Complete, Theoretical Rovibronic Spectral Characterization of the Carbon Monoxide, Water, and Formaldehyde Cations
Abstract
1. Introduction
2. Computational Methods
3. Results
3.1. CO+
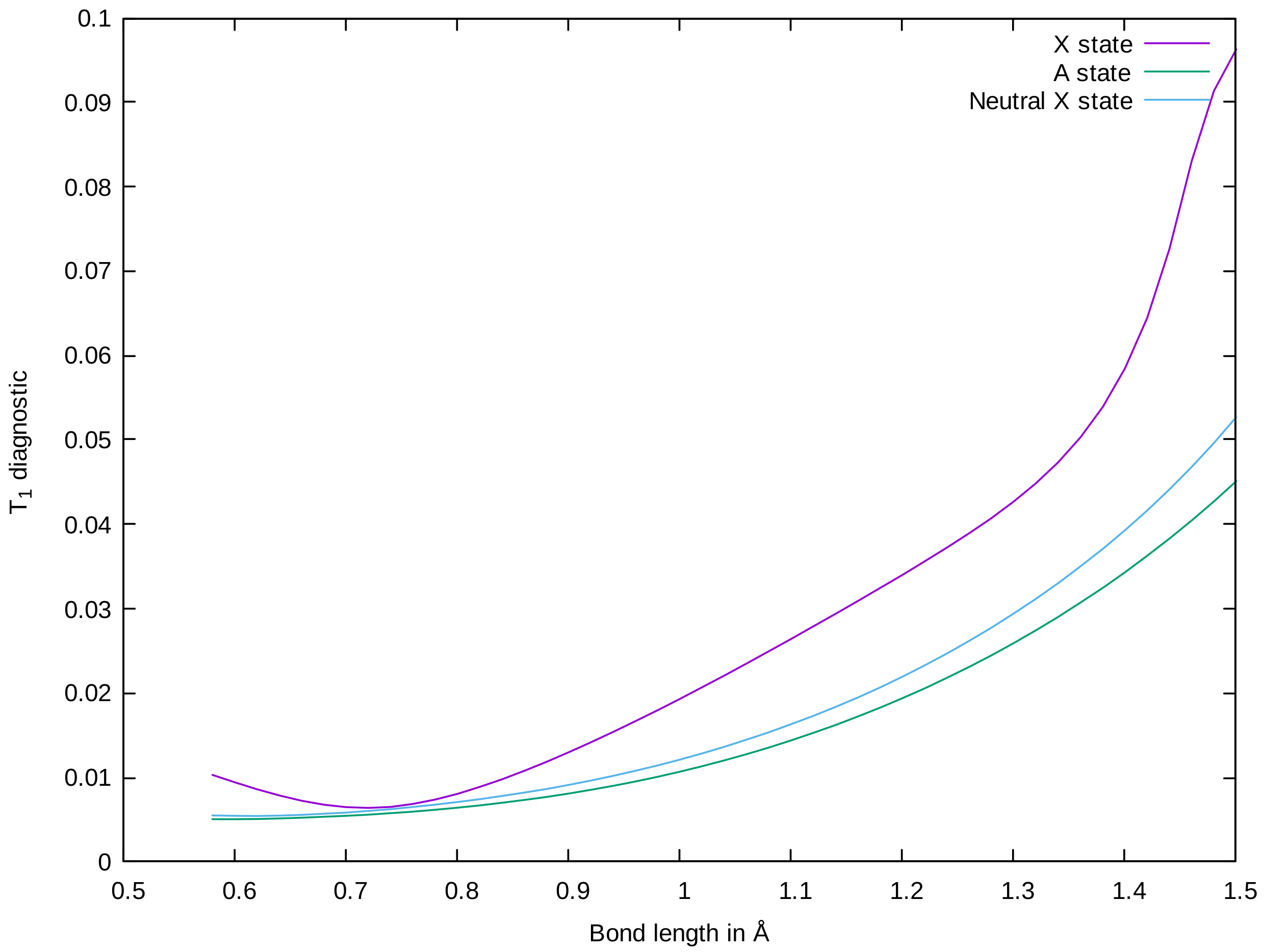
3.2. HO
3.2.1. B HO
3.2.2. A HO
3.2.3. B HO
3.3. HCO
3.3.1. B HCO
3.3.2. B HCO
3.3.3. A HCO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCSD(T) | Coupled cluster singles and doubles and perturbative triples |
| CCSDT | Coupled cluster singles doubles and triples |
| EOM-EE | Equation of motion excitation energy |
| EOM-IP | Equation of motion ionization potential |
| CBS | Complete basis set extrapolation |
| CcCT | Three-point CBS with core correlation and higher order correlation corrections |
| QFF | Quartic force field |
| MAE | Mean absolute error |
| MAD | Mean absolute difference |
| F12-TZ | QFF approach using CCSD(T)-F12 explicitly correlated methods |
| CcCR | QFF using three point CBS, core correlation and scalar relativistic corrections |
| (T)+EOM | Electronically excited state QFF using CCSD(T) and EOM-CCSD energies |
Appendix A. Symmetry Internal Coordinate Schemes
Appendix B. Rotational Constants, Distortion Constants and Geometrical Parameters
| EOM-IP-CC3 | EOM-IP-CCSDT-3 | EOM-IP-CCSDT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Const. | Units | F12-TZ | CcCR | TQcCT | CcCT | TQcCT | CcCT | TQcC | CcC |
| r(H–O) | Å | 0.999 | 0.998 | 0.998 | 0.998 | 0.995 | 0.999 | 0.998 | 0.999 |
| (H–O–H) | degrees | 109.372 | 109.458 | 109.573 | 109.577 | 109.708 | 109.567 | 109.570 | 109.577 |
| MHz | 28.045 | 28.163 | 28.19 | 28.116 | 28.185 | 28.106 | 28.18 | 28.1 | |
| MHz | 1.023 | 1.038 | 1.053 | 1.052 | 1.05 | 1.049 | 1.051 | 1.05 | |
| MHz | −129.999 | −131.243 | −132.631 | −132.188 | −132.166 | −131.659 | −132.174 | −131.678 | |
| MHz | 10.574 | 10.613 | 10.623 | 10.591 | 10.617 | 10.582 | 10.614 | 10.579 | |
| MHz | 21.091 | 21.313 | 21.48 | 21.416 | 21.544 | 21.486 | 21.561 | 21.503 | |
| kHz | 9.068 | 9.089 | 9.092 | 9.056 | 9.093 | 9.055 | 9.087 | 9.049 | |
| MHz | 4.039 | 4.152 | 4.268 | 4.258 | 4.25 | 4.24 | 4.254 | 4.245 | |
| kHz | −58.887 | −58.859 | −58.893 | −58.714 | −58.916 | −58.744 | −58.883 | −58.697 | |
| kHz | −426.562 | −439.749 | −452.737 | −450.271 | −450.597 | −448.009 | −451.065 | −448.474 | |
| kHz | 4.47 | 4.48 | 4.482 | 4.464 | 4.483 | 4.464 | 4.48 | 4.461 | |
| kHz | −9.131 | −9.129 | −9.191 | −9.15 | −9.172 | −9.13 | −9.169 | −9.12 | |
| kHz | 495.786 | 504.168 | 513.004 | 512.218 | 512.173 | 511.468 | 512.478 | 511.843 | |
| EOM-IP-CC3 | EOM-IP-CCSDT-3 | EOM-IP-CCSDT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Const. | Units | F12-TZ | CcCR | (T)+EOM/CcCR | TQcCT | CcCT | TQcCT | CcCT | TQcCT | CcCT |
| B | MHz | 256,623 | 257,362 | 257,404 | 256,998 | 256,669 | 257,004 | 256,683 | 256,976 | 256,683 |
| B | MHz | 257,183 | 257,675 | 258,240 | 256,974 | 256,756 | 256,989 | 256,782 | 256,966 | 256,772 |
| B | MHz | 251,201 | 251,670 | 252,227 | 250,913 | 250,708 | 250,926 | 250,732 | 250,901 | 250,720 |
| B | MHz | 263,302 | 263,588 | 264,681 | 262,595 | 262,476 | 262,621 | 262,516 | 262,604 | 262,498 |
| B | MHz | 252,044 | 252,480 | 253,045 | 251,748 | 251,539 | 251,759 | 251,560 | 251,737 | 251,548 |
| r(H–O) | Å | 0.988 | 0.987 | 0.987 | 0.988 | 0.988 | 0.988 | 0.988 | 0.988 | 0.988 |
| D | MHz | 6.558 | 6.59 | 6.59 | 6.592 | 6.599 | 6.593 | 6.6 | 6.595 | 6.601 |
| H | Hz | 114.068 | 113.27 | 113.276 | 112.027 | 112.767 | 111.924 | 112.606 | 112.042 | 112.608 |
| EOM-EE-CC3 | EOM-IP-CC3 | EOM-IP-CCSDT-3 | EOM-IP-CCSDT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Const. | (T)+EOM/CcCR | TQcC | CcC | TQcCT | CcCT | TQcCT | CcCT | TQcC | CcC |
| A | 856,740 | 854,842 | 854,876 | 854,475 | 854,570 | 854,171 | 854,513 | 854,046 | |
| B | 290,373 | 291,052 | 291,429 | 291,253 | 291,433 | 291,261 | 291,532 | 291,395 | |
| C | 216,870 | 217,126 | 217,338 | 217,214 | 217,320 | 217,199 | 217,372 | 217,265 | |
| A | 842,278 | 840,270 | 841,290 | 841,296 | 840,911 | 840,922 | 840,795 | 840,718 | |
| B | 287,185 | 287,966 | 288,324 | 288,128 | 288,341 | 288,148 | 288,472 | 288,315 | |
| C | 208,698 | 209,019 | 209,272 | 209,179 | 209,258 | 209,166 | 209,323 | 209,245 | |
| A | 831,448 | 828,877 | 830,261 | 830,478 | 829,862 | 830,084 | 829,773 | 829,905 | |
| B | 280,364 | 281,231 | 281,624 | 281,434 | 281,641 | 281,453 | 281,775 | 281,618 | |
| C | 204,616 | 204,926 | 205,221 | 205,144 | 205,204 | 205,128 | 205,272 | 205,205 | |
| A | 826,003 | 824,940 | 826,033 | 826,413 | 825,656 | 826,034 | 825,401 | 825,723 | |
| B | 294,686 | 295,397 | 295,777 | 295,541 | 295,800 | 295,569 | 295,981 | 295,783 | |
| C | 190,296 | 190,199 | 191,267 | 191,351 | 191,212 | 191,298 | 191,344 | 191,424 | |
| A | 840,484 | 837,876 | 840,434 | 840,666 | 839,922 | 840,177 | 839,798 | 839,896 | |
| B | 279,904 | 280,878 | 281,137 | 280,940 | 281,176 | 280,977 | 281,316 | 281,162 | |
| C | 215,039 | 215,912 | 215,392 | 215,166 | 215,428 | 215,202 | 215,452 | 215,257 | |
| EOM-EE-CC3 | EOM-IP-CC3 | EOM-IP-CCSDT-3 | EOM-IP-CCSDT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Const. | Units | (T)+EOM/CcCR | TQcC | CcC | TQcCT | CcCT | TQcCT | CcCT | TQcC | CcC |
| r(H–O) | Å | 1.125 | 1.124 | 1.123 | 1.124 | 1.123 | 1.124 | 1.123 | 1.124 | |
| (H–O–H) | degrees | 57.501 | 57.611 | 57.642 | 57.638 | 57.651 | 57.648 | 57.660 | 57.662 | |
| MHz | 18.552 | 18.313 | 18.399 | 18.318 | 18.391 | 18.311 | 18.382 | 18.305 | ||
