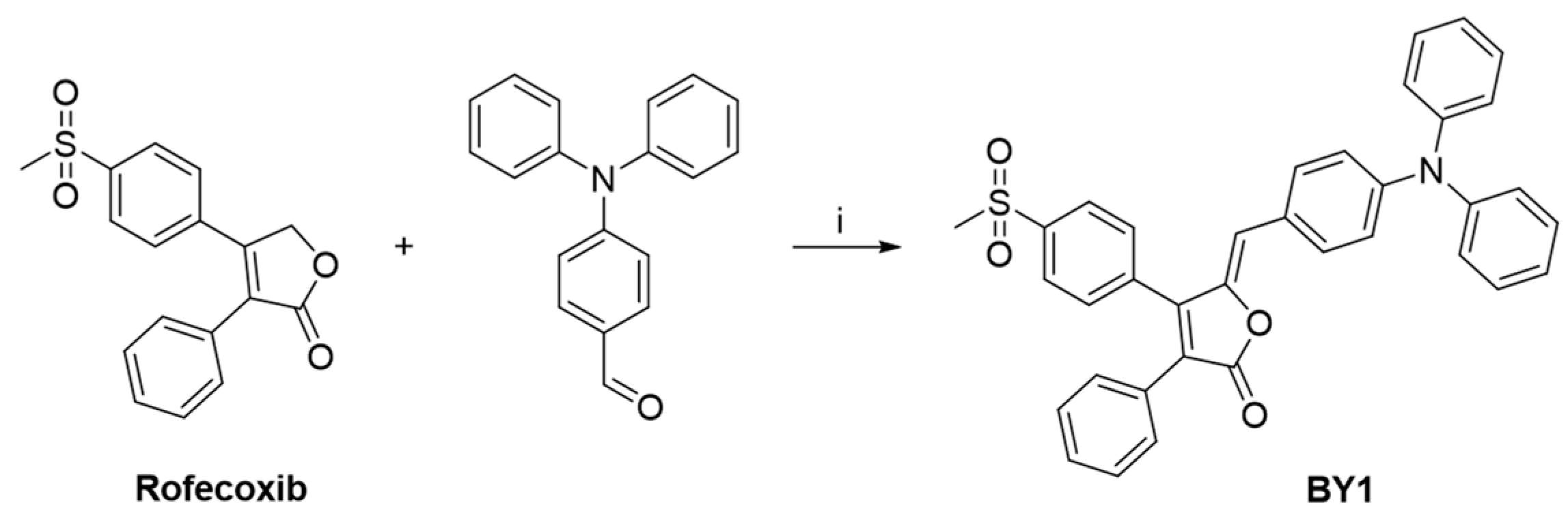
3.2. Synthesis
3.3. Fluorescent Microscope Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, X.; Wu, Y.; Liu, Q.; Li, Z.; Yan, H.; Ji, C.; Duan, J.; Liu, Z. Aggregation-induced emission (AIE) of pyridyl-enamido-based organoboron luminophores. Chem. Commun. 2015, 51, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Tang, B.Z.; Hong, Y. AIE Luminogens for Visualizing Cell Structures and Functions. In Aggregation-Induced Emission: Materials and Applications; American Chemical Society: Washington, DC, USA, 2016; Volume 1227, pp. 199–216. [Google Scholar] [CrossRef]
- Jiang, Y.; Hadjichristidis, N. Tetraphenylethene-functionalized polyethylene-based polymers with aggregation-induced emission. Macromolecules 2019, 52, 1955–1964. [Google Scholar] [CrossRef]
- Zhang, E.; Hou, X.; Zhang, Z.; Zhang, Y.; Wang, J.; Yang, H.; You, J.; Ju, P. A novel biomass-based reusable AIE material: AIE properties and potential applications in amine/ammonia vapor sensing and information storage. J. Mater. Chem. C 2019, 7, 8404–8411. [Google Scholar] [CrossRef]
- Li, W.; Ding, Y.; Tebyetekerwa, M.; Xie, Y.; Wang, L.; Li, H.; Hu, R.; Wang, Z.; Qin, A.; Tang, B.Z. Fluorescent aggregation-induced emission (AIE)-based thermosetting electrospun nanofibers: Fabrication, properties and applications. Mater. Chem. Front. 2019, 3, 2491–2498. [Google Scholar] [CrossRef]
- Panigrahi, A.; Are, V.N.; Jain, S.; Nayak, D.; Giri, S.; Sarma, T.K. Cationic organic nanoaggregates as AIE luminogens for wash-free imaging of bacteria and broad-spectrum antimicrobial application. ACS Appl. Mater. Interfaces 2020, 12, 5389–5402. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Wong, W.Y.; Ho, C.L. Functional metallophosphors for effective charge carrier injection/transport: New robust OLED materials with emerging applications. J. Mater. Chem. 2009, 19, 4457–4482. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, Y.; Cabry, C.P.; Zhou, D.; Xie, G.; Qu, Z.; Bruce, D.W.; Zhu, W. Highly efficient blueish-green fluorescent OLEDs based on AIE liquid crystal molecules: From ingenious molecular design to multifunction materials. J. Mater. Chem. C 2017, 5, 3999–4008. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Wei, Q.; Cao, L.; Wang, T.; Ge, Z. Efficient bipolar AIE emitters for high-performance nondoped OLEDs. J. Mater. Chem. C 2020, 8, 11771–11777. [Google Scholar] [CrossRef]
