Synthesis and Characterization of Curcumin-Chitosan Loaded Gold Nanoparticles by Oryctes rhinoceros’ Chitin for Cosmeceutical Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of CCG-NP
2.1.1. FTIR
2.1.2. Measurement of Zeta Potential via Zeta Sizer
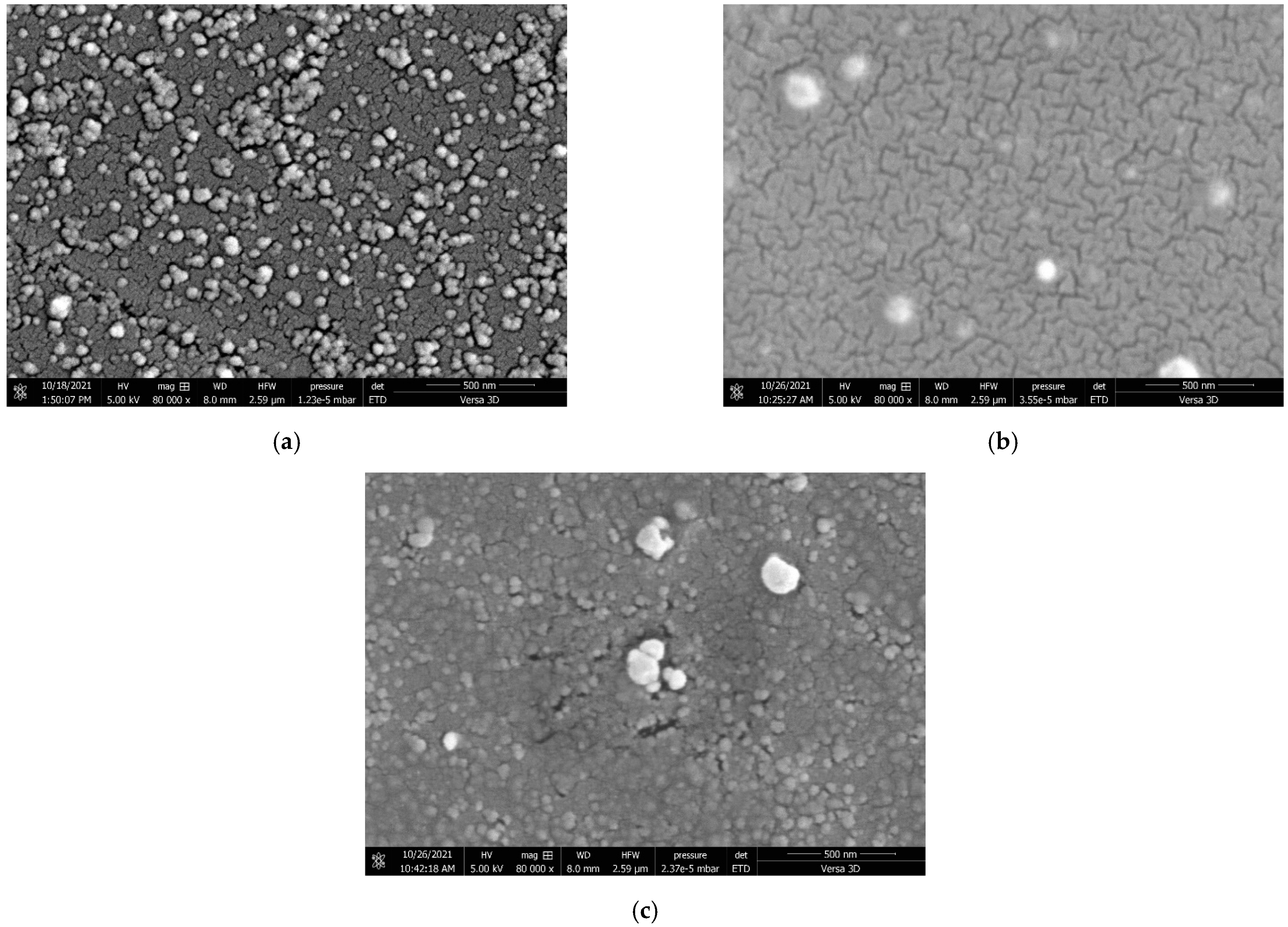
2.1.3. Morphological Properties of CCG-NP via FE-SEM
2.1.4. Particle Size Distribution Analysis via Zeta Sizer
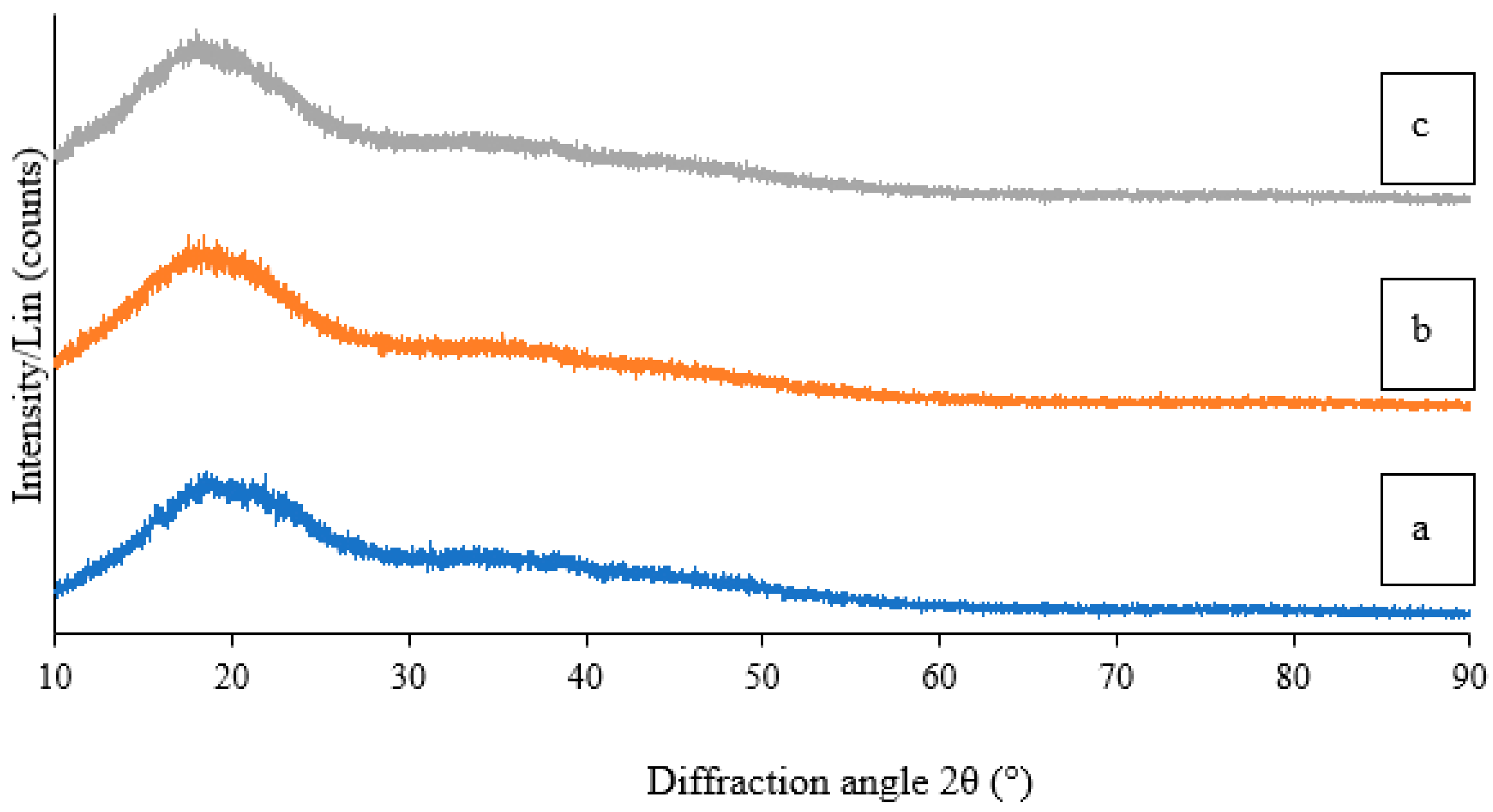
2.1.5. Crystallinity via X-ray Diffraction (XRD)
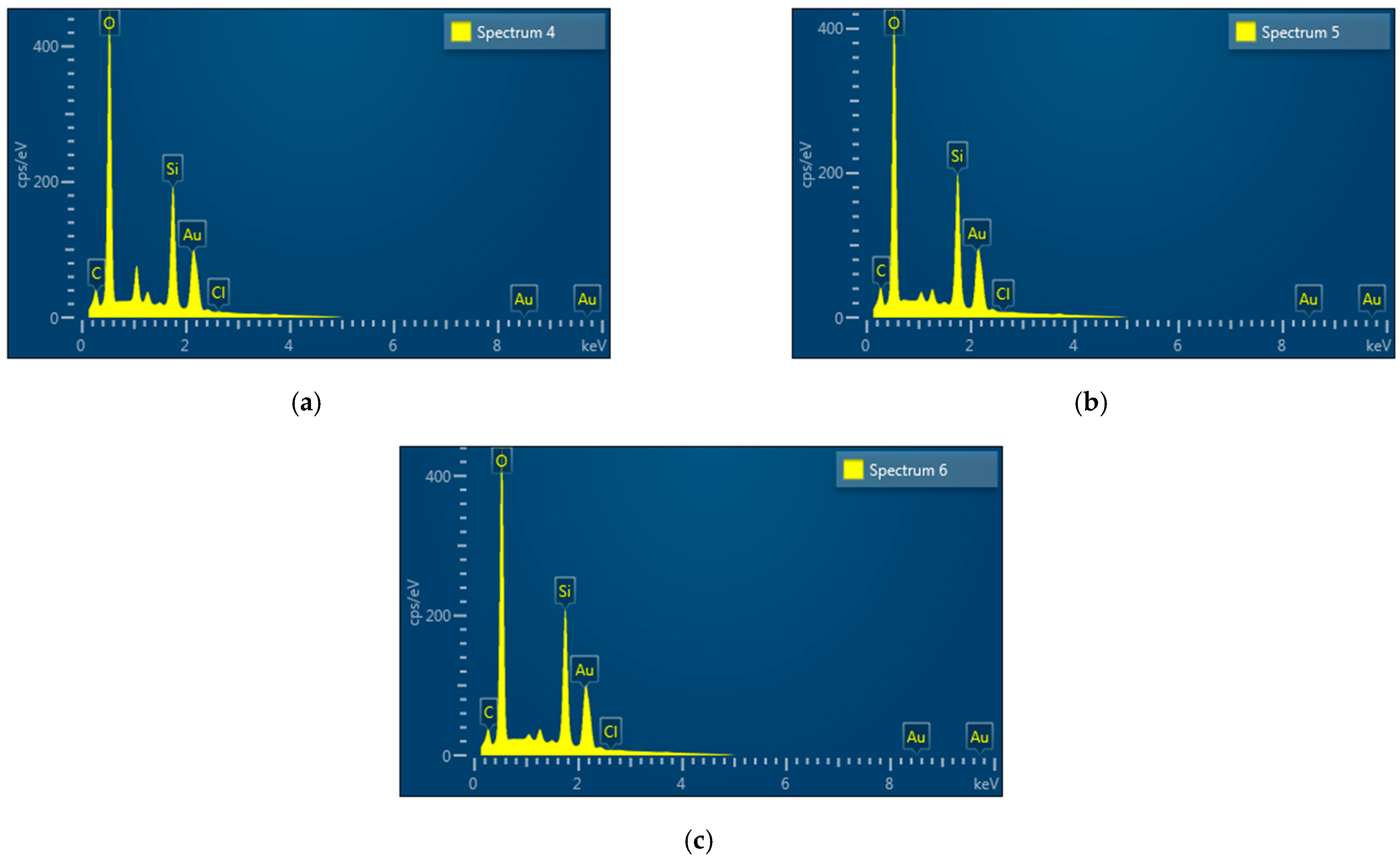
2.1.6. EDX
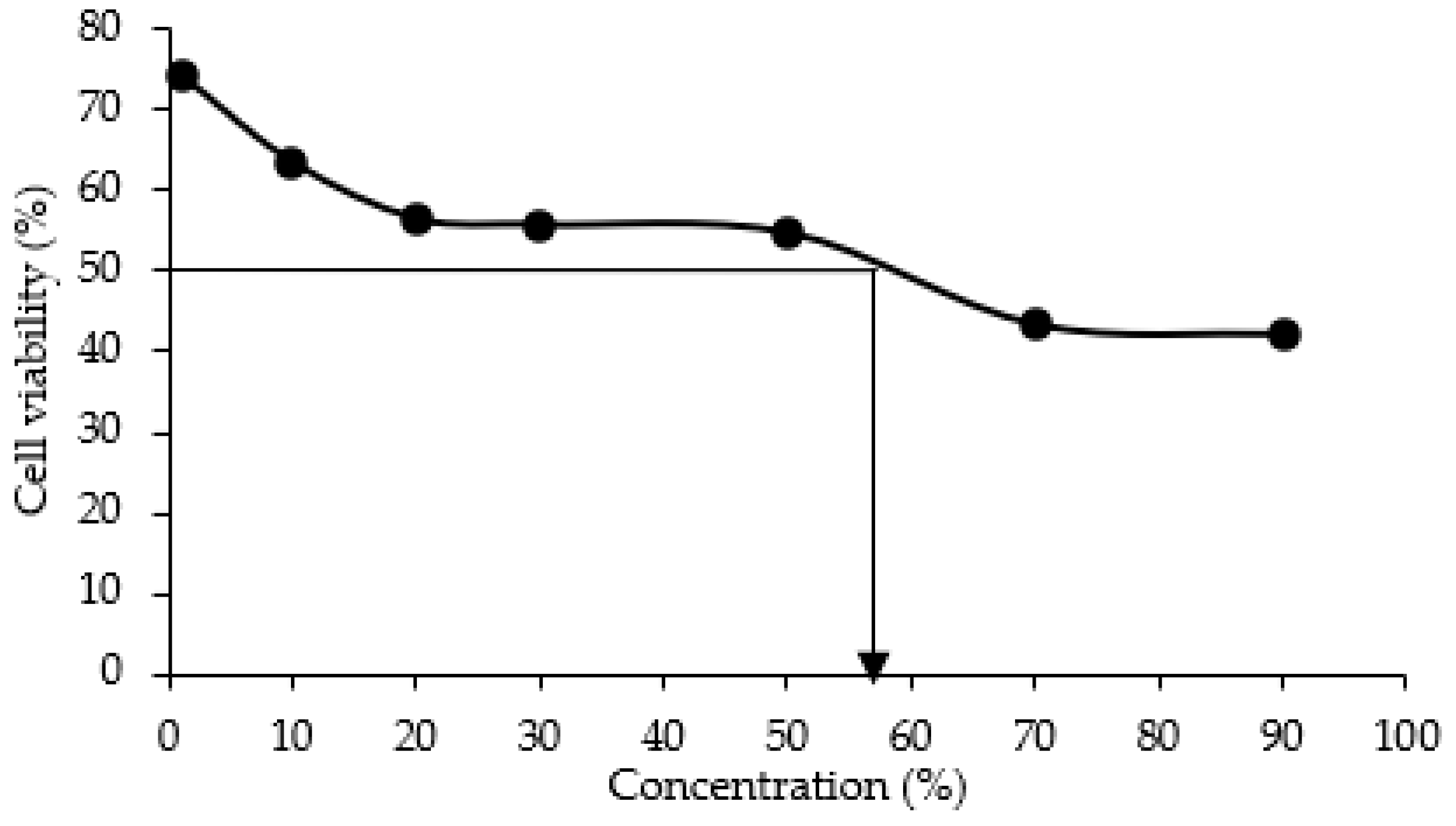
2.1.7. Toxicity Test by MTT Assay
2.1.8. Significance of the Applied Value of This Research in Cosmeceutical Application
3. Material and Methods
3.1. Preparation of Chitosan from O. rhinoceros
3.2. Preparation of CCG-NP
3.3. Characterization of CCG-NP
3.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.2. Measurement of Zeta Potential via Zeta Sizer
3.3.3. Morphological Properties of CCG-NP via FE-SEM
3.3.4. Particle Size Distribution Analysis via Zeta Sizer
3.3.5. Crystallinity via X-ray Diffraction (XRD)
3.3.6. Elemental Analysis via Energy Dispersion X-ray (EDX)
3.3.7. Toxicity Test by MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Ohnishi, K.; Suzuki, K.; Tomita, H.; Tai, B. Potential cosmetic whitening agents from insect cuticle: Tyrosinase inhibitory activity of N-acetyldopamine dimers from exuviae of cicada, Cryptotympana tustulata FABR. J. Oleo Sci. 2002, 5, 355–358. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals cosmetics. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Morganti, G.; Fabrizi, G.; Mukherjee, P.B. A new sun to rejuvenate the skin. J. Appl. Cosmetol. 2008, 26, 159–168. [Google Scholar]