| MHz | 713.318 | 703.328 | 712.235 | 710.605 | 711.331 | 709.702 | 711.308 | 709.531 | ||
| MHz | 22.464 | 25.023 | 21.547 | 21.004 | 21.754 | 21.213 | 21.582 | 21.093 | ||
| MHz | 5.576 | 5.484 | 5.54 | 5.517 | 5.536 | 5.514 | 5.535 | 5.514 | ||
| MHz | 70.21 | 69.475 | 68.705 | 68.245 | 68.704 | 68.246 | 68.577 | 68.138 | ||
| kHz | 2.955 | 2.894 | 2.922 | 2.899 | 2.922 | 2.899 | 2.929 | 2.907 | ||
| MHz | 1.094 | 1.09 | 1.097 | 1.096 | 1.097 | 1.095 | 1.095 | 1.094 | ||
| kHz | −14.575 | −13.396 | −14.111 | −14.095 | −14.027 | −14.036 | −14.136 | −14.096 | ||
| kHz | −9.603 | −12.572 | −7.459 | −6.991 | −7.955 | −7.334 | −7.311 | −7.158 | ||
| kHz | 1.445 | 1.407 | 1.422 | 1.411 | 1.422 | 1.411 | 1.425 | 1.414 | ||
| kHz | 1.062 | 1.552 | 1.043 | 0.973 | 1.098 | 1.018 | 1.106 | 1.045 | ||
| kHz | 758.931 | 737.041 | 740.336 | 733.847 | 739.376 | 732.933 | 738.163 | 731.694 | ||
| Const. | F12-TZ | CcCR | EOM-IP-CCSDT-3/TQcCT |
|---|---|---|---|
| A | 266,451 | 267,249 | 267,221 |
| B | 40,055 | 40,270 | 40,131 |
| C | 34,820 | 34,997 | 34,891 |
| A | 265,457 | 266,359 | 266,229 |
| B | 40,053 | 40,266 | 40,117 |
| C | 34,611 | 34,786 | 34,672 |
| A | 260,866 | 261,828 | 261,575 |
| B | 40,070 | 40,283 | 40,129 |
| C | 34,550 | 34,725 | 34,608 |
| A | 265,145 | 266,039 | 265,965 |
| B | 39,673 | 39,885 | 39,734 |
| C | 34,301 | 34,474 | 34,358 |
| A | 269,591 | 270,557 | 270,364 |
| B | 40,135 | 40,347 | 40,198 |
| C | 34,560 | 34,734 | 34,621 |
| A | 274,921 | 275,901 | 275,876 |
| B | 40,041 | 40,253 | 40,101 |
| C | 34,708 | 34,884 | 34,771 |
| A | 262,537 | 263,492 | 263,246 |
| B | 40,043 | 40,255 | 40,102 |
| C | 34,568 | 34,743 | 34,625 |
| A | 257,693 | 258,557 | 258,366 |
| B | 40,349 | 40,563 | 40,406 |
| C | 34,559 | 34,732 | 34,616 |
| Const. | Units | F12-TZ | CcCR | EOM-IP-CCSDT-3/TQcCT |
|---|---|---|---|---|
| r(O–C) | Å | 1.194 | 1.190 | 1.193 |
| r(C–H) | Å | 1.115 | 1.113 | 1.113 |
| r(C–O–H) | degrees | 119.510 | 119.519 | 119.515 |
| MHz | 86.027 | 86.811 | 86.876 | |
| MHz | 17.798 | 17.944 | 17.934 | |
| MHz | 2.948 | 2.973 | 2.925 | |
| MHz | 12.898 | 13.056 | 13.024 | |
| MHz | 1.827 | 1.844 | 1.82 | |
| kHz | 138.483 | 142.723 | 137.905 | |
| MHz | 5.438 | 5.532 | 5.473 | |
| kHz | 140.429 | 142.339 | 137.736 | |
| kHz | −381.316 | −388.997 | −366.657 | |
| kHz | 76.638 | 78.37 | 76.936 | |
| kHz | 72.63 | 73.622 | 71.246 | |
| kHz | 3.8 | 3.844 | 3.772 |
| Const. | Units | (T)+EOM/CcCR | EOM-IP-CCSDT-3/TQcCT |
|---|---|---|---|
| A | MHz | 269,758 | 270,948 |
| B | MHz | 32,266 | 32,886 |
| C | MHz | 28,818 | 29,326 |
| A | MHz | 266,957 | 267,989 |
| B | MHz | 32,035 | 32,673 |
| C | MHz | 28,498 | 29,016 |
| A | MHz | 262,367 | 263,253 |
| B | MHz | 32,002 | 32,642 |
| C | MHz | 28,423 | 28,938 |
| A | MHz | 268,992 | 269,927 |
| B | MHz | 32,296 | 32,936 |
| C | MHz | 28,361 | 28,873 |
| A | MHz | 266,655 | 267,812 |
| B | MHz | 31,730 | 32,393 |
| C | MHz | 28,228 | 28,766 |
| A | MHz | 263,372 | 264,300 |
| B | MHz | 31,693 | 32,335 |
| C | MHz | 28,536 | 29,061 |
| A | MHz | 263,939 | 264,868 |
| B | MHz | 31,987 | 32,624 |
| C | MHz | 28,441 | 28,956 |
| A | MHz | 270,816 | 271,856 |
| B | MHz | 32,037 | 32,681 |
| C | MHz | 28,360 | 28,879 |
| Const. | Units | (T)+EOM/CcCR | EOM-IP-CCSDT-3/TQcCT |
|---|---|---|---|
| r(O–C) | Å | 1.351 | 1.338 |
| r(C–H) | Å | 1.096 | 1.094 |
| r(C–O–H) | degrees | 118.392 | 118.411 |
| kHz | 77.129 | 77.161 | |
| MHz | 15.551 | 15.589 | |
| MHz | 1.181 | 1.191 | |
| kHz | 8.547 | 8.702 | |
| kHz | 789.912 | 801.015 | |
| mHz | −5.697 | 21.892 | |
| kHz | 2.94 | 2.914 | |
| Hz | 19.343 | 20.026 | |
| Hz | 2.044 | 1.985 | |
| mHz | 22.822 | 27.041 | |
| Hz | 10.187 | 10.576 | |
| kHz | 1.07 | 1.073 |
| Const. | Units | (T)+EOM/CcCR | EOM-IP-CCSDT-3/TQcCT |
|---|---|---|---|
| A | MHz | 245,655.9 | 242,934.4 |
| B | MHz | 36,911.9 | 36,487.7 |
| C | MHz | 32,090.1 | 31,723.1 |
| A | MHz | 241,710.3 | 238,804.3 |
| B | MHz | 36,669.8 | 36,214.1 |
| C | MHz | 31,712 | 31,319.6 |
| A | MHz | 236,953.5 | 233,905.8 |
| B | MHz | 36,679.8 | 36,239.7 |
| C | MHz | 31,648.9 | 31,266 |
| A | MHz | 240,867.6 | 239,228.5 |
| B | MHz | 36,347.2 | 36,092.6 |
| C | MHz | 31,506.7 | 31,149.6 |
| A | MHz | 243,348.2 | 239,086.7 |
| B | MHz | 36,628.6 | 35,890.9 |
| C | MHz | 31,582.5 | 31,060.7 |
| A | MHz | 238,071.2 | 235,154.9 |
| B | MHz | 36,574 | 36,131.8 |
| C | MHz | 31,699.5 | 31,295.9 |
| A | MHz | 238,231.4 | 235,191 |
| B | MHz | 36,655.2 | 36,209.7 |
| C | MHz | 31,652.7 | 31,265.4 |
| A | MHz | 244,899 | 241,998.8 |
| B | MHz | 36,648.9 | 36,171.6 |
| C | MHz | 31,426.7 | 31,074.4 |
| Const. | Units | (T)+EOM/CcCR | EOM-IP-CCSDT-3/TQcCT |
|---|---|---|---|
| r(O–C) | Å | 1.275 | 1.285 |
| r(C–H) | Å | 1.100 | 1.102 |
| r(C–O–H) | degrees | 113.349 | 112.813 |
| kHz | 83.017 | 88.493 | |
| MHz | 11.061 | 10.623 | |
| MHz | 1.375 | 1.36 | |
| kHz | 10.874 | 11.545 | |
| kHz | 845.975 | 844.286 | |
| mHz | 46.056 | −9.671 | |
| kHz | 1.651 | 1.515 | |
| Hz | 19.308 | 18.596 | |
| Hz | 70.135 | 81.113 | |
| mHz | 37.114 | 29.883 | |
| Hz | 10.652 | 10.09 | |
| Hz | 855.801 | 846.142 |
References
- Larsson, M.; Geppert, W.D.; Nyman, G. Ion Chemistry in Space. Rep. Prog. Phys. 2012, 75, 066901. [Google Scholar] [CrossRef] [PubMed]
- Fortenberry, R.C.; Bodewits, D.; Pierce, D.M. Knowledge Gaps in the Cometary Spectra of Oxygen-Bearing Molecular Cations. Astrophys. J. Suppl. Ser. 2021, 256, 6. [Google Scholar] [CrossRef]