- Zuo, B.; Liu, L.; Feng, X.; Li, D.; Li, W.; Huang, M.; Deng, Q. A novel fluorescent sensor based on triphenylamine with AIE properties for the highly sensitive detection of CN−. Dyes Pigments 2021, 193, 109534. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, L.; Zhang, S.; Shen, X.; Huang, C. Specific “light-up” sensor made easy: An aggregation induced emission monomer for molecular imprinting. Biosens. Bioelectron. 2022, 205, 114113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, H.; Lv, W.; Gai, P.; Li, F. Equipment-free and visual detection of multiple biomarkers via an aggregation induced emission luminogen-based paper biosensor. Biosens. Bioelectron. 2020, 165, 112336. [Google Scholar] [CrossRef]
- Xie, L.; Li, R.; Zheng, B.; Xie, Z.; Fang, X.; Dai, T.; Wang, X.; Li, L.; Wang, L.; Cuny, G.D.; et al. One-step transformation from rofecoxib to a COX-2 NIR probe for human cancer tissue/organoid targeted bioimaging. ACS Appl. Bio Mater. 2021, 4, 2723–2731. [Google Scholar] [CrossRef]
- He, T.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Photoluminescence: Novel Quercetin Aggregation-Induced Emission Luminogen (AIEgen) with Excited-State Intramolecular Proton Transfer for In Vivo Bioimaging. Adv. Funct. Mater. 2018, 28, 1870068. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS responsive nanoplatform with two-photon AIE imaging for atherosclerosis diagnosis and “two-pronged” therapy. Small 2020, 16, 2003253. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Q.; Zhang, Y.; Li, Y.; Feng, Z.; Zhou, J.; Qi, J.; Lin, H. Aggregation-Induced Emission (AIE) Nanoparticles-Assisted NIR-II Fluorescence Imaging-Guided Diagnosis and Surgery for Inflammatory Bowel Disease (IBD). Adv. Healthc. Mater. 2021, 10, 2101043. [Google Scholar] [CrossRef]
- Hu, J.; Liu, R.; Zhai, S.; Wu, Y.; Zhang, H.; Cheng, H.; Zhu, H. AIE-active molecule-based self-assembled nano-fibrous films for sensitive detection of volatile organic amines. J. Mater. Chem. C 2017, 5, 11781–11789. [Google Scholar] [CrossRef]
- Wu, M.Y.; Leung, J.K.; Kam, C.; Chou, T.Y.; Wang, D.; Feng, S.; Chen, S.J. A near-infrared aie probe for super-resolution imaging and nuclear lipid droplet dynamic study. Mater. Chem. Front. 2021, 5, 3043–3049. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.; Shan, G.; Leung, A.C.; Lee, M.M.; Sung, H.H.; Williams, L.D.; Lam, J.W.; Tang, B.Z. Facile synthesis of red/NIR AIE luminogens with simple structures, bright emissions, and high photostabilities, and their applications for specific imaging of lipid droplets and image-guided photodynamic therapy. Adv. Funct. Mater. 2017, 27, 1704039. [Google Scholar] [CrossRef]
- Gocze, P.M.; Freeman, D.A. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry J. Int. Soc. Analytical Cytol. 1994, 17, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef]
- Liang, J.; Tang, B.Z.; Liu, B. Specific light-up bioprobes based on AIEgen conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. [Google Scholar] [CrossRef]