- Kotze, A.F.; de Leeuw, B.J.; Lueben, H.L.; Singamuthu, K. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithlia: In vitro evaluation of Caco-2 cell monolayer. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- Andrew, C.A.; Wan, A.C.; Tai, B.C. Chitin- A promising biomaterial for tissue engineering and stem cell technologies. Biotechnol. Adv. 2013, 31, 1776–1785. [Google Scholar]
- Kuria, K. Controlled fractionation of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–623. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitin nanostructure in living organisms. In Chitin: Formation and Diagenesis; Gupta, N., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–34. [Google Scholar]
- Domard, A.; Rinaudo, M. Preparation and characterization of fully deacetylated chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Chitooligosaccharides as potential nutraceuticals: Production and bioactivities. Adv. Food Nutr. Res. 2012, 65, 321–336. [Google Scholar] [PubMed]
- Sokmen, M.; Khan, M.A. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology 2016, 24, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Huber, I.; Rozmer, Z.; Gyongyi, Z.; Budan, F.; Horvath, P.; Kiss, E.; Perjesi, P. Structuer activity relationship analysis of antiproliferative cyclic C5-curcuminoids without DNA binding: Design, synthesis, lipophilicity and biological activity. J. Mol. Stuct. 2020, 1206, 127661. [Google Scholar] [CrossRef]