- Wyckoff, S.; Heyd, R.S.; Fox, R. Unidentified Molecular Bands in the Plasma Tail of Comet Hyakutake (C/1996 B2). Astrophys. J. 1999, 512, L73–L76. [Google Scholar] [CrossRef]
- Bodewits, D.; Lara, L.M.; A’Hearn, M.F.; Forgia, F.L.; Gicquel, A.; Kovacs, G.; Knollenberg, J.; Lazzarin, M.; Lin, Z.Y.; Shi, X.; et al. Changes in the Physical Environment of the Inner Coma of 67P/Churyumov-Gerasimenko with Decreasing Heliocentric Distance. Astron. J. 2016, 152, 130. [Google Scholar] [CrossRef]
- Beth, A.; Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Combi, M.R.; Keyser, J.D.; Fiethe, B.; Fuselier, S.A.; Galand, M.; Gombosi, T.I.; et al. ROSINA Ion Zoo at Comet 67P. Astron. Astrophys. 2020, 642, A27. [Google Scholar] [CrossRef]
- Brown, M.E.; Bouchez, A.H.; Spinrad, A.H.; Johns-Krull, C.M. A High-Resolution Catalog of Cometary Emission Lines. Astron. J. 1996, 112, 1197. [Google Scholar] [CrossRef]
- Morrison, N.D.; Knauth, C.D.; Mulliss, C.L.; Lee, W. High-Resolution Optical Spectra of the Head of the Comet C/1996 B2 (Hyakutake). Publ. Astron. Soc. Pac. 1997, 109, 676. [Google Scholar] [CrossRef]
- Mumma, M.J.; McLean, I.S.; DiSanti, M.A.; Larkin, J.E.; Russo, N.D.; Magee-Sauer, K.; Becklin, E.E.; Bida, T.; Chaffee, F.; Conrad, A.R.; et al. A Survey of Organic Volatile Species in Comet C/1999 H1 (Lee) Using NIRSPEC at the Keck Observatory. Astrophys. J. 2001, 546, 1183–1193. [Google Scholar] [CrossRef]
- Cochran, A.L.; Cochran, W.D. A High Spectral Resolution Atlas of Comet 122P/de Vico. Icarus 2002, 157, 297–308. [Google Scholar] [CrossRef]
- Cremonese, G.; Capria, M.T.; Sanctis, M.C.D. Catalog of the Emission Lines in the Visible Spectrum of Comet 153P/Ikeya-Zhang. Astron. Astrophys. 2006, 461, 789–792. [Google Scholar] [CrossRef]
- Kawakita, H.; Watanabe, J.I. Revised Fluorescence Efficiencies of Cometary NH2: Ammonia Abundance in Comets. Astrophys. J. 2002, 574, L183. [Google Scholar] [CrossRef]
- Dello Russo, N.; Vervack, R.J.; Weaver, H.A.; Lisse, C.M.; Kawakita, H.; Kobayashi, H.; Cochran, A.L.; Harris, W.M.; Bockelée-Morvan, D.; Biver, N.; et al. A High-Resolution Infrared Spectral Survey of 103P/Hartley 2 on the Night of the EPOXI Closest Approach. Icarus 2013, 222, 707–722. [Google Scholar] [CrossRef]
- Opitom, C.; Fitzsimmons, A.; Jehin, E.; Moulane, Y.; Hainaut, O.; Meech, K.J.; Yang, B.; Snodgrass, C.; Micheli, M.; Keane, J.V.; et al. 2I/Borisov: A C2-Depleted Interstellar Comet. Astron. Astrophys. 2019, 631, L8. [Google Scholar] [CrossRef]
- Bomble, Y.J.; Saeh, J.C.; Stanton, J.F.; Szalay, P.G.; Kállay, M.; Gauss, J. Equation-of-Motion Coupled-Cluster Methods for Ionized States with an Approximate Treatment of Triple Excitations. J. Chem. Phys. 2005, 122, 154107. [Google Scholar] [CrossRef]
- Shi, D.; Li, W.; Sun, J.; Zhu, Z.; Liu, Y. MRCI Study of Potential Energy Curves, Spectroscopic and Molecular Properties of the CO+ Cation. Comput. Theor. Chem. 2011, 978, 126–137. [Google Scholar] [CrossRef]
- Tentscher, P.R.; Arey, J.S. Geometries and Vibrational Frequencies of Small Radicals: Performance of Coupled Cluster and More Approximate Methods. J. Chem. Theory Comput. 2012, 8, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Hakalla, R.; Szajna, W.; Piotrowska, I.; Malicka, M.I.; Zachwieja, M.; Kepa, R. Fourier-Transform Spectroscopy of the A2Πi- X2α+ System in CO+ and Depurturbation Analysis of the A2Πi(ν = 0, 1) Levels. J. Quant. Spectrosc. Rad. Transfer 2019, 234, 159–176. [Google Scholar] [CrossRef]
- Irikura, K.K. Experimental Vibrational Zero-Point Energies: Diatomic Molecules. J. Phys. Chem. Ref. Data 2007, 36, 389–398. [Google Scholar] [CrossRef]
- Das, B.; Farley, J.W. Observation of the Visible Absorption Spectrum of H2O+. J. Chem. Phys. 1991, 95, 8809–8815. [Google Scholar] [CrossRef]
- Reutt, J.E.; Wang, L.S.; Lee, Y.T.; Shirley, D.A. Molecular Beam Photoelectron Spectroscopy and Femtosecond Intramolecular Dynamics of Oxoniumyl(H2O+) and Oxoniumyl-d2(D2O+). J. Chem. Phys. 1986, 85, 6928–6939. [Google Scholar] [CrossRef]
- Huet, T.R.; Bachir, I.H.; Destombes, J.L.; Vervloet, M. The A 2A1-X 2B1 Transition of H2O+ in the Near Infrared Region. J. Chem. Phys. 1997, 107, 5645–5651. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Guo, Y.; Zhuang, H.; Liu, Y.; Chen, Y. Observation and Analysis of Two Subbands in the Absorption Spectrum of H2O+. J. Mol. Spectrosc. 2003, 219, 258–262. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Yang, X.; Guo, Y.; Liu, Y.; Li, Y.; Buenker, R.J.; Jensen, P. Vibronic Transition Moments and Line Intensities for H2O+. J. Mol. Spectrosc. 2004, 225, 96–106. [Google Scholar] [CrossRef]
- Lauzin, C.; Gans, B.; Merkt, F. High-Resolution Photoelectron-Spectroscopic Investigation of the H2O+ Cation in its Ã+ Electronic State. arXiv 2017, arXiv:1705.00974. [Google Scholar] [CrossRef]
- Truong, S.Y.; Yencha, A.J.; Juarez, A.M.; Cavanagh, S.J.; Bolognesi, P.; King, G.C. Threshold Photoelectron Spectroscopy of H2O and D2O over the Photon Energy Range 12–40 eV. Chem. Phys. 2009, 355, 183–193. [Google Scholar] [CrossRef]
- Dehareng, D.; Chapuisat, X.; Lorquet, J.C.; Galloy, C.; Raseev, G. Dynamical Study of Nonadiabatic Unimolecular Reactions: The Conical Intersection between the B2B2 and A2A1 States of H2O+. J. Chem. Phys. 1983, 78, 1246–1264. [Google Scholar] [CrossRef]
- Eroms, M.; Jungen, M.; Meyer, H.D. Nonadiabatic Nuclear Dynamics after Valence Ionization of H2O. J. Phys. Chem. A 2010, 114, 9893–9901. [Google Scholar] [CrossRef]