- Wang, E.; Zhao, E.; Hong, Y.; Lam, J.W.; Tang, B.Z. A highly selective AIE fluorogen for lipid droplet imaging in live cells and green algae. J. Mater. Chem. B 2014, 2, 2013–2019. [Google Scholar] [CrossRef]
- Jiang, M.; Gu, X.; Lam, J.W.; Zhang, Y.; Kwok, R.T.; Wong, K.S.; Tang, B.Z. Two-photon AIE bio-probe with large Stokes shift for specific imaging of lipid droplets. Chem. Sci. 2017, 8, 5440–5446. [Google Scholar] [CrossRef]
- Qin, W.; Li, K.; Feng, G.; Li, M.; Yang, Z.; Liu, B.; Tang, B.Z. Bright and photostable organic fluorescent dots with aggregation-induced emission characteristics for noninvasive long-term cell imaging. Adv. Funct. Mater. 2014, 24, 635–643. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible nanoparticles with aggregation-induced emission characteristics as far-red/near-infrared fluorescent bioprobes for in vitro and in vivo imaging applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Grabowski, Z.; Dobkowski, J. Twisted intramolecular charge transfer (TICT) excited states: Energy and molecular structure. Pure Appl. Chem. 1983, 55, 245–252. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Yang, J.; Hua, J.; Wang, B.; Qian, S.; Tian, H. Synthesis, two-photon absorption, and optical power limiting of new linear and hyperbranched conjugated polyynes based on bithiazole and triphenylamine. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1830–1839. [Google Scholar] [CrossRef]
- Sun, J.; Dai, Y.; Ouyang, M.; Zhang, Y.; Zhan, L.; Zhang, C. Unique torsional cruciform π-architectures composed of donor and acceptor axes exhibiting mechanochromic and electrochromic properties. J. Mater. Chem. C 2015, 3, 3356–3363. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Li, M.; Song, Y.; Fan, J.; Wang, C.K.; Lin, L. Theoretical Study on the Light-Emitting Mechanism of Multifunctional Thermally Activated Delayed Fluorescence Molecules. J. Phys. Chem. C 2022, 126, 2437–2446. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.; Sung, H.H.; Williams, L.D.; Zhong, Y.; Wong, K.S.; Pena-Cabrera, E.; et al. Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Albrecht, C.; Joseph, R. Lakowicz: Principles of fluorescence spectroscopy, 3rd Edition. Anal. Bioanal. Chem. 2008, 390, 1223–1224. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, W.; Lam, J.W.; Chen, S.; Sung, H.H.; Williams, I.D.; Tang, B.Z. Fluorescent pH sensor constructed from a heteroatom-containing luminogen with tunable AIE and ICT characteristics. Chem. Sci. 2013, 4, 3725–3730. [Google Scholar] [CrossRef]