- Arshad, L.; Jantan, I.; Bukhari, S.N.A.; Haque, M.A. Immunosuppressive effects of natural α,β-unsaturated carbonyl-based compounds, and their analogs and derivatives, on immune cells: A review. Front. Pharmacol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Preetha, A.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticle and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef]
- Okaraonye, C.C.; Ikewuchi, J.C. Nutritional potential of Oryctes rhinoceros larva. Pak. J. Nutr. 2009, 8, 35–38. [Google Scholar] [CrossRef]
- Chung, G.F.; Sim, S.C.; Tan, M.W. Chemical control of rhinoceros beetles in the nursery and immature oil palms. In Proceedings of the PORIM International Palm Oil Development Conference—Progress, Prospect and Challenges towards the 21st Century, Kuala Lumpur, Malaysia, 5–9 September 1991. [Google Scholar]
- Amanlou, N.; Parsa, M.; Rostamizadeh, K.; Sadighian, S.; Moghaddam, F. Enhanced cytotoxic activity of curcumin on cancer cell lines by incorporating into gold/chitosan nanogels. Mater. Chem. Phys. 2019, 226, 151–157. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; de Los Llanos Gandía, M.; Caballero, A.H. Cosmetics and Cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- O’Toole, M.G.; Henderson, R.M.; Soucy, P.A.; Fasciotto, B.H.; Hoblitzell, P.J.; Keynton, R.S.; Ehringer, W.D.; Gobin, A.S. Curcumin encapsulation in submicrometer spray-dried chitosan/tween 20 particles. Biomacromolecules 2012, 13, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Jahanizadeh, S.; Chegeny, M. Curcumin-encapsulated polysaccharide nanocomposite: Formulation design, optimization and characterization. J. Bioeng. Res. 2019, 1, 54–62. [Google Scholar]