- Baker, A.D.; Baker, C.; Brundle, C.R.; Turner, D.W. The Electronic Structures of Methane, Ethane, Ethylene and Formaldehyde Studied by High-Resolution Molecular Photoelectron Spectroscopy. Int. J. Mass Spectrom. Ion Phys. 1968, 1, 285–301. [Google Scholar] [CrossRef]
- Niu, B.; Shirley, D.A.; Bai, Y.; Daymo, E. High-Resolution He Iα Photoelectron Spectroscopy of H2CO and D2CO Using Supersonic Molecular Beams. Chem. Phys. Lett. 1993, 201, 212–216. [Google Scholar] [CrossRef]
- Niu, B.; Shirley, D.A.; Bai, Y. High Resolution Photoelectron Spectroscopy and Femtosecond Intramolecular Dynamics of H2CO+ and D2CO+. J. Chem. Phys. 1993, 98, 4377–4390. [Google Scholar] [CrossRef]
- Bruna, P. The Electronic Structure of the H2CO+ Radical and Higher Rydberg States of H2CO. Mol. Phys. 1998, 94, 917–928. [Google Scholar]
- Guan, J.; Casida, M.E.; Salahub, D.R. Time-Dependent Density-Functional Theory Investigation of Excitation Spectra of Open-Shell Molecules. J. Mol. Struct. THEOCHEM 2000, 527, 229–244. [Google Scholar] [CrossRef]
- Harbo, L.S.; Dziarzhytski, S.; Domesle, C.; Brenner, G.; Wolf, A.; Pedersen, H.B. Lifetime of Low Vibrational Levels of the Metastable B 2B2 State of H2O+ Probed by Photodissociation at 532 nm. Phys. Rev. A 2014, 89, 052520. [Google Scholar] [CrossRef]
- Jungen, C. The Renner-Teller Effect Revisited 40 Years Later. J. Mol. Spectrosc. 2019, 363, 111172. [Google Scholar] [CrossRef]
- Brommer, M.; Weis, B.; Follmeg, B.; Rosmus, P.; Carter, S.; Handy, N.C.; Werner, H.J.; Knowles, P.J. Theoretical Spin–Rovibronic 2A1(Πu)-2B1 Spectrum of the H2O+, HDO+, and D2O+. J. Chem. Phys. 1993, 98, 5222–5234. [Google Scholar] [CrossRef]
- Lew, H.; Heiber, I. Spectrum of H2O+. J. Chem. Phys. 1973, 58, 1246–1247. [Google Scholar] [CrossRef]
- Lew, H. Electronic Spectrum of H2O+. Can. J. Phys. 1976, 54, 2028–2049. [Google Scholar] [CrossRef]
- Feller, D.; Davidson, E.R. A Theoretical Study of the Adiabatic and Vertical Ionization Potentials of Water. J. Chem. Phys. 2018, 148, 234308. [Google Scholar] [CrossRef]
- Davis, M.C.; Fortenberry, R.C. (T)+EOM Quartic Force Fields for Theoretical Vibrational Spectroscopy of Electronically Excited States. J. Chem. Theory Comput. 2021, 17, 4374–4382. [Google Scholar] [CrossRef]
- Fortenberry, R.C.; Lee, T.J. Computational Vibrational Spectroscopy for the Detection of Molecules in Space. Ann. Rep. Comput. Chem. 2019, 15, 173–202. [Google Scholar]
- Morgan, W.J.; Fortenberry, R.C. Quartic Force Fields for Excited Electronic States: Rovibronic Reference Data for the 1 2A′ and 1 2A′′ States of the Isoformyl Radical, HOC. Spectrochim. Acta A 2015, 135, 965–972. [Google Scholar] [CrossRef]
- Morgan, W.J.; Fortenberry, R.C. Theoretical Rovibronic Treatment of the 2Σ+ and 2Π States of C2H & 1Σ+ State of C2H- from Quartic Force Fields. J. Phys. Chem. A 2015, 119, 7013–7025. [Google Scholar]
- Koch, H.; Christiansen, O.; Jørgensen, P.; de Meràs, A.M.S.; Helgaker, T. The CC3 Model: An Iterative Coupled Cluster Approach including Connected Triples. J. Chem. Phys. 1997, 106, 1808–1818. [Google Scholar] [CrossRef]
- Smith, C.E.; King, R.A.; Crawford, T.D. Coupled Cluster Excited Methods Including Triple Excitations for Excited States of Radicals. J. Chem. Phys. 2005, 122, 054110. [Google Scholar] [CrossRef]
- Fortenberry, R.C.; Huang, X.; Francisco, J.S.; Crawford, T.D.; Lee, T.J. The trans-HOCO Radical: Fundamental Vibrational Frequencies, Quartic Force Fields, and Spectroscopic constants. J. Chem. Phys. 2011, 135, 134301. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.F.; Gauss, J. Analytic Energy Derivatives for Ionized States Described by the Equation-of-Motion Coupled Cluster Method. J. Chem. Phys. 1994, 101, 8938–8944. [Google Scholar] [CrossRef]
- Stanton, J.F.; Bartlett, R.J. The Equation of Motion Coupled-Cluster Method—A Systematic Biorthogonal Approach to Molecular Excitation Energies, Transition-Probabilities, and Excited-State Properties. J. Chem. Phys. 1993, 98, 7029–7039. [Google Scholar] [CrossRef]
- Adler, T.B.; Knizia, G.; Werner, H.J. A Simple and Efficient CCSD(T)-F12 Approximation. J. Chem. Phys. 2007, 127, 221106. [Google Scholar] [CrossRef]
- Peterson, K.A.; Adler, T.B.; Werner, H.J. Systematically Convergent Basis Sets for Explicitly Correlated Wavefunctions: The Atoms H, He, B-Ne, and Al-Ar. J. Chem. Phys. 2008, 128, 084102. [Google Scholar] [CrossRef]
- Yousaf, K.E.; Peterson, K.A. Optimized Auxiliary Basis Sets for Explicitly Correlated Methods. J. Chem. Phys. 2008, 129, 184108. [Google Scholar] [CrossRef]
- Knizia, G.; Adler, T.B.; Werner, H.J. Simplified CCSD(T)-F12 Methods: Theory and Benchmarks. J. Chem. Phys. 2009, 130, 054104. [Google Scholar] [CrossRef]
- Agbaglo, D.; Fortenberry, R.C. The Performance of CCSD(T)-F12/aug-cc-pVTZ for the Computation of Anharmonic Fundamental Vibrational Frequencies. Int. J. Quantum Chem. 2019, 119, e25899. [Google Scholar] [CrossRef]
- Agbaglo, D.; Fortenberry, R.C. The Performance of Explicitly Correlated Wavefunctions [CCSD(T)-F12b] in the Computation of Anharmonic Vibrational Frequencies. Chem. Phys. Lett. 2019, 734, 136720. [Google Scholar] [CrossRef]
- Martin, J.M.L.; Lee, T.J. The Atomization Energy and Proton Affinity of NH3. An AbInitio Calibration Study. Chem. Phys. Lett. 1996, 258, 136–143. [Google Scholar] [CrossRef]