- Gao, S.; Wei, G.; Zhang, S.; Zheng, B.; Xu, J.; Chen, G.; Li, M.; Song, S.; Fu, W.; Xiao, Z.; et al. Albumin tailoring fluorescence and photothermal conversion effect of near-infrared-II fluorophore with aggregation-induced emission characteristics. Nat. Commun. 2019, 10, 2206. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, Z.; Dong, Y.; Qin, A.; Hong, Y.; Ji, L.; Zhu, Z.; Jim, C.K.W.; Yu, G.; Li, Q.; et al. Fluorescence enhancements of benzene-cored luminophors by restricted intramolecular rotations: AIE and AIEE effects. ChemComm 2007, 1, 70–72. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Pei, Y.; Xie, J.; Cui, D.; Liu, S.; Li, G.; Zhu, D.; Su, Z. A mechanochromic cyclemetalated cationic Ir (III) complex with AIE activity by strategic modification of ligands. Dalton Trans. 2020, 49, 13066–13071. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.W.; Jiang, K.; Li, J.X.; Luo, S.H.; Wang, Z.Y.; Jiang, H.F. 1, 1-Diphenylvinylsulfide as a Functional AIEgen Derived from the Aggregation-Caused-Quenching Molecule 1, 1-Diphenylethene through Simple Thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Liu, Y.; Yang, B.; Qiao, H.; Chai, J.; Wen, G.; Liu, B. Rational construction of AIEgens with wide color tunability and their specific lipid droplet imaging applications. J. Mater. Chem. B 2020, 8, 9533–9543. [Google Scholar] [CrossRef]

| Compound | QY (0% H2O) | QY (70% H2O) | Powder |
|---|---|---|---|
| BY1 | 1.41% | 8.64% | 11.56% |
| Compound | λpristine (nm) | λgrinding (nm) | λimmersing (nm) | λheating (nm) | ∆λ (nm) |
|---|---|---|---|---|---|
| BY1 | 626 | 658 | 616 | 626 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Liu, W.; Wang, Z.; Chen, N.; Zhou, J.; Wu, T.; Ye, Y.; Ke, Y.; Jiang, H.; Zhai, X.; et al. Facile Transformation from Rofecoxib to a New Near-Infrared Lipid Droplet Fluorescent Probe and Its Investigations on AIE Property, Solvatochromism and Mechanochromism. Molecules 2023, 28, 1814. https://doi.org/10.3390/molecules28041814
Wei Y, Liu W, Wang Z, Chen N, Zhou J, Wu T, Ye Y, Ke Y, Jiang H, Zhai X, et al. Facile Transformation from Rofecoxib to a New Near-Infrared Lipid Droplet Fluorescent Probe and Its Investigations on AIE Property, Solvatochromism and Mechanochromism. Molecules. 2023; 28(4):1814. https://doi.org/10.3390/molecules28041814
Chicago/Turabian StyleWei, Yongbo, Wei Liu, Zexin Wang, Nannan Chen, Jingming Zhou, Tong Wu, Yuqiu Ye, Yanbing Ke, Hong Jiang, Xin Zhai, and et al. 2023. "Facile Transformation from Rofecoxib to a New Near-Infrared Lipid Droplet Fluorescent Probe and Its Investigations on AIE Property, Solvatochromism and Mechanochromism" Molecules 28, no. 4: 1814. https://doi.org/10.3390/molecules28041814
APA StyleWei, Y., Liu, W., Wang, Z., Chen, N., Zhou, J., Wu, T., Ye, Y., Ke, Y., Jiang, H., Zhai, X., & Xie, L. (2023). Facile Transformation from Rofecoxib to a New Near-Infrared Lipid Droplet Fluorescent Probe and Its Investigations on AIE Property, Solvatochromism and Mechanochromism. Molecules, 28(4), 1814. https://doi.org/10.3390/molecules28041814