- Mailafiya, M.M.; Abubakar, K.; Danmaigoro, A.; Chiroma, S.M.; Rahim, E.B.A.; Moklas, M.A.M.; Zakaria, Z.A.B. Evaluation of in vitro release kinetics and mechanisms of curcumin-loaded cockle shell-derived calcium carbonate nanoparticles. Biomedpress 2019, 6, 3518–3540. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Cai, J. A novel approach of curcumin loaded chitosan/dextran nanocomposite for the management of complicated abdominal wound dehiscence. J. Clust. Sci. 2020, 31, 823–830. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An evaluation of curcumin-encapsulation chitosan nanoparticles for transdermal delivery. AAPS J. 2019, 20, 69. [Google Scholar]
- Wan, S.; Sun, Y.; Qi, X.; Tan, F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech 2012, 13, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Vandermeulen, G.; Préat, V. Combined effect of PLGA and curcumin on wound healing activity. J. Control. Release 2013, 171, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, L.M.; dos Santos, G.C.; Antonucci, G.A.; dos Santos, A.C.; Bianchi, M.D.L.P.; Antunes, L.M.G. Evaluation of the cytotoxicity and genotoxicity of curcumin in pc12 cells. Mutat. Res. 2009, 675, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physicochemical optical and biological activity of chitosan-chrome derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Percot, A.; Viton, C.; Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef]