- Martin, J.M.L.; Taylor, P.R. Basis Set Convergence for Geometry and Harmonic Frequencies. Are h Functions Enough? Chem. Phys. Lett. 1994, 225, 473–479. [Google Scholar] [CrossRef]
- Douglas, M.; Kroll, N. Quantum Electrodynamical Corrections to the Fine Structure of Helium. Ann. Phys. 1974, 82, 89–155. [Google Scholar] [CrossRef]
- De Jong, W.A.; Harrison, R.J.; Dixon, D.A. Parallel Douglas-Kroll energy and gradients in NWChem: Estimating scalar relativistic effects using Douglas-Kroll contracted basis sets. J. Chem. Phys. 2001, 114, 48–53. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Manby, F.R.; Black, J.A.; Doll, K.; Hëselmann, A.; Kats, D.; Köhn, A.; Korona, T.; Kreplin, D.A.; et al. The Molpro Quantum Chemistry Package. J. Chem. Phys. 2020, 152, 144107. [Google Scholar] [CrossRef]
- Allen, W.D.; Coworkers. INTDER 2005 is a General Program Written by W. D. Allen and Coworkers, which Performs Vibrational Analysis and Higher-Order Non-Linear Transformations. 2005. [Google Scholar]
- Gaw, J.F.; Willets, A.; Green, W.H.; Handy, N.C. SPECTRO: A Program for the Derivation of Spectrscopic Constants from Provided Quartic Force Fields and Cubic Dipole Fields. In Advances in Molecular Vibrations and Collision Dynamics; Bowman, J.M., Ratner, M.A., Eds.; JAI Press, Inc.: Greenwich, CT, USA, 1991; pp. 170–185. [Google Scholar]
- Mills, I.M. Vibration-Rotation Structure in Asymmetric- and Symmetric-Top Molecules. In Molecular Spectroscopy—Modern Research; Rao, K.N., Mathews, C.W., Eds.; Academic Press: New York, NY, USA, 1972; pp. 115–140. [Google Scholar]
- Papousek, D.; Aliev, M.R. Molecular Vibration-Rotation Spectra; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Watson, J.K.G. Aspects of Quartic and Sextic Centrifugal Effects on Rotational Energy Levels. In Vibrational Spectra and Structure; During, J.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 1–89. [Google Scholar]
- Dateo, C.E.; Lee, T.J.; Schwenke, D.W. An Accurate Quartic Force-Field and Vibrational Frequencies for HNO and DNO. J. Chem. Phys. 1994, 101, 5853–5859. [Google Scholar] [CrossRef]
- Huang, X.; Lee, T.J. Accurate Ab Initio Quartic Force Fields for NH2- and CCH- and Rovibrational Spectroscopic Constants for Their Isotopologs. J. Chem. Phys. 2009, 131, 104301. [Google Scholar] [CrossRef]
- Schwenke, D.W. Variational Calculations of Rovibrational Energy Levels and Transition Intensities for Tetratomic Molecules. J. Phys. Chem. 1996, 100, 2867–2884. [Google Scholar] [CrossRef]
- Carter, S.; Bowman, J.M.; Handy, N.C. Extensions and Tests of “Multimode”: A Code to Obtain Accurate Vibration/Rotation Energies of Many-Mode Molecules. Theor. Chem. Acc. 1998, 100, 191–198. [Google Scholar] [CrossRef]
- Carter, S.; Bowman, J.M.; Handy, N.C. Multimode Calculations of Rovibrational Energies of C2H4 and C2D4. Mol. Phys. 2012, 110, 775–781. [Google Scholar] [CrossRef]
- Aprà, E.; Bylaska, E.J.; de Jong, W.A.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Valiev, M.; van Dam, H.J.J.; Alexeev, Y.; Anchell, J.; et al. NWChem: Past, present, and future. J. Chem. Phys. 2020, 152, 184102. [Google Scholar] [CrossRef]
- Parrish, R.M.; Burns, L.A.; Smith, D.G.A.; Simmonett, A.C.; DePrince, A.E., III; Hohenstein, E.G.; Bozkaya, U.; Sokolov, A.Y.; Di Remigio, R.; Richard, R.M.; et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J. A Simple Scheme for the Direct Calculation of Ionization Potentials with Coupled-Cluster Theory That Exploits Established Excitation Energy Methods. J. Chem. Phys. 1999, 111, 8785–8788. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Harding, M.E.; Szalay, P.G.; Auer, A.A.; Bartlett, R.J.; Benedikt, U.; Berger, C.; Bernholdt, D.E.; Bomble, Y.J.; et al. CFOUR. A Quantum Chemical Program Package. Available online: http://www.cfour.de (accessed on 10 October 2022).
- Woon, D.A.; Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. V. Core-Valence Basis Sets for Boron through Neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef]
- Gronowski, M.; Koza, A.M.; Tomza, M. Ab Initio Properties of the NaLi Molecule in the a3Σ+ Electronic State. Phys. Rev. A 2020, 102, 020801. [Google Scholar] [CrossRef]
- Pyykkö, P.; Dyall, K.G.; Császár, A.G.; Tarczay, G.; Polyansky, O.L.; Tennyson, J. Estimation of Lamb-Shift Effects for Molecules: Application to the Rotation-Vibration Spectra of Water. Phys. Rev. A 2001, 63, 024502. [Google Scholar] [CrossRef]
- Lee, T.J.; Taylor, P.R. A Diagnostic for Determining the Quality of Single-Reference Electron Correlation Methods. Int. J. Quant. Chem. 1989, 36, 199–207. [Google Scholar] [CrossRef]
- Watrous, A.G.; Westbrook, B.R.; Fortenberry, R.C. F12-TZ-cCR: A Methodology for Faster and Still Highly Accurate Quartic Force Fields. J. Phys. Chem. A 2021, 125, 10532–10540. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, B.R.; Fortenberry, R.C. Anharmonic Frequencies of (MO)2 and Related Hydrides for M = Mg, Al, Si, P, S, Ca, and Ti and Heuristics for Predicting Anharmonic Corrections of Inorganic Oxides. J. Phys. Chem. A 2020, 124, 3191–3204. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, B.M.; Crofton, M.W.; Oka, T. Infrared Spectroscopy of the ν3 Band of H2O+. J. Mol. Spectrosc. 1988, 127, 1–11. [Google Scholar] [CrossRef]
- Huet, T.R.; Pursell, C.J.; Ho, W.C.; Dinelli, B.M.; Oka, T. Infrared Spectroscopy and Equilibrium Structure of H2O+ (X 2B1). J. Chem. Phys. 1992, 97, 5977–5987. [Google Scholar] [CrossRef]
- Tonkyn, R.G.; Wiedmann, R.; Grant, E.R.; White, M.G. Rotationally Resolved Photoionization of H2O+. J. Chem. Phys. 1991, 95, 7033–7040. [Google Scholar] [CrossRef]
- Brown, P.R.; Davies, P.B.; Stickland, R.J. Infrared Laser Spectroscopy of the 201 and 212 Bands of H2O+ (X2B1). J. Chem. Phys. 1989, 91, 3384–3391. [Google Scholar] [CrossRef]
- Muller, S.; Muller, H.S.P.; Black, J.H.; Beelen, A.; Combes, F.; Curran, S.; Gerin, M.; Guelin, M.; Henkel, C.; Martin, S.A.; et al. OH+ and H2O+ Absorption toward PKS 1830–211. Astron. Astrophys. 2016, 595, A128. [Google Scholar] [CrossRef]
- Liu, J.; Kim, H.T.; Anderson, S.L. Multiphoton Ionization and Photoelectron Spectroscopy of Formaldehyde via its 3p Rydberg States. J. Chem. Phys. 2001, 114, 9797–9806. [Google Scholar] [CrossRef]
- Schulenburg, A.M.; Meisinger, M.; Radi, P.P.; Merkt, F. The Formaldehyde Cation: Rovibrational Energy Level Structure and Coriolis Interaction Near the Adiabatic Ionization Threshold. J. Mol. Spectrosc. 2008, 250, 44–50. [Google Scholar] [CrossRef]
- Trabelsi, T.; Davis, M.C.; Fortenberry, R.C.; Francisco, J.S. Spectroscopic Investigation of [Al,N,C,O] Refractory Molecules. J. Chem. Phys. 2019, 151, 244303. [Google Scholar] [CrossRef]
- Domcke, W.; Cederbaum, L.S. A Many-Body Approach to the Vibrational Structure in Molecular Electronic Spectra. II. Application to Nitrogen, Carbon Monoxide, and Formaldehyde. J. Chem. Phys. 1976, 64, 612–625. [Google Scholar] [CrossRef]
| Mode | () | () | B | B | B | D | H | r | |
|---|---|---|---|---|---|---|---|---|---|
| Units | cm | cm | MHz | MHz | MHz | kHz | mHz | Å | |
| Exp. | 2183.9 | 59,270.5 | |||||||
| F12-TZ | 2213.3 | 2183.0 | 59,058 | 58,776 | 58,212 | 187.191 | 126.305 | 1.121 | |
| CcCR | 2224.6 | 2194.0 | 59,434 | 59,148 | 58,577 | 188.852 | 122.642 | 1.118 | |
| EOM-IP-CC3 | TQcCT | 2228.8 | 2198.3 | 59,405 | 59,122 | 58,557 | 187.860 | 130.221 | 1.118 |
| CcCT | 2226.3 | 2196.2 | 59,355 | 59,073 | 58,509 | 187.810 | 131.241 | 1.119 | |
| EOM-IP-CCSDT-3 | QZ | 2182.6 | 2151.0 | 58,776 | 58,482 | 57,896 | 189.746 | 92.347 | 1.124 |
| 5Z | 2187.2 | 2155.6 | 58,883 | 58,590 | 58,004 | 189.990 | 94.210 | 1.123 | |
| TQ5 | 2192.1 | 2160.3 | 58,995 | 58,702 | 58,115 | 190.213 | 94.982 | 1.122 | |
| TQ5+cC | 2201.0 | 2168.9 | 59,188 | 58,894 | 58,307 | 190.537 | 96.961 | 1.120 | |
| TQcCT | 2229.7 | 2199.2 | 59,412 | 59,130 | 58,565 | 187.787 | 130.683 | 1.118 | |
| CcCT | 2227.2 | 2197.1 | 59,363 | 59,081 | 58,518 | 187.731 | 131.768 | 1.118 | |
| EOM-IP-CCSDT | TQcC | 2232.9 | 2202.5 | 59,447 | 59,166 | 58,602 | 187.578 | 132.036 | 1.118 |
| CcC | 2230.8 | 2200.7 | 59,402 | 59,121 | 58,558 | 187.497 | 133.314 | 1.118 |
| Parameter | () | () | B | B | B | D | H | r | AEE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Units | cm | cm | MHz | MHz | MHz | kHz | mHz | Å | cm | |
| Exp. | 1534.9 | 47,649 | 20,733.3 | |||||||
| F12-TZ | 1564.8 | 1538.7 | 47,469 | 47,186 | 46,618 | 194.456 | 25.181 | 1.252 | 20,147.8 | |
| CcCR | 1569.5 | 1543.7 | 47,745 | 47,461 | 46,893 | 196.682 | 37.944 | 1.248 | 20,420.7 | |
| (T)+EOM/CcCR | 1467.6 | 1425.2 | 47,042 | 46,676 | 45,944 | 215.167 | −415.627 | 1.259 | 20,091.1 | |
| EOM-EE-CC3 | TQcC | 1496.2 | 1456.8 | 47,271 | 46,923 | 46,227 | 210.052 | −305.464 | 1.255 | 18,394.2 |
| CcC | 1495.2 | 1456.3 | 47,218 | 46,871 | 46,177 | 209.623 | −299.783 | 1.256 | 18,344.4 | |
| EOM-IP-CC3 | TQcCT | 1582.3 | 1556.1 | 47,864 | 47,581 | 47,013 | 194.969 | 34.177 | 1.247 | 20,446.7 |
| CcCT | 1581.3 | 1555.3 | 47,813 | 47,530 | 46,965 | 194.601 | 37.994 | 1.247 | 20,405.4 | |
| EOM-IP-CCSDT-3 | QZ | 1573.1 | 1546.6 | 47,541 | 47,258 | 46,692 | 193.297 | 26.122 | 1.251 | 20,568.8 |
| 5Z | 1576.1 | 1550.1 | 47,618 | 47,335 | 46,770 | 193.497 | 29.761 | 1.250 | 20,677.3 | |
| TQ5 | 1579.0 | 1552.9 | 47,706 | 47,423 | 46,857 | 193.845 | 30.579 | 1.249 | 20,786.3 | |
| TQ5+cC | 1585.8 | 1559.2 | 47,881 | 47,596 | 47,028 | 194.301 | 29.645 | 1.246 | 21,008.8 | |
| TQcCT | 1581.9 | 1555.2 | 47,860 | 47,576 | 47,008 | 195.026 | 33.348 | 1.247 | 20,410.8 | |
| CcCT | 1580.9 | 1554.9 | 47,809 | 47,526 | 46,960 | 194.653 | 36.526 | 1.247 | 20,365.3 | |
| EOM-IP-CCSDT | TQcC | 1583.4 | 1557.1 | 47,878 | 47,595 | 47,028 | 194.873 | 35.115 | 1.246 | 20,387.3 |
| CcC | 1582.6 | 1556.7 | 47,831 | 47,548 | 46,984 | 194.481 | 38.936 | 1.247 | 20,356.7 |
| Mode | () | () | B | B | B | D | H | r | AEE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Units | cm | cm | MHz | MHz | MHz | kHz | mHz | Å | cm | |
| Exp. | 1678.3 | 53,930 | 45,876.7 | |||||||
| (T)+EOM/CcCR | 1212.1 | 1195.8 | 43,868 | 44,111 | 44,597 | 255.81 | 5.262 | 1.295 | 45,293.3 | |
| EOM-EE-CC3 | TQcC | 1808.2 | 1710.2 | 54,283 | 53,832 | 52,929 | 217.777 | −0.670 | 1.172 | 42,534.7 |
| CcC | 1806.0 | 1711.7 | 54,184 | 53,733 | 52,830 | 217.129 | −0.673 | 1.173 | 44,460.8 | |
| EOM-IP-CC3 | TQcCT | 1843.7 | 1803.9 | 54,549 | 54,195 | 53,489 | 212.563 | −0.154 | 1.168 | 45,229.5 |
| CcCT | 1841.8 | 1802.3 | 54,489 | 54,136 | 53,430 | 212.305 | −0.154 | 1.169 | 45,220.3 | |
| EOM-IP-CCSDT-3 | QZ | 1831.7 | 1787.8 | 54,343 | 53,974 | 53,237 | 212.921 | −236.013 | 1.170 | 46,555.9 |
| 5Z | 1837.5 | 1793.3 | 54,444 | 54,077 | 53,343 | 212.789 | −226.247 | 1.170 | 46,636.7 | |
| TQ5 | 1843.0 | 1798.9 | 54,556 | 54,190 | 53,459 | 212.813 | −218.012 | 1.168 | 46,707.4 | |
| TQ5+cC | 1856.1 | 1812.0 | 54,774 | 54,411 | 53,684 | 212.339 | −201.694 | 1.166 | 46,725.4 | |
| TQcCT | 1844.7 | 1805.5 | 54,552 | 54,200 | 53,495 | 212.364 | −0.149 | 1.168 | 45,199.8 | |