- Kaya, M.; Seyyar, O.; Baran, T.; Bharti, L. A physicochemical characterization of fully acylated chitin structure isolated from two spider species: With new surface morphology. Int. J. Biol. Macromol. 2014, 65, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fang, Y.; Chen, L.; Lu, A.; Zhang, L. One-step synthesis of size-tunable gold nanoparticles immobilized on chitin nanofibrils via green pathway and their potential applications. Chem. Eng. J. 2017, 315, 573–582. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated alpha-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Latif, M.S.; Kormin, F.; Mustafa, M.K.; Mohamad, I.I.; Khan, M.; Abbas, S.; Ghazali, M.I.; Shafie, N.S.; Abu Bakar, M.F.; Sabran, S.F.; et al. Effect of temperature on the synthesis of Centella asiatica flavonoids extract-mediated gold nanoparticles: UV-visible spectra analyses. AIP Conf. Proc. 2018, 2016, 020071. [Google Scholar]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, B. Green biosynthesis of gold naoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 225–264. [Google Scholar]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Ahila, N.K.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 2014, 938272. [Google Scholar] [CrossRef]
- Lu, P.J.; Fu, W.E.; Huang, S.C.; Ahmad, A. Methodology for sample preparation and size measurement of commercial ZnO nanoparticles. J. Food Drug Anal. 2018, 26, 628–636. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Z.; Zhang, X.; Moklas, B. Biosynthesis of gold nanoparticles by flavonoids from Lilium casa blanca. J. Clust. Sci. 2017, 28, 3149–3158. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Haq, Z.U.; Murad, S.; Ismail, Z.; Abdullah, N.R.; Gill, H.K.; Rahman, A.U.; Yousaf, M.; Siddiqui, R.A.; Ahmad, A.; et al. Biological and molecular docking studies on coagulin-H: Human IL-2 novel natural inhibitor. Mol. Immunol. 2006, 43, 1855–1863. [Google Scholar] [CrossRef] [PubMed]

| CCG-NP | Zeta Potential ± sd (mV) |
|---|---|
| C2 | 20.2 ± 3.81 |
| C10 | −18.6 ± 3.06 |
| C16 | −16.6 ± 3.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Bakar, M.F.A.; Moujdin, I.A. Synthesis and Characterization of Curcumin-Chitosan Loaded Gold Nanoparticles by Oryctes rhinoceros’ Chitin for Cosmeceutical Application. Molecules 2023, 28, 1799. https://doi.org/10.3390/molecules28041799
Zainol Abidin NA, Kormin F, Zainol Abidin NA, Bakar MFA, Moujdin IA. Synthesis and Characterization of Curcumin-Chitosan Loaded Gold Nanoparticles by Oryctes rhinoceros’ Chitin for Cosmeceutical Application. Molecules. 2023; 28(4):1799. https://doi.org/10.3390/molecules28041799
Chicago/Turabian StyleZainol Abidin, Nurul Alyani, Faridah Kormin, Nurul Akhma Zainol Abidin, Mohd Fadzelly Abu Bakar, and Iqbal Ahmed Moujdin. 2023. "Synthesis and Characterization of Curcumin-Chitosan Loaded Gold Nanoparticles by Oryctes rhinoceros’ Chitin for Cosmeceutical Application" Molecules 28, no. 4: 1799. https://doi.org/10.3390/molecules28041799
APA StyleZainol Abidin, N. A., Kormin, F., Zainol Abidin, N. A., Bakar, M. F. A., & Moujdin, I. A. (2023). Synthesis and Characterization of Curcumin-Chitosan Loaded Gold Nanoparticles by Oryctes rhinoceros’ Chitin for Cosmeceutical Application. Molecules, 28(4), 1799. https://doi.org/10.3390/molecules28041799