| CcCT | 1843.0 | 1803.6 | 54,493 | 54,141 | 53,437 | 212.084 | −0.149 | 1.169 | 45,191.2 | |
| EOM-IP-CCSDT | TQcC | 1852.4 | 1813.3 | 54,610 | 54,262 | 53,565 | 211.286 | −0.131 | 1.167 | 45,187.6 |
| CcC | 1841.3 | 1801.1 | 54,577 | 54,253 | 53,604 | 213.45 | −0.002 | 1.167 | 45,180.8 |
| Mode | (a) | (a) | (b) | (a) | (a) | (b) | |
|---|---|---|---|---|---|---|---|
| Description | Sym. Str. | Bend | Anti Sym. Str. | Sym. Str. | Bend | Anti Sym. Str. | |
| Exp. | 3212.86 | ||||||
| Exp. | 1408.42 | ||||||
| Exp. | 3259.04 | ||||||
| Exp. | 3267 | 1435 | 3299 | ||||
| Prev theory e | 3389.7 | 1478.4 | 3440.9 | ||||
| F12-TZ | 3383.8 | 1473.1 | 3441.1 | 3211.6 | 1408.4 | 3256.2 | |
| CcCR | 3389.4 | 1473.5 | 3446.9 | 3215.4 | 1408.3 | 3260.2 | |
| EOM-IP-CC3 | TQcCT | 3387.8 | 1469.0 | 3445.7 | 3209.2 | 1403.4 | 3255.5 |
| CcCT | 3385.6 | 1468.3 | 3444.3 | 3209.9 | 1401.1 | 3256.8 | |
| EOM-IP-CCSDT-3 | QZ | 3429.1 | 1472.1 | 3373.2 | 3193.7 | 1407.2 | 3236.9 |
| 5Z | 3432.1 | 1471.1 | 3375.4 | 3200.9 | 1405.9 | 3245.0 | |
| TQ5 | 3435.7 | 1470.8 | 3378.7 | 3202.3 | 1405.5 | 3246.7 | |
| TQ5+cC | 3440.3 | 1469.7 | 3383.2 | 3206.0 | 1404.3 | 3250.5 | |
| TQcCT | 3381.5 | 1468.2 | 3438.4 | 3197.7 | 1403.1 | 3242.7 | |
| CcCT | 3378.3 | 1467.3 | 3436.0 | 3202.6 | 1401.8 | 3248.2 | |
| EOM-IP-CCSDT | TQcC | 3381.3 | 1468.2 | 3438.4 | 3202.8 | 1403.0 | 3248.0 |
| VPT2 CcC | 3378.6 | 1467.4 | 3436.6 | 3203.0 | 1402.3 | 3248.7 | |
| VCI CcC | 3378.6 | 1467.4 | 3436.6 | 3202.1 | 1399.1 | 3248.8 |
| EOM-IP-CC3 | EOM-IP-CCSDT-3 | EOM-IP-CCSDT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Const. | Units | Exp. [83] | F12-TZ | CcCR | TQcCT | CcCT | TQcCT | CcCT | TQcC | CcC |
| A | MHz | 845,854 | 850,282 | 852,770 | 852,091 | 851,738 | 850,955 | 851,909 | 851,237 | |
| B | MHz | 376,983 | 377,740 | 377,246 | 376,888 | 376,938 | 376,518 | 376,901 | 376,508 | |
| C | MHz | 260,765 | 261,547 | 261,544 | 261,308 | 261,300 | 261,024 | 261,298 | 261,046 | |
| A | MHz | 870,580.8 | 870,304 | 875,321 | 878,528 | 877,794 | 877,314 | 876,429 | 877,501 | 876,766 |
| B | MHz | 372,365.4 | 372,128 | 372,776 | 372,128 | 371,833 | 371,813 | 371,453 | 371,760 | 371,438 |
| C | MHz | 253,880.4 | 253,591 | 254,321 | 254,272 | 254,071 | 254,017 | 253,770 | 254,007 | 253,792 |
| A | MHz | 835,041.1 | 836,541 | 841,221 | 844,428 | 843,801 | 843,183 | 842,374 | 843,353 | 842,725 |
| B | MHz | 367,803.7 | 367,340 | 367,948 | 367,192 | 366,947 | 366,858 | 366,543 | 366,793 | 366,525 |
| C | MHz | 249,733.7 | 249,293 | 249,983 | 249,893 | 249,721 | 249,629 | 249,408 | 249,613 | 249,431 |
| A | MHz | 1,001,285.4 | 971,991 | 978,731 | 983,370 | 982,335 | 981,829 | 980,613 | 982,069 | 981,019 |
| B | MHz | 374,077.5 | 374,025 | 374,542 | 373,793 | 373,525 | 373,498 | 373,167 | 373,439 | 373,148 |
| C | MHz | 249,275.7 | 249,083 | 249,795 | 249,751 | 249,553 | 249,488 | 249,244 | 249,478 | 249,266 |
| A | MHz | 851,254.6 | 851,352 | 856,162 | 859,372 | 858,726 | 858,151 | 857,320 | 858,334 | 857,682 |
| B | MHz | 365,511.7 | 364,952 | 365,549 | 364,797 | 364,554 | 364,470 | 364,156 | 364,404 | 364,138 |
| C | MHz | 248,680.5 | 248,337 | 249,023 | 248,922 | 248,756 | 248,659 | 248,442 | 248,643 | 248,465 |
| Mode | (a) | (b) | (b) | (a) | (b) | (b) | AEE | |
|---|---|---|---|---|---|---|---|---|
| Description | Sym. Str. | Bend | Anti Sym. Str. | Sym. Str. | Bend | Anti Sym. Str. | ||
| Exp. | 3547 ± 16 | 876.8 | ||||||
| Exp. | 3153 ± 169 | 903 ± 80 | ||||||
| Previous theory | 3388 | 403 | 3624.3 | |||||
| Previous theory | 7886 | |||||||
| F12-TZ | 3387.1 | 392.5 | 3623.6 | 3228.9 | 1036.4 | 3416.9 | 8026.0 | |
| CcCR | 3393.3 | 405.0 | 3628.8 | 3232.1 | 995.0 | 3420.7 | 7951.0 | |
| (T)+EOM/CcCR | 3394.3 | 373.2 | 3629.8 | 3231.7 | 1056.6 | 3425.2 | 8051.4 | |
| EOM-IP-CC3 | TQcCT | 3385.6 | 430.0 | 3612.7 | 3224.5 | 689.4 | 3407.6 | 7057.6 |
| CcCT | 3377.4 | 424.5 | 3606.1 | 3217.4 | 690.4 | 3401.9 | 7060.5 | |
| EOM-IP-CCSDT-3 | QZ | 3609.8 | 419.7 | 3380.4 | 3223.9 | 906.9 | 3404.1 | 7958.9 |
| 5Z | 3607.6 | 420.9 | 3377.8 | 3222.6 | 900.5 | 3404.8 | 7918.4 | |
| TQ5 | 3608.9 | 424.4 | 3379.6 | 3224.3 | 912.9 | 3406.0 | 7892.9 | |
| TQ5+cC | 3613.4 | 427.1 | 3384.7 | 3227.2 | 888.9 | 3408.8 | 7817.2 | |
| TQcCT | 3385.5 | 428.7 | 3612.8 | 3228.0 | 1098.4 | 3406.7 | 7899.8 | |
| CcCT | 3377.5 | 423.5 | 3606.4 | 3222.2 | 1067.1 | 3405.4 | 7903.1 | |
| EOM-IP-CCSDT | TQcCT | 3384.4 | 429.2 | 3612.0 | 3223.7 | 957.5 | 3407.0 | 7865.2 |
| VPT2 CcCT | 3377.0 | 424.1 | 3606.4 | 3222.0 | 970.2 | 3405.3 | 7851.0 | |
| VCI CcCT | 3377.0 | 424.1 | 3606.4 | 3212.8 | 557.5 | 3404.8 | 7710.7 |
| Mode | () | () | () | () | () | () | AEE | |
|---|---|---|---|---|---|---|---|---|
| Description | Sym. Str. | Bend | Anti Sym. Str. | Sym. Str. | Bend | Anti Sym. Str. | ||
| Exp. | 2968 | 1596 | 36,757 ± 12 | |||||
| Exp. | 2903 ± 80 | 1532 ± 80 | 2839 ± 56 | |||||
| Previous Theory | 2613.9 | 1597.4 | 1945.1 | |||||
| (T)+EOM/CcCR | 2617.8 | 1589.0 | 1958.3 | 2383.8 | 1467.0 | 1746.1 | 33,880.0 | |
| EOM-EE-CC3 | TQcC | 2618.7 | 1602.4 | 1964.8 | 2394.9 | 1480.4 | 1760.3 | 33,919.9 |
| CcC | 2622.6 | 1603.6 | 1969.8 | 2398.1 | 1479.5 | 1764.1 | 33,881.7 | |
| EOM-IP-CC3 | TQcCT | 2627.6 | 1594.9 | 1978.8 | 2404.5 | 1479.7 | 1781.1 | 33,353.6 |
| CcCT | 2631.5 | 1596.2 | 1984.0 | 2407.8 | 1477.4 | 1783.5 | 33,313.5 | |
| EOM-IP-CCSDT-3 | QZ | 2628.4 | 1608.1 | 1969.1 | 2406.1 | 1486.2 | 1766.5 | 33,917.5 |
| 5Z | 2627.0 | 1607.2 | 1967.3 | 2404.8 | 1486.5 | 1765.1 | 33,901.7 | |
| TQ5 | 2630.7 | 1609.3 | 1970.4 | 2406.8 | 1488.7 | 1763.0 | 33,914.7 | |
| TQ5+cC | 2627.6 | 1608.2 | 1968.0 | 2405.2 | 1486.9 | 1764.4 | 33,967.5 | |
| TQcCT | 2626.9 | 1595.2 | 1978.1 | 2403.8 | 1478.8 | 1777.1 | 33,980.4 | |
| CcCT | 2630.8 | 1596.6 | 1983.2 | 2407.3 | 1477.1 | 1782.1 | 33,956.6 | |
| EOM-IP-CCSDT | TQcC | 2628.3 | 1595.7 | 1980.2 | 2406.2 | 1480.2 | 1782.2 | 34,005.7 |
| VPT2 CcC | 2632.3 | 1597.1 | 1985.1 | 2409.3 | 1478.9 | 1785.7 | 33,970.4 | |
| VCI CcC | 2632.3 | 1597.1 | 1985.1 | 2433.1 | 1561.7 | 1783.3 | 33,987.2 |
| EOM-IP-CCSDT-3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode | Description | Exp | F12-TZ | CcCR | QZ | TQ | TQ+cC | VPT2 TQcCT | VCI TQcCT |
| (a) | Sym. C–H str. | 2796.4 | 2797.7 | 2790.5 | 2785.9 | 2788.4 | 2805.6 | 2805.6 | |
| (a) | O–C str. | 1676.8 | 1683.2 | 1688.9 | 1697.1 | 1704.7 | 1677.2 | 1677.2 | |
| (a) | H–C–O sym. bend | 1257.9 | 1258.6 | 1253.7 | 1253.2 | 1253.6 | 1262.0 | 1262.0 | |
| (b) | Out-of-plane bend | 1062.1 | 1064.3 | 1063.6 | 1064.0 | 1064.9 | 1065.7 | 1065.7 | |
| (b) | Anti sym. C–H str. | 2904.3 | 2906.4 | 2892.7 | 2889.2 | 2890.7 | 2915.5 | 2915.5 | |
| (b) | H–C–O anti sym. bend | 842.8 | 844.2 | 841.0 | 841.1 | 842.1 | 847.9 | 847.9 | |
| (a) | Sym. C–H str. | 2580 ± 4 | 2616.6 | 2612.1 | 2608.9 | 2603.0 | 2604.2 | 2624.2 | 2624.6 |
| (a) | O–C str. | 1675 ± 4 | 1681.9 | 1669.9 | 1684.0 | 1690.1 | 1696.7 | 1677.3 | 1675.9 |
| (a) | H–C–O sym. bend | 1210 ± 4 | 1209.9 | 1196.3 | 1202.3 | 1200.4 | 1200.9 | 1210.8 | 1217.4 |
| (b) | Out-of-plane bend | 1036 ± 4 e | 1013.6 | 1007.9 | 1038.9 | 1036.0 | 1036.7 | 1039.2 | 1035.1 |
| (b) | Anti sym. C–H str. | 2718.24 ± | 2700.4 | 2695.1 | 2688.3 | 2682.0 | 2682.4 | 2710.4 | 2715.5 |
| (b) | H–C–O anti sym. bend | 823.7 ± 0.3 e | 825.0 | 762.9 | 819.3 | 817.7 | 819.0 | 824.4 | 820.0 |
| EOM-IP-CCSDT-3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Description | Exp | (T)+EOM/CcCR | QZ | TQ | TQ+cC | VPT2 TQcCT | VCI TQcCT | |
| (a) | Sym. C–H str. | 3041.9 | 3041.5 | 3086.0 | 3041.9 | 3041.5 | 3041.5 | |
| (a) | O–C str. | 1510.3 | 1530.3 | 1556.5 | 1510.3 | 1530.3 | 1530.3 | |
| (a) | H–C–O sym. bend | 1257.2 | 1290.9 | 1291.6 | 1257.2 | 1290.9 | 1290.9 | |
| (b) | Out-of-plane bend | 1196.0 | 1210.5 | 1235.5 | 1196.0 | 1210.5 | 1210.5 | |
| (b) | Anti sym. C–H str. | 3196.7 | 3199.0 | 3238.6 | 3196.7 | 3199.0 | 3199.0 | |
| (b) | H–C–O anti sym. bend | 1162.4 | 1177.7 | 1195.7 | 1162.4 | 1177.7 | 1177.7 | |
| (a) | Sym. C–H str. | 2870.9 | 2876.8 | 2928.1 | 2870.9 | 2876.8 | 2889.1 | |
| (a) | O–C str. | 1488 ± 4 | 1470.1 | 1489.3 | 1516.0 | 1470.1 | 1489.3 | 1505.7 |
| (a) | H–C–O sym. bend | 1250 ± 4 | 1226.4 | 1262.0 | 1260.7 | 1226.4 | 1262.0 | 1263.2 |
| (b) | Out-of-plane bend | 1179.0 | 1191.9 | 1218.3 | 1179.0 | 1191.9 | 1192.2 | |
| (b) | Anti sym. C–H str. | 3052.7 | 3050.2 | 3096.2 | 3052.7 | 3050.2 | 3050.9 | |
| (b) | H–C–O anti sym. bend | 1137.2 | 1154.9 | 1168.7 | 1137.2 | 1154.9 | 1152.7 | |
| AEE | 25,929 ± 5 | 25,248.0 | 25,748.5 | 25,935.6 | 25,975.0 | 26,469.6 | 26,471.7 | |
| EOM-IP-CCSDT-3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Description | Exp. | (T)+EOM/CcCR | QZ | TQ | TQcC | VPT2 TQcCT | VCI TQcCT | |
| (a) | Sym. C–H str. | 2897.7 | 2865.1 | 2865.6 | 2871.1 | 2877.0 | 2877.0 | |
| (a) | O–C str. | 1462.0 | 1387.8 | 1390.3 | 1394.0 | 1397.4 | 1397.4 | |
| (a) | H–C–O sym. bend | 1390.8 | 1350.2 | 1355.6 | 1361.2 | 1371.9 | 1371.9 | |
| (b) | Out-of-plane bend | 1248.2 | 1236.0 | 1238.9 | 1242.0 | 1245.6 | 1245.6 | |
| (b) | Anti sym. C–H str. | 3061.8 | 3012.8 | 3014.0 | 3019.2 | 3031.4 | 3031.4 | |
| (b) | H–C–O anti sym. bend | 1234.4 | 1213.1 | 1218.0 | 1221.8 | 1225.4 | 1225.4 | |
| (a) | Sym. C–H str. | 2764.6 | 2706.3 | 2710.8 | 2718.6 | 2733.8 | 2653.8 | |
| (a) | O–C str. | 1304 ± 4 | 1430.5 | 1348.5 | 1350.9 | 1354.6 | 1360.0 | 1374.8 |
| (a) | H–C–O sym. bend | 1352.8 | 1304.6 | 1309.6 | 1314.4 | 1326.4 | 1346.3 | |
| (b) | Out-of-plane bend | 1242.1 | 1213.5 | 1217.7 | 1218.9 | 1221.5 | 1213.4 | |
| (b) | Anti sym. C–H str. | 2858.8 | 2781.1 | 2780.3 | 2784.7 | 2803.1 | 2809.9 | |
| (b) | H–C–O anti sym. bend | 1209.4 | 1178.2 | 1182.1 | 1185.6 | 1192.6 | 1189.7 | |
| AEE | 39,928 ± 6 | 40,883.0 | 39,758.9 | 39,864.8 | 39,960.3 | 39,881.8 | 39,887.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, M.C.; Huang, X.; Fortenberry, R.C. Complete, Theoretical Rovibronic Spectral Characterization of the Carbon Monoxide, Water, and Formaldehyde Cations. Molecules 2023, 28, 1782. https://doi.org/10.3390/molecules28041782
Davis MC, Huang X, Fortenberry RC. Complete, Theoretical Rovibronic Spectral Characterization of the Carbon Monoxide, Water, and Formaldehyde Cations. Molecules. 2023; 28(4):1782. https://doi.org/10.3390/molecules28041782
Chicago/Turabian StyleDavis, Megan C., Xinchuan Huang, and Ryan C. Fortenberry. 2023. "Complete, Theoretical Rovibronic Spectral Characterization of the Carbon Monoxide, Water, and Formaldehyde Cations" Molecules 28, no. 4: 1782. https://doi.org/10.3390/molecules28041782
APA StyleDavis, M. C., Huang, X., & Fortenberry, R. C. (2023). Complete, Theoretical Rovibronic Spectral Characterization of the Carbon Monoxide, Water, and Formaldehyde Cations. Molecules, 28(4), 1782. https://doi.org/10.3390/molecules28041782






